Abstract
Aging is believed to be a nonadaptive process that escapes the force of natural selection. Here, we challenge this dogma by showing that yeast laboratory strains and strains isolated from grapes undergo an age- and pH-dependent death with features of mammalian programmed cell death (apoptosis). After 90–99% of the population dies, a small mutant subpopulation uses the nutrients released by dead cells to grow. This adaptive regrowth is inversely correlated with protection against superoxide toxicity and life span and is associated with elevated age-dependent release of nutrients and increased mutation frequency. Computational simulations confirm that premature aging together with a relatively high mutation frequency can result in a major advantage in adaptation to changing environments. These results suggest that under conditions that model natural environments, yeast organisms undergo an altruistic and premature aging and death program, mediated in part by superoxide. The role of similar pathways in the regulation of longevity in organisms ranging from yeast to mice raises the possibility that mammals may also undergo programmed aging.
Keywords: apoptosis; adaptive regrowth; programmed aging; superoxide; oxidtive stress
Introduction
Longevity in yeast, worms, flies, and mice can be extended by up to severalfold by mutations that increase protection against superoxide and other stresses (Longo and Finch, 2003). Although several mutations that extend longevity cause growth defects, some long-lived mutants grow and reproduce at normal rates. For example, certain yeast and worm mutants have doubled life spans but grow normally (Longo, 1997; Dillin et al., 2002; Fabrizio et al., 2003). If antioxidant defenses and life span can be increased without affecting reproduction, it is surprising that organisms do not express higher levels of antioxidant enzymes or acquire mutations that increase stress resistance and longevity. One explanation may be that the fitness cost of life span extension is not easily detectable. In fact, in Caenorhabditis elegans, a mutation that increases the lifespan does not cause a measurable reproductive disadvantage but impairs the ability to undergo cycles of feeding and starvation (Walker et al., 2000). It is also possible that in certain organisms the fitness cost of life span extension is at the population and not individual level (Longo, 1997; Longo et al., 1997; Skulachev, 2002). Although the rapid senescence and death of pacific salmon after spawning and the sudden death of certain organisms after reproduction (Finch, 1990) is consistent with programmed death, an altruistic program that causes accelerated aging and premature death has not been demonstrated in any organism.
Analogously to C. elegans and some hibernating mammals, yeast enter different phases based on the availability of nutrients. After growing to the maximum density by using the glucose and other nutrients available on rotting fruit, yeast populations can enter a low metabolism stationary phase and survive for weeks or generate dormant spores that can remain viable for years (Phaff et al., 1966). Moreover, when incubated in medium containing a high concentration of glucose (2% synthetic dextrose complete [SDC] medium) yeast can enter a third survival phase characterized by high metabolic rates and a shorter life span (Longo and Fabrizio, 2002; Fabrizio et al., 2003). We termed survival in these low and high metabolism phases “chronological life span” (Longo, 1997; Fabrizio and Longo, 2003). Saccharomyces cerevisiae wild-type strains DBY746 and SP1 grown in SDC medium have a mean survival of 6 d (see Fig. 1 A; Longo et al., 1996, 1997). Survival in SDC medium provides a model for natural environments such as fruits that contain a high level of glucose (rotting figs), from which ancestors of the wild-type yeast strains used in our experiments were isolated (Mortimer and Johnston, 1986). Because the death of a yeast population is mediated in part by mitochondrial superoxide (Fabrizio et al., 2001, 2003) and is delayed by overexpression of superoxide dismutases (Sods) or the human antiapoptotic protein Bcl-2, we hypothesized that it may represent a form of programmed death (Longo et al., 1997). Apoptosis has been shown to occur in yeast after treatment with hydrogen peroxide or acetate (Madeo et al., 1997, 1999; Ludovico et al., 2001; Skulachev, 2002). Analogously to mammalian cells, a caspase-related protease regulates hydrogen peroxide–induced programmed cell death in yeast (Madeo et al., 2002). Although markers of apoptosis have been detected in yeast cells undergoing replicative or chronological senescence (Laun et al., 2001; Herker et al., 2004), the existence programmed or altruistic aging has not been demonstrated in any organism.
Figure 1.
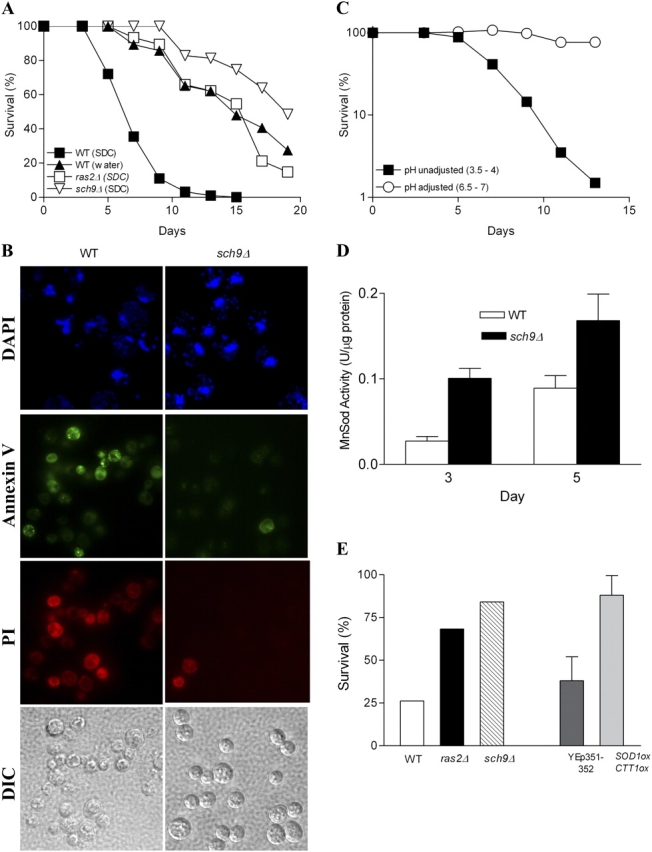
Aging yeast cells show features of apoptotic death. (A) Chronological survival of wild-type (DBY746) and long-lived mutants ras2Δ and sch9Δ populations. Life span measurement were performed by incubating wild-type (WT) cells, ras2Δ, and sch9Δ mutants either in synthetic dextrose complete (SDC) medium or in water. Viability is expressed as percent survival (100% at day 3). Results shown represent the average of 4–12 experiments. (B) Day 7 wild-type and sch9Δ mutants were stained with DAPI for analysis of nuclear morphology (top), GFP-labeled annexin V for detection of exposed phosphatidylserine and propidium iodide for detection of damaged/dead cells (middle panels). Approximately 25–30 cells for each strain were present on each field (bottom, DIC images) and were treated simultaneously with annexin V and propidium iodide. (C) Chronological survival of wild-type cells incubated in standard SDC medium (pH 3.5–4) and in SDC medium with pH adjusted and maintained at 6.5–7 from day 3 to 13. The experiment was repeated five times with similar results. A representative experiment is shown. (D) Mitochondrial Sod (Sod2) activity of wild-type and sch9Δ mutants was measured at day 3 and 5. Results shown represent the average of two experiments conducted in triplicate. Error bars show SEM. (E) Oxidative stress resistance of wild type, ras2Δ mutants, sch9Δ mutants, wild type overexpressing control vectors YEp351-YEp352, and SOD1/SOD2 overexpressors. Cells removed from day 3 in the postdiauxic phase were incubated for 60 min in phosphate buffer containing 200 μM of the superoxide H2O2-generating agent menadione. Viability was measured by plating cells onto rich medium before and after treatment. A representative experiment performed in duplicate is shown for the deletion strains. The average of two experiments is shown for the overexpressors. Error bars show SEM.
Here, we study several yeast strains isolated from grapes and three different laboratory wild-type strains under conditions that model natural environments and provide evidence for the role of superoxide in an altruistic aging and death program that kills over 90% of a yeast population to release nutrients that promote the growth of a small better-adapted mutant subpopulation.
Results
Features of apoptotic death in aging yeast
SCH9 encodes for a serine/threonine kinase that promotes aging and death in yeast (Fig. 1 A; Fabrizio et al., 2001, 2003). The deletion of RAS2 and incubation in water also prevent age-dependent death (Fig. 1 A; Longo et al., 1997; Fabrizio et al., 2003). To determine whether or not the death of the yeast population is programmed, we looked for morphological and biochemical features of apoptosis. Chromatin condensation, which is a major characteristic of mammalian cell apoptosis, is observed in yeast undergoing hydrogen peroxide– or Bax-dependent death (Madeo et al., 1997, 2002; Jin and Reed, 2002). At day 7, during the major mortality phase, chromatin staining of wild-type yeast with DAPI shows abnormal nuclear morphology including chromatin condensation and multiple stained regions (Fig. 1 B). Similar results have been reported by Herker et al. (2004). In contrast, the 7-d-old population of sch9Δ mutants maintains nuclear morphology similar to that of young cells as suggested by the absence of chromatin condensation and multiple DAPI-stained nuclear regions (Fig. 1 B). Apoptotic mammalian cells lose the ability to maintain phosphatidylserine predominantly in the inner leaflet of the plasma membrane. Consequently, phosphatidylserine is detected on the outer plasma membrane. Annexin V staining of aging yeast populations indicates that at day 7, phosphatidylserine is located in the outer phase of the cytoplasmic membrane (Fig. 1 B). The small percentage of propidium iodide–stained yeast confirmed that the annexin V did not simply stain phospatidylserines exposed after cell death (Fig. 1 B). Neither annexin V nor propidium iodide stained the long-lived sch9Δ mutants (Fig. 1 B). Another early event associated with apoptosis in mammalian cells is the alkalinization of the mitochondrial matrix and the acidification of the cytosol (Matsuyama et al., 2000). We tested if cytosolic acidification contributes to aging and death by monitoring survival after increasing the pH (Imai and Ohno, 1995). Raising the extracellular pH from 5 to 6.5 causes an increase of intracellular pH from 5.8 to 6.2 (Imai and Ohno, 1995). Death was prevented by increasing the extracellular pH at days 3–11 from the physiologic 3.5–4 pH (days 1–13) to pH 6.5 (Fig. 1 C). The effect of low pH on aging and death may be explained by a role for intracellular acidification in the activation of the Ras pathway (Thevelein and de Winde, 1999). In fact, the deletion of RAS2 doubles the life span of yeast cells (Fig. 1 A; Fabrizio et al., 2003).
Superoxide and Sods play major roles in the aging and death of yeast and higher eukaryotes (Honda and Honda, 1999; Sun and Tower, 1999; Fabrizio et al., 2001, 2003) and in the apoptosis of mammalian cells (Tanaka et al., 2002). Furthermore, mitochondrial Sod (Sod2) functions downstream of Sch9 and Ras2 to extend longevity in yeast (Fabrizio et al., 2003). To determine whether or not wild-type yeast may down-regulate Sod2 as part of an aging and death program, we assayed the activity of Sod2, which is expressed at low levels during growth in glucose medium but increases by 5–10-fold in carbon sources that require mitochondrial respiration (Maris et al., 2001). Although respiratory rates are high at day three (Fabrizio et al., 2003), mitochondrial Sod2 activity in wild-type extracts from day 3 and 5 was two- to threefold lower than in sch9Δ mutant extracts (Fig. 1 D). Furthermore, the long-lived sch9Δ and ras2Δ mutants and SOD1CTT1 overexpressors were resistant to the superoxide-generating agent menadione and to hydrogen peroxide (Fig. 1 E and not depicted), indicating that the wild-type yeast population is underprotected against oxidative toxicity during a phase in which mitochondrial respiration and oxidant generation are high. Together, these data suggest that yeast grown in SDC medium undergo an age-dependent death with characteristics of mammalian apoptosis including chromatin condensation, exposure of phosphatidylserine, cytosolic acidification, low Sod2 activity, and sensitivity to superoxide and hydrogen peroxide. These results are consistent with recent data showing chromatin condensation and exposure to phosphatidylserine in yeast cells aging chronologically (Herker et al., 2004). The ability of a switch from SDC medium to water or of mutations in the Sch9 and Ras pathways to cause a major delay in cell death (Fig. 1 A) is also consistent with the existence of programmed aging.
Budding yeast isolated from grapes undergo age-dependent death and adaptive regrowth
To determine if “programmed aging” may be limited to laboratory yeast strains, we isolated yeast cells from organically grown California red grapes. We selected cells that grew within 48 h in YPD medium, generated colonies similar to those formed by S. cerevisiae, and divided by asymmetric budding (Fig. 2 A). The survival of populations generated from cells derived from three different wild colonies was similar to that of wild-type laboratory strains (Fig. 2 B). After ∼90–99% of the “wild” population died (day 3–11), the number of viable cells in the culture increased, suggesting that adaptive regrowth is occurring. In contrast, incubation in water prevented age-dependent death and regrowth (Fig. 2 C). As observed in the laboratory wild-type strains, the pH of wild yeast grown in SDC medium decreases to approximately pH 3 by day 1. Adjustment of the extracellular pH from 3 to 6.5 prevented age-dependent death (Fig. 2 D). These data confirm our results with laboratory strains and suggest that programmed aging occurs in wild strains isolated from natural environments.
Figure 2.
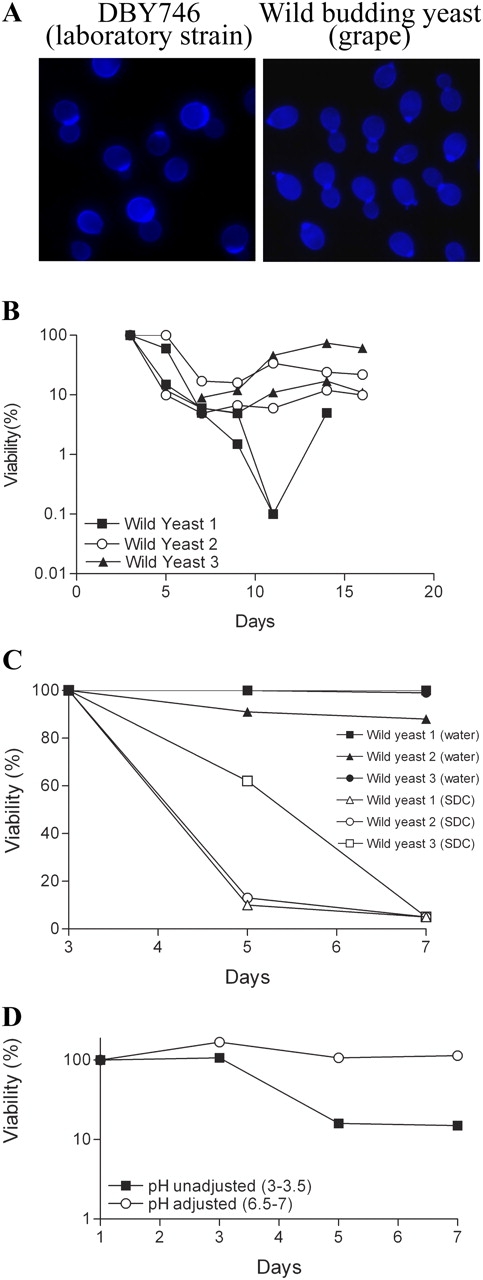
Adaptive regrowth in wild yeast. (A) Wild-type DBY746 and wild yeast isolated from grapes were stained with Calcofluor to analyze their morphology and budding pattern. Chronological survival/regrowth of wild yeast cells incubated in SDC medium (B), water (C), and standard SDC medium (pH 3–3.5) and SDC medium with pH adjusted and maintained at 6.5–7 from day 1 to 7 (D). Viability was measured every 2 d. These experiments were performed twice in duplicate with similar results.
Effect of superoxide and hydrogen peroxide in promoting adaptive regrowth
We observed that in many laboratory wild-type cultures, the number of viable organisms (colony-forming units) increases after 90–99% of the population dies (Fig. 3 A). This adaptive regrowth, which is reminiscent of the growth (gasping) of bacterial populations maintained in stationary phase (Zambrano and Kolter, 1996), is important for adaptation to starvation conditions. To determine whether or not early death may be adaptive, we monitored the ability of various wild-type, short-, and long-lived populations to regrow during stationary phase by using nutrients available in the expired medium (Fig. 3 A and Table I). In ∼50% of wild-type cultures, a small subpopulation regrew after day 10, when the majority of the population had lost viability (88 flasks; Table I and Fig. 3). Increased levels of cytosolic superoxide, which we have previously shown to play a major role in the chronological aging of yeast, caused increased adaptation as evidenced by adaptive regrowth in 89% of populations of yeast lacking SOD1 (sod1Δ) and 75% of those lacking both SOD1 and catalase (sod1Δ ctt1Δ; Fig. 3, A and B; and Table I). In most studies, sod1Δ and sod1Δ ctt1Δ mutants died within 24 h and were able to grow back within the first 7 d (Fig. 3, A and B). The deletion of SOD1 also caused early growth in the parental strain BY4700, which is more closely related to the strains originally isolated on rotting figs (Mortimer and Johnston, 1986). Strain BY 4700/sod1Δ exhibited an early adaptation advantage compared with wild-type strain BY4700 (Fig. 3 C), confirming our results with strains DBY746. However, the strain lacking SOD1 eventually died, whereas the wild-type strain reached and maintained a viability of ∼1% of the initial viability for over 60 d (Fig. 3 C and not depicted). sod1Δ mutants in the DBY746 genetic background also died after more than 40 d in culture (unpublished data). These results suggest that the beneficial effect of greatly elevated superoxide on adaptive regrowth is only temporary and may explain in part why natural selection has prevented loss-of-function mutations in the SOD1 gene.
Figure 3.
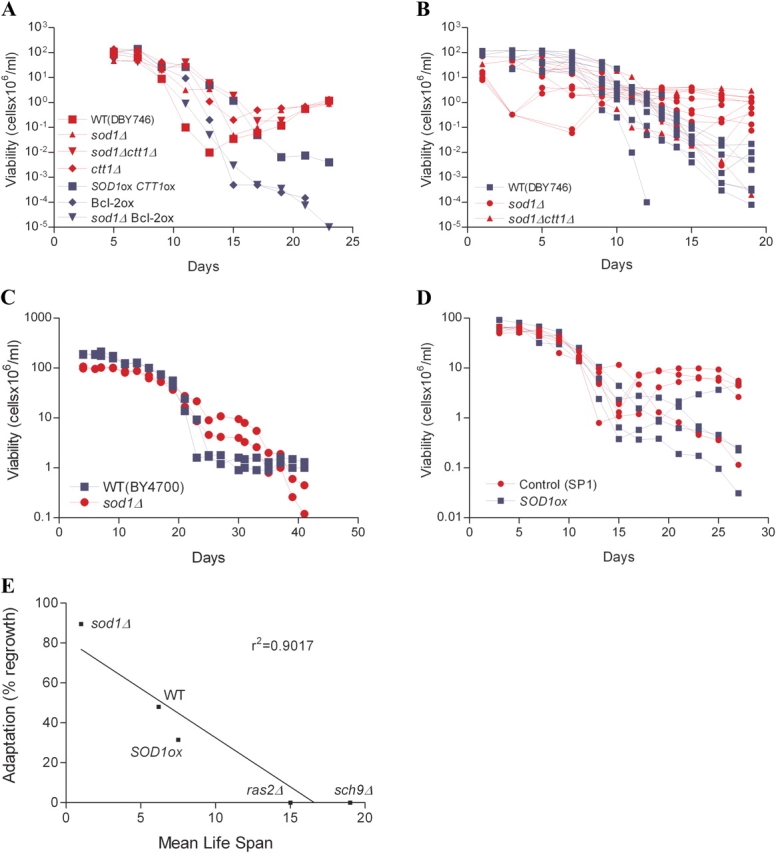
Adaptive regrowth in short- and long-lived strains. Red lines and symbols are used for strains that have a regrowth advantage relative to the other strain(s) (blue) in that experiment. (A) The ability to regrow after the majority of the population had died was monitored in various wild-type (WT DBY746 and control strain YEp351-YEp352), short-lived mutants lacking cytosolic Sod (sod1Δ and sod1Δ ctt1Δ), and long-lived strains overexpressing antioxidant enzymes or the antiapoptotic mammalian protein Bcl-2 (SOD1ox CTT1ox and Bcl-2ox). sod1Δ mutants overexpressing Bcl-2 and ctt1Δ mutants were also included in the study. A single representative experiment is shown (see Table I for all experiments). (B) Adaptive regrowth was monitored in DBY746 wild-type (blue line) and short-lived strains (sod1Δ and sod1Δ ctt1Δ; red line). Results from four different experiments are plotted. Each line represents a single sample. A total of 8 wild-type samples, 11 sod1Δ, and 3 sod1Δ ctt1Δ is shown. (C) Adaptive regrowth of wild-type strain BY4700 and of a sod1Δ mutant originated in the same background. The experiment was performed three times in duplicate. A representative experiment is shown. (D) Adaptive regrowth of wild-type strain SP1 and of SOD1 overexpressors originated in the same background. A representative experiment is shown. (E) Correlation between adaptive regrowth and mean life span (r = 0.90).
Table I.
Adaptive regrowth in yeast with different life span
| Strain | Adaptive regrowth
|
Mean Life Span |
||
|---|---|---|---|---|
| Yes | No | Percentage of regrowth |
||
| DBY746 (WT) | 42 | 46 | 48 | 6.2 |
| sod1Δ | 17 | 2 | 89c | 1 |
| sod1Δ ctt1Δ | 3 | 1 | 75 | 1 |
| ctt1Δ | 1 | 1 | 50 | 6.2 |
| sod1Δ Bcl-2 | 4 | 6 | 40 | 6.2 |
| SOD1ox SOD2ox | 0 | 9 | 0c | 7.8 |
| SOD1ox CTT1ox | 0 | 13 | 0c | 7.5 |
| SOD1ox | 6 | 13 | 31.5c | 7.2 |
| SOD2ox | 0 | 6 | 0c | 6.65 |
| CTT1ox | 3 | 7 | 30c | 6.2 |
| Bcl-2ox | 0 | 9 | 0c | 7 |
| ras2Δ | 0 | 15 | 0c | 15 |
| sch9Δ | 0 | 14 | 0c | 19 |
| yap1Δ | 1 | 10 | 10c | 6 |
| skn7Δ | 2 | 10 | 20c | 6 |
| SP1 (WT) | 3 | 1 | 75 | 7.8 |
| SOD1ox | 1 | 3 | 25 | 9 |
| Summary | ||||
| WT | 45 | 47 | 49c | 7 |
| Long-liveda | 6 | 82 | 6.8c | 9.9 |
| Short-livedb | 20 | 3 | 87c | 1 |
Superoxide resistant: SOD1ox SOD2ox, SOD1ox CTT1ox, SOD1ox, SOD2ox, Bcl-2ox, ras2Δ, and sch9Δ.
Superoxide sensitive: sod1Δ and sod1Δ ctt1Δ.
Significantly different versus wild type (WT). When four or less samples were available, the corresponding strains were excluded from statistical analysis. Chi square test (χ2 = 64, df = 10, P < 0.05) followed by Scheffé's post-test for multiple comparisons (P < 0.05).
To further test the hypothesis that the oxidant-dependent adaptation is related to mammalian apoptosis, we overexpressed the human antiapoptotic protein Bcl-2 in sod1Δ mutants and wild-type yeast. Bcl-2 overexpression, which extends survival (Longo et al., 1997), caused a major decrease in the frequency of adaptive regrowth of both sod1Δ mutants and wild-type cultures (Fig. 3 A and Table I). The overexpression of SOD1 in sod1Δ mutants also prevented adaptive regrowth (unpublished data).
In contrast to wild-type and sod1Δ mutants, long-lived yeast overexpressing antioxidant enzymes (SOD2ox, SOD1ox SOD2ox, and SOD1ox CTT1ox), lacking RAS2 (ras2Δ) or lacking SCH9 (sch9Δ), exhibited a major reduction of regrowth frequency (Fig. 3 A and Table I). Adaptive regrowth was not observed in any of the cultures overexpressing both SOD1 and catalase (SOD1ox CTT1ox; Fig. 3 A, and Table I, 13 cultures) and was reduced from a 48 to 31% frequency by the overexpression of SOD1 (19 cultures; Table I). Overexpression of SOD1 also caused a major adaptive regrowth disadvantage in another parental strain (SP1; Fig. 3 D and Table I).
Growth under starvation condition was not observed in any of the 29 independent cultures of the long-lived ras2Δ and sch9Δ (Table I). This effect of mutations in the RAS2 or SCH9 genes on stationary phase regrowth may be mediated, in part, by decreased superoxide and hydrogen peroxide levels. In fact, deletion mutations in RAS2 or SCH9 increase resistance to both superoxide and hydrogen peroxide (Fig. 1 E), decrease the level of superoxide, and extend longevity by inducing the expression of Sod2 and other stress resistance genes (Fabrizio et al., 2001, 2003).
In summary, regrowth was observed in 90% of the cultures of short-lived mutants deficient in SOD1, in 50% of wild-type cultures, but in <10% of long-lived yeast overexpressing antioxidant enzymes (Table I). The strong inverse correlation between mean life span and the ability to grow in stationary phase (r = 0.90; Fig. 3 E) is consistent with a role for superoxide and hydrogen peroxide in a death program that increases the chance of adapting to a nutrient-poor environment. These results are also consistent with the initiation of caspase-dependent apoptosis in yeast exposed to hydrogen peroxide (Madeo et al., 2002). However, a major increase in longevity may not be required to prevent adaptive growth. In fact, the overexpression of SOD1CTT1 or SOD1SOD2 prevents growth in stationary phase cultures but only causes a 10–30% increase in life span.
Adaptive regrowth in media that model natural environments
To test whether or not the age-dependent adaptive regrowth may be an artifact of the laboratory medium, we monitored viability in yeast cultures aged in medium obtained by processing organic red grapes or in medium containing 10% of the ammonium sulfate compared with the standard SDC medium. This decrease in ammonium sulfate can serve to model the low levels of nitrogen, characteristic of certain natural environments (e.g., grapes). The S. cerevisiae DBY746 strain grown in grape extracts died at rates similar to those observed in SDC medium (Fig. 4 A). A leveling off in mortality and then adaptive regrowth was observed after day 15 (Fig. 4 A and not depicted). In contrast, adaptive regrowth in sod1Δ cultures maintained in “grape extracts” was observed much earlier (day 3; Fig. 4 A).
Figure 4.
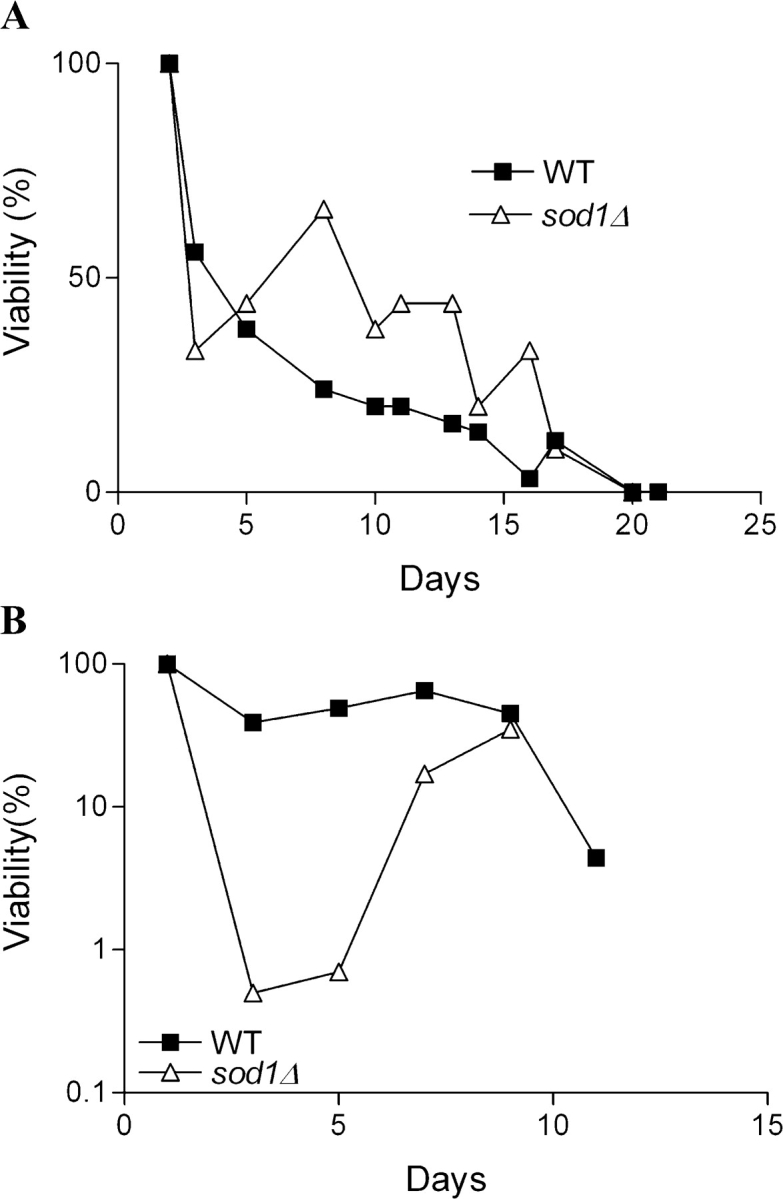
Survival of yeast in media that mimic natural environments. Chronological life span of wild-type DBY746 and sod1Δ mutants incubated in filtered extracts of California red grapes (A) and in SDC medium containing 10% of the standard concentration of ammonium sulfate (B). Both experiments were performed twice in duplicate.
Growth and incubation of wild-type and sod1Δ mutants in “low nitrogen” medium resulted in an “adaptive regrowth” pattern similar to that in SDC and grape extract media, with sod1Δ mutants growing back at day 3 (Fig. 4 B). Thus, sod1Δ mutants showed an early “regrowth” in all the media tested. Notably, the low nitrogen medium extended survival by ∼2–3 d (Fig. 4 B).
Competition experiments in mixed cultures
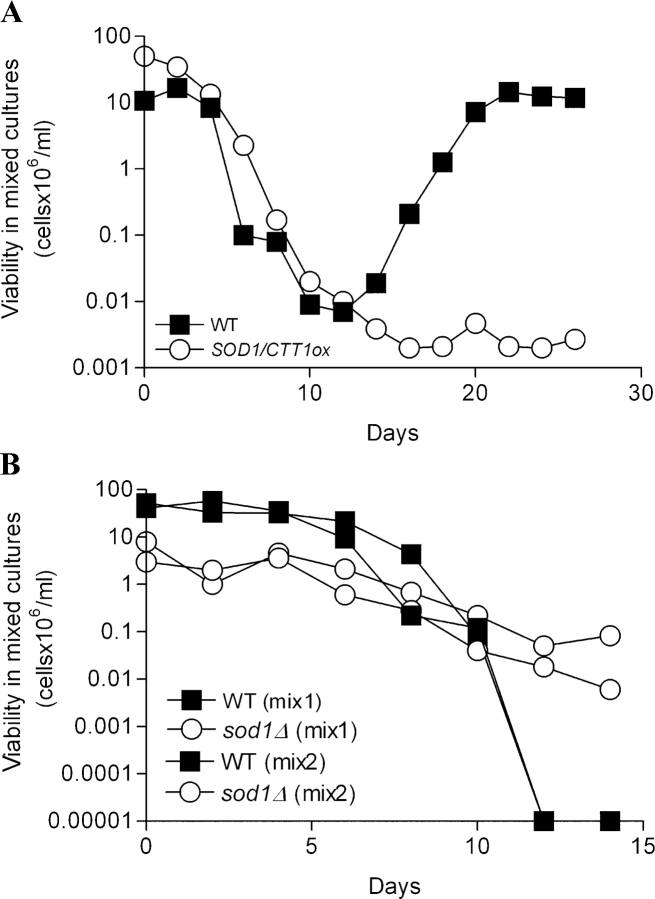
To test further if cytosolic superoxide and hydrogen peroxide can provide an adaptive regrowth advantage, we mixed in the same flasks wild-type yeast with mutants either lacking SOD1 or overexpressing both SOD1 and CTT1. To distinguish between the wild-type and mutant populations, we monitored the presence of specific biosynthetic markers present on the SOD1 and CTT1 overexpression plasmids (Fabrizio et al., 2003), which do not affect either survival or adaptive regrowth (unpublished data). Although the SOD1CTT1 population initially survived better than wild-type yeast, after day 13 only wild-type organisms grew back by using nutrients released by dead cells (Fig. 5 A and not depicted). By day 21, the ratio between SOD1CTT1 overexpressors and wild-type yeast in the flasks was 1:10,000, confirming that overexpressors of Sod1 and catalase have an adaptive growth disadvantage compared with wild-type yeast. We also monitored the survival of mixed cultures of wild-type yeast and sod1Δ mutants. Because sod1Δ mutants die very rapidly (Longo et al., 1996), to avoid an early takeover of the culture by wild-type yeast, we used sod1Δ mutants that have acquired additional mutations that reverse the initial survival defects. These mutants were isolated during chronological survival and maintain viability comparable to that of wild-type yeast during the first 2 d in culture. The ratio between sod1Δ mutants and wild type was low during the initial 9 d, but the sod1Δ mutants slowly took over the culture and reached a 1,000:1 ratio by day 14 (Fig. 5 B). These results confirm that superoxide provides an adaptive regrowth advantage but a survival disadvantage. To determine whether or not the mutation that reverses survival defects in sod1Δ mutants (Fig. 5 B) causes an adaptive regrowth advantage, we tested if yeast lacking the PMR1 gene regrow early compared with wild type. We determined the mutation that reverses the survival defects of our sod1Δ mutant isolated after regrowth to be in the PMR1 gene after determining that the mutants (Fig. 5 B) grow in the absence of lysine and are sensitive to manganese (not depicted; Lapinskas et al., 1995). The two major mutations that reverse the lysine and methionine auxotrophies of sod1Δ mutants are in the BSD2 and PMR1 genes (Lapinskas et al., 1995). It is possible to distinguish between these mutations by testing for sensitivity to 5 mM MnCl2, which is only associated with the accumulation of cytosolic manganese caused by loss of Pmr1 activity. The pmr1Δ mutation did not cause an early adaptive regrowth, suggesting that increased superoxide levels and not “survival mutations” are responsible for the adaptive advantage of sod1Δ mutants in mixed sod1Δ/wildtype cultures (unpublished data).
Figure 5.
Competition between long and short-lived strains and wild type. (A) DBY746 WT and SOD1CTT1 overexpressors were mixed in the same flask at a number of cells ratio of 1:1 (day 0). SOD1CTT1 overexpressors were chosen to avoid a complete takeover of the culture during the early survival phases, which occurs with longer lived mutants such as ras2Δ and sch9Δ. The viability of wild-type cells and of cells overexpressing SOD1 and/or CTT1 was monitored at the days indicated by counting the number of cells containing leucine and uracil biosynthetic markers inserted in the SOD1 and CTT1 overexpression plasmids. These markers do not affect either survival or adaptive regrowth (not depicted). Open circles show the cells containing one or both overexpressing plasmids. A representative experiment is shown. (B) Competition between a short-lived strain and wild type. DBY746 WT and cells deficient in cytosolic SOD1 (sod1Δ) were mixed in the same flask at a 1:1 number of cells ratio. To avoid an early takeover of the culture by wild type, we mixed wild-type cells with sod1Δ mutants isolated at day 20 of a previous experiment (the sod1Δ isolated at day 20 acquire additional mutations that prevent death during the initial days). A representative experiment performed in duplicate is shown.
Evidence for a role of nutrients released by dying yeast in promoting adaptation to starvation
To understand how superoxide promotes adaptive regrowth, we tested whether or not it functions as a signaling molecule that stimulates growth in stationary phase. The sod1 mutants did not exhibit a growth advantage, and the SOD1 overexpressors were not disadvantaged compared with wild-type populations when grown in the medium removed from wild-type or mutant cultures at days 5 to 17 (unpublished data). To determine if superoxide may instead promote adaptive regrowth by increasing the release of nutrients, we measured the concentration of proteins in the medium. A major increase in the concentration of proteins was observed in the medium removed from sod1Δ cultures at day 5 and 9 relative to wild-type cultures (Fig. 6 A). In contrast, ras2 and sch9 mutations and SOD1CTT1 or SOD1SOD2 overexpression caused a decrease in the age-dependent release of proteins into the medium (Fig. 6, A and B). We also evaluated how the nutrients released by wild-type and sod1Δ mutants would support the growth of a population isolated after regrowing in stationary phase. The medium collected from wild-type cultures of different ages allowed growth to levels that increased with increasing age of the culture (regrown yeast grew poorly in the medium removed from day 5 wild-type cultures but grew well in that removed from day 17 cultures; Fig. 6 C). In contrast, the medium collected from sod1Δ cultures at day 5 could support growth to a 10-fold higher density compared with that removed from wild-type cultures (Fig. 6 C). Thus, the superoxide-dependent death and release of nutrients into the medium creates an environment that can increase the growth of a subpopulation.
Figure 6.
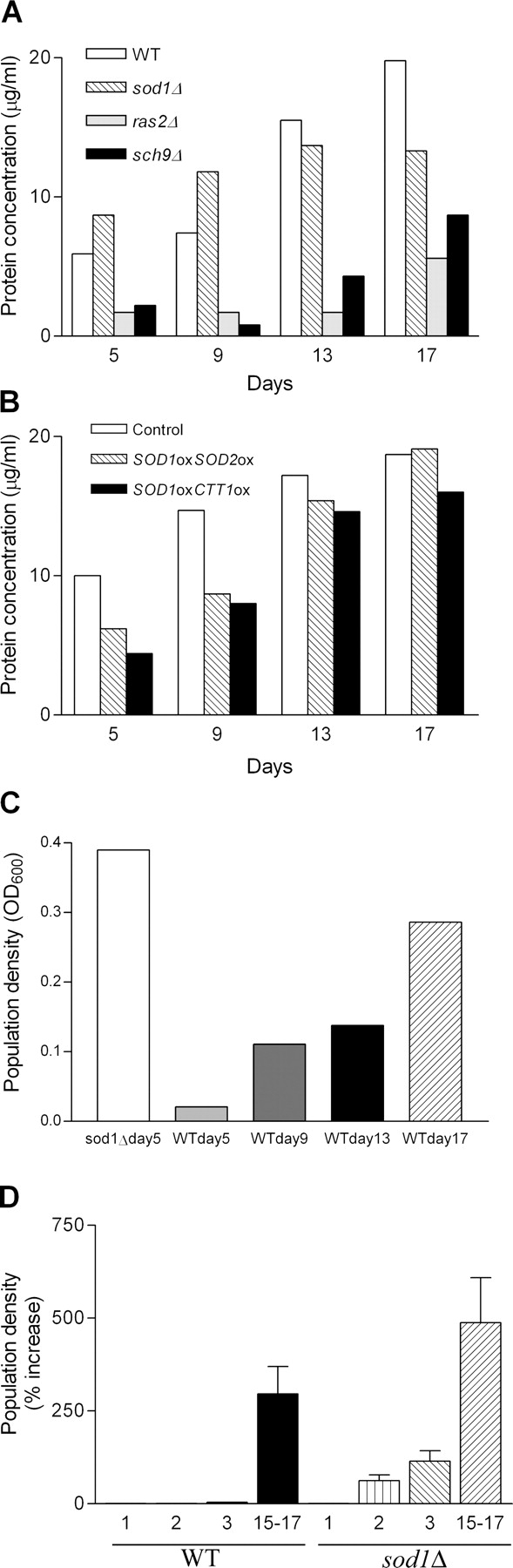
Release of nutrients by wild-type, short-, and long-lived mutants. Protein concentration in the medium removed from wild-type DBY746, sod1-, ras2-, and sch9-deficient mutants (A) or control strain YEp351-YEp352, SOD1ox SOD2ox, and SOD1ox CTT1ox (B) at days 5 to 17. A representative experiment is shown. The experiment was repeated three times with similar results. (C) Regrowth mutant adr-5 (see Materials and methods) was incubated in the medium removed from sod1Δ cultures at day 5 or wild-type cultures at day 5 to 17 for 4 d. The final population density is shown (OD600). A representative experiment is shown. (D) DBY746 was inoculated (OD600 0.1) in the medium removed from its own cultures at day 1, 2, 3, and 15 and from sod1Δ cultures at day 1, 2, 3, and 17 and incubated for 6 d. The percentage of increase of the population density is shown. The average of two experiments is shown. Error bars show SEM.
To determine if the early regrowth of sod1Δ mutants may result not from an early death but from the inability of these mutants to absorb all the nutrients during log phase, we measured the level of nutrients available in cultures of sod1Δ mutants and wild-type cells at days 1 to 3. Neither the medium removed from wild-type cultures at days 1–3 nor the medium removed from sod1Δ mutants at day 1 supported the growth of wild-type cells (Fig. 6 D). A small increase in cell density was observed in the medium removed from sod1Δ cultures at days 2 and 3. These results confirm that mutations in SOD1 promote early regrowth by causing age-dependent death and the release of nutrients.
Mutation frequency in sod1Δ and long-lived mutants
To determine if genes that regulate aging and protection against superoxide/hydrogen peroxide toxicity may affect adaptive regrowth by also regulating mutation rates, we monitored the age-dependent frequency of mutations in the CAN1 gene (Huang et al., 2003). At day 3, the frequency of can R mutations was approximately fivefold higher in sod1Δ mutants compared with DBY746 wild-type controls (Fig. 7 A). These results are consistent with those published by Gralla and Valentine (1991) and Huang et al. (2003) for sod1Δ mutants. Notably, a genomewide screen of 4,870 yeast gene deletion mutants identify sod1Δ mutants as one of the top 25 mutator strains (Huang et al., 2003). In contrast, the age-dependent increase in can R mutation frequency was delayed by ∼2–4 d in SOD1CTT1 overexpressors and by ∼10 d in sch9Δ mutants compared with DBY746 controls (Fig. 7, B and C). These results are consistent with a role for the rate of spontaneous mutations in adaptive regrowth.
Figure 7.
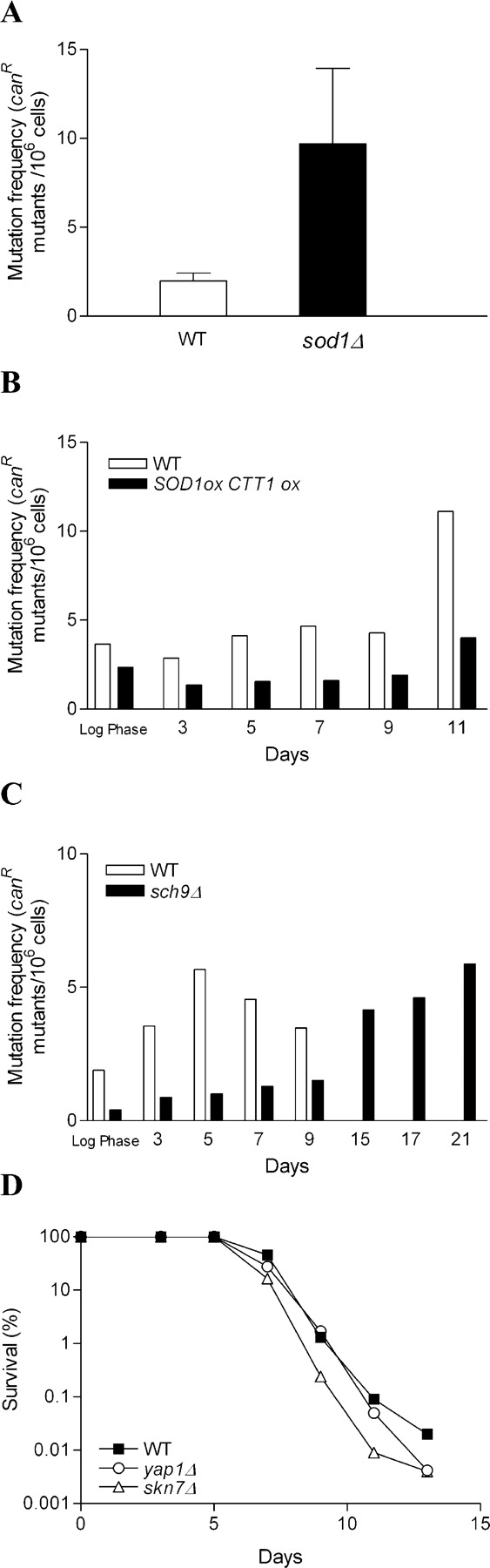
Spontaneous mutation frequency of short- and long-lived strains. (A) Mutation frequency measured as can R mutants/106 cells of wild-type (WT) and sod1Δ mutants at day 3. The average of four experiments is shown. Error bars show SEM. Mutation frequency measured as can R mutants/106 cells of wild-type and sch9Δ (B) and wild-type and SOD1ox CTT1ox (C) at the indicated ages. For B and C, a representative experiment is shown. The experiments were performed four times with similar results. (D) Survival of wild-type and mutants lacking either the YAP1 or the SKN7 gene. YAP1 and SKN7 are involved in the regulation of protection against oxidative stress. Their deletion causes a high can R mutation rate. A representative experiment is shown.
To test whether or not higher spontaneous mutation rates are sufficient to cause the early adaptive regrowth advantage in sod1Δ mutants, we monitored viability in two deletion strains also identified in the genomewide screen of mutants with the highest rate of spontaneous mutations, yap1Δ and skn7Δ. The mutation rate in these deletion mutants is approximately three times that of wild-type cells (Huang et al., 2003). In addition, these mutations are known to decrease resistance to oxidative damage, but only cause minor changes in the basal or stress-induced expression of SOD1 (Garay-Arroyo et al., 2003; Zhang et al., 2003). Thus, yap1Δ and skn7Δ mutants can also serve to determine whether adaptive regrowth in sod1Δ mutants is superoxide-dependent or can be achieved by simply causing a general reduction in the resistance to oxidative stress. Neither yap1Δ nor skn7Δ mutations produced the early adaptive regrowth advantage observed in sod1Δ (Fig. 7 D). However, both yap1Δ and skn7Δ mutants, despite their increased mutation frequency, showed reduced adaptive regrowth after day 13, suggesting that the stress resistance genes activated by Yap1/Skn7 are fundamental for yeast chronological survival (Table I).
Isolation of regrowth mutants
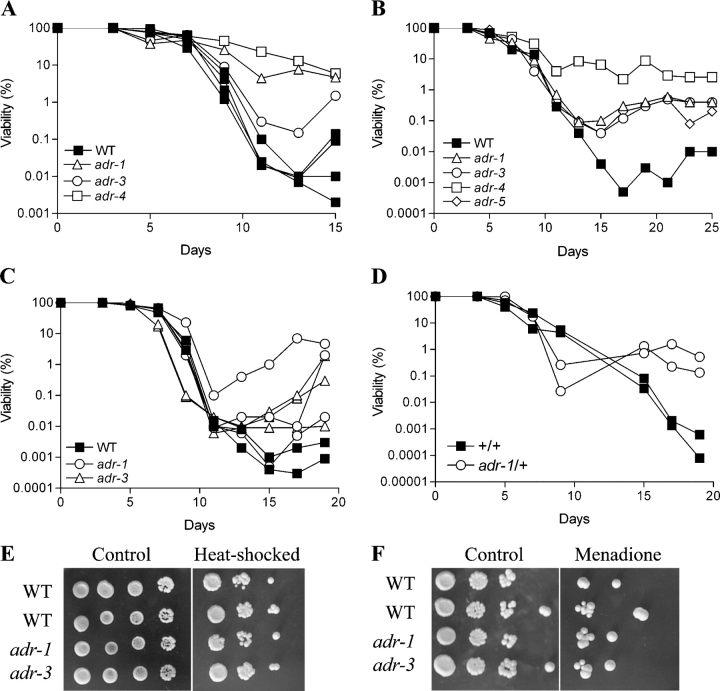
The death of Escherichia coli populations maintained in stationary phase is followed by growth of a better-adapted mutant subpopulation, which rapidly takes over the culture (Zambrano et al., 1993). To test if the yeast population undergoes programmed death to favor the growth of a mutant sub-population, we isolated regrown organisms and compared their survival and regrowth to that of wild type. In six studies with four independent clones (adr-1, adr-3, adr-4, and adr-5), we observed that organisms isolated after regrowth would adapt and grow back earlier than wild-type organisms during survival in stationary phase (Fig. 8, A–C), suggesting that the subpopulations that regrow have acquired a mutation that improves adaptation to the new environment containing nutrients released by dead cells. To confirm the presence of a “regrowth mutation,” we generated diploid cells by mating the adr-1 mutant with the wild-type DBY747 strain. The diploid strain maintained the ability to regrow early, suggesting that the regrowth mutation is dominant (Fig. 8 D). After sporulation of the adr-1/+, diploid tetrads were dissected and cultures from individual spores were generated and monitored for their survival/regrowth phenotype. 48 spores derived from 12 tetrads were analyzed. Although the adaptive regrowth phenotype is difficult to monitor, the survival pattern of the 48 cultures is consistent with the presence of a single regrowth mutation as suggested by a higher age-dependent viability in ∼50% of the cultures (unpublished data). Therefore, the altruistic behavior of the population favors organisms that are not genetically identical to the dying population, but that have evolved to adapt to the changing environment.
Figure 8.
Adaptive regrowth and stress resistance of wild-type strain DBY746 and of regrowth mutants. (A–C) Survival and adaptive regrowth of wild-type cells and four different haploid regrowth mutants (adr-1, adr-3, adr-4, and adr-5). Representative experiments are shown. (D) Survival and adaptive regrowth of diploid DBY746/DBY747 (+/+) and heterozygous adr1/+ mutant. A representative experiment performed in duplicate is shown. (E) Heat-shock resistance of wild-type (DBY746) and regrowth mutants adr-1 and adr-3. Serial dilutions of day 3 postdiauxic phase cultures were spotted onto YPD plates and incubated at 55°C for 90 min. (F) Oxidative stress resistance of wild-type, adr-1, and adr-3 mutants. Day 3 postdiauxic phase cells were diluted in K-phosphate buffer and treated with 250 μM of menadione for 1 h. After treatment, cells were serially diluted, spotted onto YPD plates, and incubated at 30°C for 3–4 d. Both heat-shock and oxidative stress resistance experiments were repeated four times with similar results.
To test whether decreased stress resistance is associated with adaptive regrowth, we determined the resistance of the mutants to the superoxide-generating agent menadione, hydrogen peroxide, or heat shock. Adaptive regrowth mutants were not sensitive to heat or menadione (which increases the generation of both superoxide and hydrogen peroxide; Fig. 8, E and F), suggesting that superoxide regulates adaptive regrowth indirectly by favoring either spontaneous or specific mutations and by providing the nutrients for regrowth.
Computational simulation of adaptation to changing environments in S. cerevisiae undergoing programmed or “stochastic” aging
We describe a computational model and simulation to compare the ability of yeast cells that undergo either programmed or stochastic aging and death to adapt to changing environments. We have coded and run two models for two situations: (1) We modeled the early death and higher mutation frequency of a population of 109 wild-type DBY746 cells (based on our experiments with 109 cells/flask; Fig. 9 A). (2) We modeled the longer life span and lower mutation frequency of sch9Δ mutants (Fig. 9 B). For the simulation of programmed aging in wild-type cells we assumed that the population follows survival Eq. 1:
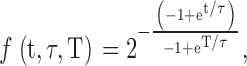 |
(1) |
where f is the fraction of viable yeast cells, T is the expected mean life span of our wild-type cells and taken to be 5.7 d, and τ is 0.3135/G, where G is the slope of the mortality rate (G = 0.0627; Fig. 9 D) known as the Gompertz mortality rate coefficient. τ replaces the A0 intrinsic vulnerability constant and the Mo age-independent mortality rate constant of Makeham (Finch, 1990) based on fitting the average survival curve of strain DBY746.
Figure 9.
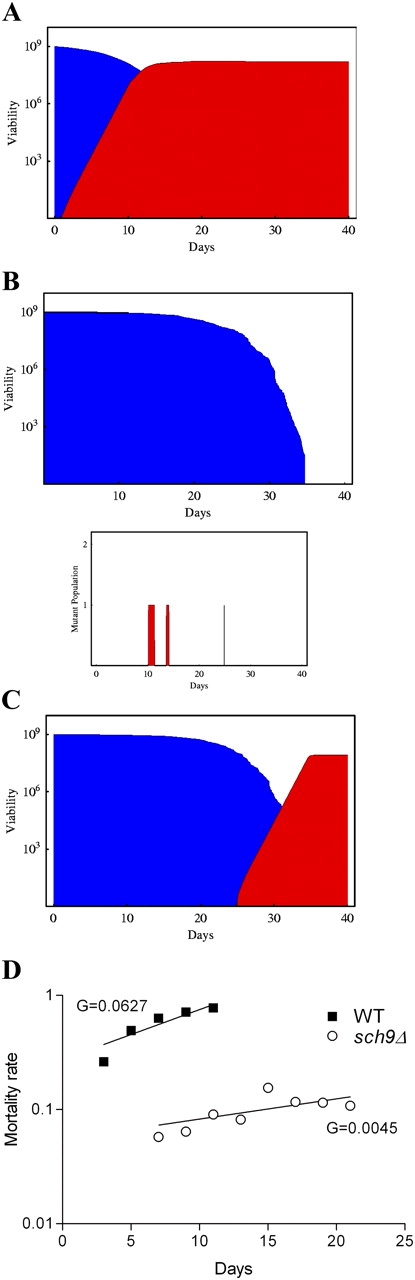
Computational simulation of adaptation to changing environments in S. cerevisiae undergoing programmed or stochastic aging. (A) Results of a computational simulation for programmed aging (model 1) showing the survival of wild-type yeast (blue) and the regrowth of a mutant population (red) that uses the nutrients released by dead cells. Initial population of 109 cells (based on the experimental population size). This result was observed in 52% of the 100 realizations (see text). (B) Results of a computational simulation for stochastic aging (model 2) showing the survival of a long-lived population (blue; sch9Δ) and the regrowth of a mutant population (bottom, red). A typical single realization starting with an initial population of 109 long-lived yeast cells is shown. This result was observed in 99.6% of the 1,000 realizations. The graph on the bottom shows the age-dependent generation/survival of regrowth mutants in sch9Δ cultures. (C) Simulation result for model 2 (B) showing the late regrowth that occurred in 0.4% of the realizations (red). (D) Slopes of the mortality rates (G) for wild-type cells or long-lived mutants (smoothed by using 6-d windows; i.e., mortality at day 5 is the average of the death rates between days 3–5, 5–7, and 7–9).
We assumed that the frequency of mutations that cause an adaptive regrowth advantage is 10−9 by day 5 and increases according to the age-dependent increase in the frequency of canavanine mutations (Fig. 7). To model this, we use a logistic probability density function with mean of μ = 134 and scaling parameter β = 6.21 to mimic the real mutation frequency of the wild-type yeast cell (based on extrapolation from the slope of regrowth of five mutant populations). The cumulative probability function C(t, μ, β) for this is
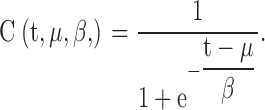 |
(2) |
From this function we find that between days 0 and 5 each living yeast cell has one chance in 109 to have undergone a mutation, and between days 0 and 11 this chance has increased by 2.6-fold, and then it remains stable until death (Fig. 7).
We did not use the frequency of mutations in the CAN1 gene (2 × 10−6) based on the observation that 50% of the wild-type DBY746 cultures do not regrow (Table I). In fact, based on the simulations described in the following paragraphs, a frequency of adaptive mutations above 10−8 is expected to cause regrowth in nearly all cultures.
When a yeast cell dies, it releases its nutrients into the environment in the new form we call type B. Based on the leveling off of the viability of regrowth mutants (R) after regrowth at a sixth of the initial (day 1) maximum viability (unpublished data), we assumed that R requires the B nutrients released by six wild-type yeast after death to reproduce and generate a young R. Based on the slope of regrowth we calculated that the R population doubles every ∼13.5 h. For these simulations, we assumed that R no longer follows the programmed death pattern of DBY746 because it switches to a growth mode.
In 52% of 100 independent realizations of the computational model described in the previous paragraphs, we observed the growth pattern shown in Fig. 9 A. In the simulations where regrowth was observed, the first young R was generated at day 1 and the viability of the R population surpassed that of wild-type cells by day 12 (Fig. 9 A). These simulations are consistent with the experimental data (Table I and Figs. 2–5). When we doubled the mutation frequency at all time points (2 × 10−9), regrowth was observed in 82% of the simulations. When we decreased mutation frequency by fivefold (2 × 10−10), regrowth was observed in only 18% of the population. Thus, the combination of high mutation frequency mortality and release of nutrients promotes adaptive regrowth.
For the simulation of stochastic aging, we used the mean life span and mortality pattern of the long-lived sch9Δ mutants. We used Eq. 1, where T (mean life span) is taken to be 20 d and τ is 0.0205/G (based on G = 0.0045; Fig. 9 D). For age-dependent mutation frequencies in populations of sch9Δ mutants, we used Eq. 2 with μ = 105 and β = 4.34. These parameters are based on the lower age-dependent CAN1 mutation frequency for sch9Δ mutants (Fig. 7). For these simulations, we assumed that when a cell dies it releases nutrients, which are divided and used equally by the surviving population. Furthermore, we assumed that R, which is now aging stochastically, will continue to follow the death rate described in Eq. 1 even after acquiring the regrowth mutation.
In contrast to the aforementioned simulation for wild-type DBY746 mutants, regrowth occurred in only 4 of 1,000 independent realizations of the computational model for long-lived strains (Fig. 9, B and C). In the long-lived cultures, R mutants appeared as early as day 10 but died before generating a young R (Fig. 9 B, bottom). In the 0.4% of the cultures where regrowth occurred, the growth of R mutants was observed late, in 25-d-old cultures (Fig. 9 C). Thus, the computational simulations suggest that programmed aging and the consequent early death, together with a relatively high mutation frequency, can result in a major advantage in adaptation to changing environments.
Discussion
Around 1870 Alfred Wallace, the codiscoverer of natural selection, wrote: “when one or more individuals have provided a sufficient number of successors they themselves, as consumer of nourishment in a constantly increasing degree, are an injury to those successors. Natural selection therefore weeds them out, and in many cases favours such races as die almost immediately after they have successors” (Rose, 1991). This group selection theory lost support in favor of evolutionary theories based on the age-dependent decline on the force of natural selection (Rose, 1991). The argument against Wallace's programmed aging theory was that accidental mortality is sufficiently high to prevent organisms from reaching “old age” in their natural environments (Medawar, 1952), and therefore many organisms may not benefit from a death program. Because senescence is predicted from our data to be reached frequently by yeast in natural environments, a death program may be advantageous for this microorganism. In a previous work, we showed that incubation in water extends longevity and suggested that yeast undergo an aging program (Longo et al., 1997). Here, we provide conclusive evidence for an altruistic premature aging program, which depends in part on superoxide. It will be important to determine if the gasping cycles observed in bacterial populations may also depend on an aging and death program (Zambrano and Kolter, 1996). Frequent death and growth cycles may promote the selection of populations of mutants better adapted to a particular environment. Naturally, a death program may not be advantageous under all conditions. In fact, yeast incubated in water live much longer than in glucose medium (Figs. 1 and 2). S. cerevisiae exposed to water in natural environments are likely to be separated from the rest of the colony. Thus, a yeast organism washed off a grape by rain may not provide any advantage to isogenic organisms by undergoing a suicide program. In fact, it is sufficient to raise the extracellular pH from that of day 3 cultures (pH 3.5) to that of water to block the death program (Figs. 1 C and 2 D), suggesting that low pH signals to yeast organisms in the culture that the population density is high, that nutrients are low, and that a death program should be activated. The rapid age-dependent death in SDC medium but not in water of both laboratory S. cerevisiae and of yeast strains isolated from grapes indicates that programmed death may be common in microorganisms aging in natural environments. Features of apoptotic death in yeast aging chronologically have been recently detected by Herker et al. (2004). In that article, the authors propose that apoptotic death promotes the survival of the remaining population. Our results instead suggest that the premature apoptotic death promotes the regrowth of a subpopulation of better-adapted mutants rather than life span extension in the surviving population. This altruistic aging program, which depends in part on superoxide levels, may have evolved to promote adaptation to changing environments.
The computational simulation of survival and regrowth based on the experimental age-dependent mutation frequency and death rate of the wild-type and long-lived cells is consistent with our theory that early death and high mutation frequency have evolved to promote early adaptation (Fig. 9 A). The simulations with population size equivalent to that used experimentally (1 billion/flask), confirm that the early aging, death, and nutrient release in wild-type cultures (programmed aging) cause an increase in mutation frequency and the generation of R mutants by day 5, which regrow in 52 of 100 realizations. In contrast, the simulation based on the lower mutation frequency and longer life span of sch9Δ mutants (stochastic aging) indicates that yeast that age slowly and attempt to survive as long as possible have a major adaptive disadvantage, assuming that the nutrients released by the dying population are redistributed equally to the survivors. The latter simulation also shows that some adaptive regrowth mutants are generated, but are too old and die before they can acquire sufficient nutrients to reproduce (Fig. 9 B). Notably, we do not know whether sch9Δ mutants age stochastically or simply activate the “aging program” at a later point. In either scenario, our data and simulations suggest that, as first hypothesized by Wallace, natural selection favors such races that die after they have successors. Mutations that increase longevity and antioxidant protection or the overexpression of Sods and catalase negatively affect adaptive regrowth, raising the possibility that natural selection has prevented alleles that confer longevity extension from becoming fixed. In fact, mutations that cause inactivation of Ras2 or higher levels of Sod and catalase, which do not cause detectable growth defects, are predicted to be frequent and could easily become fixed if they did not negatively affect adaptation to changing environments.
The simulations indicate that minor changes in mutation frequency can cause major changes in the ability of a population to adapt and regrow. The association between mutation frequency and adaptive regrowth in wild-type, SOD1CTT1 overexpressors, sch9Δ, and sod1Δ mutants is consistent with the outcome of these simulations (Fig. 9). However, increased mutation frequency is not sufficient to explain the early adaptive regrowth of sod1Δ. In fact, yap1Δ and skn7Δ mutants have high mutation rates but do not adapt early (Fig. 7 D), suggesting that an early release of nutrients is also required to maximize regrowth. The central role of Sods in the aging and death of yeast and the ability of Sod1 to cause major changes in adaptive regrowth suggests that superoxide and possibly hydrogen peroxide are important mediators of the Ras2- and Sch9-dependent aging/adaptive regrowth program (Longo, 1997; Fabrizio et al., 2001, 2003). Notably, superoxide is an important mediator of mammalian apoptosis (Hockenbery et al., 1993).
Although it is not known whether or not higher eukaryotes would benefit from an altruistic aging and death program, the role of similar genes and pathways in the regulation of longevity in organisms ranging from yeast to mice (Longo and Finch, 2003) raises the possibility that aging is also programmed in mammals.
Materials and methods
Strains and plasmids
The wild-type strains used in this study were DBY746 (MATα leu 2–3, 112 his3Δ1 trp1-289 ura3–52 GAL +), DBY747 (MATα leu2–3, 112 his3Δ1 trp1-289 ura3–52 GAL +), SP1 (MATα leu2, his3, ura3), and BY4700 (MATα ura3Δ0). The diploid strain DBY746/DBY747 was obtained by mating the two haploid wild types described above. Mutants lacking SCH9, RAS2, SOD1, and CTT1 and strains overexpressing SOD1, SOD2, CTT1, and Bcl-2 originated in the DBY746 background have been previously described (Longo et al., 1997; Fabrizio et al., 2001, 2003). The yap1Δ mutant in the DBY746 background was provided by E.B. Gralla (University of California, Los Angeles, Los Angeles, CA). The deletion of the SKN7 gene in the DBY746 background and of SOD1 in the BY4700 background were produced by one-step gene replacement using plasmids skn7::TRP1 (provided by H. Bussey, McGill University, Montreal, Canada) and pSOD1::URA3 (Gralla and Valentine, 1991), respectively. The gene disruptions were verified by PCR analysis. The SP1 SOD1ox strain was generated by transformation of SP1 with high copy plasmid YEp352SOD1 (Fabrizio et al., 2003).
Isolation of regrowth mutants and wild yeast
We considered a population “regrown” (adaptive regrowth) if the viability increased by at least 100% and remained at that level or higher for at least two data points. The “frequency of regrowth” refers to the number of cultures in which regrowth was observed compared with the total number of cultures studied for a particular strain.
To obtain regrowth mutants adr-1, adr-3, adr-4, and adr-5, a small aliquot of cells was isolated at day 34 from four different DBY746 cultures (during the regrowth phase) and frozen. A new study was started with the regrown populations and, at day 25 (during another regrowth phase), four single colonies were isolated and frozen. Isolation of the clones after two cycles of survival and regrowth should favor the selection of better-adapted mutants.
Wild yeast strains (1–3) were isolated by plating a few drops of unfiltered grape extract onto YPD plates. Several colonies grew after 2–3 d. Those that most resembled budding yeast colonies were frozen for further analysis. To observe the morphology and budding pattern of the wild strains, cells were fixed with 70% ethanol, stained with Calcofluor (25 μM in water), and observed by fluorescence microscopy.
Chronological survival and apoptotic markers detection
Chronological lifespan of cells incubated in both SDC medium and water was measured as described previously (Longo et al., 1996; Fabrizio et al., 2001). Viability was measured every 2 d starting at day 1–3.
Experiments in medium containing low nitrogen were performed by incubating yeast in an SDC medium containing 1/10 of the standard ammonium sulfate concentration. Chronological life span of yeast incubated in filtered extracts obtained from organically grown California red grapes was also measured.
For nuclear staining, yeast cells were fixed with 70% ethanol, washed with water, incubated in 50 ng/ml DAPI in water, and examined by fluorescence microscopy. Exposed phosphatidylserine was detected by reaction with a GFP-coupled annexin V (ApoAlert Annexin V Apoptosis Kit; CLONTECH Laboratories, Inc.) as described previously (Madeo et al., 1997). Yeast were simultaneously stained also with 0.5 mg/ml propidium iodide to identify damaged/dead cells.
The effect of cytosolic alkalinization on cell death was studied by increasing the extracellular pH from the normal pH at day 1–3 (3– 4) to pH 6.5. The increase in extracellular pH causes intracellular alkalinization.
Fluorescence microscopy
For image acquisition, we used a fluorescence microscope (model DM IRB; Leica) equipped with a PL fluotar100×/1.30 objective, a high-resolution digital camera (SPOT RT slider), and SPOT RT software v3.4.
Stress resistance assays and Sod measurements
Heat-shock resistance was tested by spotting serial dilutions of cells removed from day 3 SDC postdiauxic phase cultures onto YPD plates and incubating at 55°C (heat-shocked) or at 30°C (control) for 90 min. Pictures were taken after 3–4 d of incubation at 30°C.
For oxidative stress resistance assays, day 3 postdiauxic cells were diluted to an OD600 of 0.1 in K-phosphate buffer, pH 7.4, and treated with 200–250 μM of menadione for 60 min. Viability was measured by plating the cells onto YPD plates before and after treatment. Alternatively, serial dilutions of untreated and menadione-treated cells were spotted onto YPD plates and incubated at 30°C. Pictures were taken after 3–4 d.
Sod activity was measured according to the method of auto-oxidation of 6-hydroxydopamine (Heikkila and Felicitas, 1976). For separate measurements of mitochondrial Sod activity, 1 mM NaCN, which inhibits 95% of the cytoplasmic Sod, was added to the reaction mix.
Competition studies
For wild type/SOD1ox CTT1ox competition study, 100-μl aliquots of yeast cells of the two strains were removed from day 20 cultures and frozen. The frozen aliquots were later expanded by growing the cells in SDC medium for 3 d and mixed at a ratio of 1:1. The viability of the mixed culture was measured every 2 d by plating onto SDC plates. To distinguish between the wild type and the overexpressors, we monitored the ability of cells to grow in the absence of leucine and uracil or on SDC plates (the YEp352-SOD1 and YEp351-CTT1 overexpression plasmids contain, respectively, the URA3 and LEU2 genes). Control plasmids expressing uracil and leucine biosynthetic enzymes did not affect either survival or adaptive regrowth.
The wild type/sod1Δ competition study was performed analogously to the previous one except that, at day 20, postdiauxic sod1Δ population was mixed with a nonpreselected wild-type population. This was done to avoid the early takeover of the sod1Δ mutants, which die very rapidly, by the wild-type yeast. sod1Δ mutants isolated at day 20 survive similarly to the wild type.
Spontaneous mutation frequency
To measure spontaneous mutation frequency, overnight inoculations were diluted in 25 ml of liquid SDC medium and incubated at 30°C. Cell viability was measured during the exponential growth phase and every 2 d starting at day 3 by plating appropriate dilutions onto YPD plates. To identify the canavanine resistant mutants (can R) in the liquid culture, an appropriate number of cells was harvested by centrifugation, washed once with sterile water, and plated on selective medium (SD-ARG, 60 mg/l L-canavanine sulfate). Colonies were counted after 3–4 d.
Computational simulations for the growth of yeast
We have coded and run two similar models for two different situations described in this section.
Both of our models start with 109 original (O) yeast cells at day 0, each of which holds and retains one nutrient unit of type A for the duration of its lifetime. The simulation proceeds by advancing the time by steps of units of Δt = 1/16 of a day (the value of 1/16 d or 1.5 h represents the doubling rate of a wild-type yeast cell and was chosen because it is the fastest possible biological process we consider in this model). During each time step, each yeast cell may undergo death, a mutation, or may continue living as it is.
The cellular data
The computer simulation maintains a list of the living cells present in the population for both the original (O) and the mutant (R) population. This population list is structured and changes by forming, updating, and eventually removing sub-lists organized by generation. At time t our list has the following general form:
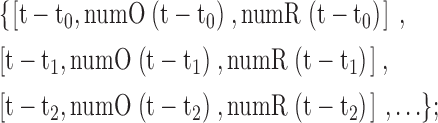 |
and will contain a maximum of t/Δt sub-lists. Here, t−ti is the age of the (i+1)th generation; ti is the birth time of cells belonging to that particular generation; numO(t−ti) is the number of cells of the original type O cells of age t−ti; numR(t−ti) is the number of cells of the mutant type R cells of age t−ti. Whenever both numO and numR belonging to the same generation fall to zero, its related sub-list is removed from the population list. Because in both our simulations the original type cell O dies and never grows, we have only one generation for this type. Therefore, before the creation of any mutant, the list will be of the form {[Age, numO(age), 0]}. Once the mutant R is formed and starts growing, the number of generations and thus sub-lists start to grow linearly.
Death
We assume that our yeast cells follow a survival equation f (t,T,t) given in Eq. 1. This equation allows us to derive and compute the expected number of cells of age t that will have died over the next time step. This is found by differentiating f (t,T,t) with respect to time and multiplying this by the time step Δt. From this, we deduce that if we start with N(t) alive wild-type cells at time t, then the expected number of deaths D(N(t)) during the following time step is
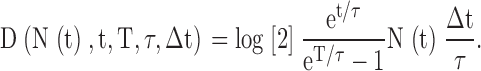 |
(3) |
Using Eq. 3 above, we compute the expected number of dying cells for each generation. If this number is larger than 30 then the number of cells removed is computed by generating a random integer variable with mean and variance of D(N(t)) taken from a negative exponential distribution. Otherwise, we use this expectation to compute the probability for each cell to die. Therefore, by generating 30 uniform random variables in the range of 0 and 1 and comparing these to the computed probability, we stochastically remove the cells.
Once an original (O) yeast cell dies, it releases its nutrients back to the environment in the new form we call type B. It requires the death of six original yeast cells to produce one unit of nutrient B. Likewise, the death of one mutant R releases the one unit of nutrient B it retained for its life span.
Mutation
Each of the original O yeast cells has a chance to mutate to cell form R during its life span. This mutation occurs with a time-dependent probability. We use a cumulative logistic probability function given in Eq. 2, with a mean of μ and scaling parameter β to mimic the real mutation frequency observed experimentally. This function is only used up to and including the day for which the mutations were experimentally recorded. After this time, we impose there to be no more additional mutations. If n mutations take place for a cell belonging to the (i+1)th generation, we modify the related sub-list by subtracting n to numO(t−ti) and adding n to numR(t−ti).
Reproduction
The ability for reproduction is also determined by an age-dependent function. In both our models, the mutant yeast cell R requires to be fit and young enough in order to start reproducing. If the mutation occurs very late in life, the mutant is likely not to be fit enough to use its nutrients for reproduction. This fitness function is also determined via the function f (t,T,t) with the same parameters that determine its survival chance.
The conditions for a mutant cell to reproduce depends on the availability of nutrient of type B. Here, the two models described differ.
In the first model, which starts off with an original population of the programmed aging yeast type, once the mutant R is formed and absorbs one unit of nutrient B, it is from then on active to reproduce and every 13.5 h. This is because, in this model, our original yeast cell does not use any of the nutrients of type B.
In the second model, which starts off with an original population of long-lived yeast type, once the mutant R is formed it will need to wait until the average amount of released nutrients B per total number of present cells increases to one (or six deaths). In this model, we assume that both the original long-lived yeast cell and its mutant consume nutrients of type B. Thus, when six original O cells die producing one unit of nutrient B, this gets equally shared between all the cells in the population (regrowth mutants or original cells).
Acknowledgments
We thank Caleb Finch and Steve Finkel for the careful reading of the manuscript and helpful comments. We thank Roland Suri for help in statistical analysis.
This work was supported in part by an American Federation for Aging Research grant and by National Institutes of Health grant AG 20642 to V.D. Longo.
Abbreviations used in this paper: SDC, synthetic dextrose complete; Sod, superoxide dismutase.
References
- Dillin, A., D.K. Crawford, and C. Kenyon. 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 298:830–834. [DOI] [PubMed] [Google Scholar]
- Fabrizio, P., and V.D. Longo. 2003. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2:73–81. [DOI] [PubMed] [Google Scholar]
- Fabrizio, P., F. Pozza, S.D. Pletcher, C.M. Gendron, and V.D. Longo. 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 292:288–290. [DOI] [PubMed] [Google Scholar]
- Fabrizio, P., L.L. Liou, V.N. Moy, A. Diaspro, J. SelverstoneValentine, E.B. Gralla, and V.D. Longo. 2003. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 163:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch, C.E. 1990. Longevity, Senescence, and the Genome. University Press, Chicago, IL. 922 pp.
- Garay-Arroyo, A., F. Lledias, W. Hansberg, and A.A. Covarrubias. 2003. Cu,Zn-superoxide dismutase of Saccharomyces cerevisiae is required for resistance to hyperosmosis. FEBS Lett. 539:68–72. [DOI] [PubMed] [Google Scholar]
- Gralla, E.B., and J.S. Valentine. 1991. Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J. Bacteriol. 173:5918–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila, R.E., and C. Felicitas. 1976. A sensitive assay for superoxide dismutase based on the autoxidation of 6-hydroxydopamine. Anal. Biochem. 75:356–362. [DOI] [PubMed] [Google Scholar]
- Herker, E., H. Jungwirth, K.A. Lehmann, C. Maldener, K.U. Frohlich, S. Wissing, S. Buttner, M. Fehr, S. Sigrist, and F. Madeo. 2004. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery, D., Z.N. Oltvai, X. Yin, C.L. Milliman, and S.J. Korsmeyer. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 75:241–251. [DOI] [PubMed] [Google Scholar]
- Honda, Y., and S. Honda. 1999. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 13:1385–1393. [PubMed] [Google Scholar]
- Huang, M.E., A.G. Rio, A. Nicolas, and R.D. Kolodner. 2003. A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc. Natl. Acad. Sci. USA. 100:11529–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, T., and T. Ohno. 1995. Measurement of yeast intracellular pH by image processing and the change it undergoes during growth phase. J. Biotechnol. 38:165–172. [DOI] [PubMed] [Google Scholar]
- Jin, C., and J.C. Reed. 2002. Yeast and apoptosis. Nat. Rev. Mol. Cell Biol. 3:453–459. [DOI] [PubMed] [Google Scholar]
- Lapinskas, P.J., K.W. Cunningham, X.F. Liu, G.R. Fink, and V.C. Culotta. 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun, P., A. Pichova, F. Madeo, J. Fuchs, A. Ellinger, S. Kohlwein, I. Dawes, K.U. Frohlich, and M. Breitenbach. 2001. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 39:1166–1173. [PubMed] [Google Scholar]
- Longo, V. 1997. The chronological life span of Saccharomyces cerevisiae. Studies of superoxide dismutase, Ras and Bcl-2. Ph.D. thesis. University of California, Los Angeles, CA. 179 pp.
- Longo, V.D., and P. Fabrizio. 2002. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell. Mol. Life Sci. 59:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo, V.D., and C.E. Finch. 2003. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 299:1342–1346. [DOI] [PubMed] [Google Scholar]
- Longo, V.D., E.B. Gralla, and J.S. Valentine. 1996. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 271:12275–12280. [DOI] [PubMed] [Google Scholar]
- Longo, V.D., L.M. Ellerby, D.E. Bredesen, J.S. Valentine, and E.B. Gralla. 1997. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 137:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico, P., M.J. Sousa, M.T. Silva, C. Leao, and M. Corte-Real. 2001. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 147:2409–2415. [DOI] [PubMed] [Google Scholar]
- Madeo, F., E. Frohlich, and K.U. Frohlich. 1997. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Frohlich, M. Ligr, M. Grey, S.J. Sigrist, D.H. Wolf, and K.U. Frohlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lachelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K.U. Frohlich. 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 9:911–917. [DOI] [PubMed] [Google Scholar]
- Maris, A.F., A.L. Assumpcao, D. Bonatto, M. Brendel, and J.A. Henriques. 2001. Diauxic shift-induced stress resistance against hydroperoxides in Saccharomyces cerevisiae is not an adaptive stress response and does not depend on functional mitochondria. Curr. Genet. 39:137–149. [DOI] [PubMed] [Google Scholar]
- Matsuyama, S., J. Llopis, Q.L. Deveraux, R.Y. Tsien, and J.C. Reed. 2000. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2:318–325. [DOI] [PubMed] [Google Scholar]
- Medawar, P. 1952. An Unsolved Problem in Biology. HK Lewis, London. 23 pp.
- Mortimer, R.K., and J.R. Johnston. 1986. Genealogy of principal strains of the yeast genetic stock center. Genetics. 113:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaff, H.J., M.W. Miller, and E.M. Mrak. 1966. The Life of Yeasts. Harvard University Press, Cambridge, MA. 186 pp.
- Rose, M. 1991. Evolutionary Biology of Aging. Oxford University Press, Oxford. 221 pp.
- Skulachev, V.P. 2002. Programmed death in yeast as adaptation? FEBS Lett. 528:23–26. [DOI] [PubMed] [Google Scholar]
- Sun, J., and J. Tower. 1999. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 19:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H., I. Matsumura, S. Ezoe, Y. Satoh, T. Sakamaki, C. Albanese, T. Machii, R.G. Pestell, and Y. Kanakura. 2002. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-κB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell. 9:1017–1029. [DOI] [PubMed] [Google Scholar]
- Thevelein, J.M., and J.H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904–918. [DOI] [PubMed] [Google Scholar]
- Walker, D.W., G. McColl, N.L. Jenkins, J. Harris, and G.J. Lithgow. 2000. Evolution of lifespan in C. elegans. Nature. 405:296–297. [DOI] [PubMed] [Google Scholar]
- Zambrano, M.M., and R. Kolter. 1996. GASPing for life in stationary phase. Cell. 86:181–184. [DOI] [PubMed] [Google Scholar]
- Zambrano, M.M., D.A. Siegele, M. Almiron, A. Tormo, and R. Kolter. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 259:1757–1760. [DOI] [PubMed] [Google Scholar]
- Zhang, L., K. Onda, R. Imai, R. Fukuda, H. Horiuchi, and A. Ohta. 2003. Growth temperature downshift induces antioxidant response in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 307:308–314. [DOI] [PubMed] [Google Scholar]