Abstract
Loss of tissue polarity and increased proliferation are the characteristic alterations of the breast tumor phenotype. To investigate these processes, we used a three-dimensional (3D) culture system in which malignant human breast cells can be reverted to a normal phenotype by exposure to inhibitors of phosphatidylinositol 3-kinase (PI3K). Using this assay, we find that Akt and Rac1 act as downstream effectors of PI3K and function as control points of cellular proliferation and tissue polarity, respectively. Our results also demonstrate that the PI3K signaling pathway is an integral component of the overall signaling network induced by growth in 3D, as reversion affected by inhibition of PI3K signaling also down-modulates the endogenous levels of β1 integrin and epidermal growth factor receptor, the upstream modulators of PI3K, and up-regulates PTEN, the antagonist of PI3K. These findings reveal key events of the PI3K pathway that play distinct roles to maintain tissue polarity and that when disrupted are instrumental in the malignant phenotype.
Keywords: three-dimensional cultures; Akt; Rac1; tumor reversion; tissue polarity
Introduction
Class I phosphatidylinositol 3-kinase (PI3K) is activated by growth factor–responsive tyrosine kinases such as epidermal growth factor receptor (EGFR; Grant et al., 2002) and integrin-responsive kinases such as focal adhesion kinase (FAK; Chen and Guan, 1994). Activated PI3K leads to the production of membrane-associated phosphatidylinositol 3,4,5-trisphosphate (PIP3), which in turn causes the recruitment to the cell membrane and subsequent activation of a number of signaling molecules (Vivanco and Sawyers, 2002). PI3K is a key mediator in processes that regulate cell orientation; for both Dictyostelium and cultured human leukocytes, directionality of chemotaxis is controlled by polarization of PIP3 to the leading edge of the cell (Servant et al., 2000; Funamoto et al., 2002; Wang et al., 2002b). PI3K has been found to be constitutively up-regulated in a substantial fraction of human breast cancers (Vivanco and Sawyers, 2002), and overexpression of PI3K in cultured nonmalignant human mammary epithelial cells is sufficient to confer a malignant phenotype (Zhao et al., 2003).
During tumor progression, tissue polarity is lost and control of proliferation is compromised (Fish and Molitoris, 1994; Reichmann, 1994; Bissell and Radisky, 2001), and although these two phenomena have been suggested to be linked, previous investigations have not revealed the extent to which the increased cellular proliferation in tumors can directly produce tissue disorganization, and to what extent loss of polarity is an independent function of deregulated signaling pathways downstream of the oncogenic signal(s). To dissect the molecular mediators of these processes we have used an assay (Petersen et al., 1992) in which human mammary epithelial cells from the HMT-3522 tumor progression series are cultured in a physiologically relevant, three-dimensional (3D) laminin-rich basement membrane (lrBM). When cultured in 3D lrBM, the phenotypically normal, nonmalignant HMT-3522 S-1 (S-1) cells undergo growth arrest, produce an endogenous basement membrane, and form polarized acinus-like structures, very similar to primary cells from reduction mammoplasty. In contrast, the malignant HMT-3522 T4-2 (T4-2) cells continue to proliferate into apolar, amorphous structures, similar to structures formed by primary tumor cells in this assay (Petersen et al., 1992). In comparison to S-1 cells, expression levels of EGFR and β1 integrin in T4-2 cells are greatly increased, and down-regulation of these signaling pathways in T4-2 cells grown in 3D lrBM can restore the formation of polarized acinus-like structures, resulting in a reversion similar to the normal phenotype of the S-1 cells (Weaver et al., 1997; Wang et al., 1998).
As PI3K is activated downstream of both EGFR and β1 integrin (Chen and Guan, 1994; Lee and Juliano, 2000; Grant et al., 2002), we hypothesized that the phenotypic reversion affected by down-modulation of EGFR/β1 integrin signaling in T4-2 cells was due to attenuation of PI3K activity. We showed previously that even highly malignant metastatic cancer cells, cultured in 3D lrBM, could be reverted to a normal phenotype by inhibition of PI3K, if treatment with PI3K inhibitors was performed in combination with appropriate manipulation of other signaling pathways (Wang et al., 2002a). Here we use inhibition of PI3K alone to dissect the signaling pathways that control proliferation and polarity in breast tumor cells. Our results reveal a new functional link between extracellular signaling mediators and tissue function that provides insight into processes that control the malignant phenotype if imbalanced. We also show that the PI3K and its lipid product, PIP3, are relocalized to the basal surface of the acini when the malignant cells are reverted in lrBM, a process that may play a role in integration of signaling pathways in reformation of polarity.
Results
Down-modulation of PI3K activity results in phenotypic reversion of human mammary tumor cells
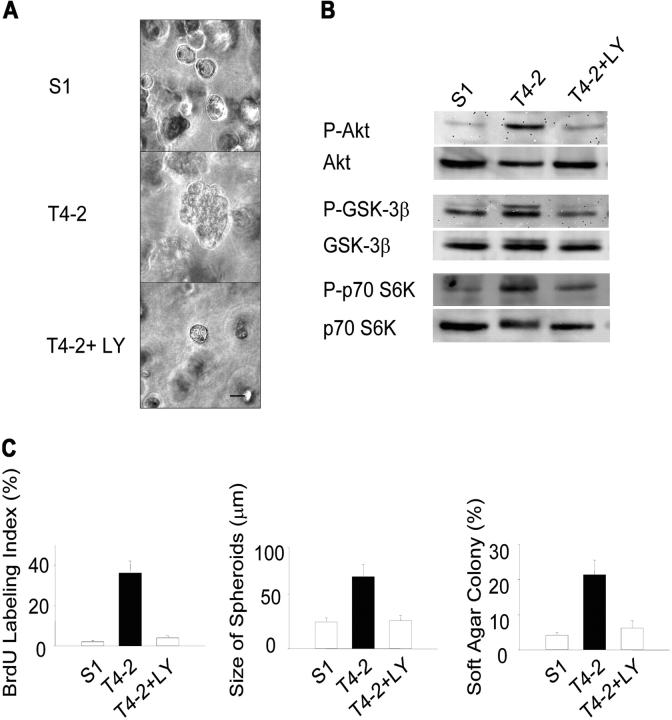
We found previously that malignant T4-2 cells showed increased signaling from EGFR and β1 integrin, relative to their nonmalignant predecessors, and that down-modulation of either EGFR or β1 integrin activity in cells grown in 3D lrBM caused the cells to form growth-arrested, polarized acinus-like structures (Weaver et al., 1997; Wang et al., 1998). As PI3K is an effector of both of these signaling pathways (Chen and Guan, 1994; Lee and Juliano, 2000; Grant et al., 2002), we hypothesized that inhibition of PI3K signaling would also revert these cells. We found that treatment of T4-2 cells with 8 μM of the PI3K inhibitor LY294002 (which prevented phosphorylation of downstream Akt at serine 473, as well as other downstream signaling mediators glycogen synthase kinase-3β [GSK-3β] and p70S6K; Fig. 1 B) did cause phenotypic reversion, as characterized by inhibition of proliferation, decreased colony size, and reduced growth in soft agar cultures (Fig. 1, A and C). Using indirect immunofluorescence, we found that the LY294002-reverted T4-2 cells regained the polarization of the apicolateral tight junction marker ZO-1, the basal marker α6 integrin, and the reorganization of the actin cytoskeleton (Fig. 2 A). Similar data were obtained in cells treated with the alternative PI3K inhibitor, wortmannin (unpublished data). These results demonstrate that down-regulation of the PI3K pathway in T4-2 mammary tumor cells restores an intrinsic property of forming polarized, growth-arrested structures in response to a physiologically relevant microenvironment.
Figure 1.
Attenuation of PI3K activity results in phenotypic reversion of HMT-3522 T4-2 human mammary tumor cells cultured in 3D BM. (A) Phase contrast micrographs of 10-d 3D lrBM cultures of phenotypically normal (S-1), malignant (T4-2), and T4-2 cells treated with 8 μM PI3K inhibitor, LY294002 (T4-2+LY). Bar, 20 μm. (B) Cell lysates from 10-d 3D lrBM cultures were analyzed for phosphorylated Akt (serine 473)/total, phosphorylated GSK-3β (serine 9)/total, and phosphorylated p70 S6 kinase (threonine 389)/total by Western blot. (C) Inhibition of PI3K causes a reduction in cellular proliferation (left, BrdU labeling assay, n = 3), colony size (center, 50 colonies assessed for each experiment, n = 3), and anchorage-independent growth (right, soft agar assay, colonies scored positive when >50 μm, n = 3).
Figure 2.
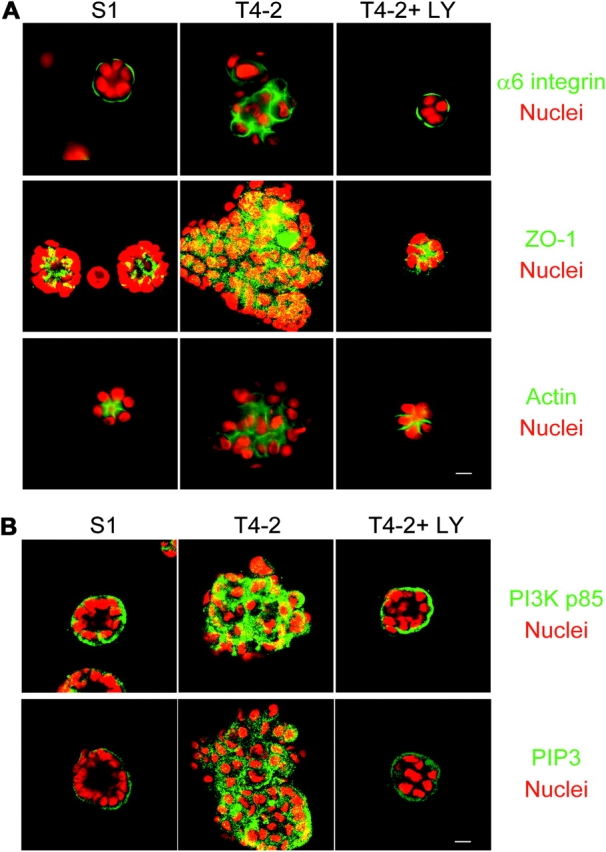
Inhibition of PI3K results in reestablishment of tissue polarity. (A) Down-modulation of PI3K activity of T4-2 cells was sufficient to repolarize the apicolateral tight junction protein ZO-1 and the basal ECM receptor, α6 integrin, and to result in the reorganization of the actin cytoskeleton. (B) PI3K and its phospholipid product, PIP3, are basolaterally localized in S-1 acini, apolarly distributed in the T4-2 colonies, and normalized in the reverted T4-2 structures. For both A and B, S-1, T4-2, and T4-2+ LY (revertants) were cultured for 10 d in 3D lrBM. α6 integrin, ZO-1, actin, PI3K p85 subunit, and PIP3 were stained by specific antibodies and phalloidin-FITC, and imaged by confocal fluorescence microscopy. Bars, 10 μm.
Phenotypic reversion is accompanied by repolarization of PI3K and its phospholipid product
Proper interpretation of extracellular signaling cues requires asymmetric distribution of intracellular signaling molecules (Comer and Parent, 2002; Wedlich-Soldner and Li, 2003). Recently, signaling asymmetry of PI3K and its lipid product, PIP3 has been shown to control the directionality of chemotactic migration in human neutrophils and in single Dictyostelium cells (Servant et al., 2000; Funamoto et al., 2002; Wang et al., 2002b), and PIP3 has been found to become polarized to the basal surface of MDCK cells grown as monolayers on filters or in 3D collagen gels (Watton and Downward, 1999; Yu et al., 2003). We found that both PI3K (p85 subunit) and PIP3 are polarized to the basal surface of phenotypically normal S-1 cells grown in 3D lrBM, and that this asymmetric distribution is lost in T4-2 cells (Fig. 2 B). However, phenotypic reversion of the T4-2 cells through attenuation of PI3K signaling led to repolarization of these signaling components (Fig. 2 B). Given that the S-1 and reverted T4-2 cells show correct tissue polarity, whereas the untreated T4-2 cells are apolar, these results provide the first evidence that polarized distribution of PI3K and PIP3 is an intrinsic property of phenotypically normal acini that is lost during tumor progression.
Phenotypic reversion of T4-2 cells by treatment with LY294002 results in cross-modulation of multiple signaling pathways
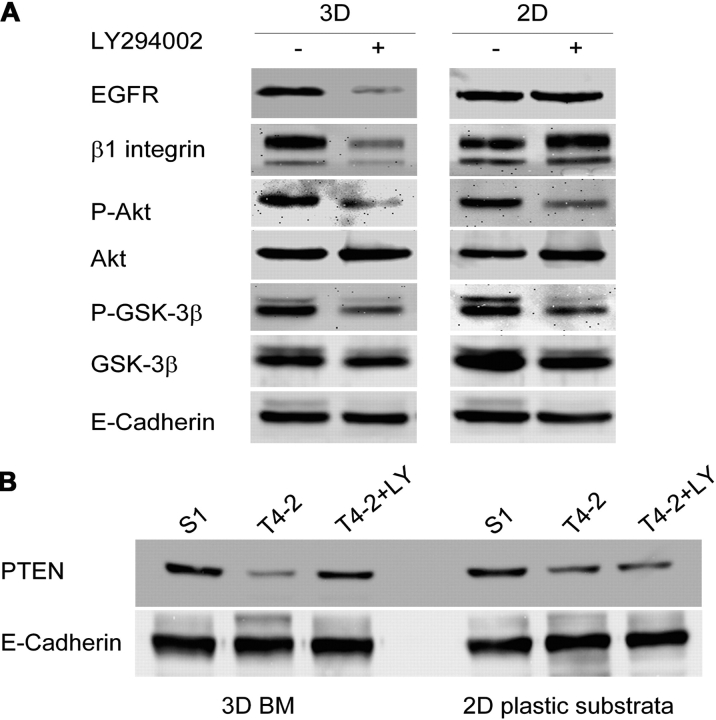
We had shown previously that inhibition of either EGFR or β1-integrin results in phenotypic reversion of T4-2 cells associated with down-modulation of the total levels of both signaling molecules, and that this activity influences and is influenced by the MAPK signaling pathway (Wang et al., 1998). We now show that PI3K signaling is also an integral component of this cross-modulated signaling network. T4-2 cells treated with LY294002 show reduced levels of EGFR and β1 integrin (Fig. 3 A). This effect depended upon 3D lrBM as it is not observed in cells cultured on two-dimensional (2D) plastic substrata (it should be noted that inhibition of PI3K activity, as measured by activation of downstream mediators Akt and GSK-3β, was equally effective in cells on 2D or in 3D; Fig. 3 A). In addition, our results revealed that PTEN, the antagonist of PI3K that acts to dephosphorylate PIP3 and which becomes down-regulated in many carcinomas (Simpson and Parsons, 2001; Yamada and Araki, 2001), is also a component of the cross-modulated signaling network, as treatment of T4-2 cells with LY294002 resulted in an increase of PTEN to the level of the nonmalignant cells; this modulation, too, was seen only in cells cultured on 3D lrBM (Fig. 3 B). Taken together, these results demonstrate the existence of a retrodirectional control network that exists only when cells are cultured in a proper tissue context.
Figure 3.
Attenuation of PI3K activity results in cross-modulation of other signaling pathways and intermediates. Cell lysates from S-1, T4-2, and T4-2+LY grown in 3D lrBM or on 2D plastic substrata for 10 d were analyzed for expression of (A) EGFR, β1 integrin, phosphorylated Akt (serine 473)/total, phosphorylated GSK-3β (serine 9)/total, and (B) PTEN (n = 3); E-cadherin was used as the loading control. It was shown previously that the total level of E-cadherin does not change under these conditions (Weaver et al., 1997).
Increased proliferation and loss of tissue polarity are functionally separable consequences of increased PI3K signaling
PI3K has been found to control a wide variety of downstream signal transduction pathways, the number and composition of which vary according to cell and tissue type (Chan et al., 1999; Vanhaesebroeck et al., 2001). The best-studied effector of PI3K is Akt, a regulator of cellular proliferation and apoptosis (Scheid and Woodgett, 2001). We examined the possibility that the PI3K inhibitor-mediated reversion was due to reduction of Akt activity by expressing a dominant active Akt construct (Myr-Akt) in the T4-2 cells (Fig. 4 A). Expression of this construct blocked the effect of PI3K inhibitor on phosphorylation of Akt and downstream mediators of Akt activity (Fig. 4 B), and substantially increased the proliferation of T4-2 cells (Fig. 4, C and D). However, examination of colony polarity revealed that the LY294002-treated T4-Myr-Akt cell spheroids largely retained basal tissue polarity despite their considerably larger size (Fig. 4, E and F). This result revealed that increased proliferation alone was not sufficient to disrupt tissue polarity in the reverted T4-2 cells (which retain all of their phenotype-altering genetic mutations), and suggested that other effectors of PI3K might be responsible for disruption of tissue polarity.
Figure 4.

Expression of Akt increases proliferation but does not affect polarity. (A) Expression of constitutively active Akt in T4-2 cells (Myr-Akt), detected by Western analysis of cells infected with Myr-Akt (+) or vector control (−), and probed with anti-Akt (left) or anti-HA antibodies (right). (B) Activity of Myr-Akt mutant and its downstream target were not affected by PI3K inhibitor LY294002; cell lysates from T4-2 and T4-2+LY expressing Myr-Akt or vector grown in 3D lrBM for 10 d were analyzed for phosphorylated Akt (serine 473)/total and GSK-3β (serine 9)/total. (C) T4+Myr-Akt colonies were larger than control (T4-2+Vector) colonies, both in the presence and absence of LY294002, as assessed by phase contrast microscopy. Bar, 20 μm. (D) T4-2 + Myr-Akt colonies had more nuclei per spheroid cross section. Total nuclear number at spheroid cross section and spheroid numbers were counted and are presented as cell number per spheroid cross section. Statistical analyses revealed significant differences between Myr-Akt and vector control (mean ± SD, P values calculated using Student's t test; more than 500 colonies from 5 independent experiments were analyzed for each condition). (E) Constitutively active Akt signaling did not affect the basal tissue repolarization when T4-2+Myr-Akt cells were reverted by PI3K inhibitor, as assessed by basal localization of α6 integrin relative to DAPI-stained nuclei. Bar, 10 μm. (F) Quantitative analysis of polarity by percentage of spheroids without polarized distribution of basal α6 integrin. No significant difference was found between Myr-Akt and vector control for each condition (mean ± SD, P > 0.05, Student's t test; more than 600 colonies were analyzed for each condition from 3 independent experiments).
Recent investigations of acinus-like cysts of MDCK cells grown in 3D collagen gels have demonstrated a role for Rac1 in control of cellular polarity (O'Brien et al., 2001). When we examined the activity of Rac1 in S1, T4-2, and LY294002-reverted T4-2 cells using pull-down assays, we found a high correlation of active Rac1 levels with loss of tissue polarity (Fig. 5 A). To test the role of Rac1 in the reversion phenotype, we expressed a dominant active Rac1 construct in T4-2 cells (Rac1L61; Fig. 5 B). We found that this construct did not greatly affect the rate of cellular proliferation (Fig. 5, C and D), but did inhibit the restoration of polarity in response to LY294002 (Fig. 5, E and F). Although the inhibition of reversion by the Rac1L61 was incomplete, this was most likely due to the heterogenous expression of this construct in the target cell population (assessed by immunofluorescence; unpublished data). As a complementary method to validate this result, we also found that infection with an adenovirus containing Rac1V12, which was expressed in a much higher proportion of the cells, showed much greater resistance to LY294002-mediated reversion of polarity (Fig. S1 C, available at http://www.jcb.org/cgi/content/full/jcb.200306090/DC1).
Figure 5.
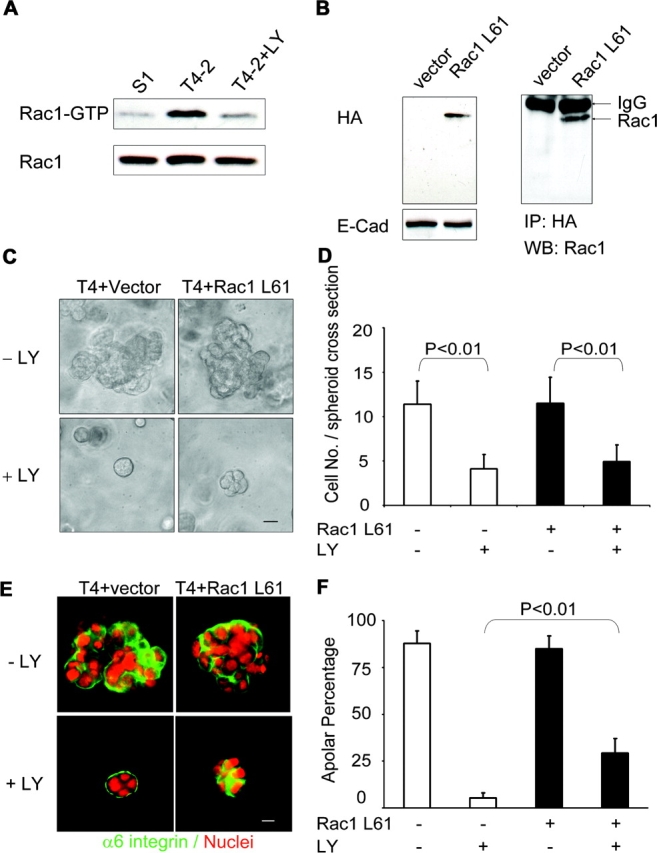
Expression of Rac1 disrupts tissue polarity but does not prevent proliferation arrest. (A) Rac1 activity correlates with PI3K activity, analyzed by using recombinant PAK-GST-CD fusion protein pulldown from lysates of 10-d 3D lrBM cultures of S-1, T4-2, and T4-2+LY cells. (B) Expression of constitutively active Rac1 L61, detected from lysates of cells infected with Rac1L61 or vector control and probed with anti-HA antibody (left) or immunoprecipitated with anti-HA antibody and blotted by anti-Rac1 antibody (right). (C) Inhibition of PI3K attenuates growth of both T4-2+Rac1 L61 colonies and control (T4-2+Vector) colonies, as assessed by phase contrast microscopy. Bar, 20 μm. (D) Analysis of total nuclear number at spheroid cross section reveals that treatment with LY294002 causes statistically signinficant decrease of colony cell number, but expression of Rac1L61 causes no substantial difference (mean ± SD, P values calculated using Student's t test; more than 300 colonies were analyzed from 3 independent experiments for each condition). (E) Attenuation of PI3K activity does not restore tissue polarity in T4-2 cells that express Rac1 L61, as assessed by immunofluorescence of vector control or transfected cells, stained with antibody against α6 integrin and with DAPI. Bar, 10 μm. (F) Statistical analysis of data in E, in which polarity was assessed by percentage of spheroids without polarized distribution of α6 integrin at basal surface (mean ± SD, P < 0.01, vector control versus Rac1 L61 cells treated with LY294002, Student's t test; more than 700 colonies were analyzed for each condition from 3 independent experiments).
These results demonstrated that the increased PI3K signaling in T4-2 cells, relative to the same cells treated with LY294002 or to the nonmalignant S-1 cells, leads to activation of both Rac1 and Akt, and that these effectors signal to two functionally distinct phenotypes: activation of Rac1 causes the loss of cellular polarity, and activation of Akt causes increased proliferation. To determine whether together these two effectors were sufficient to recapitulate the effects of PI3K, T4-2 cells were infected with both constructs (Fig. 6 A). Colonies derived from cells transfected with these constructs were considerably larger than from control vector-infected cells. Treatment with PI3K inhibitor reduced the increased proliferation only to the level of the malignant vector-transfected cells (Fig. 6, B and C), but it had no repolarizing effect (Fig. 6 D). To determine whether these effects were also manifested in a different surrogate tumor malignancy assay, the four cell types (T4-2, T4+Myr-Akt, T4+Rac1L61, and T4+MyrAkt+Rac1L61) were cultured in 3D methylcellulose in the absence or presence of LY294002 (Fig. 6 E, −LY and +LY). In this assay as well, we found that combined expression of both constructs completely abrogated the effects of LY294002 treatment.
Figure 6.

Simultaneous expression of both Akt and Rac1 is sufficient to prevent reversion induced by attenuation of PI3K activity. (A) Expression of both constitutively active Akt (Myr-Akt) and Rac1 (Rac1 L61) in T4-2 cells was detected by probing Western blots of cell lysates with anti-HA antibody. (B) PI3K inhibition fails to decrease proliferation and to restore tissue organization in Myr-Akt and Rac1 L61-double transfectants, as assessed by phase contrast microscopy and (C) by increased nuclei per spheroid cross section (mean ± SD, P values calculated using Student's t test; more than 300 colonies were analyzed for each condition from three independent experiments). Bar in B, 20 μm. (D) Restoration of basal tissue polarity is disrupted in double transfectants, as assessed by aberrant location of α6 integrin in LY294002-treated T4-2 Myr-Akt+Rac1 L61 cells grown in 3D lrBM for 10 d. Representative images from three independent experiments are shown. Bar, 10 μm. (E) Myr-Akt and Rac1 L61 double transfectants collaborate to increase anchorage-independent cell growth and overcome the inhibitory effects of treatment with PI3K inhibitor. Transfectants were grown in methyl cellulose for 3 wk in the absence or presence of PI3K inhibitor. Representative images are shown from duplicate experiments.
Discussion
A number of studies have shown that the PI3K signaling pathway becomes dysregulated in many types of carcinoma (Vivanco and Sawyers, 2002). The results presented here define mechanisms by which the high activity of PI3K in malignant T4-2 human breast cells contributes to their malignant phenotype. We find that down-modulation of PI3K activity in the T4-2 cells grown in 3D lrBM causes structural repolarization and reversion to a nonmalignant phenotype (Fig. 1) similar to the effects observed previously from inhibition of EGFR and β1 integrin (Weaver et al., 1997; Wang et al., 1998), and we additionally show that both the normal and the normalized reverted acinus-like structures had basal polarization of PI3K and its lipid signaling product, PIP3 (Fig. 2 B). The evidence that inhibition of PI3K can affect crossmodulation of a number of distinct signaling pathways is a demonstration that pathways downstream of PI3K are integrated into transduction networks when cells are grown in the physiological 3D lrBM; consistent with this model, we found that reversion of the tumor cells to a normal phenotype was associated with increased expression of PTEN, the PI3K antagonist (Fig. 3 B). Looking for the signaling effectors that controlled the increased proliferation and decreased polarity downstream of PI3K in the T4-2 cells led to identification of Akt as a mediator of increased proliferation (Fig. 4) and Rac1 as an inhibitor of polarization (Fig. 5), and we found that these two genes, when expressed in combination, were sufficient to overcome the inhibition of PI3K (Fig. 6). These results suggest a model in which the key aspects of the early malignant phenotype, growth and disorganization, can be controlled through disruption of signaling pathways that become interconnected and integrated in 3D lrBM (Fig. 7).
Figure 7.
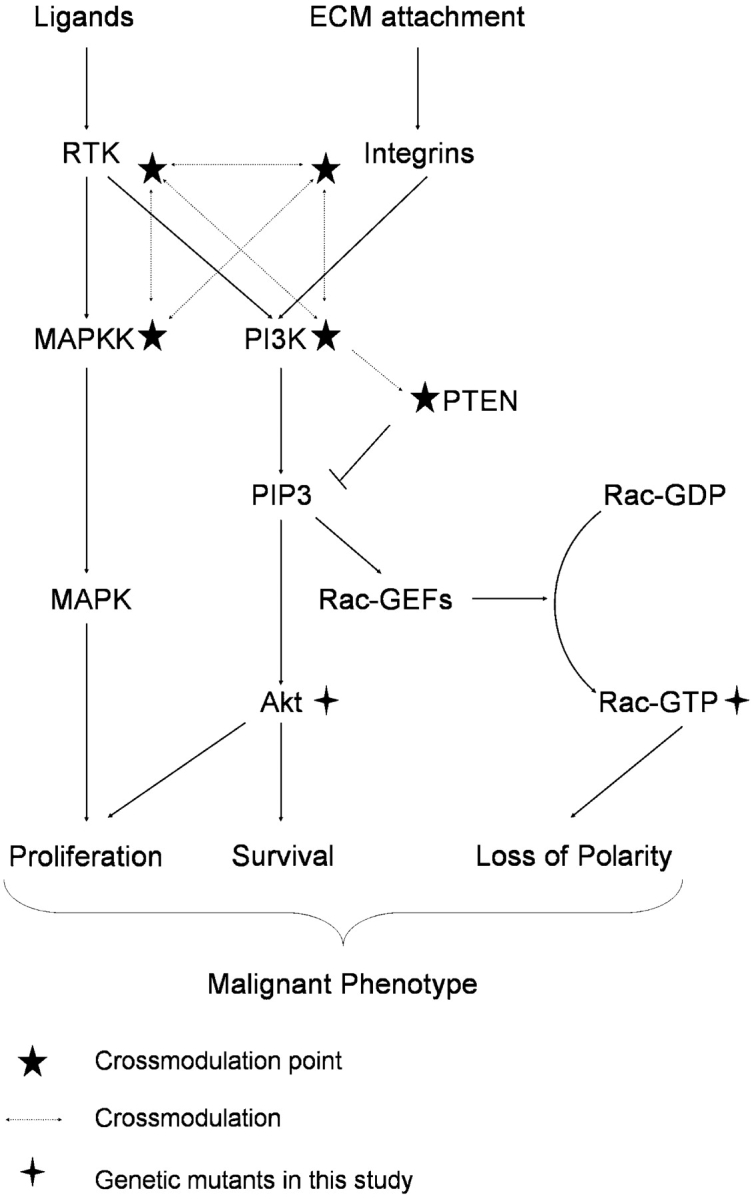
Scheme of proposed tumor cell signaling network to control polarity, proliferation, and apoptosis. T4-2 signaling network. PI3K activity is increased in tumor cells as a result of aberrant signaling from cell–ECM and cell–growth factor receptor interactions. The consequent activation of Akt contributes to the increased cellular proliferation through downstream pro-proliferation and anti-apoptotic pathways. Increased PIP3 also leads to up-regulation of Rac1 through activation of Rac1-specific GEF activity, resulting in altered organization of the actin cytoskeleton, formation and maintenance of tight junctions, and directionality of vesicle trafficking, effects that combine to disorganize the tissue structure. Thus, each pathway independently affects cellular behavior, but the synergistic effect leads to the tumor phenotype. (★) Cross-modulation in 3D lrBM. Inhibition at any of these pressure points results in normalization of the expression and activity at each of the other points, with concomitant normalization of downstream signaling pathways.
It is an important consideration, however, that the progressively increased proliferation and disorganization that typify acquisition of malignancy in mammary epithelial cells is due to more than just PI3K-dependent activation of Rac1 and Akt (although combined activation of these two pathways has been shown to be sufficient to confer malignancy in an experimental model of premalignant human mammary epithelial cells; Zhao et al., 2003). During tumor progression there are many genetic and epigenetic alterations that together contribute to produce malignancy; for the progression of S-1 to T4-2 cells, these include induction of many pathways likely not directly related to PI3K signaling. Reversion of the T4-2 tumor cells grown in 3D suppresses most (if not all) of these other pathways, reducing the proliferation and restoring polarity, but does not change the fact that the reverted cells still retain all of the same genetic alterations and abnormalities. Thus, reversion (whether by LY294002 or by any other T4-2 reversion reagent) results in a cell system that is genetically malignant but biochemically normal, and the consequences of manipulating a single signaling pathway in reverted cells provides information directly relevant to basic characteristics of malignant cell behavior. Using this model, we now show how the altered signaling pathways in T4-2 tumor cells may contribute to the tumor phenotype, how the functions of master regulators (such as PI3K) may be dissected in terms of downstream signaling pathways (in this case, Rac1 and Akt), and how manipulation of these pathways can cause the tumor cells to adopt a normal phenotype.
Normalization of signaling pathways in T4-2 cells in response to inhibition of PI3K is dependent upon culture in 3D lrBM, as T4-2 cells grown on 2D tissue culture plastic do not show the dramatic downmodulation of β1 integrin and EGFR (Fig. 3 A), up-regulation of PTEN (Fig. 3 B), or the alterations in cellular morphology in response to treatment with inhibitors of PI3K (Figs. 2 and 3). Also, for T4-2 cells grown in 3D lrBM, the reduction in PI3K signaling is paralleled by a reorganization of signaling orientation, as both PI3K and its phospholipid product, PIP3, became repolarized to the basolateral surface of the reorganized T4-2 cell structures (Fig. 2). This basolateral distribution of PI3K and PIP3 might indeed reflect the localization of active cell surface receptors, e.g., integrins and receptor tyrosine kinases, many of which have particular functions when localized to the basal or basolateral surfaces (Playford et al., 1996; Weaver et al., 1997; Vermeer et al., 2003). Basolateral polarization of PIP3 has been suggested to be a critical determinant of differentiated tissue behavior in polarized MDCK cells grown as monolayers on filters (Watton and Downward, 1999) or as cysts in 3D collagen gels (Yu et al., 2003), and PIP3 becomes apolarly distributed in the plasma membrane during branching morphogenesis (Yu et al., 2003), a process believed to involve the transitory dedifferentiation to a migratory and invasive state that is highly reminiscent of the malignant phenotype. PI3K signaling polarization is also an essential component of chemotactic migration in neutrophils and Dictyostelium (Servant et al., 2000; Funamoto et al., 2002; Wang et al., 2002b), and the directionality of neuronal axon growth is controlled by spatially localized PI3K activity (Shi et al., 2003). Our observations in mammary epithelial cells do not reveal the extent to which the polarized distribution of PI3K and PIP3 causes, or is the consequence of, tissue polarity, but previous observations with these cells in 3D lrBM and with MDCK cysts have suggested that formation of cell–cell contacts is an essential component of ECM-induced cell polarity (Weaver et al., 1997, 2002; Yeaman et al., 1999). If so, then formation of tight junctions at points of cell–cell contact may provide boundaries for localization of PI3K and other signaling effectors such as integrins and growth factors that then provide the polarizing principle. These possibilities are under investigation.
We have found that the 3D presentation of lrBM is essential for coupling the expression levels and activity of EGFR and β1 integrin in cultured mammary epithelial cells (Wang et al., 1998), and evidence in other systems also implicates reorganization of signaling pathways in cells cultured in 3D lrBM (Cukierman et al., 2001, 2002; Muthuswamy et al., 2001). We now show that components of the PI3K signaling pathway are involved in this cross-modulation process, as phenotypic reversion by inhibition of PI3K is associated with, and presumably, supported by, up-regulation of the PI3K antagonist, PTEN (Fig. 3). This also requires the establishment of organized structures in 3D lrBM, as treatment of T4-2 cells with PI3K inhibitors does not result in up-regulation of PTEN when cells are grown on 2D plastic substrata (Fig. 3 B). Given that the signaling reorganization associated with reversion of T4-2 cells is associated with global repolarization of signaling molecules, we suggest that directional orientation of signaling is an essential component of the cross-modulation process. In this regard, it is tempting to speculate that the 3D lrBM–directed basal localization of PI3K and PIP3 may explain why the cells in the outer layer of acini are more resistant to apoptosis than those not in contact with 3D lrBM (Debnath et al., 2002).
High expression of PI3K is commonly found in cancers and cancer cell lines (Vivanco and Sawyers, 2002; Wang et al., 2002a), and there is considerable evidence that the activity of this enzyme is a key component of the tumorigenic process. Cowden syndrome (an autosomal-dominant cancer predisposition syndrome caused by inherited mutations in PTEN) causes elevated risk of breast, thyroid, and skin tumors (Liaw et al., 1997); mice made heterozygous for expression of PTEN develop cancers at multiple sites (Di Cristofano et al., 1998), and transgenic mice deficient for PTEN expression in the mammary gland developed tumors at early stage (Li et al., 2002). Recent experiments using immortalized human mammary epithelial cells has shown that early passage cells require transfection of additional oncogenes along with PI3K (or Rac1/Akt) to become malignant, whereas late passage cells (which presumably accumulate more alterations) can be transformed with only PI3K (or Rac1/Akt) (Zhao et al., 2003); these results are also consistent with our model in which additional abnormalities must exist in addition to PI3K activation in order for HMT3522 mammary epithelial cells to become tumorigenic. Overexpression of constitutively active Akt in T lymphocytes, pancreatic cells, and the mammary gland increases cellular proliferation and promotes survival but does not induce cellular transformation or increase tumor incidence (Vivanco and Sawyers, 2002), suggesting that additional factors must be required. Rac1 isoforms are overexpressed in cancers of the breast and other organs, and increased activity of Rac1 or Rac3 has been found in breast carcinoma cell lines and Ras-transformed breast epithelial cells (Mira et al., 2000; Sahai and Marshall, 2002). Here, we have unified the roles of elevated Rac1 and Akt activities in a simple mechanistic framework: we have found that the high levels of PI3K in T4-2 cells contribute to the loss of polarity and increased proliferation through Akt and Rac1, and we find that these pathways and phenomena are functionally separable. We find that PI3K-Akt signaling is responsible for an appreciable increase in cell proliferation (Fig. 4), whereas the PI3K-Rac1 signaling is responsible for the loss of basal tissue polarity (Fig. 5), and that expression of both can completely prevent reversion by LY294002. These results show that overactive PI3K signaling activates these two effectors for separate but collaborative regulation of the distinct cellular behaviors of tumor tissues (Fig. 7). Significantly, our results also reveal that increased cell proliferation (in the absence of a polarity-disrupting signal) is not sufficient to result in loss of tissue organization (Fig. 4 E), a finding that may explain why Akt overexpression by itself is not sufficient to increase tumor incidence as well.
In conclusion, we have used the 3D lrBM assay to determine the role of PI3K signaling in the tumorigenic phenotype, signaling reorganization, and tissue polarity of mammary epithelial cells. Our discovery of asymmetric distribution of PI3K and PIP3 in these polarized acinus-like structures strongly implies that they might act as spatial determinants to regulate mammary epithelial polarity. Our elucidation of the events downstream of PI3K sheds light on the process by which increased proliferation and loss of tissue polarity act collaboratively to produce the malignant phenotype.
Materials and methods
Reagents and cell culture
lrBM from Englebreth-Holm-Swarm tumors (matrigel), Vitrogen (rat tail collagen type I), and the antibodies for E-cadherin, β1-integrin, EGFR, α6 integrin, and Ki-67 were described previously (Weaver et al., 1997; Wang et al., 1998). The other antibodies used in this study are: PTEN (clone 2), Akt (clone 7), Rac1 (clone 102), PI3K p85 (clone 4), and p110 subunit (clone 19) (Transduction Laboratory), HA (12CA5; Roche), Phospho-Akt (serine 473 or threonine 308), total and phospho-GSK-3β (serine 9), total and phospho-p70 S6 kinase (threonine 389; Cell Signaling Technology), PIP3 monoclonal antibody (Chen et al., 2002; clone RC6F8; Echelon Corp.), and ZO-1 antibody (gift of Shoichiro Tsukita, Kyoto University Faculty of Medicine, Kyoto, Japan). HMT-3522 mammary epithelial cells were cultured as described previously (Weaver et al., 1997; Wang et al., 1998). The PI3K inhibitor LY294002 or wortmannin (Calbiochem) was dissolved in DMSO and added to culture medium at the final concentration of 8 μM or 2.5 nM after cells were plated; control cultures were treated with vehicle only. Cells were treated with inhibitor or vehicle inside matrigel or on tissue culture plates for 10 d and inhibition was maintained by replacing with fresh medium containing inhibitor or vehicle every 2 d. No toxicity was found during the 10-d treatment with LY294002 or wortmannin.
DNA constructs and gene transfection
A construct containing Rac1 L61–HA (gift of Tung C. Chan, Thomas Jefferson University, Philadephia, PA) was digested with EcoRI and BamHI and cloned into the pLXSN–Neo retroviral construct (CLONTECH Laboratories, Inc.). The myr-Akt pWZL retroviral construct myrΔ4–129 (Kohn et al., 1998) was provided by Richard Roth (Stanford University, Stanford, CA). Transfection of Phoenix packaging cells (gift of Garry P. Nolan, Stanford University) and production of retroviral stock were according to standard protocols. The HMT-3522 mammary epithelial cells were infected at 40–50% confluence. Myr-Akt and Rac1 L61-double transfectants were produced by sequentially infecting cells with each construct. The stably expressing cells were selected in the presence of neomycin (500 μg/ml) or hygromycin B (50 μg/ml) and surviving clones were pooled.
Immunoblotting, immunoprecipitation, and indirect immunofluorescence
Immunoblotting and indirect immunofluorescence were performed as described previously (Weaver et al., 1997; Wang et al., 1998). For immunoprecipitation, cells were lysed in IP buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris/HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 2 mM NaF, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml E 64, and 1 mM Pefabloc) and centrifuged at 16,000 g at 4°C. Equal amounts of protein lysates were precleared by 50 μl of protein G plus–conjugated agarose beads (Santa Cruz Biotechnology, Inc.) before the addition of 1 μg of primary antibody. Samples were incubated at 4°C with gentle rotation for 1 h. Subsequently, samples were incubated with 30 μl of protein G plus–conjugated beads for 1 h at 4°C. The beads were washed three times with IP buffer before being heated with sample buffer at 95°C for 5 min and analyzed by SDS-PAGE and Western blotting.
For Rac1 activity assay, the cells from 10-d culture in 3D BM were lysed in GST-Fish buffer (10% glycerol, 50 mM Tris, pH 7.4, 100 mM NaCl, 1% NP-40, 2 mM MgCl2, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml E 64, and 1 mM Pefabloc) and centrifuged at 16,000 g at 4°C. Equal amounts of protein supernatants were incubated with recombinant GST-PAK-CD fusion protein (containing the Rac and Cdc42 binding region from human PAK1; Sander et al., 1998), bound to glutathione-coupled Sepharose beads (Amersham Biosciences) at 4°C for 30 min. The beads were washed with an excess of lysis buffer, eluted in sample buffer, and then analyzed by SDS-PAGE and Western blotting using antibody against Rac1.
For PIP3 immunofluorescence, the isolated colonies were fixed with 3.7% formaldehyde, washed with CSK buffer (10 mM Hepes, 138 mM KCl, 3 mM MgCl2, 1 mM EDTA), permeabilized by 0.1% Triton X-100 in CSK buffer, and blocked with 3% skim milk in blocking buffer (50 mM Tris/HCl, pH 7.5, 1 mM CaCl2). Primary antibody diluted 1:100 in blocking buffer was incubated with samples for 1 h in room temperature followed by FITC-conjugated secondary antibody.
Nuclei were counterstained with DAPI (Sigma-Aldrich). Control sections were stained with secondary antibodies only. The slides were sealed with Vectashield (Vector Laboratories). The images were collected with Zeiss 410 LSM confocal microscope (Zeiss Pluar 40× oil objective lenses; Carl Zeiss MicroImaging, Inc.) or RT SLIDER SPOT digital camera (SPOT RT v3.2 software; Diagnostic Instruments) attached to Zeiss Photomicroscope III (Zeiss Plan-Neofluar 40× oil objective lenses; Carl Zeiss MicroImaging, Inc.). Images for figures were colored and resized with Adobe Photoshop 7.0 software.
Anchorage-independent growth assays
For soft agar assay, 5,000 cells were plated in 1 ml of DME/F12 containing 0.3% agarose, overlaid with 1 ml of 1% agarose, and then exposed to treatment as indicated. Cultures were maintained for 15 d. Colonies from duplicated wells were measured and scored positive when the colony sizes exceeded a diameter of 50 μm.
For methyl cellulose anchorage–independent growth assay, 100,000 cells were seeded per 60-mm dishes in 5 ml of DME/F12 containing 1.5% methyl cellulose (Fisher Scientific) with inhibitor or vehicle only. Colonies were scored after 3 wk.
Online supplemental material
Supplemental figures show the following results; treatment of T4-2 cells with PI3K inhibitor LY294002 at 8-μM concentration when grown in 3D lrBM for 10 d does not affect cell viability or lead to increased cell death (Fig. S1 A); the same treatment decreases cell proliferation of T4-2 cells when cultured on 2D plastic (Fig. S1 B); expression of constitutively active Rac1 V12 in T4-2 cells transduced by adenovirus inhibits LY294002-induced repolarization of basal marker α6 integrin in 3D lrBM (Fig. S1 C); treatment of T4-2 cells with PI3K inhibitor does not change its expression levels (Fig. S2 A); overexpression of constitutively active Akt in T4-2 cells greatly increases anchorage-independent growth of T4-2 cells in soft agar (Fig. S2 B); and PIP3 staining intensity is significantly reduced when treating T4-2 cells with PI3K inhibitor (Fig. S2 C). Figs. S1 and S2 are available at http://www.jcb.org/cgi/content/full/jcb.200306090/DC1.
Acknowledgments
We thank Senthil Muthuswamy, Kunxin Luo, G. Steven Martin, Valerie M. Weaver, Zena Werb, and members of the Bissell laboratory (Paraic A. Kenny, Jimmie E. Fata, Virginia Novaro, and Aylin Rizki) for critical reading of the manuscript and for helpful comments; we also thank Jimmie E. Fata, David W. Knowles, Dinah D. Levy, and Sun-Young Moonlee for assistance with confocal microscopy and other techniques. We thank Richard A. Roth for providing expression constructs.
This work was supported by National Institutes of Health (NIH; grant CA64786-08), the Department of Energy (OBER DEAC0376SF00098), and by an Innovator Award from the Department of Defense (DAMD17-02-1-0438) to M.J. Bissell. H. Liu is the recipient of a predoctoral fellowship from Breast Cancer Research Program of the Department of Defense. D.C. Radisky is the recipient of a postdoctoral fellowship from NIH and the American Cancer Society.
The online version of this article contains supplemental material.
F. Wang's present address is Department of Cellular and Molecular Pharmacology and the Cardiovascular Research Institute, University of California, San Francisco, San Francisco, CA 94143.
Abbreviations used in this paper: 2D, two dimensional; 3D, three dimensional; EGFR, epidermal growth factor receptor; GSK 3β, glycogen synthase kinase-3β; lrBM, laminin-rich basement membrane; Myr-Akt, myristoylated Akt (constitutively active Akt); PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol 3,4,5-trisphosphate.
References
- Bissell, M.J., and D. Radisky. 2001. Putting tumours in context. Nat. Rev. Cancer. 1:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, T.O., S.E. Rittenhouse, and P.N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68:965–1014. [DOI] [PubMed] [Google Scholar]
- Chen, H.C., and J.L. Guan. 1994. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 91:10148–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R., V.H. Kang, J. Chen, J.C. Shope, J. Torabinejad, D.B. DeWald, and G.D. Prestwich. 2002. A monoclonal antibody to visualize PtdIns(3,4,5)P(3) in cells. J. Histochem. Cytochem. 50:697–708. [DOI] [PubMed] [Google Scholar]
- Comer, F.I., and C.A. Parent. 2002. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 109:541–544. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., R. Pankov, D.R. Stevens, and K.M. Yamada. 2001. Taking cell-matrix adhesions to the third dimension. Science. 294:1708–1712. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., R. Pankov, and K.M. Yamada. 2002. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 14:633–639. [DOI] [PubMed] [Google Scholar]
- Debnath, J., K.R. Mills, N.L. Collins, M.J. Reginato, S.K. Muthuswamy, and J.S. Brugge. 2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 111:29–40. [DOI] [PubMed] [Google Scholar]
- Di Cristofano, A., B. Pesce, C. Cordon-Cardo, and P.P. Pandolfi. 1998. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19:348–355. [DOI] [PubMed] [Google Scholar]
- Fish, E.M., and B.A. Molitoris. 1994. Alterations in epithelial polarity and the pathogenesis of disease states. N. Engl. J. Med. 330:1580–1588. [DOI] [PubMed] [Google Scholar]
- Funamoto, S., R. Meili, S. Lee, L. Parry, and R.A. Firtel. 2002. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 109:611–623. [DOI] [PubMed] [Google Scholar]
- Grant, S., L. Qiao, and P. Dent. 2002. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front. Biosci. 7:d376–d389. [DOI] [PubMed] [Google Scholar]
- Kohn, A.D., A. Barthel, K.S. Kovacina, A. Boge, B. Wallach, S.A. Summers, M.J. Birnbaum, P.H. Scott, J.C. Lawrence, Jr., and R.A. Roth. 1998. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273:11937–11943. [DOI] [PubMed] [Google Scholar]
- Lee, J.W., and R.L. Juliano. 2000. alpha5beta1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol. Biol. Cell. 11:1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G., G.W. Robinson, R. Lesche, H. Martinez-Diaz, Z. Jiang, N. Rozengurt, K.U. Wagner, D.C. Wu, T.F. Lane, X. Liu, et al. 2002. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 129:4159–4170. [DOI] [PubMed] [Google Scholar]
- Liaw, D., D.J. Marsh, J. Li, P.L. Dahia, S.I. Wang, Z. Zheng, S. Bose, K.M. Call, H.C. Tsou, M. Peacocke, et al. 1997. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16:64–67. [DOI] [PubMed] [Google Scholar]
- Mira, J.P., V. Benard, J. Groffen, L.C. Sanders, and U.G. Knaus. 2000. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc. Natl. Acad. Sci. USA. 97:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy, S.K., D. Li, S. Lelievre, M.J. Bissell, and J.S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, L.E., T.S. Jou, A.L. Pollack, Q. Zhang, S.H. Hansen, P. Yurchenco, and K.E. Mostov. 2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3:831–838. [DOI] [PubMed] [Google Scholar]
- Petersen, O.W., L. Ronnov-Jessen, A.R. Howlett, and M.J. Bissell. 1992. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA. 89:9064–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford, R.J., A.M. Hanby, S. Gschmeissner, L.P. Peiffer, N.A. Wright, and T. McGarrity. 1996. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 39:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann, E. 1994. Oncogenes and epithelial cell transformation. Semin. Cancer Biol. 5:157–165. [PubMed] [Google Scholar]
- Sahai, E., and C.J. Marshall. 2002. RHO-GTPases and cancer. Nat. Rev. Cancer. 2:133–142. [DOI] [PubMed] [Google Scholar]
- Sander, E.E., S. van Delft, J.P. ten Klooster, T. Reid, R.A. van der Kammen, F. Michiels, and J.G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, M.P., and J.R. Woodgett. 2001. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol. 2:760–768. [DOI] [PubMed] [Google Scholar]
- Servant, G., O.D. Weiner, P. Herzmark, T. Balla, J.W. Sedat, and H.R. Bourne. 2000. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 287:1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S.H., L.Y. Jan, and Y.N. Jan. 2003. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 112:63–75. [DOI] [PubMed] [Google Scholar]
- Simpson, L., and R. Parsons. 2001. PTEN: life as a tumor suppressor. Exp. Cell Res. 264:29–41. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., S.J. Leevers, K. Ahmadi, J. Timms, R. Katso, P.C. Driscoll, R. Woscholski, P.J. Parker, and M.D. Waterfield. 2001. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70:535–602. [DOI] [PubMed] [Google Scholar]
- Vermeer, P.D., L.A. Einwalter, T.O. Moninger, T. Rokhlina, J.A. Kern, J. Zabner, and M.J. Welsh. 2003. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 422:322–326. [DOI] [PubMed] [Google Scholar]
- Vivanco, I., and C.L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2:489–501. [DOI] [PubMed] [Google Scholar]
- Wang, F., V.M. Weaver, O.W. Petersen, C.A. Larabell, S. Dedhar, P. Briand, R. Lupu, and M.J. Bissell. 1998. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA. 95:14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., R.K. Hansen, D. Radisky, T. Yoneda, M.H. Barcellos-Hoff, O.W. Petersen, E.A. Turley, and M.J. Bissell. 2002. a. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J. Natl. Cancer Inst. 94:1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., P. Herzmark, O.D. Weiner, S. Srinivasan, G. Servant, and H.R. Bourne. 2002. b. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4:513–518. [DOI] [PubMed] [Google Scholar]
- Watton, S.J., and J. Downward. 1999. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9:433–436. [DOI] [PubMed] [Google Scholar]
- Weaver, V.M., O.W. Petersen, F. Wang, C.A. Larabell, P. Briand, C. Damsky, and M.J. Bissell. 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, V.M., S. Lelievre, J.N. Lakins, M.A. Chrenek, J.C. Jones, F. Giancotti, Z. Werb, and M.J. Bissell. 2002. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., and R. Li. 2003. Spontaneous cell polarization: undermining determinism. Nat. Cell Biol. 5:267–270. [DOI] [PubMed] [Google Scholar]
- Yamada, K.M., and M. Araki. 2001. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 114:2375–2382. [DOI] [PubMed] [Google Scholar]
- Yeaman, C., K.K. Grindstaff, and W.J. Nelson. 1999. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79:73–98. [DOI] [PubMed] [Google Scholar]
- Yu, W., L.E. O'Brien, F. Wang, H. Bourne, K.E. Mostov, and M.M. Zegers. 2003. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol. Biol. Cell. 14:748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J.J., O.V. Gjoerup, R.R. Subramanian, Y. Cheng, W. Chen, T.M. Roberts, and W.C. Hahn. 2003. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 3:483–495. [DOI] [PubMed] [Google Scholar]