Abstract
Single-layered epithelia are the first differentiated cell types to develop in the embryo, with columnar and squamous types appearing immediately after blastocyst implantation. Here, we show that mouse embryonic stem cells seeded on hensin or laminin, but not fibronectin or collagen type IV, formed hemispheric epithelial structures whose outermost layer terminally differentiated to an epithelium that resembled the visceral endoderm. Hensin induced columnar epithelia, whereas laminin formed squamous epithelia. At the egg cylinder stage, the distal visceral endoderm is columnar, and these cells begin to migrate anteriorly to create the anterior visceral endoderm, which assumes a squamous shape. Hensin expression coincided with the dynamic appearance and disappearance of columnar cells at the egg cylinder stage of the embryo. These expression patterns, and the fact that hensin null embryos (and those already reported for laminin) die at the onset of egg cylinder formation, support the view that hensin and laminin are required for terminal differentiation of columnar and squamous epithelial phenotypes during early embryogenesis.
Keywords: epithelium; extracellular matrix proteins; cell size; embryonic development; stem cell
Introduction
Epithelia are generally classified into simple (monolayered) or stratified (multilayered) cells, with simple epithelia being further divided into squamous, cuboidal, or columnar cells. These descriptions are based on cell height and shape, and it is likely that these are only the most obvious of the differences among epithelial cells. The molecular mechanisms that cause these kinds of morphological variations are largely unknown.
Generation of epithelia is one of the critical events during early mouse embryogenesis, where the first differentiation event at embryonic day 3.5 (E3.5) forms the blastocyst, a structure that consists of an epithelium, the trophectoderm, and the inner cell mass (ICM; see Fig. 7; for review see Hogan et al., 1994). The polarized cells of the trophectoderm transport fluid into the interior, generating the balstocoel cavity. The trophectoderm terminally differentiates to produce the mural trophectoderm located at the pole opposite the ICM. The second differentiation event involves the conversion of the outer layer of the ICM to another epithelium, the primitive endoderm, which later terminally differentiates into the visceral and parietal endoderms. These latter endoderms eventually become the extraembryonic yolk sac and Reichert's membrane, respectively. The transcription factors GATA-6 and HNF4 begin to be expressed in the primitive endoderm, and their deletion results in a failure in endoderm development with consequent periimplantation lethality (Chen et al., 1994; Morrisey et al., 1998). At the same time, a flat cluster of the ICM expands its mass and extends to the abembryonic pole, resulting in the formation of the egg cylinder, which includes the epiblast (the future embryo proper) surrounded by the visceral and parietal endoderms. The resultant egg cylinder allows gastrulation to occur.
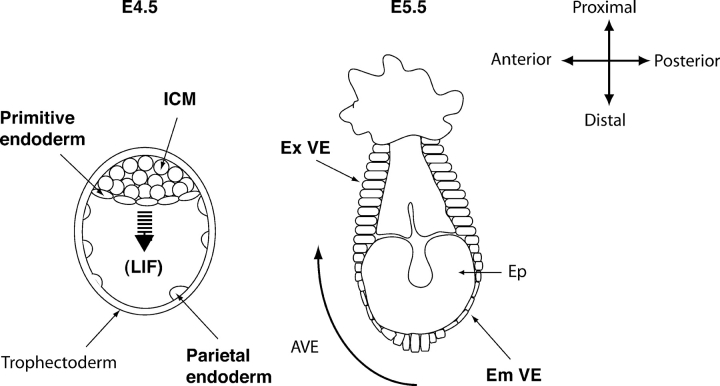
Figure 7.
A proposed model for egg cylinder morphogenesis. Expansion of the ICM to the abembryonic pole occurs in the mouse blastocyst between E4.5 and E5.5. Because hemisphere formation on the extracellular matrices did not occur in the absence of LIF (Fig. 3), we propose that LIF provides the driving force for the egg cylinder morphogenesis. The surface cells of the ICM (primitive endoderm) differentiate to two types of epithelia. Most epithelia in the embryonic region are squamous embryonic visceral endoderm, whereas cells in the extraembryonic region are columnar extraembryonic visceral endoderm. A few cells at the distal end of visceral endoderm become columnar at E5.5 and migrate to the embryonic/extraembryonic boundary to form AVE. Experimental results in vitro suggested that hensin specified the columnar cells; in contrast, laminin induced the squamous cells (Figs. 5 and 6). Thus, hensin and laminin are involved in the regionalization of the egg cylinder and possibly in the establishment of the anteroposterior axis that is mediated by AVE formation. Em VE, embryonic visceral endoderm; Ep, epiblast; Ex VE, extraembryonic visceral endoderm.
Although the visceral endoderm is a proper epithelium, the shape of the cells exhibits dynamic and region-specific changes during the development of the egg cylinder, starting at E5.0. Epithelia in the extraembryonic region are columnar (Reinius, 1965; Solter et al., 1970), but those that cover the epiblast exhibit many changes. Before gastrulation begins, the embryonic visceral endoderm develops a columnar shape at its most distal portion, whereas the rest of the epithelium retains its squamous shape (Rivera-Perez et al., 2003). This development is accompanied by the expression of specific markers, including the homeo domain protein Hex (Thomas and Beddington, 1996; Beddington and Robertson, 1998; Thomas et al., 1998). Remarkably, this columnar epithelium migrates over the next few hours to assume an anterior position and become the anterior visceral endoderm (AVE), an epithelium critical for establishment of the anteroposterior axis (for review see Beddington and Robertson, 1999). During its migration, it assumes a more flattened, squamous shape (Srinivas et al., 2004).
The differentiation of visceral endoderm in vitro has been studied using embryoid bodies, which are free floating spheres of embryonic stem (ES) cells that eventually exhibit phenomena similar to those of the early embryo, including cavitation (Li et al., 2003). Deletion of the laminin gene resulted in periimplantation embryonic lethality, and in vitro studies showed that laminin was required for the formation of the visceral endoderm in embryoid bodies (Smyth et al., 1999). Disruption of the expression of integrin β1, a laminin receptor, resulted in a similar phenotype in in vivo and in vitro embryoid bodies (Fassler and Meyer, 1995; Stephens et al., 1995). However, the effect of such disruption on the morphological distinction between the embryonic and the extraembryonic cell types was not studied.
While studying the generation and regulation of epithelial polarity in a kidney cell line, we discovered that an ECM protein induced their terminal differentiation (van Adelsberg et al., 1994; Takito et al., 1996). Development of epithelia can be thought of as occurring in at least two steps: (1) conversion of nonepithelial stem cells to “generic” or protoepithelia, followed by (2) terminal differentiation (Al-Awqati, 2003). Protoepithelia have all the basic characteristics of these cells, including polarity, transepithelial transport, and tight and adherent junctions, but they lack several tissue-specific features. Terminally differentiated epithelia have many apical structures, such as microvilli, that are produced by a subapical actin network that also includes several actin-binding proteins and differentiated cytokeratins. Unlike protoepithelia, terminally differentiated epithelia show specialized apical functions, including vigorous apical endocytosis and apical secretory granules that can fuse by a process of regulated exocytosis. These epithelia also have characteristic shapes; for example, single-layered epithelia often become columnar. We identified and isolated an ECM protein that induced this phenotype change and termed it hensin (van Adelsberg et al., 1994; Takito et al., 1996). Hensin caused the terminal differentiation of epithelia, changing the kidney cell line from a flat into a columnar epithelium both in vitro (Vijayakumar et al., 1999) and in vivo (Schwartz et al., 2002). Its sequence showed that it was a large multidomain protein with many repeated sequences (Takito et al., 1999). Secreted hensin monomer underwent a complex series of modification until it was deposited as a fiber in the ECM, where it could induce terminal differentiation (Hikita et al., 1999, 2000). In most epithelia, hensin was expressed in many tissue-specific alternately spliced forms (Takito et al., 1999). The putative tumor suppressor DMBT1, which is the human orthologue of hensin, was deleted in a large fraction of gliomas and glioblastomas (Mollenhauer et al., 1997) and in lung, gastric, and colon cancers (Mori et al., 1999).
In the present work, we show that hensin null mouse embryos died between E4.5 and E5.5, when the first columnar epithelia appeared. Hensin and laminin, but not fibronectin and collagen type IV, were able to induce visceral endoderm from mouse ES cells in vitro. The epithelia generated by hensin were columnar, whereas those induced by laminin assumed a squamous shape.
Results
Targeted deletion of hensin
We designed a targeting construct that replaced the first exon encoding the signal peptide with lacZ and neomycin cassette (Fig. 1 A). The heterozygotes obtained by homologous recombination showed no apparent abnormality in growth, fertility, or bone structure. Genotyping of heterozygote crosses showed no hensin knockouts in newborn pups or in embryos at E8.5 or E6.5, but we found many empty deciduas, which showed no detectable embryonic structures (Fig. 1 C). A quarter of E3.5 blastocysts showed the null allele. Histological analysis of E5.5 embryos revealed that the embryos were absorbed (Fig. 1 D). Therefore, hensin knockout embryos died between E4.5 and E5.5.
Figure 1.
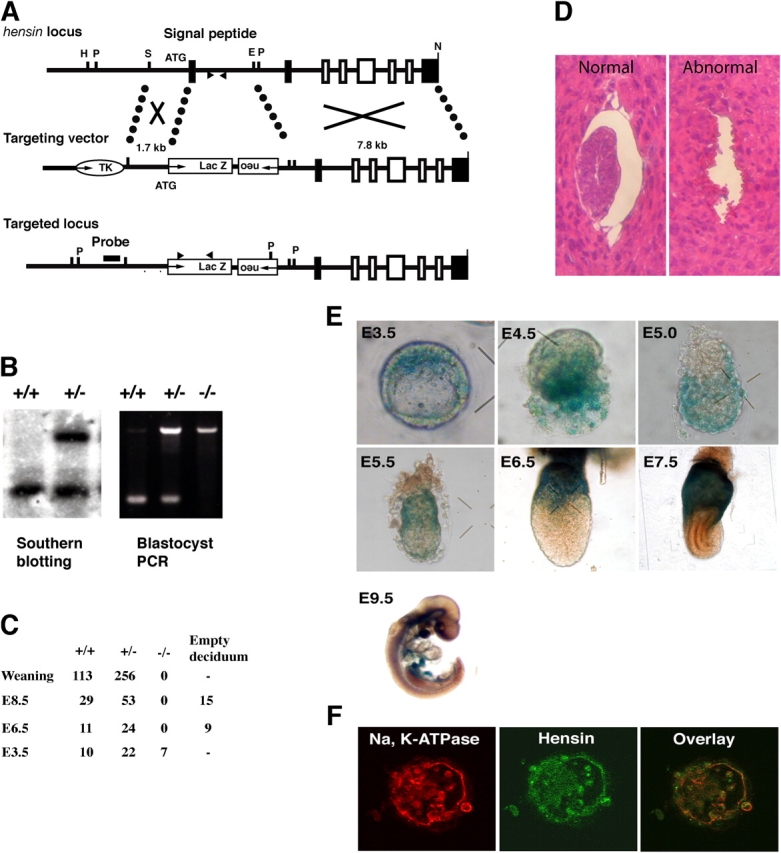
Targeted disruption of the mouse hensin gene and expression of hensin in early embryos. (A) A partial restriction map of the mouse hensin locus and the design of the target construct for homologous recombination. Exons found in the mouse hensin/CRP-ductin cDNA (Cheng et al., 1996) are represented by shaded rectangles, whereas those not previously reported are shown as open rectangles. Arrowheads indicate the primer sites for PCR genotyping. Restriction sites are as follows: E, EcoRI; H, HindIII; N, NotI; P, PstI; and S, SacI. (B) Genotyping by Southern blot (left) and by PCR (right). PstI-digested genomic DNA from the tail of a weaning mouse was hybridized with a probe shown in A. Genomic DNA from the E3.5 blastocyst was amplified by PCR with the primer sets that recognized the deleted intronic sequence and the lacZ-coding sequence shown in A. (C) Results of genotyping. (D) Hematoxylin and eosin staining of normal and abnormal E5.5 embryos. (E) Expression of hensin in the early embryo. Freshly isolated embryos at various developmental stages were stained for lacZ activity. (F) Confocal images of a blastocyst stained with Na, K-ATPase or hensin antibody. Note the basal staining in the mural trophectoderm and the cytoplasmic staining in the ICM.
Using the inserted lacZ as a marker for hensin expression, we found that the earliest time of expression was in the E3.5 blastocyst, where hensin was expressed in the ICM (Fig. 1 E). Expression later appeared in the mural trophectoderm at E4.0, and in its descendants, the giant trophoblasts. The pattern of expression at E4.5 is consistent with expression in the primitive endoderm. At E5.0, hensin was expressed in all regions of the visceral endoderm, which is a descendant of the primitive endoderm. Then, at E5.5, expression decreased in the embryonic visceral endoderm, remaining only at the distal visceral endoderm (Fig. 1 E). Later, although all the embryonic visceral endoderm became devoid of hensin (lacZ) expression, expression increased in the extraembryonic visceral endoderm. The signal disappeared from the giant trophoblasts. At E9.5, faint staining was observed in the midbrain, notochord, liver primordium, midgut, and hindgut. These areas are precursors of the adult brain, liver, intestine, and colon where hensin is highly expressed. Hensin expression in the blastocyst was confirmed by antibody staining (Fig. 1 F), which showed that it was present in an ECM pattern underneath the mural trophectoderm but was more intracellular in the other structures.
We used the blastocyst outgrowth assay to monitor the phenotypes of the presumed hensin knockouts in vitro. E3.5 blastocysts in culture started the outburst after 2–3 d, and then formed the cylinder-shaped ICM, which was surrounded by the flattened giant trophoblasts (Fig. 2 A). 96% of blastocysts (95/99) from wild-type crosses showed a normal phenotype. But of heterozygote crosses, only 71% (76/107) had a normal phenotype; where tested (n = 20), all of these had either a +/− or a +/+ genotype. The remainder showed gross defects, including 11% (12/107) with no detectable ICM, 13% (14/107) with a degenerating ICM, and 5% (5/107) with a small ICM at the time of the outburst. This latter phenotype was also observed in the control crosses at the same percentage (3/99). The genotype of blastocyst outgrowths that failed to grow was a −/− genotype in seven out of the eight blastocysts tested. All outgrowths examined had well-developed giant trophoblasts.
Figure 2.
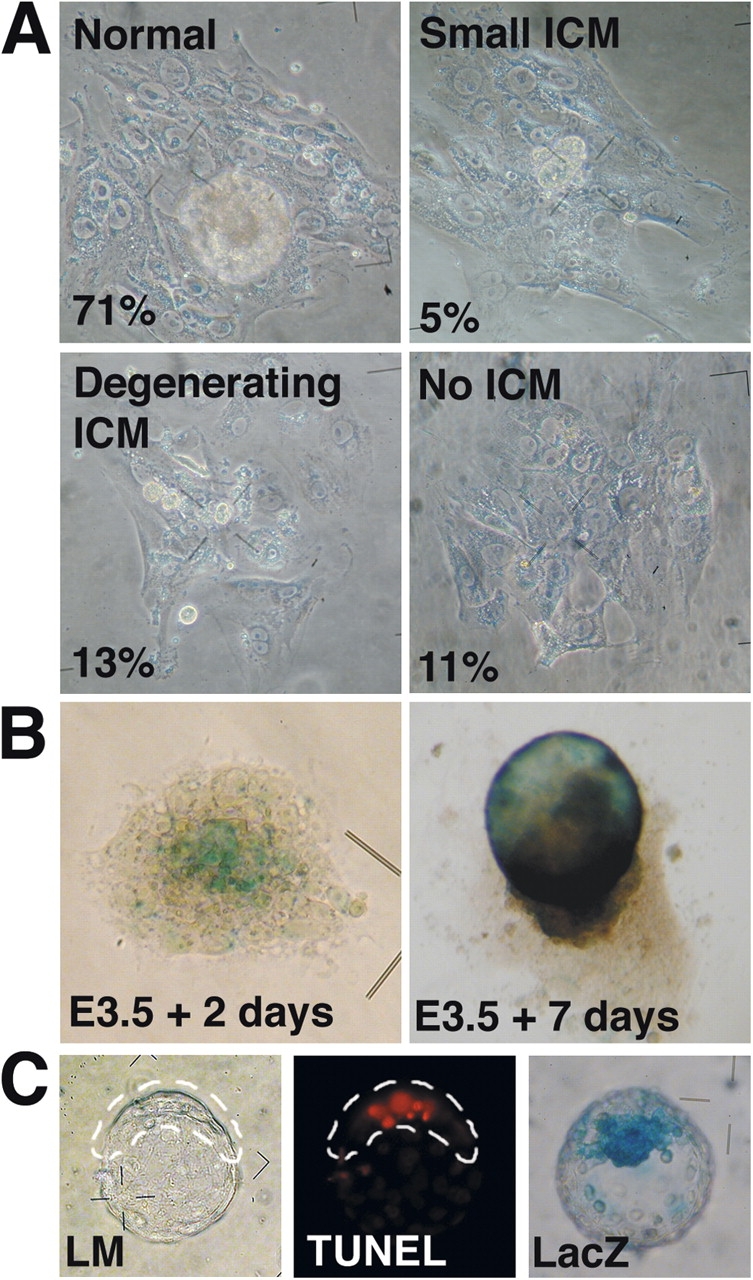
Phenotypes of hensin knockouts in culture. (A) E3.5 blastocysts were cultured in the outgrowth medium for 7 d. The phenotypes of the outgrowth were classified as normal, small ICM type, degenerating ICM type, or no ICM type. (B) LacZ staining of the outgrowth 2 d (when the outburst starts) and 7 d after culture. (C) Apoptosis in the blastocyst. (left) Light microscopic image of the TUNEL-stained blastocyst. ICM is enclosed within the dashed line. (middle) Fluorescence image of the same blastocyst. (right) Age-matched blastocyst stained for lacZ activity.
It has been shown that differentiation of the trophectoderm does not require the participation of basement membranes (Smyth et al., 1999). The mural trophectoderm at the late blastocyst stage acquires vigorous phagocytic activity and internalizes beads in vitro (Rassoulzadegan et al., 2000). We exposed blastocysts to beads at E3.5 and cultured them in the outgrowth medium to examine their properties. Phagocytosis was evident even in the blastocysts that later showed ICM failures (i.e., they were likely derived from the knockout; unpublished data), suggesting that hensin was not required for terminal differentiation of mural trophectoderm, the first epithelia in mice.
LacZ staining of the embryo, in the outgrowth assay, indicated that hensin was expressed at the central ICM region at the time of outburst and in the presumptive visceral endoderms after 7 d in culture (Fig. 2 B). Thus, hensin knockout embryos seemed to die because of their inability to form the egg cylinder in the outgrowth assay. To identify the mechanism of ICM failure, we stained blastocysts with the TUNEL reagent to detect the apoptosis. Freshly isolated E3.5 blastocysts had no TUNEL-positive cells. Control blastocysts cultured overnight in M16 showed TUNEL-positive cells near the surface of the ICM. LacZ staining indicated that hensin was expressed in the primitive endoderm and/or the ICM in the blastocyst cultured overnight (Fig. 2 C). 95% (83/87) of the blastocysts from wild-type crosses had less than six TUNEL-positive cells. In contrast, 27% (27/99) of the blastocysts recovered from hensin heterozygote crosses had 7–16 TUNEL-positive cells. These results suggested that the disintegration of the ICM occurred via apoptosis.
Because the pattern of expression suggests that at the time of embryonic death (i.e., ∼E4.5), hensin is expressed in the primitive endoderm (the precursor of visceral endoderm), it is likely that embryonic lethality is due to a defect in either the formation or the terminal differentiation of the primitive endoderm layer.
Hemisphere formation from ES cells on ECM filters
Hensin's early expression in the ICM and/or primitive endoderm and high expression in the visceral endoderm (Fig. 1 E), and the ICM failure due to apoptosis (Fig. 2 C), suggested that hensin might play a critical role in endoderm terminal differentiation. To directly test the effect of polymerized hensin on the basal surface of ES cells, we seeded the cells on filters. ES cells were plated at low density and cultured for 5 d on a filter coated with fibronectin, collagen type IV, or laminin, or on a hensin-conditioned filter, with or without leukemia inhibitory factor (LIF; Fig. 3 A). In the presence of LIF, ES cells plated on fibronectin formed an epithelial-like monolayer. Unexpectedly, ES cells plated on laminin- or hensin-conditioned filters had slower proliferation and expansion in the x-y plane, but started to grow in the z axis, forming hemispheres. ES cells plated on collagen type IV produced small hemispheres at first; but, within 5 d of culture, the structures fused with each other, forming a monolayer. Without LIF, we did not observe hemispheres on any matrix; instead, the structures were amorphous multilayers of cells. The number of hemispheres formed on hensin or laminin was several hundred per filter (Fig. 3 B). The dimension of each hemisphere was ∼100–400 μm in diameter and ∼30–90 μm in height. Hemispheres were composed of approximately six to seven layers of cells, with an empty space in the center next to the filter (Fig. 3 C), a phenomenon that seemed similar to cavitation (Coucouvanis and Martin, 1995). These hemispheres could survive over 10 d in culture and sometimes fused with each other. These results indicated that ES cells received a distinct signal from each ECM.
Figure 3.
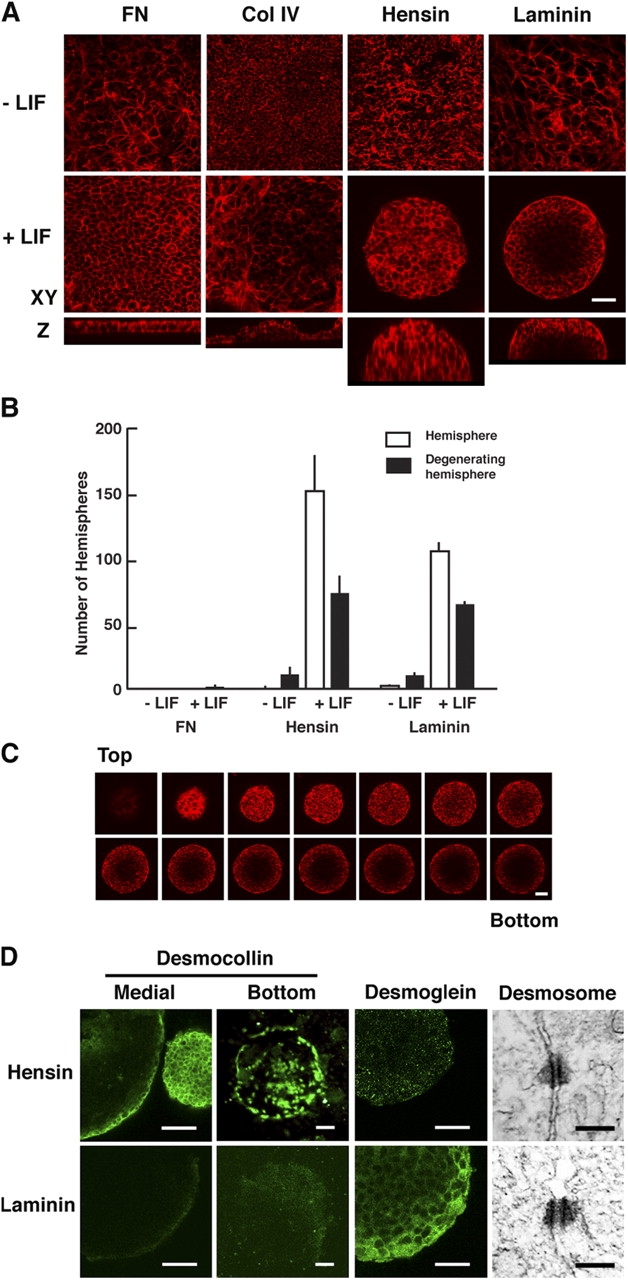
Hemisphere formation of ES cells on extracellular matrices. (A) Confocal images of ES cells cultured for 5 d on filters coated with fibronectin (FN), collagen type IV (Col IV), hensin, or laminin with and without LIF. Actin filaments were stained with rhodamine phalloidin. Top and middle panels show the images in the x-y plane, and bottom panels show the corresponding images in the z axis. (B) The number of hemispheres after 7 d in culture on fibronectin, hensin, or laminin. Error bars indicate SD; n = 4. (C) Z-sections of hemispheres. A hemisphere cultured for 5 d on laminin with LIF was stained with rhodamine phalloidin and optically sectioned with a 6-μm interval using a confocal microscope. Note that the phalloidin-negative area at the center bottom in the hemisphere is probably due to cavitation. (D) Desmosomes and hemidesmosomes. Hemispheres formed 5 d after culture on hensin or laminin with LIF were stained with desomocollin or desmoglein antibodies. Images at the bottom of hemispheres were taken after removing the hemispheres from the filter. Images of desmosomes with radiating intermediate filaments were obtained by transmission electron microscopy in the outermost cells of hemispheres cultured for 10 d with LIF. Bars: (A, C, and D) 20 μm; (electron microscopy) 200 nm.
The hemispheres on laminin were larger and taller than those on hensin, but were easily detached from the filter on changing the medium or other manipulations. Because the interaction between the basal surface of cells and ECM can be mediated by hemidesmosomes, we stained the hemispheres with antibodies against the desmosomal components desmocollin and desmoglein (Fig. 3 D). Desomocollin was detected in the outermost layer of hemispheres formed on hensin. The antibody detected a ringlike structure at the base of the hemisphere as well as scattered large dots, suggesting the establishment of hemidesmosomes. In contrast, the expression of desmocollin was very low in the hemisphere formed on laminin. Desmoglein was highly expressed, but mostly in the cell–cell border of these laminin-induced hemispheres; it was not expressed in the hensin-induced hemispheres. Although the two junctional molecules were detected in both hemispheres 7 d after culture, the results suggested a qualitative difference of desmosomes in the two types of hemispheres. The establishment of desmosomes in the hemispheres was confirmed by electron microscopy (Fig. 3 D).
Antibody-blocking experiments verified the specificity of matrix-induced generation of hemispheres. Addition of hensin-blocking antibodies decreased the number of hemispheres to 50% on the hensin-conditioned filters, suggesting that there might be other factors involved in the hemisphere formation.
Effect of LIF on morphogenesis and differentiation of ES cells
The time course of gross morphological changes in ES cells plated on laminin was monitored by phalloidin binding to actin filaments (Fig. 4 A). Most ES cells appeared as round single cells 4 h after seeding and started cell division 18 h later. There was no detectable effect of LIF on individual cell morphology at 2 d after plating. ES cell colonies cultured with LIF showed a smooth and round surface after 66 h. In the absence of LIF, many peripheral cells migrated out from the ES aggregate. Some peripheral cells developed lamellipodia and formed abundant actin stress fibers, suggesting the loss of ES cell phenotypes. These cells showed minimal E-cadherin expression. Between 3 and 5 d after culture without LIF, most ES cell colonies collapsed and formed amorphous multilayers. Because LIF is used for the maintenance of ES cells, the initial hemisphere formation in the absence of LIF might reflect a “memory” effect of the earlier exposure.
Figure 4.
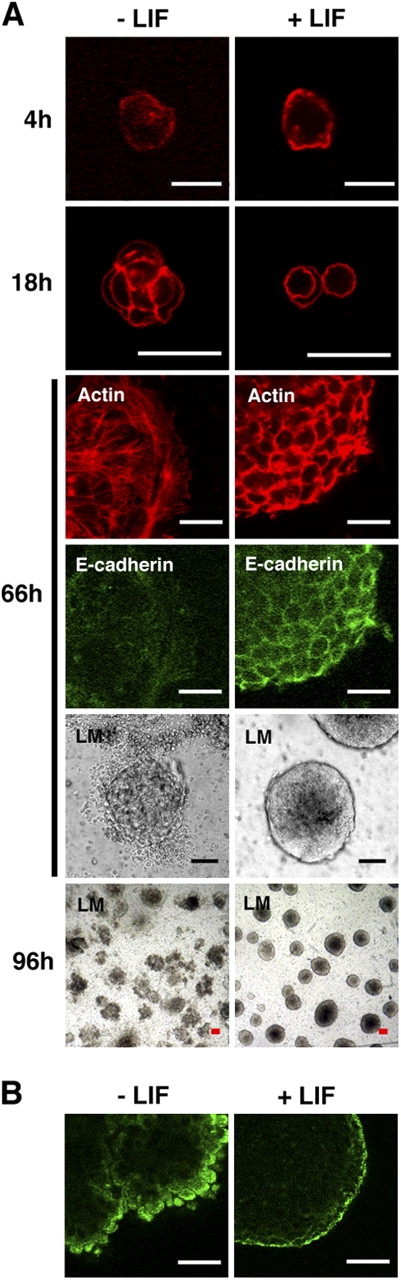
Effects of LIF on hemisphere morphogenesis and differentiation. (A) Time course of morphological changes in ES cells seeded on laminin with and without LIF. ES cells at the indicated times were stained with rhodamine phalloidin to detect actin filaments and E-cadherin antibody. The gross morphology was observed with light microscope (LM). (B) Hemispheres cultured for 7 d on laminin, with and without LIF, were stained with α-fetoprotein antibody. Note the rough surface of the hemispheres cultured without LIF. Bars: (A, 4h) 7 μm; A, 18–96h, and B) 20 μm.
α-Fetoprotein, a definitive marker of visceral endoderm (Dziadek and Adamson, 1978), was expressed in the outermost monolayer of hemispheres cultured on laminin in the presence of LIF (Fig. 4 B). In the absence of LIF, some rare hemispheres remained 7 d after plating (Fig. 3 B) and had ragged surfaces, but still expressed α-fetoprotein. This indicates that LIF had little effect on the differentiation of visceral endoderm from ES cells. A similar conclusion was reached regarding the embryoid body system (Murray and Edgar, 2001b). Thus, LIF seems to be involved in hemisphere morphogenesis, but not in the differentiation of visceral endoderms.
Epithelialization in the hemisphere
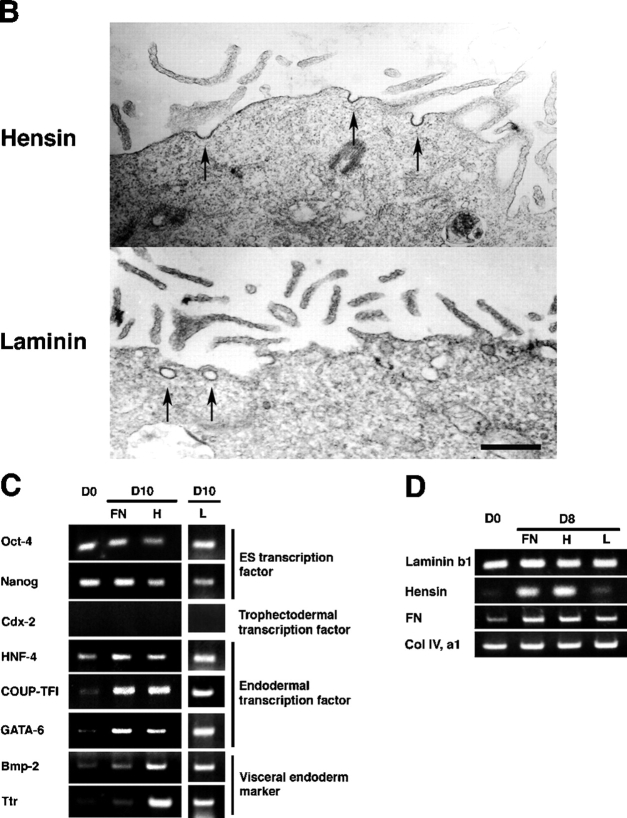
Surface cells of the ICM in the blastocyst differentiate to primitive endoderm and then to visceral endoderm in vivo. We examined whether similar epithelialization occurred in the ES cell–derived hemispheres induced by hensin and laminin. Cells at the outermost layer of the hemisphere expressed E-cadherin, a cell–cell adhesion molecule, at their lateral membranes (Fig. 5 A), as well as the tight junction protein ZO-1. Markers of terminal differentiation of the visceral endoderm, such as cytokeratin 19 (Tamai et al., 2000), were expressed at the cell border of epithelia, including the apical membrane, at day 5. Similarly, villin, an actin-binding protein critical for formation of microvilli (Friederich et al., 1990), was highly expressed in the apical cell periphery on day 7. Although monolayers of ES cells seeded on fibronectin-coated filters also expressed these proteins, their distribution was largely cytoplasmic. In ES cells grown on hensin and laminin, villin and cytokeratin 19 were correctly localized to the apical surface. By electron microscopy, long microvilli were observed at the apical membrane of the surface cells of hemispheres (Fig. 5 B). ES cells grown on hensin or laminin were able to endocytose apically applied FITC-dextran, whereas cells grown on fibronectin could not (Fig. 5 A).
Figure 5.

Induction of terminal differentiation of epithelia from ES cells by hensin and laminin, but not by fibronectin. (A) ES cells cultured for 5 d on hensin, laminin, or fibronectin were stained with antibodies against E-cadherin, ZO-1, and cytokeratin 19 (CK-19). Arrows indicate the tight junction in ZO-1 staining. Expression of villin, GATA-6, and apical endocytosis was tested on ES cells cultured for 7 d. Endocytosis was assessed by the uptake of FITC-dextran at 37°C (Vijayakumar et al., 1999). Arrows indicate the dextran internalized at the apical membrane. Bar, 20 μm. (B) Microvilli were observed by transmission electron microscopy at the apical membrane of the surface cell of the hemisphere that was cultured for 10 d. Arrows indicate endocytic vesicles. The size of the vesicles was 93 ± 9 nm (n = 9). Bar, 500 nm. (C) RT-PCR analyses of endodermal gene expression in ES cells cultured for 10 d on fibronectin, hensin, or laminin. The data presented are the combined results of two separate experiments: in the first, we compared the gene expression among ES cells (day 0) with fibronectin and hensin (day 10); in the second, among ES cells (day 0) with hensin and laminin (day 10). FN, fibronectin; H, hensin; L, laminin. (D) RT-PCR analyses of ECM gene expression in ES cells cultured for 8 d on fibronectin, hensin, or laminin.
Induction of differentiation of ES cells to visceral endoderm was examined by RT-PCR (Fig. 5 C). All ES cells cultured on fibronectin, hensin, and laminin induced the expression of the early endoderm markers COUP-TFI, GATA-6, and HNF-4. The visceral endoderm markers transthyretin and Bmp-2 increased after 10 d in ES cells cultured on hensin and laminin but not in those cultured on fibronectin. These results suggest that the outermost monolayer of hemispheres generated by hensin or laminin had properties of the terminally differentiated form of visceral endoderms, whereas the monolayer differentiated on fibronectin did not.
Before seeding, ES cells did not express hensin but expressed laminin and collagen IV and low levels of fibronectin (Fig. 5 D). Seeding the cells on laminin had no effect on expression of hensin, laminin, or fibronectin. However, seeding the cells on fibronectin or hensin increased fibronectin and hensin expression, but had no effect on laminin expression. These results demonstrate that the composition of the ECM can have profound effects on the expression of matrix proteins. Although a more comprehensive analysis will be needed, the results suggest that the response of ES cells to various ECM proteins is complex and includes positive feedback control of expression, a phenomenon that is likely to be involved in fixing the cell identity.
Columnar cells on hensin but squamous cells on laminin
We confirmed others' findings (Reinius, 1965; Solter et al., 1970) that in E7.5 embryo, the visceral endoderm in the extraembryonic region becomes columnar. At this age, the visceral endoderm that covers the embryo has been replaced by the definitive endoderm, which appears to also be squamous (Fig. 6 A). The dimensions of ES cells grown on the three matrices were measured, after the cells were stained with rhodamine phalloidin, using confocal microscopy (Fig. 6 B). The cell height of ES cells grown on fibronectin was 4.4 ± 0.7 μm, whereas that of the outermost monolayer of hemispheres formed on hensin was 8.1 ± 1.4 μm, and the height of those grown on laminin was 4.7 ± 0.9 μm (mean ± SD; the cell height of cells grown on hensin was significantly different from the heights of those grown on the two other matrices; P < 0.01, n = 15 each). The cell widths of ES cells grown on fibronectin, hensin, and laminin were 4.8 ± 1.0 μm, 4.4 ± 0.8 μm, and 10.3 ± 2.8 μm, respectively. The results indicated that the outermost epithelia of the hemispheres generated by hensin are columnar cells, whereas those produced by laminin are squamous ones.
Figure 6.
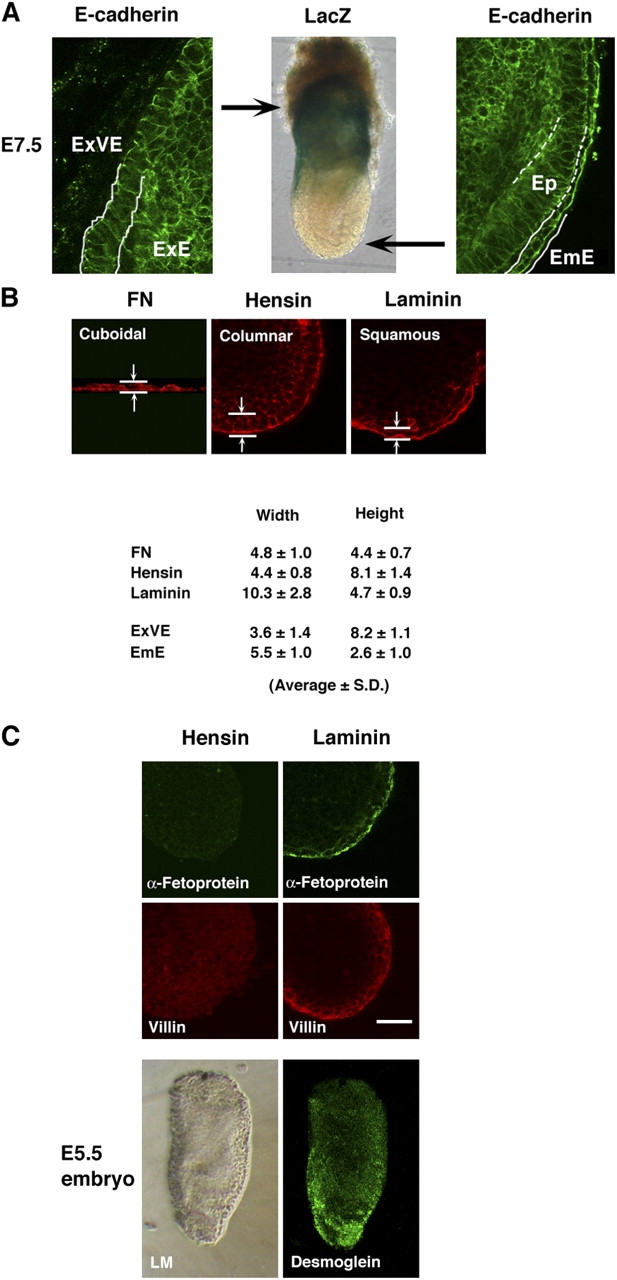
Generation of columnar epithelia by hensin and squamous cells by laminin. (A) An E7.5 embryo was stained with E-cadherin antibody to delineate the cell shape. (left) Columnar epithelia in the extraembryonic region. ExVE, extraembryonic visceral endoderm; ExE, extraembryonic ectoderm. (middle) LacZ staining indicates that E7.5 embryos express hensin in the extraembryonic cells. (right) Squamous epithelia in the embryonic region. EmE, embryonic endoderm; Ep, epiblast. Arrows indicate the relative positions of images in the egg cylinder. (B) Measurements of the cell width and cell height of ES cells cultured for 8 d on fibronectin, hensin, or laminin and of extraembryonic visceral endoderm and embryonic endoderm, in E7.5 embryo. (C) Distinction between hemispheres formed on hensin and laminin. Expression of α-fetoprotein (day 7) and villin (day 5) in hemispheres formed on hensin or laminin. Desmoglein was predominantly expressed in the embryonic cells in E5.5 embryo. LM, phase-contrast light microscopy. Bar, 20 μm.
α-Fetoprotein, a widely accepted marker of visceral endoderm, is vigorously secreted by epithelia in the embryonic region. However, its production is suppressed in the extraembryonic cells (Dziadek, 1978). The apical membranes of the outermost monolayer of hemispheres formed by laminin were positive for α-fetoprotein 7 d after plating (Fig. 6 C). But hemispheres induced by hensin were negative for this marker during the period of observation from days 5 to 12. The expression of α-fetoprotein in vivo correlates more with the position of the visceral endoderm than with the morphology of the epithelium (Waldrip et al., 1998). Juxtaposition of visceral endoderm to extraembryonic ectoderm inhibits expression of α-fetoprotein. These data raise the possibility that the ECM in the vicinity of the extraembryonic ectoderm differs from that adjacent to the epiblast or mesoderm.
Although villin was expressed in the outermost monolayer of hemispheres generated by both hensin and laminin 7 d after plating, it had yet to be expressed in hensin-induced hemispheres by day 5. A similar delay in villin expression was seen in in vivo studies, in the extraembryonic cells after blastocyst implantation (Ezzell et al., 1989).
Finally, desmoglein was expressed at day 5 in the hemispheres generated by laminin, but not in those generated by hensin (Fig. 3 D). Desmoglein was predominantly distributed in the embryonic region in E5.5 embryo (Fig. 6 C).
Together, these results demonstrate that hensin was able to induce terminal differentiation of ES cells to form extraembryonic visceral endoderm, whereas laminin was able to convert ES cells into embryonic visceral endoderm. In contrast, fibronectin could not convert these cells to the terminally differentiated visceral endoderm.
Discussion
Shortly after implantation, the embryo is composed of three epithelial cell types: the primitive endoderm (derived from the ICM closest to the blastocoel), the epiblast (also derived from the ICM), and the trophectoderm. The primitive endoderm will later form the embryonic and extraembryonic visceral endoderms. The epiblast will form the embryo proper and is columnar in shape. It exists in apposition to the embryonic visceral endoderm. The epiblast is radially symmetrical but has proximal (i.e., closer to the uterus) and distal poles. The visceral endoderm cells at the distal pole become “thickened” due to their conversion to columnar epithelia, which is likely a result of inductive signals from the distal epiblast (Viebahn et al., 1995; Rivera-Perez et al., 2003). Expression of the homeobox gene Hex occurs simultaneously with this transformation (Thomas and Beddington, 1996; Thomas et al., 1998). Remarkably, this columnar epithelium migrates, over the next few hours, to assume an anterior position, thereby becoming the AVE. During its migration, it assumes a more flattened, squamous shape (Srinivas et al., 2004). The appearance of the distal visceral endoderm and its movement as the AVE have achieved great importance, as recent results demonstrate that this epithelium is critical for the generation of the anteroposterior axis of the embryo (for review see Beddington and Robertson, 1998). Deletion of many of the genes expressed in this structure results in defects in the patterning of future head structures. Interestingly, hensin's expression during the stages that precede gastrulation is dynamic. Hensin is first expressed in the primitive endoderm (Fig. 1 E), and later throughout the entire visceral endoderm. It then becomes localized to the distal portion of the embryonic visceral endoderm (and perhaps in its anterior portion), and later disappears from the embryonic visceral endoderm. Its expression parallels the appearance of the columnar epithelial phenotype. Given the fact that deletion of hensin results in embryonic lethality at the time of its early appearance in the primitive endoderm or early visceral endoderm, we suggest that hensin might play a critical role in the differentiation of these columnar epithelia and, hence, in mediating their function in establishing the anteroposterior axis.
The deposition of the basement membrane between the primitive endoderm and the ICM precedes the terminal differentiation of visceral endoderm in vivo (Enders et al., 1978; Salamat et al., 1995). Deletion of γ1 laminin or its receptor (β1 integrin) results in a phenotype similar to that of the hensin null mice (Fassler and Meyer, 1995; Stephens et al., 1995; Smyth et al., 1999). We recently found that tyrosine-phosphorylated β1 integrin was necessary for the polymerization of hensin in the kidney epithelial cell line (unpublished data). Only polymerized hensin can activate the terminal differentiation program (Hikita et al., 1999, 2000). Thus, we propose that hensin and laminin, deposited in the ECM, specify the development of the columnar and squamous epithelia of the egg cylinder stage of the embryo (Fig. 7). This hypothesis implies that the deposition of specific ECM proteins provides instructive cues for regionalization of the embryo; this regionalization likely influences the later development of the embryo, because the differentiation of epiblasts is known to depend on signals provided by the basement membrane that is located between the epiblast and the visceral endoderm (Rossant and Ofer, 1977; Li et al., 2002). Our finding that laminin and hensin determined the distinct type of simple epithelia opens the way for the identification of the pathways that determine epithelial terminal differentiation, including the regulation of cell shape.
The formation of embryoid bodies from ES cells or from teratocarcinoma F9 cells in vitro (Hogan et al., 1981) has been used to study the factors involved in the development of the embryo at the egg cylinder stage. The ICM, a precursor of ES cells, isolated from a blastocyst by immunosurgery, can also generate an egg cylinder–like structure in culture (Solter and Knowles, 1975). Hensin and laminin null blastocysts died during the phase of egg cylinder morphogenesis and showed ICM failure in the outgrowth assay, whereas the phenotypes of fibronectin knockout embryos (George et al., 1993) and collagen type IV α3 knockouts (Cosgrove et al., 1996) died after gastrulation, suggesting that these latter proteins were not required for egg cylinder morphogenesis. ES cells cultured on hensin- or laminin-coated filters developed hemispherical structures, with visceral endoderm developing on their curved surfaces and cavities inside. We suggest that in vitro hemisphere formation bears much resemblance to egg cylinder development.
Hemidesmosomes are junctions that allow epithelial cells to tightly attach to the ECM. Hemispheres formed on hensin developed desmocollin-containing junctions, but those seeded on laminin-coated filters did not (Fig. 3 D). This likely explains why the laminin hemispheres often floated away from the filter. Studies have recently shown that when the distal visceral endoderm begins to migrate, it loses its columnar shape and becomes flattened; i.e., it becomes squamous (Rivera-Perez et al., 2003; Srinivas et al., 2004). We speculate that columnar cells produced by hensin in the distal visceral endoderm develop hemidesmosomes, similar to what we found in ES cells, that would prevent them from anterior migration. Their conversion to squamous epithelia might result from the onset of a laminin-mediated absence of these structures that would allow migration. Direct studies will need to be performed to test these hypotheses. In addition, we suggest that the morphology and development of the yolk sac might also result from this phenomenon. The proximal yolk sac is tightly attached to the uterus and the embryo by hemidesmosomes, whereas the distal yolk sac readily detaches from the embryo. These features of the yolk sac may be explained by the continuing expression of hensin at the proximal part of the visceral endoderm (Fig. 1 E).
The LIF knockout study revealed that maternal LIF was needed for normal implantation of the blastocyst, suggesting that LIF may be needed for some types of differentiation events rather than for the maintenance of progenitors of germline cells (Stewart et al., 1992). In the E3.5 blastocyst, LIF and its receptor are expressed at both the trophectoderm and the ICM, and continue to be expressed in extraembryonic tissues after implantation (Conquet and Brulet, 1990; Nichols et al., 1996). LIF is secreted by endometrial glands on the fourth day of pregnancy, just before the implantation of blastocysts (Bhatt et al., 1991). Although cytokine is thought to prepare the uterine receptivity of the blastocyst by acting on the trophectoderm (Cheng et al., 2001), the presence of the LIF receptor in the ICM suggests an additional role. We showed here that external LIF was needed for the hemisphere formation from ES cells by hensin and laminin (Figs. 3 A and 4 A). Removal of LIF did not prevent the expression of α-fetoprotein, but prevented the formation of hemispheres (Fig. 4 B). These results are most compatible with a role for LIF in the priming of ES cells (and, by implication, of the ICM and egg cylinder) for morphogenesis rather than for a direct effect on the terminal differentiation of epithelia, which is a role played by hensin and laminin (Fig. 7).
Materials and methods
Gene targeting
We obtained a 15.9-kb mouse hensin genomic DNA that contained the 5′-UTR and the signal sequences of mouse CRP-ductin cDNA (Cheng et al., 1996) by screening the mouse 129/SvJ genomic library with a 400-bp cDNA-encoding rabbit hensin SRCR1 domain (Takito et al., 1999). The whole sequence of the genomic clone exactly matched that of a BAC sequence deposited in GenBank/EMBL/DDBJ (available under accession no. AC087063). We designed a targeting construct to replace the first exon and the subsequent intronic sequence (1 kb) with the lacZ having a nuclear localization signal and PGKneo cassette (Fig. 1 A). A pKO scrambler (model NTKV-1904; Stratagene) was used as a backbone-targeting vector. The targeting vector was linearized with NotI and electroporated into 129/SvJ ES cells. The 5′ outside of the short arm of the targeting vector was designed as a Southern probe to detect an 11-kb band for the recombinant and a 7-kb band for the wild-type allele after PstI digestion. The recombinant ES cells were transferred to C57BL/6J blastocyst to produce the chimeras. One of four male chimeras was fertile, and the recombined allele was transferred to C57BL/6J and SW mice. Both strains showed similar results. Because the first pregnancy of heterozygous females mated with heterozygous males produced over 90% heterozygotes, we used heterozygous females after first or second delivery for experiments among F2–F5. The developmental age of the embryo was designated E0.5 when the vaginal plug was found in the mother (at noon). Genotyping of the blastocysts was performed by PCR. The primer pairs used were 5′-GCACGCTGAT TGAAGCAGAAG-3′ and 5′-AAACCGCCAA GACTGTTACCC-3′ for recombined LacZ detection, and 5′-ATCTCAGTCC TGGTGGGTTG-3′ and 5′-TGGGTTACAC AGGGTGTCCT-3′ for wild-type detection. Animal experiments were performed in accordance with Columbia University guidelines on animal care (IACUC protocol 2898).
Blastocysts
Mouse embryos were manipulated according to standard procedures (Hogan et al., 1994). For outgrowth assay, E3.5 blastocysts were treated with pronase to remove the zona pellucida. The denuded blastocysts were cultured in a droplet of DME with 15% FBS for 7 d. Apoptotic cells were detected by the TUNEL method. The blastocyst isolated at E3.5 was cultured in M16 overnight and stained using the In Situ Cell Death Detection Kit (TMR red; Roche) according to the protocol provided. Images of blastocysts were obtained on Nikon SMZ800 and TMS objective lenses (4×/0.1; 10×/0.25) and were recorded using digital cameras (Coolpix models 950 and 4500 [Nikon]; EOS Digital Rebel [Canon]).
Immunocytochemistry and histology
Immunofluorescence confocal microscopy was done at room temperature with Axiovert 100 and 200M equipped with LSM 5 for image capture (models LSM 410 and 510 META; objective lenses, 20×/0.4 oil, 40×/1.3 oil, 100×/1.4 oil; Carl Zeiss MicroImaging, Inc.). Immersol (518F; Carl Zeiss MicroImaging, Inc.) was used as immersion oil. ES cells cultured on filters were fixed with 4% paraformaldehyde for 1 h and permeabilized with 0.1% NP-40 for 15 min before being stained with antibodies. Antibodies used were α-fetoprotein (NeoMarkers), E-cadherin (Zymed Laboratories), villin (CHEMICON International), and ZO-1 (Santa Cruz Biotechnology, Inc.). Antibodies against cytokeratin 19 (TROMA-3) and Na, K-ATPase were gifts from R. Kemler (Max Planck Institute, Freiburg, Germany) and M. Caplan (Yale University, New Haven, CT). Mouse hensin CUB antibody was generated as described previously (Takito et al., 1999). Fluorescein- or rhodamine-labeled secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Transmission electronmicroscopy was done on JOEL 1200 at 80 kV according to the standard procedures. All images presented were digitally converted and were processed with Adobe Photoshop CS.
ES cell culture
An ES cell line, 129/SvEv MM13, was a gift of M. Mendelsohn (Columbia University, New York, NY). The cells were maintained on mouse embryonic fibroblast feeder layers in DME, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 0.03 mM nucleosides, 0.5 mM 2-mercaptoethanol (all from Specialty Media), 15% ES cell-qualified FBS (HyClone), 1,000 IU/ml ESGRO (CHEMICON International), and penicillin-streptomycin (GIBCO BRL), as described previously (Mombaerts et al., 1996). For differentiation experiments, ES cells were seeded at 10,000 cells/6.4-mm-diam filter in the same media used for the maintenance. Permeable supports precoated with fibronectin, collagen type IV, and laminin were obtained from Becton Dickinson. The hensin-conditioned filter was prepared as described previously (van Adelsberg et al., 1994; Vijayakumar et al., 1999). For antibody-blocking experiments, the hensin-conditioned filter was preincubated with the hensin CUB domain antibody at a 1:100 dilution overnight. The next day, the ES cells were plated and cultured in the presence of the antibody. For RT-PCR, total RNA was recovered from the filter with TRIzol (Invitrogen) and reverse transcribed with SuperScript II (Invitrogen). The primer sequences used were adopted from others (Cosgrove et al., 1996; Murray and Edgar, 2001a; Fujikura et al., 2002; Mitsui et al., 2003).
Measurements of cell dimension
Cell dimension was determined using NIH ImageJ software. ES cells cultured for 8 d on fibronectin, laminin, or hensin were fixed and stained with phalloidin for confocal microscopy. The width and height of the outermost cells in hemispheres grown on hensin or laminin were determined with images taken in the x-y plane of the hemispheres. The width of cells cultured on fibronectin was measured with images of the x-y plane, and the cell height was determined with z axis images. For measurements of the cell dimensions of visceral endoderm, E7.5 embryos were isolated from the uterus, fixed with 100% methanol, and stained with E-cadherin antibody. Confocal images were collected from whole mount embryos. The width and height of the embryonic and extraembryonic visceral endoderms were determined with images of the x-y plane.
Acknowledgments
We thank M. Mendelsohn and A. Nemes for the manipulation of blastocysts and ES cells; V.E. Papaioannou for discussion; and M. Caplan and R. Kemler for providing the Na, K-ATPase and TROMA-3 antibodies. We are especially grateful to the anonymous reviewer who pointed out the possible involvement of hensin in the AVE migration.
This work was supported by the National Institutes of Health (NIH) grant DK20999. Columbia's Optical Microscopy Facility is supported by NIH grant RR-10506.
Abbreviations used in this paper: AVE, anterior visceral endoderm; E, embryonic day; ES, embryonic stem; ICM, inner cell mass; LIF, leukemia inhibitory factor.
References
- Al-Awqati, Q. 2003. Terminal differentiation of intercalated cells: the role of hensin. Annu. Rev. Physiol. 65:567–583. [DOI] [PubMed] [Google Scholar]
- Beddington, R.S., and E.J. Robertson. 1998. Anterior patterning in mouse. Trends Genet. 14:277–284. [DOI] [PubMed] [Google Scholar]
- Beddington, R.S., and E.J. Robertson. 1999. Axis development and early asymmetry in mammals. Cell. 96:195–209. [DOI] [PubMed] [Google Scholar]
- Bhatt, H., L.J. Brunet, and C.L. Stewart. 1991. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc. Natl. Acad. Sci. USA. 88:11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.S., K. Manova, D.C. Weinstein, S.A. Duncan, A.S. Plump, V.R. Prezioso, R.F. Bachvarova, and J.E.J. Darnell. 1994. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gasturation of mouse embryos. Genes Dev. 8:2466–2477. [DOI] [PubMed] [Google Scholar]
- Cheng, H., M. Bjerknes, and H. Chen. 1996. CRP-ductin: a gene expressed in intestinal crypts and in pancreatic and hepatic ducts. Anat. Rec. 244:327–343. [DOI] [PubMed] [Google Scholar]
- Cheng, J.-G., J.R. Chen, L. Hernandez, W.G. Alvord, and C.L. Stewart. 2001. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA. 98:8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet, F., and P. Brulet. 1990. Developmental expression of myeloid leukemia inhibitory factor gene in preimplantation blastocysts and in extraembryonic tissue of mouse embryos. Mol. Cell. Biol. 10:3801–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove, D., D.T. Meehan, J.A. Grunkemeyer, J.M. Kornak, R. Sayers, W.J. Hunter, and G.C. Samuelson. 1996. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 10:2981–2992. [DOI] [PubMed] [Google Scholar]
- Coucouvanis, E., and G.R. Martin. 1995. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 83:279–287. [DOI] [PubMed] [Google Scholar]
- Dziadek, M. 1978. Modulation of alphafoetoprotein synthesis in the early postimplantation mouse embryo. J. Embryol. Exp. Morphol. 46:135–146. [PubMed] [Google Scholar]
- Dziadek, M.A., and E. Adamson. 1978. Localization and synthesis of alphafoetoprotein in postimplantation mouse embryos. J. Embryol. Exp. Morphol. 43:289–313. [PubMed] [Google Scholar]
- Enders, A.C., R.L. Givenand, and S. Schlafke. 1978. Differentiation and migration of endoderm in the rat and mouse at implantation. Anat. Rec. 190:65–77. [DOI] [PubMed] [Google Scholar]
- Ezzell, R.M., M.M. Chafel, and P.T. Matsudaira. 1989. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 106:407–419. [DOI] [PubMed] [Google Scholar]
- Fassler, R., and M. Meyer. 1995. Consequences of lack of b1 integrin gene expression in mice. Genes Dev. 9:1896–1908. [DOI] [PubMed] [Google Scholar]
- Friederich, E., E. Pringault, E. Arpin, and D. Louvard. 1990. From the structure to the function of villin, an actin-binding protein of the brush border. Bioessays. 12:403–408. [DOI] [PubMed] [Google Scholar]
- Fujikura, J., E. Yamato, S. Yonemura, K. Hosoda, S. Masui, K. Nakao, J. Miyazaki, and H. Niwa. 2002. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16:784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, E.L., E.N. Gorges-Labouesse, R.S. Patel-King, H. Rayburn, and R.O. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 119:1079–1091. [DOI] [PubMed] [Google Scholar]
- Hikita, C., J. Takito, S. Vijayakumar, and Q. Al-Awqati. 1999. Only multimeric hensin located in the extracellular matrix can induce apical endocytosis and reverse the polarity of intercalated cells. J. Biol. Chem. 274:17671–17676. [DOI] [PubMed] [Google Scholar]
- Hikita, C., S. Vijayakumar, J. Takito, H. Erdjument-Bromage, P. Tempst, and Q. Al-Awqati. 2000. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J. Cell Biol. 151:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, B.L.M., A. Taylor, and E.D. Adamson. 1981. Cell interaction modulates embryonal carcinoma cell differentiation into parietal visceral endoderm. Nature. 291:235–237. [DOI] [PubMed] [Google Scholar]
- Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY. 497 pp.
- Li, S., D. Harrison, S. Carbonetto, R. Fassler, N. Smyth, and D. Edgar. 2002. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., D. Edgar, R. Fassler, W. Wadsworth, and P.D. Yurchenco. 2003. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell. 4:613–624. [DOI] [PubMed] [Google Scholar]
- Mitsui, K., Y. Tokuzawa, H. Itoh, K. Segawa, M. Murakami, K. Takahashi, M. Maruyama, M. Maeda, and S. Yamanaka. 2003. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 113:631–642. [DOI] [PubMed] [Google Scholar]
- Mollenhauer, J., S. Wiemann, W. Schurlen, B. Korn, Y. Hayashi, K.K. Wilgenbus, A.V. Deimlingand, and A. Poustka. 1997. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3-26.1 is deleted in malignant brain tumors. Nat. Genet. 17:32–39. [DOI] [PubMed] [Google Scholar]
- Mombaerts, P., F. Wang, C. Dulac, S.K. Chao, A. Nemes, M. Mendelsohn, J. Edmondson, and R. Axel. 1996. Visualizing an olfactory sensory map. Cell. 87:675–686. [DOI] [PubMed] [Google Scholar]
- Mori, M., T. Shiraishi, S. Tanaka, M. Yamagata, K. Mafune, Y. Tanaka, H. Ueo, G. Barnard, and K. Sugimachi. 1999. Lack of DMBT1 expression in oesophageal, gastric, and colon cancers. Br. J. Cancer. 79:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey, E.E., Z. Tang, K. Sigrist, M.M. Lu, F. Jiang, H.S. Ip, and M.S. Parmacek. 1998. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 15:3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, P., and D. Edgar. 2001. a. The regulation of differentiation and behaviour of extra-embryonic endodermal cells by basement membranes. J. Cell Sci. 114:931–939. [DOI] [PubMed] [Google Scholar]
- Murray, P., and D. Edgar. 2001. b. The regulation of embryonic stem cell differentiation by leukaemia inhibitory factor (LIF). Differentiation. 68:227–234. [DOI] [PubMed] [Google Scholar]
- Nichols, J., D. Davidson, T. Taga, K. Yoshida, I. Chambers, and A. Smith. 1996. Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis. Mech. Dev. 57:123–131. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan, M., B.S. Rosen, I. Gillot, and F. Cuzin. 2000. Phagocytosis reveals a reversible differentiated state early in the development of the mouse embryo. EMBO J. 19:3295–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius, S. 1965. Morphology of the mouse embryo, from the time of implantation to mesoderm formation. Z. Zellforsch. Mikrosk. Anat. 68:711–723. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez, J.A., J. Mager, and T. Magnuson. 2003. Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev. Biol. 261:470–487. [DOI] [PubMed] [Google Scholar]
- Rossant, J., and L. Ofer. 1977. Properties of extra-embryonic ectoderm isolated from postimplantation mouse embryos. J. Embryol. Exp. Morphol. 39:183–194. [PubMed] [Google Scholar]
- Salamat, M., N. Miosge, and R. Herken. 1995. Development of Reichert's membrane in the early mouse embryo. Anat. Embryol. (Berl.). 192:275–281. [DOI] [PubMed] [Google Scholar]
- Schwartz, G.J., S. Tsuruoka, S. Vijayakumar, S. Petrovic, A. Mian, and Q. Al-Awqati. 2002. Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J. Clin. Invest. 109:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, N., S.H. Vatansever, P. Murray, M. Meyer, C. Frie, M. Paulsson, and D. Edgar. 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter, D., and B.B. Knowles. 1975. Immunosurgery of mouse blastocysts. Proc. Natl. Acad. Sci. USA. 72:5099–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter, D., I. Damjanov, and N. Skreb. 1970. Ultrastructure of mouse egg-cylinder. Z. Anat. Entwicklungsgesch. 132:291–298. [DOI] [PubMed] [Google Scholar]
- Srinivas, S., T. Rodrigues, M. Clements, J.C. Smith, and R.S.P. Beddington. 2004. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 131:1157–1164. [DOI] [PubMed] [Google Scholar]
- Stephens, L.E., A.E. Sutherland, I.V. Klimanskaya, A. Andrieux, J. Meneses, R.A. Pedersen, and C.H. Damsky. 1995. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9:1883–1895. [DOI] [PubMed] [Google Scholar]
- Stewart, C.L., P. Kaspar, L.J. Brunet, H. Bhatt, I. Gadi, F. Kontgen, and S.J. Abbondanzo. 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 359:76–79. [DOI] [PubMed] [Google Scholar]
- Takito, J., C. Hikita, and Q. Al-Awqati. 1996. Hensin, a new collecting duct protein involved in the in vitro plasticity of intercalated cell polarity. J. Clin. Invest. 98:2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takito, J., L. Yan, J. Ma, C. Hikita, S. Vijayakumar, D. Warburton, and Q. Al-Awqati. 1999. Hensin, the polarity reversal protein, is encoded by DMBT1, a gene frequently deleted in malignant gliomas. Am. J. Physiol. Renal Physiol. 46:F277–F289. [DOI] [PubMed] [Google Scholar]
- Tamai, Y., T. Ishikawa, M.R. Bösl, M. Mori, M. Nozaki, H. Baribault, R.G. Oshima, and M.M. Taketo. 2000. Cytokeratins 8 and 19 in the mouse placental development. J. Cell Biol. 151:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, P., and R. Beddington. 1996. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr. Biol. 6:1487–1496. [DOI] [PubMed] [Google Scholar]
- Thomas, P.Q., A. Brown, and R.S. Beddington. 1998. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 125:85–94. [DOI] [PubMed] [Google Scholar]
- van Adelsberg, J., J.C. Edwards, J. Takito, B. Kiss, and Q. Al-Awqati. 1994. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell. 76:1053–1061. [DOI] [PubMed] [Google Scholar]
- Viebahn, C., B. Mayer, and M. Hrabe de Angelis. 1995. Signs of the principle body axes prior to primitive streak formation in the rabbit embryo. Anat. Embryol. (Berl.). 192:159–169. [DOI] [PubMed] [Google Scholar]
- Vijayakumar, S., J. Takito, C. Hikita, and Q. Al-Awqati. 1999. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation. J. Cell Biol. 144:1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip, W.R., E.K. Bikoff, P.A. Hoodless, J.L. Wrana, and E.J. Robertson. 1998. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 92:797–808. [DOI] [PubMed] [Google Scholar]