Abstract
During the past years, yeast has been successfully established as a model to study mechanisms of apoptotic regulation. However, the beneficial effects of such a cell suicide program for a unicellular organism remained obscure. Here, we demonstrate that chronologically aged yeast cultures die exhibiting typical markers of apoptosis, accumulate oxygen radicals, and show caspase activation. Age-induced cell death is strongly delayed by overexpressing YAP1, a key transcriptional regulator in oxygen stress response. Disruption of apoptosis through deletion of yeast caspase YCA1 initially results in better survival of aged cultures. However, surviving cells lose the ability of regrowth, indicating that predamaged cells accumulate in the absence of apoptotic cell removal. Moreover, wild-type cells outlast yca1 disruptants in direct competition assays during long-term aging. We suggest that apoptosis in yeast confers a selective advantage for this unicellular organism, and demonstrate that old yeast cells release substances into the medium that stimulate survival of the clone.
Keywords: aging; apoptosis; oxygen stress; Saccharomyces cerevisiae; YCA1
Introduction
Apoptosis is a form of cellular suicide that leads to the rapid removal of unwanted or damaged cells. As misregulation of apoptosis can result in human diseases such as neurodegenerative disorders, spread of viral infections, AIDS, and cancer, apoptosis is of particular importance for medical research (Steller, 1995). Recent analyses have established yeast as a model to study mechanisms of apoptotic regulation. In the yeast Saccharomyces cerevisiae, we detected cell death with typical markers of apoptosis, such as DNA fragmentation, phosphatidylserine externalization, and chromatin condensation (Madeo et al., 1997). Apoptosis was observed in association with a mutation in the AAA-ATPase gene CDC48 (cdc48 S565G; Madeo et al., 1997), whose mammalian orthologue was subsequently implicated in regulation of apoptosis (Shirogane et al., 1999). In addition, several other pathways crucial for mammalian apoptosis are conserved in yeast, e.g., a newly discovered proteasomal apoptotic pathway leading to destruction of Cdc6p (Blanchard et al., 2002). Exposure to low doses of H2O2 or accumulation of reactive oxygen species (ROS) by depletion of glutathione induces apoptosis in wild-type yeast cells, indicating that, like in metazoans, ROS are key regulators of yeast apoptosis (Madeo et al., 1999). In fact, most apoptotic scenarios that have been explored in yeast are related to oxygen stress (Laun et al., 2001; Ludovico et al., 2002; Mazzoni et al., 2003).
Previously, we demonstrated that a caspase-like protease (Yca1p) mediates oxygen stress-dependent apoptosis in yeast (Madeo et al., 2002). However, it remained unclear why low doses of oxygen radicals can trigger yeast apoptosis, as yeast harbor many potent oxygen stress sensors and defense mechanisms. An example is Yap1p, which is a basic-region leucine zipper (bZIP) transcription factor (Moye-Rowley et al., 1989) that mediates oxidative stress responses by regulating target genes encoding proteins involved in oxidant defense. In brief, though a variety of apoptotic pathways are known in yeast, the beneficial effects of a cell suicide program such as apoptosis for a single-celled organism remained unexplained.
Here, we demonstrate that chronologically aged yeast cultures die exhibiting typical markers of apoptosis. Old yeast cultures release substances into the medium that stimulate survival of other old cells. Disruption of the apoptotic machinery by deletion of YCA1 is disadvantageous for the population, as in direct competition assays the wild-type cells outlast the disruptants during long-term aging. We propose that yeast cells commit altruistic suicide to provide nutrients for other, probably younger and fitter cells. This provides for the first time an explanation on how apoptosis can be an advantage even for a unicellular organism.
Results and discussion
Chronologically aged yeast cells die exhibiting an apoptotic phenotype
Aging yeast cells in long-term cultivation are considered to be a model for the aging process in human postmitotic tissues (MacLean et al., 2001). This kind of long-term cultivation leads to death of a majority of the cell population (Longo et al., 1997).
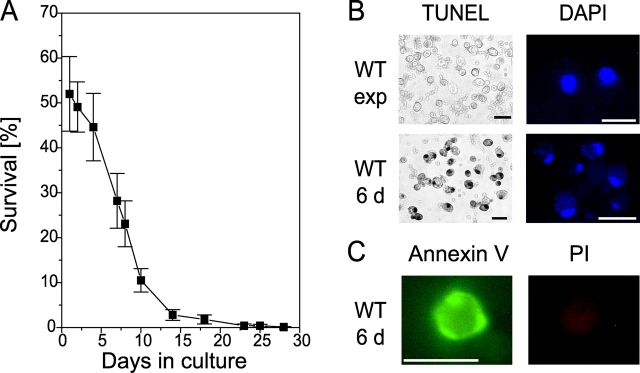
We cultured wild-type yeast (strain S288C) in synthetic complete (SC) medium continuously for 30 d, and monitored the portion of living cells using a plating assay (Fig. 1 A). After 3 d, the survival rate started to decrease, and in 14-d-old cultures only a few cells stayed alive. Different wild-type strains (W303, S288C, BY4741, and diploid strain DBY39) all showed this substantial loss of surviving cells upon long-term cultivation, although the exact time course of survival rate varied. To investigate whether apoptosis might contribute to cell loss, we analyzed apoptotic markers in chronologically aged and exponentially growing cultures.
Figure 1.
Chronologically aged yeast cells die exhibiting typical markers of apoptosis. (A) Survival (colony formation; 100% represents the number of plated cells) of wild-type cultures during chronological aging. Data represent mean ± SEM. (B) Cells from 6-d chronologically aged and exponentially grown wild-type cultures were fixed and stained for DNA cleavage (TUNEL) and chromatin condensation (DAPI). Bars, 5 μm. (C) Cells from 6-d chronologically aged wild-type cultures show externalization of phosphatidylserine visualized by annexin V staining. Cells were stained with propidium iodide (PI) for integrity control. Bars, 5 μm.
Previously, yeast apoptosis has been shown to include cleavage of chromosomal DNA, which can be detected by TUNEL assay (Madeo et al., 1997). After a 6-d culture in SC liquid medium, yeast cells became TUNEL positive. 50% of the cells exhibited an intense black nuclear staining, indicating massive DNA fragmentation (Fig. 1 B). On the other hand, exponentially growing cells remained TUNEL negative (Fig. 1 B).
20% of the yeast cells grown for 6 d on SC medium showed apoptotic chromatin morphology upon DAPI staining, with chromatin fragments forming a semicircle (Fig. 1 B). In contrast, in exponentially growing cultures, chromatin appeared as a single round spot in the middle of the cell (Fig. 1 B).
An early morphological marker of apoptosis is the exposure of phosphatidylserine at the outer leaflet of the cytoplasmic membrane, which is conserved from yeast to mammalian cells (Martin et al., 1995; Madeo et al., 1997). In yeast, phosphatidylserine can be detected by FITC-labeled annexin V staining upon cell wall digestion. Concomitantly, cells were checked for membrane integrity by incubation with propidium iodide. 10–20% of the cells grown for 6 d on SC medium showed strong fluorescence around the whole circumference of the cell upon staining with annexin V but did not take up propidium iodide, indicating an intact membrane (Fig. 1 C). Exponentially growing cultures did not exhibit staining with annexin V (unpublished data).
In summary, chronological aging of yeast leads to the occurrence of the typical membranous and nuclear markers of apoptosis. We conclude that long-term cultivation-induced mortality is indeed of apoptotic nature.
Overexpression of YAP1 improves survival during chronological aging
ROS have been shown to be both necessary and sufficient for inducing apoptosis in yeast (Madeo et al., 1999). Chronologically aged cells were tested for the production of ROS by incubation with dihydrorhodamine 123 (DHR). In the cell, this dye can be oxidized to the fluorescent chromophore rhodamine 123 by ROS. We monitored the formation of ROS in a time course during aging. Exponentially growing and 1-d-old wild-type cells showed no fluorescence upon staining. From d 3–12 the portion of ROS-accumulating cells increased; after 12 d all cells were DHR positive (Fig. 2 A). The percentage of DHR-stained cells corresponds to cell death observed in the plating assay (Fig. 1 A). To examine whether ROS formation occurs only because yeasts in stationary phase have to switch from fermentation to oxidative phosphorylation, we analyzed cultures that were growing exponentially on nonfermentable medium for formation of ROS. Those cells showed nearly no staining with DHR, indicating that ROS formation during chronological aging cannot simply be explained by the diauxic shift (Fig. 2 A).
Figure 2.
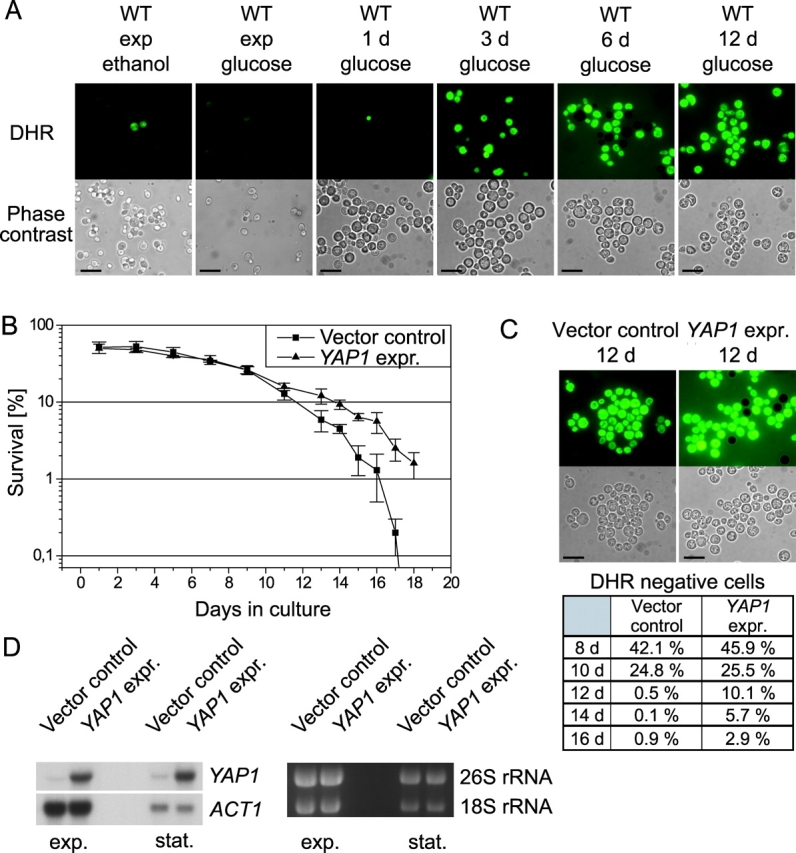
Generation of ROS triggers age-induced apoptosis. (A) Cells from chronologically aged and exponentially grown wild-type cultures on glucose and ethanol medium were analyzed for accumulation of ROS visualized by DHR staining and viewed through FITC-channel. Bars, 10 μm. (B) Survival of cells with YAP1 overexpression and vector control. Data represent mean ± SEM. (C) Cells from aged YAP1 overexpression and vector control strains were stained with DHR. Bars, 10 μm. The percentage of stained cells of both strains is displayed in the chart. For quantification, we counted at least 1,000 cells. (D) Northern hybridization of YAP1 overexpressor and vector control.
Yap1p mediates the general response of yeast cells to stress and is functionally homologous to the human apoptosis regulator AP-1 (Moye-Rowley et al., 1989). We found that overexpression of YAP1 leads to significantly prolonged survival of a chronologically aged culture (Fig. 2 B). Starting from d 10, the YAP1 overexpressor displayed better survival than the vector control (e.g., 4.8% better survival on d 14) and was still viable after 18 d, when the control strain completely had lost its viability. Consistently, a subpopulation of the YAP1 overexpressor showed no ROS accumulation during late stages of chronological aging (e.g., 5.7% on d 14), whereas all cells of the vector control displayed ROS formation as visualized through DHR staining. Cells were counted for quantification (Fig. 2 C).
Overexpression of YAP1 was verified by Northern hybridization (Fig. 2 D). We conclude that internal production of ROS is tightly associated with chronological aging-induced cell death in yeast, and protection from internal ROS might well be responsible for increased viability of YAP1-overexpressing cultures.
Yeast caspase Yca1p and other cysteine proteases promote apoptosis in aging yeast cells
Previously, we have shown that oxygen stress induces yeast apoptosis via activation of the yeast caspase Yca1p (Madeo et al., 2002). Disruption of YCA1 also increased the survival rate of chronologically aged cultures (Madeo et al., 2002).
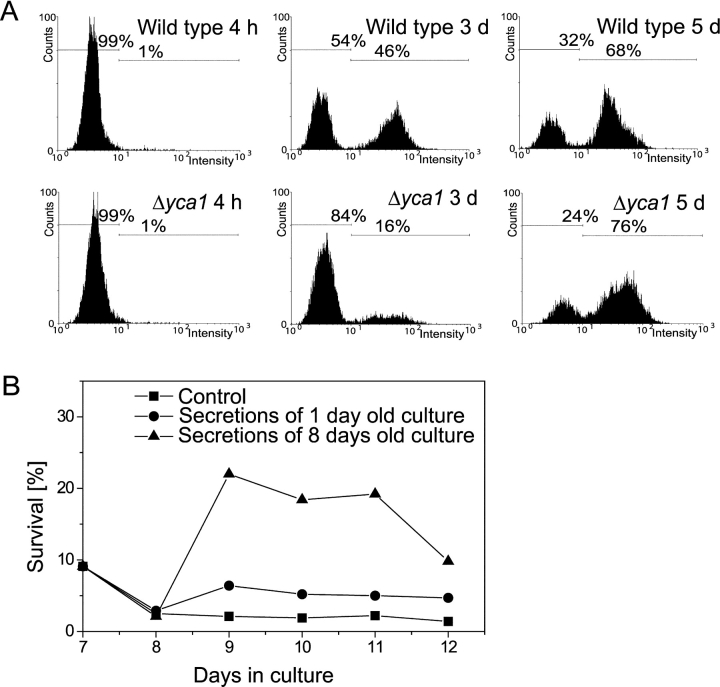
Here, we demonstrate in vivo that aging cells show caspase activity using a fluorogenic caspase substrate (Fig. 3 A). During exponential growth, no caspase activity was detectable with our assay. However, after 3 d in culture, nearly half of the wild-type cells displayed caspase activity compared with only 16% of the yca1 null mutant, indicating that most of the caspase activity during aging is due to Yca1p. Interestingly, in 5-d-old cultures, ∼70% of the cells of wild type as well as the yca1 null mutant showed caspase activity, indicating that yet another caspase or protease capable of processing caspase substrates is active during chronological aging. It should be noted that a range of cysteine proteases are sensitive to caspase inhibitors, and are probably able to cut fluorogenic caspase substrates as well (Rozman-Pungercar et al., 2003).
Figure 3.
Aging yeast cells show caspase activation and release substances that can stimulate survival of other old cells. (A) Chronologically aged wild-type and yca1 disruption strains were analyzed in vivo for caspase activity by FITC-VAD-fmk staining using flow cytometry for quantification. This experiment was performed three times independently with similar results. (B) Survival of chronologically aged wild-type yeast in the presence of secretions released by 1- or 8-d-old cultures (final concentration 2 mg/ml, addition on d 7 and 11) or without addition. Experiments were performed three times independently with different secretions and gave similar results.
Old yeast cultures release substances that improve survival of aged cells
We addressed the question of whether physiological death of a majority of cells in a yeast culture provides more advantages than just to spare the nutrients for the surviving cells. To find out whether an aged culture releases substances into the surrounding, we grew yeast on SC medium until massive cell death set in (8 d), transferred the culture to distilled water, incubated for 3 d, dried the culture supernatant, and analyzed its contents. It should be noted that yeast cells do not lose viability when incubated in pure water (Longo et al., 1997).
The supernatant contains a mixture of mostly low mol wt molecules. Individual proteins could not be detected by HPLC mass spectrometry (unpublished data). The composition, appearance (bright yellow color), and smell of this mixture differs completely from commercial yeast extract or from freshly made yeast whole-cell extracts, indicating that this extract is not the result of cell lysis.
To analyze their effect on cells in different growth phases, these secretions were added to exponentially growing cultures and to cultures grown on SC medium for 7 d. Treatment of exponentially growing cultures had no effect on survival (unpublished data). However, survival rates of the aged culture increased more than eightfold in comparison to untreated cultures from the same batch (Fig. 3 B). Starting at d 12, viability of the supplemented culture again diminished. This was not caused by exhaustion of the added secretions, as a second addition on d 11 did not further improve survival. Secretions from young cultures had only a slight (less than threefold) effect on survival (Fig. 3 B). The experiments were performed three times with independently gained secretions, and similar results were obtained.
As we detected mostly low mol wt molecules, we performed amino acid analysis and found a reproducible pattern of amino acids in every dried supernatant (unpublished data). Addition of single amino acids that were found to be increased in the secretions didn't improve survival of aged cultures (unpublished data). To further characterize the secretions, we analyzed their heat stability and separated them by size. Addition of heated (for 30 min at 100°C) secretions did improve the survival rates of aged wild-type cells similar to untreated secretions. Separation into different mol wt fractions by ultrafiltration and addition of these fractions to aged cultures revealed that the active compounds have a mol wt >5,000 (unpublished data).
We conclude that the observed improvement of survival with secretions released from older cultures might partly be due to nutrient effects, as whole-cell extracts (autolyzed at high cell density; Difco) also resulted in a slight (about twofold; unpublished data) improvement of survival. Released yeast secretions are probably easier to use for other yeast cells than are whole yeast cell extracts. The observation that secretions released from older cell cultures increase the viability of aged cells indicates an additional beneficial effect of cell death in old cultures. It has been shown that yeast cells can influence other cells by releasing soluble substances into the media, e.g., during sporulation (Jakubowski and Goldman, 1988) and mating. Our results suggest that during chronological aging, cells release substances into their surrounding that stimulate survival of other cells, probably the replicative younger and fitter ones.
Disruption of YCA1 is disadvantageous for the population
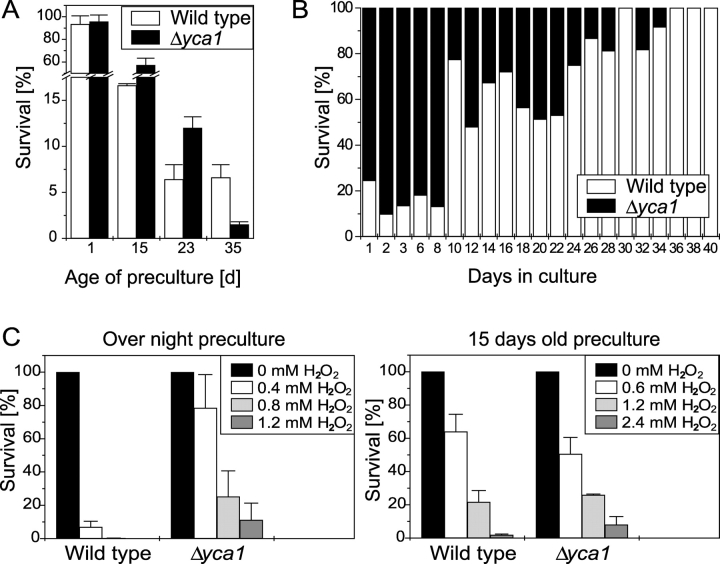
We have shown that chronologically aged cultures die exhibiting apoptotic markers, accumulate oxygen radicals, and show caspase activity. This is consistent with our observation that disruption of the yeast caspase YCA1 delays age-induced cell death and enhances oxygen stress resistance (Madeo et al., 2002). Therefore, lack of Yca1p should be an advantage for survival. We addressed the question of whether this might only be a short-term benefit. When nutrients are exhausted in wildlife, yeast cells lucky to reach a new food source (fruit of flower) have to switch from hunger metabolism to use of high concentrations of nutrients. To mimic this situation experimentally, we used wild-type and yca1 null mutant cultures and determined the survival rate after aging (“scarce nutrients”). Consistent with previous results, the yca1 mutant stain showed a substantial higher survival rate in a plating assay (unpublished data; Madeo et al., 2002). Then, we tested whether aged cultures are able to adapt to a new environment containing fresh medium (“rich nutrients”). We inoculated the aged wild-type and yca1 null mutant into fresh medium at low density (3 × 105 cells/ml), and examined the survival after 6 h during exponential growth phase. Taking non-aged precultures, almost all cells of both strains survived (Fig. 4 A). With slightly older precultures, the yca1 null mutant strain exhibited better survival after regrowth (57% with 15-d-old and 12% with 23-d-old precultures) compared with the wild type (17% with 15-d-old and 6.5% with 23-d-old precultures; see Fig. 4 A). However, with 35-d-old precultures, in the culture of the yca1 null mutant only 1% of the transferred cells was viable in a plating assay, whereas the wild type remained at 7% survival (Fig. 4 A). Obviously, an aged yca1 null mutant strain is not able to properly regrow in fresh liquid culture. We conclude that Yca1p-mediated apoptosis might have developed to eliminate cells that are not able to adapt to a new environment, e.g., predamaged or replicative older cells.
Figure 4.
The apoptotic program represents a selective advantage for yeast cultures. (A) Survival of wild-type and yca1 null mutant strain during exponential growth (6 h after inoculation) in dependency of the age of the preculture. Data represent mean ± SEM. (B) Competition assay between wild-type and yca1 null mutant strain. Equal amounts of cells of exponentially growing cultures of wild-type and yca1 null mutant strains were mixed, and the percentage of living cells of both strains was monitored by plating assay (100% is the total number of living cells). Experiments were performed four times with similar results. (C) Survival of wild-type and yca1 null mutant strain after treatment with H2O2 at low cell density. Aged or overnight precultures were inoculated at 3 × 105 cells/ml in fresh medium, grown for 3 h, treated with H2O2, and analyzed for survival after 3 h. Data represent mean ± SEM.
To find out whether wild-type or yca1 null mutant cells are dominant in a direct competition, we mixed the same amount of cells of both strains during exponential growth and monitored survival during chronological aging. To discriminate between both strains, aliquots were plated on YPD and YPD-geneticin plates (only yca1 null mutant cells are able to survive on both). During the first 8 d, more viable cells were of the yca1 null mutant genotype (Fig. 4 B). Afterwards, the wild type did prevail over the yca1 null mutant, and after 36 d only wild-type cells did survive (Fig. 4 B). Hence, cells that are disrupted in the apoptotic machinery through deletion of YCA1 have a short-term advantage, but are not able to survive when they have to compete against wild-type cells during aging.
Another advantage of the yca1 null mutant strain is the increased resistance to H2O2-induced apoptosis, as was shown previously (Madeo et al., 2002). To test whether preaging of yca1 null mutants has effects on H2O2 resistance, we inoculated low density cultures (3 × 105 cells/ml) from 15-d-old precultures, added H2O2 in varying concentrations during exponential growth, and examined survival. Although mutant cells from young cultures showed better survival (than wild-type cells) after H2O2 treatment, this advantage was completely lost in 15-d-old precultures (Fig. 4 C; Madeo et al., 2002). Therefore, a major advantage of the yca1 null mutant, its resistance to oxygen stress, seems to be lost after aging. It should be noted that cells derived from stationary phase have an increased resistance to oxygen stress (Jamieson et al., 1994). This might explain the higher H2O2 concentration necessary for induction of cell death with older precultures. To test the specificity of the apoptotic response, we used nystatin as a necrotic death stimulus. Nystatin leads to formation of pores in the plasma membrane and should induce nonapoptotic death. Treatment with nystatin revealed no differences in survival rates between wild-type and yca1 null mutant strains (LD50 = 7 μg/ml for both strains).
How can apoptosis during stationary phase provide an evolutionary advantage?
The important role of ROS in the regulation of apoptosis may provide an explanation for the origin and primary function of the suicide process. ROS occur during respiration, UV radiation, or chemical and enzymatic reactions in every organism. Because ROS are highly reactive and modify proteins, lipids, and nucleic acids, ROS-induced cell damage is a frequent event. Cells have developed mechanisms to detoxify ROS and to repair oxidative damage, but this can only reduce (not completely prevent) fatal cell damage. Most damaged cells will continue to metabolize for some time, even when they have lost the ability to proliferate.
For a monoclonal population of cells, it may be evolutionarily advantageous to spare most of the dwindling resources for a few healthy cells, rather than waste these resources on potentially ROS-damaged or old cells that have a reduced chance of long-term survival. Survival of yeast in the wild depends on their ability to cope with dramatic changes in the nutrient environment that include simultaneous lack of nitrogen and carbon sources, or an excess of nutrient sources, such as sugar. Apoptosis may be an important mechanism for yeast to adapt to these adverse environmental conditions in a manner that ensures survival of the clone. The better adapted cells are able to survive longer with substances released by dying cells. Moreover, death of aging yeast seems to be an active process and not an unavoidable fate. Due to protection against internal ROS via YAP1 expression or disruption of the yeast caspase YCA1, some cells in the population are able to survive significantly longer. However, we also show that the advantage to avoiding cell death during aging turns to a disadvantage in the long run. Mutants that survive better at earlier stages of the aging process, like the yca1 null mutant, lose their potential to found new populations. These yca1 mutants probably lack the capability to adapt to new environments, perhaps due to damage that normally would cause these cells to be eliminated by apoptosis, thus leaving only the youngest, fittest, and best adapted cells. Consistently, when yca1 disruptants have to compete against wild-type cells, the latter ones show better survival in the end.
Materials and methods
Strains, plasmids, and growth conditions
Experiments were performed in three wild-type backgrounds, DBY2068 (Mat a; ura3-52; leu2-3,112; his4-619) derived from S288C, BY4741 (Mat a; his3Δ1; leuΔ0; met15Δ0; ura3Δ0; from EUROSCARF), and W303 (Mat a; ura3-52; trp1Δ2; leu2-3,112; his3-11; ade2-1; can1-100). Experiments with the yca1 disruption strain were performed in the BY4741 background (yca1::kanMX4). For the experiments with YAP1, expression pFW300 and the corresponding empty vector YEp351 in W303 background were used (Wendler et al., 1997). In this vector, YAP1 is under the control of its own promoter region. Northern hybridization was performed as described previously (Jungwirth et al., 2001).
Strains were grown on SC medium consisting of 0.17% yeast nitrogen base (Difco), 5% (NH4)2SO4, and 2% glucose or 3% ethanol, supplemented with 30 mg/l of all amino acids (except 80 mg/l histidine and 200 mg/l leucine), 30 mg/l adenine, and 320 mg/l uracil. The YAP1 expression strains were grown in SC medium without leucine.
For aging experiments, cultures were inoculated from fresh overnight cultures (culture volume should be 10% of the flask volume), and aliquots were taken out to perform survival plating and tests for apoptotic markers.
Test for apoptotic markers
For survival platings, cell cultures were diluted, cell concentration was determined with a CASY cell counter, and aliquots containing 1,000 cells were plated on YPD plates. The number of colonies was determined after 2 d at 28°C.
Annexin V labeling (CLONTECH Laboratories, Inc.), DHR and DAPI staining, TUNEL assay (In Situ Cell Death Detection Kit, POD; Boehringer), and the measurement of caspase activity (CaspACE™; Promega) were performed as described previously (Madeo et al., 1997, 1999, 2002). As cells from stationary phase have thicker cell walls, we modified the protocol for cell wall digestion for all cultures using 120 U lyticase (Sigma-Aldrich) and 75 μl β-glucuronidase/arylsulfatase (Roche) per ml cell suspension for 2 h at 30°C. To determine frequencies of phenotypes (TUNEL, annexin V, DAPI, and DHR), at least 300 cells of three independent experiments were evaluated. For image acquisition, we used a fluorescence microscope (model BH-2RFCA; Olympus), a digital camera (model c35AD-4; Olympus), and analySIS® software (Soft Imaging System GmbH).
Isolation of cell secretions
For the isolation of secretions released from cells, 4 l SC medium was inoculated with strain S288C, grown for 1 or 8 d, harvested (for 12 min at 8,000 rpm in Heraeus Suprafuge 22, rotor HFA 12.500), washed three times with 1 l bidistilled water, and resuspended in 4 l bidistilled water. After 3 d shaking at 28°C in bidistilled water, the cells were harvested as before. To remove residual cells, the supernatant was centrifuged again and filter sterilized (0.22-μm pore size). It was then concentrated in a rotation evaporator (Rotavapor R110; Büchi) and lyophilized. The average amount of dried secretions we gained was ∼0.6 g out of 4 l culture volume. The dried secretions were stored at −20°C. It was suspended in bidistilled water to a final concentration of 200 mg/ml.
Acknowledgments
We are grateful to William C. Burhans for critical reading of the manuscript and for helpful discussion.
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds zur Förderung der wissenschaftlichen Forschung (Austria).
E. Herker and H. Jungwirth contributed equally to this paper.
Abbreviations used in this paper: DHR, dihydrorhodamine 123; ROS, reactive oxygen species; SC, synthetic complete.
References
- Blanchard, F., M.E. Rusiniak, K. Sharma, X. Sun, I. Todorov, M.M. Castellano, C. Gutierrez, H. Baumann, and W.C. Burhans. 2002. Targeted destruction of DNA replication protein Cdc6 by cell death pathways in mammals and yeast. Mol. Biol. Cell. 13:1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski, H., and E. Goldman. 1988. Evidence for cooperation between cells during sporulation of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 8:5166–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson, D.J., S.L. Rivers, and D.W. Stephen. 1994. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology. 140:3277–3283. [DOI] [PubMed] [Google Scholar]
- Jungwirth, H., H. Bergler, and G. Högenauer. 2001. Diazaborine treatment of Baker's yeast results in stabilization of aberrant mRNAs. J. Biol. Chem. 276:36419–36424. [DOI] [PubMed] [Google Scholar]
- Laun, P., A. Pichova, F. Madeo, J. Fuchs, A. Ellinger, S. Kohlwein, I. Dawes, K.U. Fröhlich, and M. Breitenbach. 2001. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 39:1166–1173. [PubMed] [Google Scholar]
- Longo, V.D., L.M. Ellerby, D.E. Bredesen, J.S. Valentine, and E.B. Gralla. 1997. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 137:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico, P., F. Rodrigues, A. Almeida, M.T. Silva, A. Barrientos, and M. Corte-Real. 2002. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell. 13:2598–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, M., N. Harris, and P.W. Piper. 2001. Chronological lifespan of stationary phase yeast cells; a model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms. Yeast. 18:499–509. [DOI] [PubMed] [Google Scholar]
- Madeo, F., E. Fröhlich, and K.U. Fröhlich. 1997. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Fröhlich, M. Ligr, M. Grey, S.J. Sigrist, D.H. Wolf, and K.U. Fröhlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lächelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K.U. Fröhlich. 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 9:911–917. [DOI] [PubMed] [Google Scholar]
- Martin, S.J., C.P. Reutelingsperger, A.J. McGahon, J.A. Rader, R.C. van Schie, D.M. LaFace, and D.R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, C., P. Mancini, L. Verdone, F. Madeo, A. Serafini, E. Herker, and C. Falcone. 2003. A truncated form of KlLsm4p and the absence of factors involved in mRNA decapping trigger apoptosis in yeast. Mol. Biol. Cell. 14:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye-Rowley, W.S., K.D. Harshman, and C.S. Parker. 1989. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 3:283–292. [DOI] [PubMed] [Google Scholar]
- Rozman-Pungercar, J., N. Kopitar-Jerala, M. Bogyo, D. Turk, O. Vasiljeva, I. Stefe, P. Vandenabeele, D. Bromme, V. Puizdar, M. Fonovic, et al. 2003. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 10:881–888. [DOI] [PubMed] [Google Scholar]
- Shirogane, T., T. Fukada, J.M. Muller, D.T. Shima, M. Hibi, and T. Hirano. 1999. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 11:709–719. [DOI] [PubMed] [Google Scholar]
- Steller, H. 1995. Mechanisms and genes of cellular suicide. Science. 267:1445–1449. [DOI] [PubMed] [Google Scholar]
- Wendler, F., H. Bergler, K. Prutej, H. Jungwirth, G. Zisser, K. Kuchler, and G. Högenauer. 1997. Diazaborine resistance in the yeast Saccharomyces cerevisiae reveals a link between YAP1 and the pleiotropic drug resistance genes PDR1 and PDR3. J. Biol. Chem. 272:27091–27098. [DOI] [PubMed] [Google Scholar]