Abstract
Apoptosis-inducing factor (AIF), a key regulator of cell death, is essential for normal mammalian development and participates in pathological apoptosis. The proapoptotic nature of AIF and its mode of action are controversial. Here, we show that the yeast AIF homologue Ynr074cp controls yeast apoptosis. Similar to mammalian AIF, Ynr074cp is located in mitochondria and translocates to the nucleus of yeast cells in response to apoptotic stimuli. Purified Ynr074cp degrades yeast nuclei and plasmid DNA. YNR074C disruption rescues yeast cells from oxygen stress and delays age-induced apoptosis. Conversely, overexpression of Ynr074cp strongly stimulates apoptotic cell death induced by hydrogen peroxide and this effect is attenuated by disruption of cyclophilin A or the yeast caspase YCA1. We conclude that Ynr074cp is a cell death effector in yeast and rename it AIF-1 (Aif1p, gene AIF1).
Keywords: apoptosis-inducing factor; YNR074C; YCA1; cyclophilin A; aging
Introduction
Apoptosis-inducing factor (AIF) is a flavoprotein with oxidoreductase activity localized in the mitochondrial intermembrane space (Susin et al., 1999; Miramar et al., 2001). Upon apoptosis induction, AIF translocates to the nucleus, where it leads to chromatin condensation and DNA degradation (Susin et al., 1999). AIF has been suggested to control a caspase-independent pathway of apoptosis, important for neurodegeneration and normal development (Susin et al., 1999; Cregan et al., 2002).
Recently, the yeast Saccharomyces cerevisiae has become a useful model organism for the study of apoptosis. Apoptotic markers were observed in association with a mutation in the AAA-ATPase gene CDC48 (Madeo et al., 1997), whose metazoan orthologues were subsequently implicated in the regulation of apoptosis (Shirogane et al., 1999; Wu et al., 1999). Yeast apoptosis is often accompanied by the generation of oxygen radicals (Laun et al., 2001; Mazzoni et al., 2003; Weinberger et al., 2003), and orthologues of core regulators of mammalian apoptosis such as caspases, HtrA2/Omi, and the proteasomal death pathways have been shown to be conserved in yeast (Blanchard et al., 2002; Madeo et al., 2002; Fahrenkrog et al., 2004). In addition, physiological scenarios of yeast apoptosis have been described during aging processes (Laun et al., 2001; Herker et al., 2004).
Here, we describe an orthologue of AIF in yeast cells. Yeast Aif1p shows the same localization and exhibits similar death executing pathways as mammalian AIF. We demonstrate that yeast Aif1p is dependent on cyclophilin A (CypA) and partially on caspase action.
Results and discussion
Ynr074cp (Aif1p) is the yeast homologue of AIF
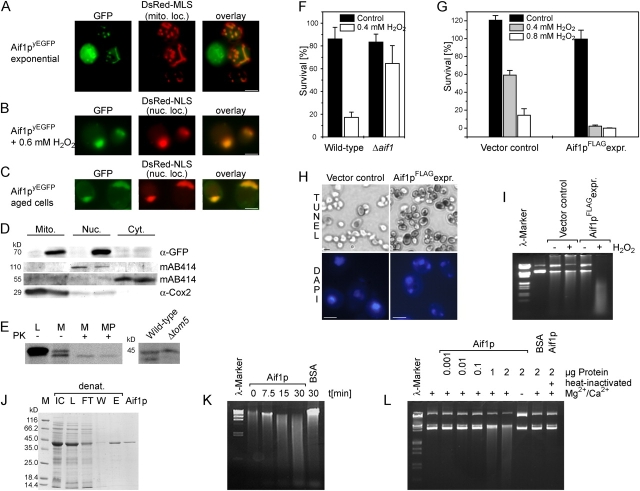
Sequence comparison revealed that ORF YNR074C of S. cerevisiae encodes a protein of 41.3 kD, showing significant similarity with both AIF as well as AMID (AIF-homologous mitochondrion-associated inducer of death; Wu et al., 2002). Ynr074cp displays 22% identity and 41% similarity with human AIF (Fig. 1). Human AIF is a flavoprotein with NADH oxidase activity and contains a mitochondrial localization sequence in the NH2 terminus and in the COOH terminus a nuclear localization sequence, as well as a putative DNA binding domain composed by positively charged amino acids. Although the COOH-terminal domain is moderately conserved in yeast Aif1p, the central oxidoreductase domain exhibits a high degree of similarity. Although Aif1p is lacking a typical mitochondrial localization sequence we observe mitochondrial localization for Aif1p during cell fractionation (Fig. 2 D) and in fluorescence microscopy as demonstrated by colocalization with the mitochondrial marker DsRed Su1-69 (Fig. 2 A). The mitochondrial localization of Aif1p was confirmed by import experiments of a 35S-labeled Aif1p precursor (Fig. 2 E) in vitro. Upon incubation with isolated yeast mitochondria, a proteolytically processed mature form of Aif1p was generated that remained protease resistant in mitochondria and mitoplast (Fig. 2 E), indicating that yeast Aif1p is attached to the inner mitochondrial membrane or alternatively located in the matrix.
Figure 1.
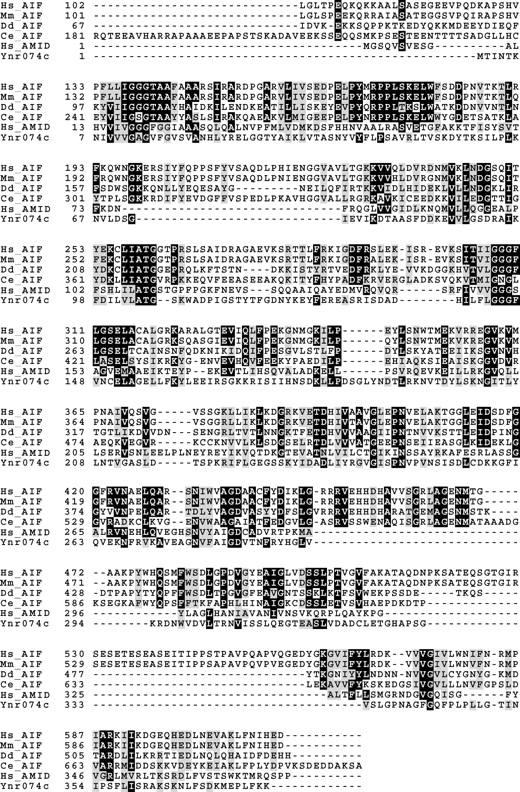
Ynr074cp (Aif1p) is the yeast homologue of AIF. Alignment of Homo sapiens (Hs_AIF), Mus musculus (Mm_AIF), Dictyostelium discoideum (Dd_AIF), Caenorhabditis elegans (Ce_AIF) AIF, and Homo sapiens AMID (Hs_AMID) amino-acid sequences with the protein encoded by ORF YNR074C. Black boxes indicate amino acid identity and gray boxes indicate amino acid similarity.
Figure 2.
AIF translocates from mitochondria to the nucleus under apoptotic conditions, degrades DNA, and induces apoptosis in yeast. (A) Fluorescence microscopy of cells expressing Aif1pyEGFP during exponential growth. Mitochondria were visualized with mitochondrial marker DsRed Su1-69. (B) Fluorescence microscopy of exponentially growing cells expressing Aif1pyEGFP and DsRed-NLS (nuclear staining) after an apoptotic stimulus with 0.6 mM H2O2 for 5 h on SCD. (C) Fluorescence microscopy of chronological aged yeast cells expressing Aif1pyEGFP and DsRed-NLS (nuclear staining) after 5 d growing on SCD. (D) Immunoblot of cellular fractions of untreated cells during exponential growth expressing endogenous yEGFP-tagged AIF1 expressing (lanes 2, 4, 6) and controls (lanes 1, 3, 5). Blot probed with antibodies against GFP, or Cox2p, or with mAB 414, a mAb immunoreacting with both p110, a nuclear pore protein and with a 55-kD cytosolic protein (Aris and Blobel, 1989). (E) Import of in vitro–synthesized 35S-labeled Aif1p precursor into isolated mitochondria. Wild-type and Δtom5 mitochondria were incubated with Aif1p precursor protein. Wild-type mitochondria (M) and mitoplasts (MP) were treated with proteinase K (PK). (F) Survival of Δaif1 and wild type after treatment with 0.4 mM H2O2 for 4 h during early exponential growth. Data represent mean ± SEM. (G) Survival of Aif1pFLAG overexpressor and vector control after a 20-h induction on galactose with and without H2O2. Data represent mean ± SEM. (H) TUNEL and DAPI staining of Aif1pFLAG overexpressor and vector control after a 20-h induction on galactose with H2O2. (I) Degradation of 1 μg purified plasmid DNA by 2.5 μg cell extracts from Aif1pFLAG overexpressor. (J) Purification of Aif1p after recombinant expression in E. coli under denaturing conditions, and after refolding via dialysis. IC, induced control; L, lysate; M, marker; FT, flow through; W, wash; E, eluate; and refolded Aif1p. (K) Time course of the degradation of isolated yeast nuclei by purified refolded Aif1p. (L) Degradation of 1 μg plasmid DNA with different concentrations of purified refolded Aif1p. Bars, 5 μm.
Aif1p translocates from mitochondria to the nucleus upon apoptosis induction
To determine the cellular localization of Aif1p in yeast cells, we expressed GFP-tagged Ynr074cp (Aif1pyEGFP). Fluorescence microscopy revealed the colocalization of the Aif1p-GFP construct with a mitochondria marker DsRed Su1-69 (Fig. 2 A).
Low doses of reactive oxygen species are necessary and sufficient to induce yeast apoptosis (Madeo et al., 1999). After treatment with 0.6 mM H2O2 for 5 h, Aif1pyEGFP relocalized from mitochondria to the nucleus, as revealed by colocalization of Aif1pyEGFP with the nuclear marker DsRed-NLS (Fig. 2 B). Recently, we have shown that chronological aging is a physiological trigger for apoptosis in yeast (Herker et al., 2004). Consistently, we observe a predominantly nuclear localization of Aifp1yEGFP in aged yeast cells (Fig. 2 C). These data confirm that, like mammalian AIF, S. cerevisiae Aif1p translocates from mitochondria into the nucleus during apoptosis.
Subcellular fractionation of yeast cells with chromosomally GFP-tagged Aif1p confirmed the distribution of Aif1pyEGFP (Fig. 2 D). It should be noted that in untreated cells a nuclear Aif1pyEGFP localization is observable in cell fractionation but not in in vivo fluorescence. This might be due to the conditions during fractionation, in particular the digestion of the cell wall seems to be stressful for the cells, as it leads to a nuclear localization of Aif1pyEGFP (unpublished data).
Aif1p induces apoptosis in yeast
To explore the contribution of Aif1p to yeast cell death, wild-type and AIF1 disruptant (Δaif1) cells were exposed to H2O2 or to acetic acid, which also induces apoptosis in budding yeast (Madeo et al., 1999; Ludovico et al., 2001). Δaif1 cells treated with 0.4 mM H2O2 (Fig. 2 F) or 200 mM acetic acid (not depicted) showed a significantly higher survival rate compared with similarly treated isogenic wild type. Therefore, Aif1p is required for efficient apoptotic cell death in budding yeast.
Next, we investigated whether overexpression of Aif1p might sensitize yeast to apoptotic stimuli. Overexpression of AIF1 for 20 h did not compromise cell survival in the absence of additional apoptotic stimuli (Fig. 2 G). However, exposure of Aif1p overexpressing cells to low doses of H2O2 resulted in massive cell death after 20 h compared with H2O2-treated isogenic controls (Fig. 2 G). Due to the incubation time required for overexpression (20 h), cell densities are much higher than in Fig. 2 F and hence low doses of H2O2 do not induce massive cell death in the isogenic control strains. To test whether this Aif1p-facilitated cell death is of apoptotic nature, subcellular markers of apoptosis were examined. Chromatin condensation indicated by DAPI staining was detectable in 30% of the Aif1p overexpressing cells treated with H2O2 (Fig. 2 H). Also, overexpression of Aif1p leads to DNA fragmentation in 80% of the cells, as revealed by TUNEL staining (Fig. 2 H). Moreover, plasmid DNA was completely degraded after incubation with cell extracts from strains overexpressing yeast Aif1p but not with control extracts from isogenic strains (Fig. 2 I). Interestingly, the Aif1p overexpressing yeast need peroxide treatment to yield lysates capable of digesting plasmid DNA. Immunoblotting of Aif1p overexpressor reveals a fivefold accumulation of Aif1p after treatment with H2O2 (unpublished data). This could be due to the different localization of Aif1p or changed transcription/translation of apoptotic cells.
We next asked whether purified recombinant Aif1p (Fig. 2 J) from E. coli is capable of degrading DNA. Indeed, Aif1p shows a DNase activity on purified yeast nuclei (Fig. 2 K) and plasmid DNA (Fig. 2 L). This function was dose dependent, as well as dependent on divalent cations (Fig. 2 L). DNase activity of Aif1p requires refolding of the protein and is destroyed by heat (100°C; Fig. 2 L). But refolding and the ability of DNA degradation did not require the presence of FAD, indicating that the predicted FAD binding domain and the NADH oxidoreductase activity are not involved, similar to mammalian AIF (Susin et al., 1999). The required concentration of Aif1p for degradation of plasmid DNA is ∼1 μg protein per 1 μg plasmid DNA indicating that Aif1p works together with other cofactors for catalyzing nucleolytic attack of DNA in vivo. Purified yeast nuclei are degraded to random fragments and not internucleosomally. This may be caused by the S. cerevisiae chromatin structure with approximately no linker DNA between the nucleosomes (Lowary and Widom, 1989).
The apoptotic function of Aif1p is partially caspase dependent
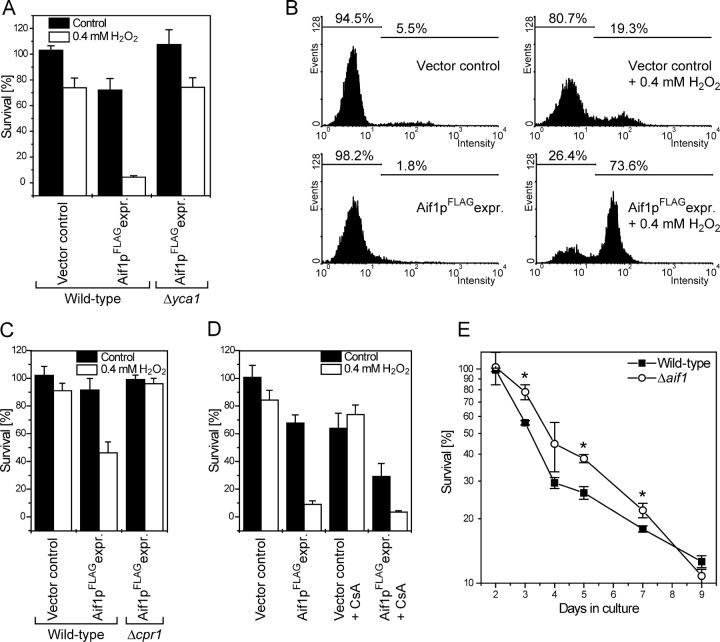
We next addressed the question of whether the mechanism of Aif1p-induced cell death is conserved between mammals and fungi. Although early reports described human AIF as a mediator of a caspase-independent way of cellular suicide (Susin et al., 1999), the release of AIF from mitochondria may be subordinated to earlier caspase activation (Arnoult et al., 2003), supporting the notion that caspases and AIF would be engaged in cooperative or redundant pathways. As described above, Aif1p overexpression stimulates apoptotic cell death only in synergy with mild oxygen stress. The yeast caspase (Yca1p) is activated by the same apoptotic stimulus (Madeo et al., 2002). We thus examined the caspase dependency of Aif1p mediated cell death in yeast. Treatment of wild-type cells overexpressing Aif1p with 0.4 mM H2O2 killed ∼90% of these cells (Fig. 2 G and Fig. 3 A). In contrast, 0.4 mM H2O2 killed only ∼30% of cells overexpressing Aif1p when the YCA1 gene had been deleted from these cells (Fig. 3 A). Therefore, apoptosis potentized by overexpression of Aif1p is mostly, but not entirely, caspase dependent. Consistently, in an in vivo caspase assay, yeast cells overexpressing Aif1p and treated with 0.4 mM H2O2 demonstrated an elevated caspase activity (Fig. 3 B) in wild-type background that is attenuated in the Δyca1 strain (not depicted).
Figure 3.
Putative pathways of Aif1p death functions. (A) Survival of Aif1pFLAG overexpressor and vector control in wild type and yca1 disruptant background after a 20-h induction on galactose with and without 0.4 mM H2O2. Data represent mean ± SEM. (B) Aif1pFLAG overexpressor and vector control grown for 20 h on galactose with or without 0.4 mM H2O2 were analyzed in vivo for caspase activity by FITC-VAD-fmk staining using flow cytometry for quantification. (C) Survival of yeast cells upon moderate overexpression of Aif1pFLAG and vector control in wild type and Δcpr1 background after a 20-h induction with or without 0.4 mM H2O2. Data represent mean ± SEM. (D) Survival of Aif1pFLAG overexpressor and vector control in wild type after a 20-h induction on galactose with or without 0.4 mM H2O2 and with 50 μg/ml Cyclosporin A (CsA) as indicated. Data represent mean ± SEM. (E) Chronological aging of wild type and Δaif1 cells. Asterisks indicate a significant difference in an independent t test for days 3 and 5 at 0.02 and for day 7 at 0.075 level.
CypA is required for Aif1p-induced cell death
AIF function in apoptosis has been shown to critically depend on cyclophilins (Candé et al., 2004), a large family of highly conserved proteins acting as peptidylprolyl cis-trans-isomerases throughout protein folding. It has been suggested that CypA has a latent nuclease activity (Montague et al., 1997) and Kroemer and colleagues (Candé et al., 2004) recently discovered that human AIF interacts with CypA to induce lysis of chromatin. Therefore, we investigated whether yeast Aif1p function also depends on cyclophilin action. Aif1p was overexpressed in two yeast strains in which either the CPR1 or CPR2 genes, which encode cyclophilin homologues, had been disrupted. It appears of note, that Aif1p overexpression resulted in a lower expression level in the CPR1 disrupted strain in comparison to the wild type (unpublished data). Therefore, we adjusted the Aif1p overexpression in the wild type to a comparable level (see Materials and methods). Disruption of CPR1, the yeast homologue of human CypA (Dolinski et al., 1997; Fig. 3 C), but not disruption of the cyclophilin B homologue CPR2 (not depicted) abrogated cell death induced by overexpression of Aif1p. In mammals, the cooperative effects of CypA and AIF in apoptosis occur independently of the peptidylprolyl cis-trans-isomerase activity of CypA (Candé et al., 2004). We also observe that cyclosporin A (CsA), which inhibits the peptidylprolyl cis-trans-isomerase activity of CypA did not increase survival of cells overexpressing Aif1p (Fig. 3 D).
AIF1-deficient cells survive better during chronological aging
Long-term cultivation causes an aging processes in the whole yeast culture, called chronological aging (Fabrizio and Longo, 2003), which leads to physiological-induced apoptosis in yeast (Herker et al., 2004). Therefore, we investigated whether Aif1p participates in chronological aging. We observed that disruption of AIF1 significantly delayed the onset of age-induced cell death, which is consistent with the proapoptotic role of Aif1p (Fig. 3 E). This time course of survival was reproduced in 12 independent experiments, which showed that the AIF1-deficient strain survived better from day 3 to 7 than the wild type in each single experiment.
Together, these results indicate that Aif1p is an effector of apoptotic cell death and is a bona fide AIF homologue in budding yeast. The existence of a yeast homologue of human AIF with similar subcellular dynamics and function emphasizes the common evolutionary origin of yeast and animal apoptosis. Therefore, budding yeast may provide a suitable model system for tracing the evolutionary roots of apoptosis and, by extension, for identifying additional AIF substrates and regulators.
Materials and methods
Strains and plasmids
Experiments were performed in BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), obtained from Euroscarf. Yeast strains BY4741, Δaif1 (YNR074C), Δcpr1, Δcpr2, and Δyca1 were obtained from Euroscarf and were grown on SC medium containing 0.17% yeast nitrogen base (Difco), 0.5% (NH4)2SO4, 30 mg/l of all amino acids (except 80 mg/l histidine and 200 mg/l leucine), 30 mg/l adenine, and 320 mg/l uracil with 2% glucose as carbon source (SCD). For adaptation of protein levels of Aif1p in wild type and Δcpr1 cells we used SCD/G media containing 1% glucose, respectively, 1% galactose for the wild type.
Genomic DNA was isolated from BY4741. To construct Aif1pFLAG, AIF1 was amplified by PCR, cut with EcoR1 and Not1 and ligated into vector pESC-His (Stratagene). The construct codes for a COOH terminally flag-tagged protein and was expressed under the control of an inducible Gal1 promoter. Chromosomal COOH terminally yEGFP-tagged AIF1 was generated using the protocol of Knop et al. (1999). Vector pYM12 was used as template and kanMX6-yEGFP-tag cassette was amplified by PCR with primers containing homologous regions to AIF1. The amplified cassette was transformed into BY4741. To generate an Aif1p/yEGFP fusion under the control of the MET25 promoter, AIF1 was amplified by PCR from plasmid pYCG_YNR074c (EUROSCARF) cut with BamH1–EcoR1 and ligated into vector pUG35.
Test for apoptotic markers
Overexpression of Aif1p with and without hydrogen peroxide for survival assays was performed as described for yeast strain FMY21 (Madeo et al., 2002), survival platings of Δaif1 strains incubated with or without hydrogen peroxide were performed as described previously for a YCA1 disruptant strain (Madeo et al., 2002). DAPI staining and TUNEL test were performed as described (Madeo et al., 1997). To determine the frequency of morphological phenotypes, at least 300 cells of five independent experiments were evaluated. For image acquisition we used a fluorescence microscope (model BH-2RFCA; Olympus), a digital camera (model c35AD-4; Olympus) and analySIS software (Soft Imaging System GmbH). In vivo staining of caspase activity by flow cytometric analysis was performed as described previously (Madeo et al., 2002).
Immunoblotting
Cell extracts were prepared and blots were treated as described previously (Madeo et al., 2002), probed with murine mAbs against GFP (Roche), murine mAbs against nuclear pore complex proteins (clone MAb414; Convance), or rabbit pAbs against Cox2 (a gift from A. Barrientos, Columbia University, New York, NY).
Staining procedures and epifluorescence microscopy analysis
Chimeric Ynr074-GFP fusion protein localization in mitochondria was shown by colocalization with the vector pYX142 containing a DsRed Su1-69 protein under a TPI promoter (Jakobs et al., 2003). For determination of localization of the fusion proteins in the nuclei, cells were transformed with vector pUR36-NLS (Rodrigues et al., 2001), nuclei were in vivo labeled with DsRed-NLS protein. A microscope (model Axiovert 200; Carl Zeiss MicroImaging, Inc.) equipped with filter wheels to control excitation and emission wavelength, with a cooled charge-coupled device (CCD) video camera (model AxioCam HRm; Carl Zeiss MicroImaging, Inc.), and software for image archival and management (AXIOVISION 4) was used for examination of cell populations. The excitation source was a 100 W/DC Hg vapor arc lamp (Carl Zeiss MicroImaging, Inc.). In each case the appropriate fluorescence filter was used.
Spheroblasts and cell fractionation
Spheroblasts were isolated from lyticase-treated cells as described previously (Jazwinski, 1990). For preparation of nuclei, mitochondria, and cytosolic fraction, cells were inoculated to an OD600nm = 0.1, and grown on SCD for 4 h. Nuclei were prepared with a 50% percoll gradient as described previously (Shermann et al., 1986). Preparation of mitochondria was modified according to Daum et al. (1982).
Purification of recombinant Aif1p
AIF1 was expressed from pQE-60 expression vector (QIAGEN) and purified following the manual instructions for purification under denaturing conditions. Refolding was achieved by excessive dialysis against 1 mM EDTA, 0.5 mM EGTA, 50 mM glycine, 0.1% NP-40, 150 mM NaCl, 10% glycerol, and 50 mM Hepes, pH 7.9. After concentration using Centriprep YM-30 (Millipore) Aif1p was stored at −80°C.
DNase activity
Cell extracts were prepared as described previously (Madeo et al., 2002), with a modified lysis buffer (150 NaCl, 50 mM Tris-HCl, pH 7.4, 5 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM PMSF). To the indicated amount (micrograms) of cell extract 1 μg of plasmid DNA (pESC-His; Stratagene) and 2 mM MgCl2/CaCl2 was added. The reaction mix was incubated for 90 min at 37°C and DNA fragments were separated on a 1% agarose gel.
The indicated amount of purified recombinant Aif1p or BSA was added to 1 μg plasmid DNA (pYES2; Invitrogen), incubated at 30°C for 30 min in the presence or absence of 2 mM MgCl2/CaCl2 followed by gel electrophoresis. Heat-inactivated Aif1p was incubated for 10 min at 100°C. 16 μg of purified recombinant Aif1p or BSA was added to purified yeast nuclei (200 μg of protein) for the indicated time. After incubation the genomic DNA was isolated by incubation in 1% SDS, 1% Triton X-100, 50 mM NaCl, 50 mM MES, pH 6.4, and 0.66 μg proteinase K for 2 h, phenol/chloroform extraction, ethanol precipitation, and incubation in 1 mg/ml RNase. Samples were analyzed by gel electrophoresis.
In vitro mitochondrial import
Radiolabeled preproteins were obtained by in vitro transcription and translation reactions using TNT T7 coupled reticulocyte lysate (Promega) in the presence of redivue l-[35S]methionine (Amersham Biosciences), creating an Aif1p with a NH2 terminally c-Myc tag. Import mixtures contained 1–5% reticulocyte lysate (vol/vol) in 1% BSA (wt/vol), 600 mM sorbitol, 50 mM KCl, 2 mM potassium phosphate, 10 mM MgCl2, 50 mM Hepes-KOH, pH 7.4, 2 mM NADH, and 2 mM ATP, and were incubated with mitochondria (25–50 mg protein) at 25°C for 1 h. Mitochondria were converted to mitoplasts by 10-fold dilution in ice-cold 20 mM Hepes, pH 7.4. Protease treatment was performed by addition of 50 μg/ml proteinase K to the reaction and incubation for 30 min at 0°C. The protease was inactivated by the addition of 1 mM PMSF. Mitochondria or mitoplast were centrifuged at 14,000 g for 15 min at 4°C washed with ice-cold 600 mM sorbitol, 80 mM KCl, 20 mM Hepes, pH 7.4, resuspended in Laemmli buffer, loaded on a 12% SDS polyacrylamide gel, electroblotted onto an Immobilon PVDF membrane (Millipore), and detected by autoradiography.
Acknowledgments
We thank Manuel T. Silva and B. Burhans for critical reading of the manuscript and all the helpful comments and suggestions. We are grateful to Patrick Rockefeller for technical assistance.
This project was supported by the Deutsche Forschungsgemeinschaft (to S. Wissing, E. Herker, and F. Madeo) and supported by grants from European Commission (Trans-Death, to C. Candé and G. Kroemer) and Ligue Contre le Cancer (to G. Kroemer).
S. Wissing and P. Ludovico contributed equally to this paper.
M. Corte-Real and F. Madeo contributed equally to this paper.
Abbreviations used in this paper: AIF, apoptosis-inducing factor; CypA, cyclophilin A.
References
- Aris, J.P., and G. Blobel. 1989. Yeast nuclear envelope proteins cross react with an antibody against mammalian pore complex proteins. J. Cell Biol. 108:2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult, D., B. Gaume, M. Karbowski, J.C. Sharpe, F. Cecconi, and R.J. Youle. 2003. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 22:4385–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard, F., M.E. Rusiniak, K. Sharma, X. Sun, I. Todorov, M.M. Castellano, C. Gutierrez, H. Baumann, and W.C. Burhans. 2002. Targeted destruction of DNA replication protein Cdc6 by cell death pathways in mammals and yeast. Mol. Biol. Cell. 13:1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candé, C., N. Vahsen, I. Kouranti, E. Schmitt, E. Daugas, C. Spahr, J. Luban, R.T. Kroemer, F. Giordanetto, C. Garrido, et al. 2004. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene. 23:1514–1521. [DOI] [PubMed] [Google Scholar]
- Cregan, S.P., A. Fortin, J.G. MacLaurin, S.M. Callaghan, F. Cecconi, S.W. Yu, T.M. Dawson, V.L. Dawson, D.S. Park, G. Kroemer, and R.S. Slack. 2002. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J. Cell Biol. 158:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum, G., P.C. Bohni, and G. Schatz. 1982. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J. Biol. Chem. 257:13028–13033. [PubMed] [Google Scholar]
- Dolinski, K., S. Muir, M. Cardenas, and J. Heitman. 1997. All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 94:13093–13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio, P., and V.D. Longo. 2003. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2:73–81. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog, B., U. Sauder, and U. Aebi. 2004. The Saccharomyces cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 117:115–126. [DOI] [PubMed] [Google Scholar]
- Herker, E., H. Jungwirth, K.A. Lehmann, C. Maldener, K.U. Frohlich, S. Wissing, S. Buttner, M. Fehr, S. Sigrist, and F. Madeo. 2004. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs, S., N. Martini, A.C. Schauss, A. Egner, B. Westermann, and S.W. Hell. 2003. Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p. J. Cell Sci. 116:2005–2014. [DOI] [PubMed] [Google Scholar]
- Jazwinski, S.M. 1990. Preparation of extracts from yeast. Methods in Enzymology. Academic Press, Inc., San Diego, CA. 154–174. [DOI] [PubMed]
- Knop, M., K. Siegers, G. Pereira, W. Zachariae, B. Winsor, K. Nasmyth, and E. Schiebel. 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast. 15:963–972. [DOI] [PubMed] [Google Scholar]
- Laun, P., A. Pichova, F. Madeo, A. Ellinger, S.D. Kohlwein, K.U. Frohlich, I. Dawes, and M. Breitenbach. 2001. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 39:1166–1173. [PubMed] [Google Scholar]
- Lowary, P.T., and J. Widom. 1989. Higher-order structure of Saccharomyces cerevisiae chromatin. Proc. Natl. Acad. Sci. USA. 86:8266–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico, P., M.J. Sousa, M.T. Silva, C. Leao, and M. Corte-Real. 2001. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 147:2409–2415. [DOI] [PubMed] [Google Scholar]
- Madeo, F., E. Frohlich, and K.U. Frohlich. 1997. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139:729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Frohlich, M. Ligr, M. Grey, S.J. Sigrist, D.H. Wolf, and K.U. Frohlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lachelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K.U. Frohlich. 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 9:911–917. [DOI] [PubMed] [Google Scholar]
- Mazzoni, C., P. Mancini, L. Verdone, F. Madeo, A. Serafini, E. Herker, and C. Falcone. 2003. A truncated form of KlLsm4p and the absence of factors involved in mRNA decapping trigger apoptosis in yeast. Mol. Biol. Cell. 14:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miramar, M.D., P. Costantini, L. Ravagnan, L.M. Saraiva, D. Haouzi, G. Brothers, J.M. Penninger, M.L. Peleato, G. Kroemer, and S.A. Susin. 2001. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 276:16391–16398. [DOI] [PubMed] [Google Scholar]
- Montague, J.W., F.M. Hughes Jr., and J.A. Cidlowski. 1997. Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis-trans-isomerase activity. Potential roles of cyclophilins in apoptosis. J. Biol. Chem. 272:6677–6684. [DOI] [PubMed] [Google Scholar]
- Rodrigues, F., M. van Hermet, H.Y. Steensma, M. Corte-Real, and C. Leao. 2001. Red fluorescent protein (DsRed) as a reporter in Saccharomyces cerevisiae. J. Bacteriol. 183:3791–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shermann, F., G.R. Fink, and J.B. Hicks. 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 139–142.
- Shirogane, T., T. Fukada, J.M. Muller, D.T. Shima, M. Hibi, and T. Hirano. 1999. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 11:709–719. [DOI] [PubMed] [Google Scholar]
- Susin, S.A., H.K. Lorenzo, N. Zamzami, I. Marzo, B.E. Snow, G.M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, et al. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 397:441–446. [DOI] [PubMed] [Google Scholar]
- Weinberger, M., L. Ramachandran, and W.C. Burhans. 2003. Apoptosis in yeasts. IUBMB Life. 55:467–472. [DOI] [PubMed] [Google Scholar]
- Wu, D., P.J. Chen, S. Chen, Y. Hu, G. Nunez, and R.E. Ellis. 1999. C. elegans MAC-1, an essential member of the AAA family of ATPases, can bind CED-4 and prevent cell death. Development. 126:2021–2031. [DOI] [PubMed] [Google Scholar]
- Wu, M., L.G. Xu, X. Li, Z. Zhai, and H.B. Shu. 2002. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem. 277:25617–25623. [DOI] [PubMed] [Google Scholar]