Abstract
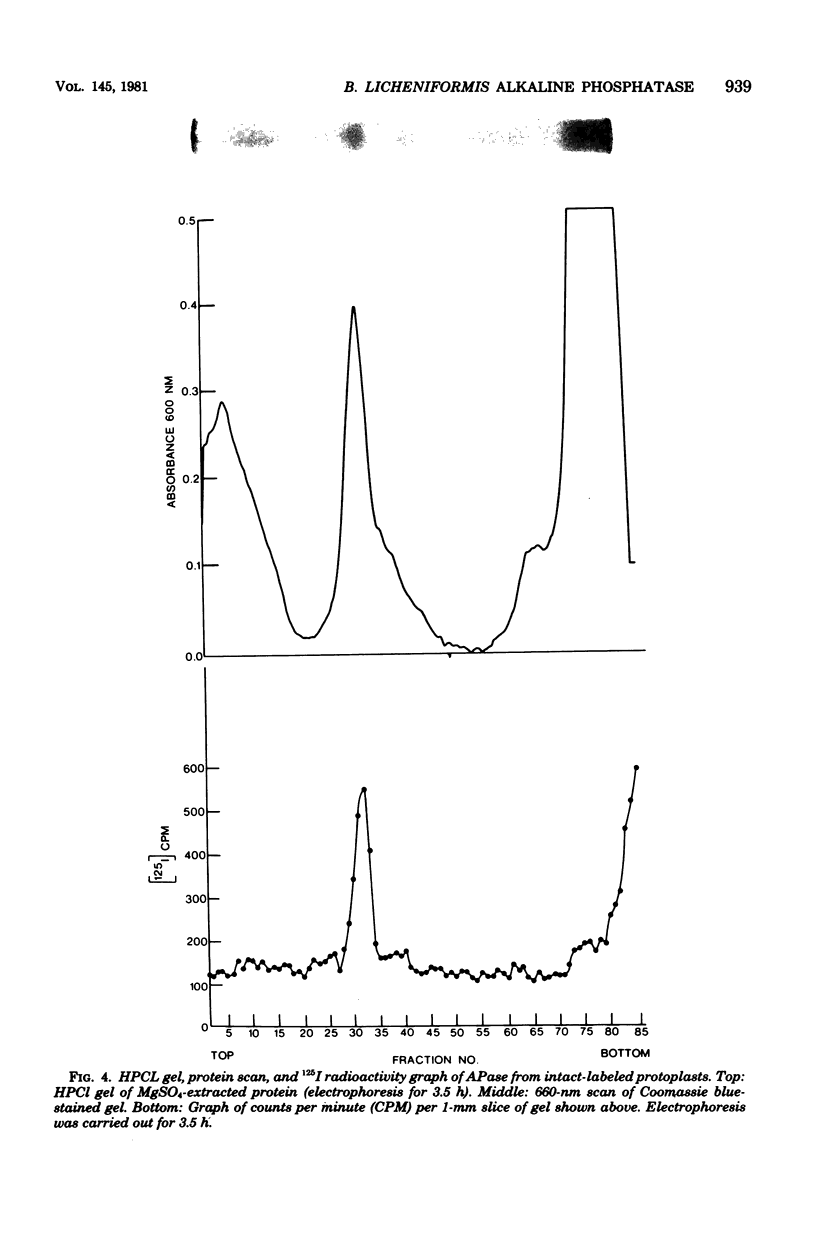
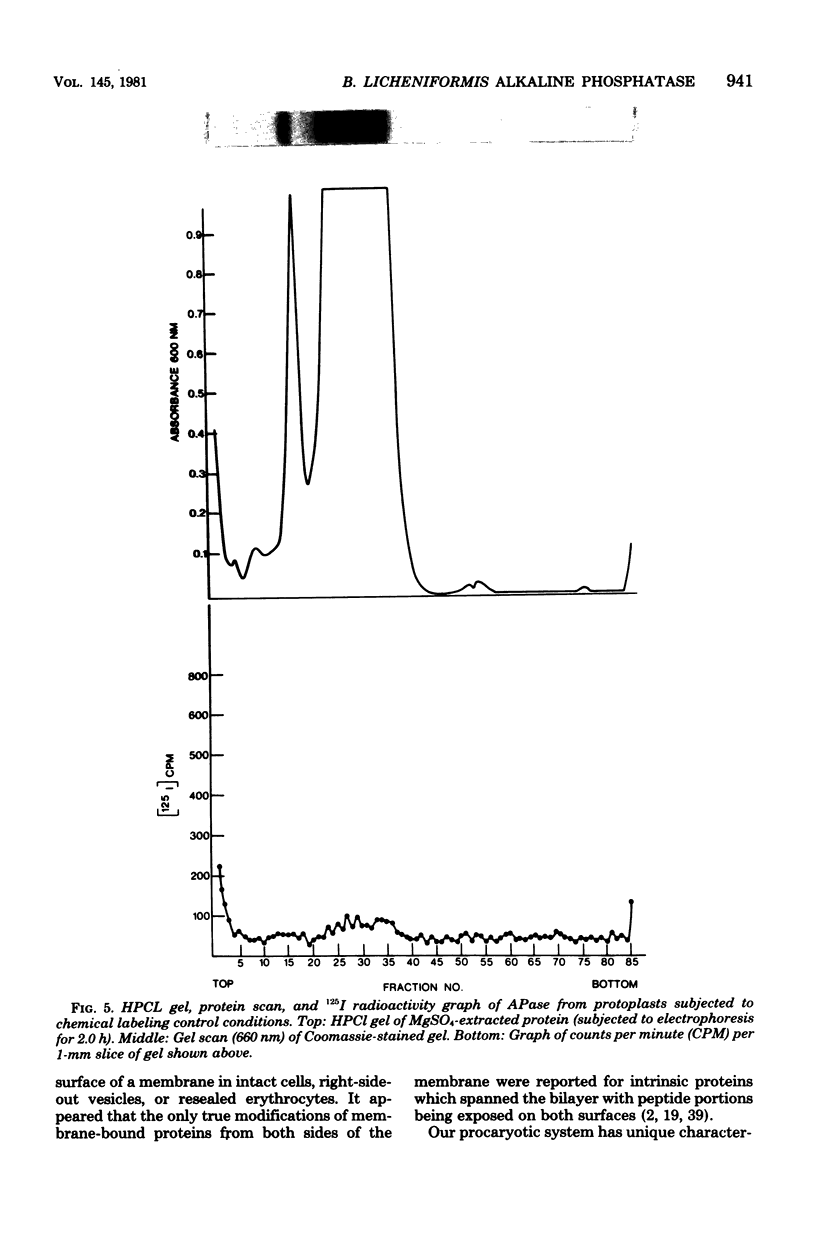
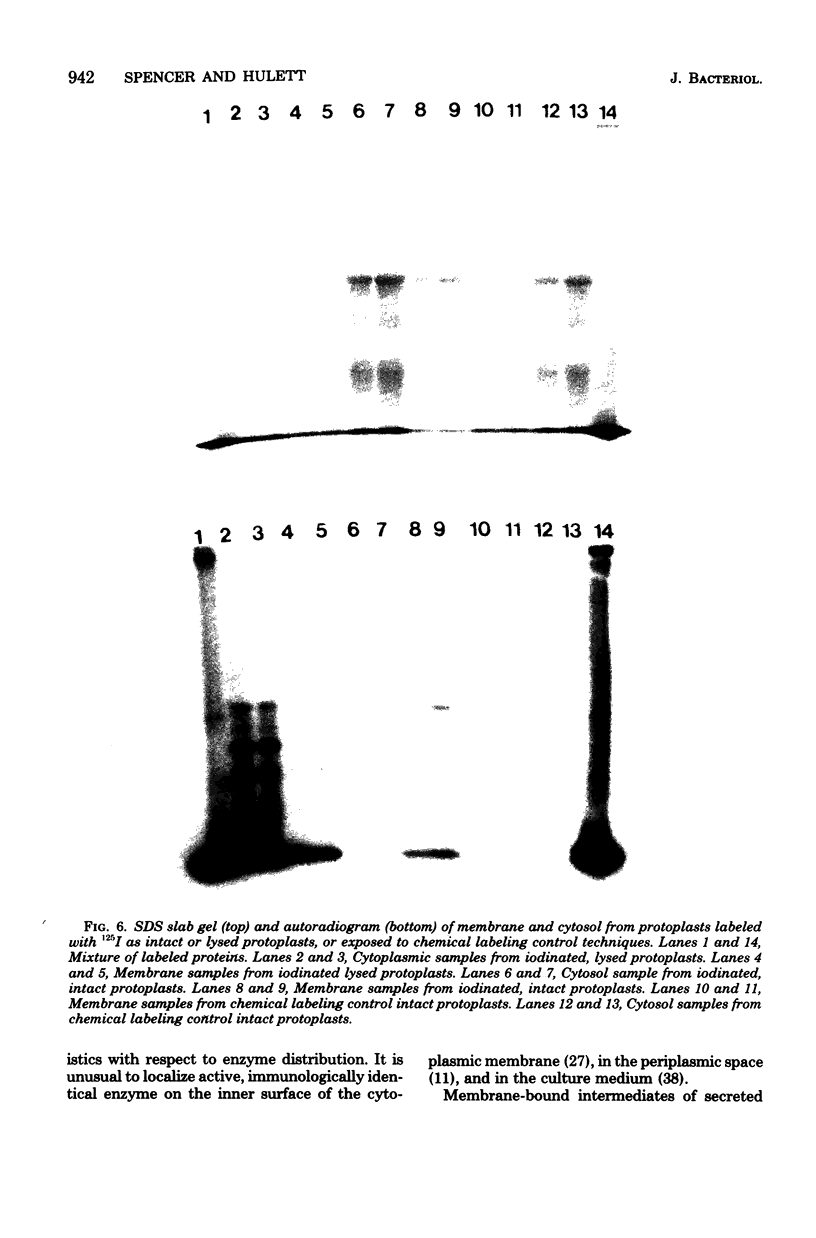
Previous histochemical and biochemical localizations of alkaline phosphatase in Bacillus licheniformis MC14 have shown that the membrane-associated form of the enzyme is located on the inner surface of the cytoplasmic membrane, and soluble forms are located in the periplasmic space and in the growth medium. The distribution of salt-extractable alkaline phosphatase on the surfaces of the cytoplasmic membrane of B. licheniformis MC14 was determined by using lactoperoxidase-125I labeling techniques. Cells harvested during rapid alkaline phosphatase production were converted to protoplasts or lysed protoplasts and labeled. Analysis of the data obtained indicated that 30% of the salt-extractable, membrane-associated alkaline phosphatase was located on the outer surface of the cytoplasmic membrane, whereas 70% of the membrane-associated enzyme was localized on the inner surface. Controls for protoplast integrity (release of tritiated thymidine or examination of cytoplasmic proteins for label content) indicated excellent protoplast stability. Controls indicated that chemical labeling was not a factor in the apparent distribution of alkaline phosphatase on the membrane. These results support the previously reported histochemical localization of alkaline phosphatase on the membrane inner surface. The presence of alkaline phosphatase on the membrane outer surface is reasonable, considering the soluble forms of the enzyme found in the periplasmic region and in the culture medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Carraway K. L. Covalent labeling of membranes. Biochim Biophys Acta. 1975 Dec 29;415(4):379–410. doi: 10.1016/0304-4157(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Cashel M., Freese E. Excretion of alkaline phosphatase of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):541–544. doi: 10.1016/0006-291x(64)90189-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Ghosh B. K. Immunoelectron microscopic localization of penicillinase in Bacillus licheniformis. J Bacteriol. 1979 Mar;137(3):1374–1385. doi: 10.1128/jb.137.3.1374-1385.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Wouters J. T., Lampen J. O. Distribution of the sites of alkaline phosphatase(s) activity in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):928–937. doi: 10.1128/jb.108.2.928-937.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Ghosh A., Ghosh B. K. Properties of the membrane-bound alkaline phosphatase from glucose- and lactate-grown cells of Bacillus subtilis SB 15. J Biol Chem. 1977 Oct 10;252(19):6813–6822. [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. I. Isolation and characterization. J Biol Chem. 1971 Mar 25;246(6):1556–1565. [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Glynn J. A., Schaffel S. D., McNicholas J. M., Hulett F. M. Biochemical localization of the alkaline phosphatase of Bacillus licheniformis as a function of culture age. J Bacteriol. 1977 Feb;129(2):1010–1019. doi: 10.1128/jb.129.2.1010-1019.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D. Cell wall teichoic acid as a reserve phosphate source in Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):35–43. doi: 10.1128/jb.137.1.35-43.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Molecular weight and subunits of the alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1371–1376. doi: 10.1021/bi00784a015. [DOI] [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Purification and properties of an alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1364–1371. doi: 10.1021/bi00784a014. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., Schaffel S. D., Campbell L. L. Subunits of the alkaline phosphatase of Bacillus licheniformis: chemical, physicochemical, and dissociation studies. J Bacteriol. 1976 Nov;128(2):651–657. doi: 10.1128/jb.128.2.651-657.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. Phosphate utilization and alkaline phosphatase activity in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):23–28. doi: 10.1007/BF00428340. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Resolution and purification of three periplasmic phosphatases of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):399–410. doi: 10.1128/jb.130.1.399-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Hubbard A. L., Sabatini D. D. On the spatial arrangememt of proteins in microsomal membranes from rat liver. J Cell Biol. 1974 Mar;60(3):616–627. doi: 10.1083/jcb.60.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K., Pratt C., Torriani A. Genetic analysis of regulatory mutants of alkaline phosphatase of E. coli. Genetics. 1975 Nov;81(3):459–468. doi: 10.1093/genetics/81.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampen J. O. Movement of extracellular enzymes across cell membranes. Symp Soc Exp Biol. 1974;(28):351–374. [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas J. M., Hulett F. M. Electron microscope histochemical localization of alkaline phosphatase(s) in Bacillus licheniformis. J Bacteriol. 1977 Jan;129(1):501–515. doi: 10.1128/jb.129.1.501-515.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Hubbard A., Ferris B. Iodination of the human platelet membrane. Studies of the major surface glycoprotein. J Biol Chem. 1973 Apr 25;248(8):2928–2936. [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichstein E., Blostein R. Asymmetric iodination of the human erythrocyte membrane. Biochem Biophys Res Commun. 1973 Sep 18;54(2):494–500. doi: 10.1016/0006-291x(73)91448-4. [DOI] [PubMed] [Google Scholar]
- Schaffel S. D., Hulett F. M. Alkaline phosphatase from Bacillus licheniformis. Solubility dependent on magnesium, purification and characterization. Biochim Biophys Acta. 1978 Oct 12;526(2):457–467. doi: 10.1016/0005-2744(78)90137-7. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]
- Schneider D. L., Kagawa Y., Racker E. Chemical modification of the inner mitochondrial membrane. J Biol Chem. 1972 Jun 25;247(12):4074–4079. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Spencer D. B., Chen C. P., Hulett F. M. Effect of cobalt on synthesis and activation of Bacillus licheniformis alkaline phosphatase. J Bacteriol. 1981 Feb;145(2):926–933. doi: 10.1128/jb.145.2.926-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staros J. V., Richards F. M. Photochemical labeling of the cytoplasmic surface of the membranes of intact human erythrocytes. J Biol Chem. 1975 Oct 25;250(20):8174–8178. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Traficante L. J., Lampen J. O. Vesicle penicillinase of Bacillus licheniformis: existence of periplasmic-releasing factor(s). J Bacteriol. 1977 Jan;129(1):184–190. doi: 10.1128/jb.129.1.184-190.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A. S. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):616–623. doi: 10.1128/jb.110.2.616-623.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. A., Tristram H. Localization in the Cell and Extraction of Alkaline Phosphatase from Bacillus subtilis. J Bacteriol. 1970 Dec;104(3):1045–1051. doi: 10.1128/jb.104.3.1045-1051.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]