Abstract
We investigated whether or not the topographic regulation of melanocyte differentiation is determined by mesenchymal–epithelial interactions via fibroblast-derived factors. The melanocyte density in palmoplantar human skin (i.e., skin on the palms and the soles) is five times lower than that found in nonpalmoplantar sites. Palmoplantar fibroblasts significantly suppressed the growth and pigmentation of melanocytes compared with nonpalmoplantar fibroblasts. Using cDNA microarray analysis, fibroblasts derived from palmoplantar skin expressed high levels of dickkopf 1 (DKK1; an inhibitor of the canonical Wnt signaling pathway), whereas nonpalmoplantar fibroblasts expressed higher levels of DKK3. Transfection studies revealed that DKK1 decreased melanocyte function, probably through β-catenin–mediated regulation of microphthalmia-associated transcription factor activity, which in turn modulates the growth and differentiation of melanocytes. Thus, our results provide a basis to explain why skin on the palms and the soles is generally hypopigmented compared with other areas of the body, and might explain why melanocytes stop migrating in the palmoplantar area during human embryogenesis.
Keywords: pigmentation; regulation; dickkopf; β-catenin; MITF
Introduction
Melanocytes in the integument, inner ear, and choroid of vertebrates are derived from the neural crest during development (Erickson and Reedy, 1998; Dorsky et al., 2000a). The formation of the neural crest requires several signals, including members of the Wnt (Dorsky et al., 2000b; Wilson et al., 2001; Garcia-Castro et al., 2002), fibroblast growth factor (Trainor et al., 2002), and bone morphogenetic protein families (Wilson et al., 2001). Neural crest cells migrate via two pathways during embryogenesis: a ventral path between the neural tube and somites for cells that will differentiate into neurons and glial cells of the peripheral nervous system, and a dorsolateral path between the ectoderm and dermamyotome of the somites for cells that will differentiate into melanocytes (Erickson and Reedy, 1998; Dorsky et al., 2000a). Two hypotheses have been proposed to explain the migration and differentiation of neural crest cells into melanocytes (Erickson and Reedy, 1998): the pathway-directed model, by which neural crest cells are exposed to factors from the ectoderm or dermamyotome that direct their differentiation into melanocytes (Dorsky et al., 2000a), and the premigration-directed model, by which neural crest cells that enter the dorsolateral path phenotypically differ from those migrating ventrally. In addition to further characterizing processes involved in normal development, studying the migration and differentiation of human melanocytes is critical to elucidating the mechanisms of pigmentary disorders such as piebaldism and Waardenburg syndrome (Spritz, 1997). Piebaldism results from mutations in the KIT protooncogene (Syrris et al., 2002), and Waardenburg syndrome type 2 results from mutations of microphthalmia-associated transcription factor (MITF; Takeda et al., 2000a). However, virtually nothing is known about mechanisms by which melanocytes stop migrating in the skin in palmoplantar areas during human embryogenesis and why the palms and the soles are generally hypopigmented.
Although fibroblasts were originally thought to be homogeneous, there is increasing evidence that they are heterogeneous in terms of cell replication and senescence (Bordin et al., 1984), synthesis of collagen and other matrix proteins (Yamaguchi et al., 2000), and cytokine production (Koumas et al., 2001). One recent work showed that adult human fibroblasts maintain key expression patterns of HOX genes, which are important for the regulation of patterning in the primary and secondary axes of the developing embryo, suggesting that HOX genes may regulate topographic differentiation and positional memory (Chang et al., 2002). Mesenchymal–epithelial interactions play crucial roles not only during embryogenesis but also in the maintenance of tissue homeostasis in adult skin and during carcinogenesis (Arias, 2001). Keratinocytes cocultured with c-Jun-null fibroblasts show decreased proliferation and differentiation due to the decreased expression of keratinocyte growth factor and granulocyte-macrophage colony-stimulating factor by fibroblasts, whereas keratinocytes cocultured with JunB-null fibroblasts show increased proliferation and differentiation due to the increased expression of those growth factors by fibroblasts (Szabowski et al., 2000). Those results suggest that c-Jun and JunB, members of the AP-1 family of transcription factors, antagonistically control cytokine-regulated mesenchymal–epithelial interactions in adult mouse skin. Wnt signaling pathways, including the stability of β-catenin and its association with lymphoid enhancer binding factor 1/T-cell–specific factor (LEF1/TCF) in the nucleus, also play pivotal roles in the induction of the epithelial mesenchymal transition (Eger et al., 2000).
We previously reported that adult human palmoplantar fibroblasts are not only topographically different from nonpalmoplantar fibroblasts but also that they induce a palmoplantar phenotype, determined by the expression of keratin 9 (Knapp et al., 1986), in nonpalmoplantar keratinocytes through heterotypic mesenchymal–epithelial interactions in vitro (Yamaguchi et al., 1999). We also reported that pigmented nonpalmoplantar epidermis becomes hypopigmented when it is grafted onto palmoplantar wounds (Yamaguchi and Yoshikawa, 2001). We now report that the topographic regulation of melanocyte differentiation is differentially regulated via mesenchymal–epithelial interactions by fibroblasts derived from palmoplantar and nonpalmoplantar skin.
Results
Melanocyte density and melanin distribution in skin on the palms and soles differ from other sites of the body
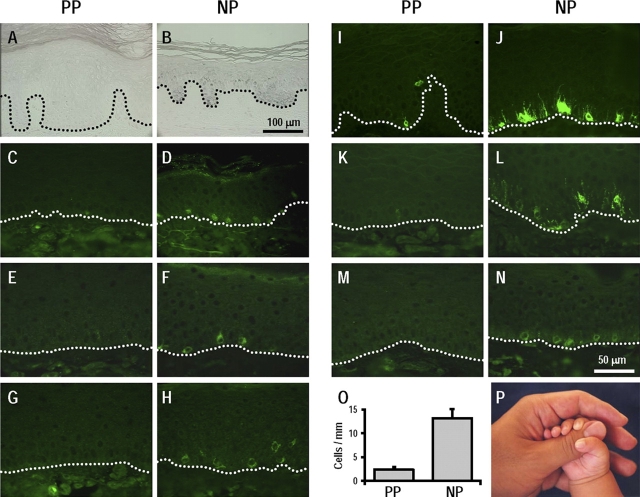
Topographical differences in melanocyte distribution have been poorly understood since Szabo (1954) first studied them using the 3,4-dihydroxyphenylalanine reaction method. We used Fontana-Masson silver staining and immunohistochemistry to compare the melanin distribution, the expression of melanosomal proteins, and the melanocyte number between palmoplantar and nonpalmoplantar areas. Fontana-Masson staining showed that melanin distribution in palmoplantar epidermis (Fig. 1 A) is much less than that in the nonpalmoplantar epidermis (Fig. 1 B), there being no detectable melanin in skin on the palms or soles. The intensity of staining for various melanocyte-specific markers, such as MITF (Fig. 1, C and D), tyrosinase (TYR; Fig. 1, E and F), dopachrome tautomerase (DCT; Fig. 1, G and H), MART1 (Fig. 1, I and J), and gp100 (Fig. 1, K–N) in nonpalmoplantar epidermis was dramatically higher than in palmoplantar epidermis. The density of melanocytes in palmoplantar epidermis, as measured by the number of cells positive for melanosomal proteins, was more than fivefold lower than in nonpalmoplantar epidermis (Fig. 1 O), suggesting that palms and soles are hypopigmented (Fig. 1 P) because of these differences in melanin distribution and in melanocyte function.
Figure 1.
Melanocyte function in palmoplantar (PP) and in nonpalmoplantar (NP) skin. (A and B) Fontana-Masson staining for melanin. Bar, 100 μm. (C–N) Immunohistochemical staining for MITF (C and D), TYR (E and F), DCT (G and H), MART1 (I and J), and gp100 (K–N). HMB45 (K and L) and αPEP13h (M and N) specifically stain gp100 in stage II–IV melanosomes and in stage I melanosomes, respectively. Bar, 50 μm. (O) Melanocyte density measured by the number of cells positive for melanosomal proteins. Data are reported as means ± SD. (P) Macroscopic view of hypopigmented palm (palmoplantar) skin and hyperpigmented arm (nonpalmoplantar) skin.
Palmoplantar fibroblasts express high levels of dickkopf 1 (DKK1), whereas nonpalmoplantar fibroblasts express higher levels of DKK3
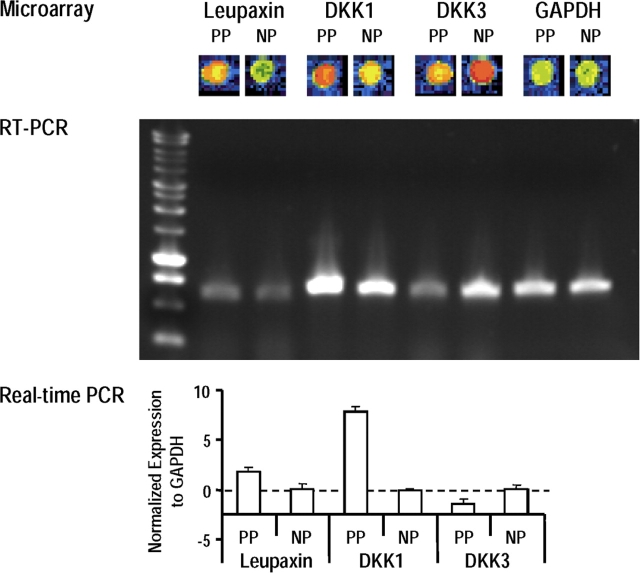
We hypothesized that these differences might result from the effects of fibroblasts in the dermis of those tissues. To check differences in gene expression patterns between palmoplantar fibroblasts and nonpalmoplantar fibroblasts, cDNA microarray assays were performed using cultures obtained from the same subjects. Among the 10,177 human genes investigated, the most dramatic differences were that palmoplantar fibroblasts expressed 4.4-fold higher levels of leupaxin, 3.6-fold higher levels of DKK1, and 2.4-fold lower levels of DKK3 than did nonpalmoplantar fibroblasts (Fig. 2; and Tables I and II). To confirm those cDNA microarray results, RT-PCR and quantitative real-time PCR analyses of the different fibroblast populations were performed. After normalization against levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in nonpalmoplantar fibroblasts, palmoplantar fibroblasts expressed a 1.7-fold higher expression of leupaxin, a 7.8-fold higher expression of DKK1, and a 1.4-fold lower expression of DKK3. Because of these dramatically different patterns of DKK expression, we focused our work on DKK1 and 3 secretion by fibroblasts. Normal human melanocytes and MNT-1 melanoma cells in culture did not express either of those genes, as measured by RT-PCR (unpublished data).
Figure 2.
Differential expression of leupaxin, DKK1, and DKK3 by palmoplantar (PP) and by nonpalmoplantar (NP) fibroblasts. Representative differences in gene expression patterns of leupaxin, DKK1, and DKK3 between palmoplantar fibroblasts and nonpalmoplantar fibroblasts as measured by microarray (top; quantitative results are summarized in Tables I and II). (middle) RT-PCR confirms the expression patterns of leupaxin, DKK1, and DKK3 in palmoplantar and in nonpalmoplantar fibroblasts. These data are representative of five independent experiments. (bottom) Real-time PCR to quantitate the expression of leupaxin, DKK1, and DKK3 after normalization of the target gene to GAPDH. Data are reported as means ± SD.
Table I. Genes highly expressed by palmoplantar fibroblasts detected by cDNA microarrays.
| Fold difference | Accession no. | Gene name |
|---|---|---|
| 4.4 | NM_004811 | leupaxin |
| 3.6 | NM_012242 | dickkopf (X. laevis) homologue 1 |
| 2.9 | NM_002730 | protein kinase, cAMP-dependent, catalytic, α |
| 2.8 | AL550163 | serine (or cysteine) proteinase inhibitor, clade B (ovalbumin), member 2 |
| 2.7 | NM_002421 | matrix metalloproteinase 1 (interstitial collagenase) |
| 2.7 | M57736 | ectonucleotide pyrophosphatase/phosphodiesterase 1 |
| 2.6 | BG541572 | caveolin 1, caveolae protein, 22 kD |
| 2.5 | BE812329 | serine (or cysteine) proteinase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) |
| 2.5 | Z23022 | B-cell CLL/lymphoma 1 |
| 2.5 | D29810 | Human mRNA for unknown product, partial cds |
| 2.5 | R52795 | interleukin 13 receptor, α 2 |
| 2.3 | BE257647 | ribonucleotide reductase M1 polypeptide |
| 2.3 | BF239180 | SMC4 (structural maintenance of chromosomes 4, yeast)-like 1 |
| 2.2 | NM_004670 | 3′-phosphoadenosine 5′-phosphosulfate synthase 2 |
| 2.2 | AV714379 | RAB6 interacting, kinesin-like (rabkinesin6) |
| 2.1 | NM_001150 | alanyl (membrane) aminopeptidase (aminopeptidase N, aminopeptidase M, CD13, p150) |
| 2.1 | R99207 | ESTs, highly similar to SMHU1B metallothionein 1B (H. sapiens) |
| 2.1 | BF031192 | metallothionein 1L |
| 2.1 | NM_000627 | latent transforming growth factor β binding protein 1 |
| 2.1 | AL048540 | Microfibril-associated glycoprotein 2 |
| 2 | AU124962 | minichromosome maintenance deficient (S. cerevisiae) 7 |
| 2 | NM_002658 | plasminogen activator, urokinase |
| 2 | BE858855 | progesterone membrane binding protein |
| 2 | NM_006867 | RNA-binding protein gene with multiple splicing |
| 2 | AA235116 | H. sapiens cDNA FLJ11245 fis, clone PLACE1008629 |
Table II. Genes highly expressed by nonpalmoplantar fibroblasts detected by cDNA microarrays.
| Fold difference | Accession no. | Gene name |
|---|---|---|
| 3.6 | NM_005940 | matrix metalloproteinase 11 (stromelysin 3) |
| 2.4 | AB033421 | dickkopf (X. laevis) homologue 3 |
| 2.4 | X56160 | hexabrachion (tenascin C, cytotactin) |
| 2.2 | NM_001135 | aggrecan 1 (chondroitin sulfate proteoglycan 1) |
| 2.2 | L36033 | stromal cell–derived factor 1 |
| 2.1 | BC001263 | serum/glucocorticoid-regulated kinase |
| 2.1 | NM_002188 | interleukin 13 |
| 2 | BE790903 | hepatocellular carcinoma–associated antigen 112 |
Nonpalmoplantar fibroblasts stimulate the growth and pigmentation of melanocytes more than palmoplantar fibroblasts
We tested the effects of fibroblasts on melanocytes using cocultures of human melanocytes with fibroblasts derived from palmoplantar skin or from nonpalmoplantar skin. Those cocultures were performed using the collagen gel model in which fibroblasts are embedded in a collagen gel matrix underneath a porous filter with melanocytes growing on the upper surface in a physiologically relevant context (Yamaguchi et al., 1999). Effects on melanocyte growth and pigmentation were assessed after various times of coculture (Table III). Coculture with palmoplantar fibroblasts significantly inhibited the proliferation of melanocytes compared with their coculture with nonpalmoplantar fibroblasts. The effects of fibroblasts on TYR enzyme activity and on melanin production in melanocytes were also measured (Table III). Coculture of melanocytes with palmoplantar fibroblasts significantly decreased those markers of differentiation below levels found in coculture with nonpalmoplantar fibroblasts.
Table III. Effects of fibroblasts on melanocyte proliferation and differentiation in culture.
| Palmoplantar fibroblasts
|
Nonpalmoplantar fibroblasts
|
|||||
|---|---|---|---|---|---|---|
| Untreated | Normal IgG | Anti-DKK1 IgG | Untreated | Normal IgG | Anti-DKK1 IgG | |
| Proliferation | 0.125 ± 0.007 | 0.128 ± 0.011 | 0.231 ± 0.034 | 0.192 ± 0.022 | 0.202 ± 0.011 | 0.200 ± 0.033 |
| vs. nonpalmoplantar | P < 0.001 | P < 0.001 | NS | — | — | — |
| vs. untreated | — | NS | P < 0.001 | — | NS | NS |
| TYR | 378 ± 15 | 319 ± 30 | 574 ± 53 | 812 ± 134 | 782 ± 145 | 793 ± 145 |
| vs. nonpalmoplantar | P < 0.05 | P < 0.05 | NS | — | — | — |
| vs. untreated | — | NS | P < 0.05 | — | NS | NS |
| Melanin | 137 ± 34 | 131 ± 26 | 249 ± 31 | 222 ± 24 | 203 ± 23 | 244 ± 5 |
| vs. nonpalmoplantar | P < 0.01 | P < 0.01 | NS | — | — | — |
| vs. untreated | — | NS | P < 0.001 | — | NS | NS |
Melanocytes were cocultured with palmoplantar or nonpalmoplantar fibroblasts for 5 d (in the presence or absence of normal or anti-DKK1 IgG, where noted) after which cell growth, TYR, and melanin were measured, as detailed in Materials and methods. Proliferation is reported as A562; TYR is reported as counts per minute per microgram of total protein per hour; and melanin is reported as nanogram of melanin per microgram of total protein. All experiments were repeated three times in duplicate. Data are reported as means ± SD.
That DKK1 secreted from the fibroblasts was in fact responsible for the observed effects was confirmed in experiments where we added a specific monoclonal antibody to DKK1 that inhibits its function (Tian et al., 2003). We used an ELISA to measure the DKK1 concentration in palmoplantar fibroblasts and found that it was below the limit of sensitivity (i.e., <1 ng/ml; unpublished data). We added an excess of the DKK1-neutralizing antibody (at 50 ng/ml) 1 d before the coculture and daily during the 5 d of coculture. Addition of that DKK1-neutralizing antibody to the cocultures abrogated the inhibition of proliferation and pigmentation markers elicited by coculture with palmoplantar fibroblasts but had no effect on cocultures of melanocytes and nonpalmoplantar fibroblasts (the IgG control had no effect).
Thus, these results demonstrate that palmoplantar fibroblasts inhibit the growth and pigmentation of melanocytes compared with nonpalmoplantar fibroblasts and that those effects are modulated by secreted DKK1.
DKK1 decreases the growth and pigmentation of melanocytes by inactivating MITF
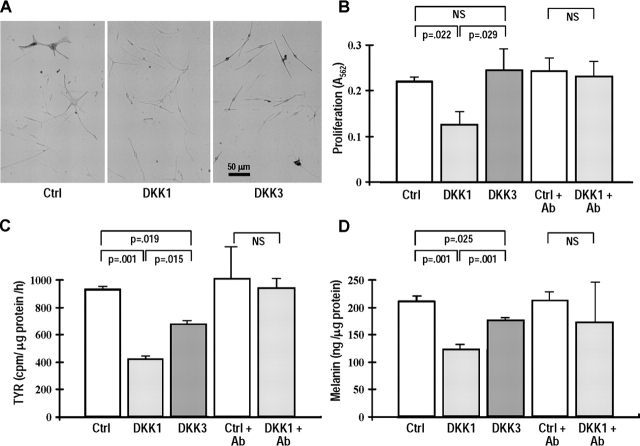
To investigate the effects of DKK1 and 3 on melanocyte function more directly, we cocultured melanocytes with DKK-transfected fibroblasts (Fig. 3). Morphologically, melanocytes cocultured with DKK1-transfected fibroblasts had reduced dendricity and melanin production (Fig. 3 A). DKK1 significantly decreased melanocyte growth by ∼40% compared with untreated controls, whereas DKK3 had no significant effect on proliferation (Fig. 3 B). DKK1 significantly decreased TYR activity by ∼50% and melanin production by ∼40%, whereas DKK3 decreased those by only ∼25% (Fig. 3, C and D). Together, these results show that DKK1 secreted by fibroblasts is able to decrease melanocyte proliferation and function, whereas DKK3 had no effect on proliferation and only slight effects on differentiation.
Figure 3.
DKK1 decreases the growth and pigmentation of melanocytes. To investigate the effects of DKK1 and 3 on melanocyte function, studies using melanocytes cocultured with transfected fibroblasts were performed. (A) Phase-contrast micrographs of melanocytes cocultured with control-, DKK1-, or DKK3-transfected fibroblasts. (B) Proliferation of melanocytes cocultured with fibroblasts transfected with control, DKK1, or DKK3 as measured by the MTT assay. (C) TYR activity in melanocytes cocultured with transfected fibroblasts as measured by the C14-tyrosine assay. (D) Melanin production by melanocytes cocultured with transfected fibroblasts as detected by the melanin assay. These data are representative results of five independent experiments. (B–D) +Ab indicates cocultures performed in the presence of DKK1-inhibitory antibody for 5 d. Data are reported as means ± SD.
To confirm that DKK1 secreted from the fibroblasts was responsible for these observed effects, we used the monoclonal antibody specific for DKK1 to inhibit its function. We used an ELISA to measure the DKK1 concentration in the conditioned medium from DKK1-transfected fibroblasts and found that it ranged from 2.5 to 10 ng/ml (unpublished data). Then, we added an excess of the DKK1-neutralizing antibody (at 50 ng/ml) before the insert was placed on the collagen gel and every day for 5 d; then, we measured effects on proliferation and pigmentation. Addition of that DKK1-neutralizing antibody abrogated the inhibition of proliferation and pigmentation markers induced in melanocytes by DKK1 (Fig. 3, B–D), confirming that the effects were due to DKK1 (antibody had no effect on untreated controls).
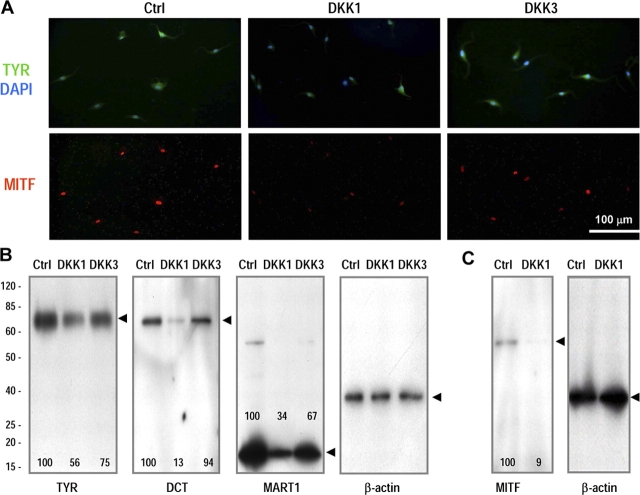
To clarify the mechanisms by which DKK1 decreases melanocyte function, immunohistochemistry and Western blot analyses were performed (Fig. 4). DKK1 remarkably decreased the expression of TYR (Fig. 4 A, top) and of MITF (Fig. 4 A, bottom), whereas DKK3 had no detectable effect on the expression of TYR or MITF.
Figure 4.
Regulation of melanocyte function by fibroblasts expressing DKK1 or DKK3. (A) Immunohistochemistry of TYR (top, green) or MITF (bottom, red) expression by melanocytes cocultured with control-, DKK1-, or DKK3-transfected fibroblasts. (B) Western blotting to examine expression of melanogenic proteins; cell extracts obtained from melanocytes cocultured for 5 d with transfected fibroblasts. Expression of β-actin was used as a loading control. (C) Expression of MITF was analyzed in extracts of nuclei obtained from melanocytes after 5 d of coculture. β-actin is shown as a loading control. The numbers on the blots in B and C represent their quantitation as a percentage of control, corrected against the β-actin loading control. These data are representative results of four independent experiments.
Extracts of melanocytes cocultured for 5 d with fibroblasts transfected with the control vector, with DKK1, or with DKK3 were analyzed by Western blot (Fig. 4 B). DKK1 decreased the expression of TYR, DCT, and MART1 in melanocytes, whereas DKK3 had little or no effect on those melanogenic proteins. Extracts of nuclei obtained from melanocytes cocultured for 5 d with DKK1-transfected fibroblasts showed dramatically decreased expression of MITF by DKK1 compared with controls (Fig. 4 C). The decreased expression of MITF elicited by DKK1 correlates well with the decreased expression of TYR, DCT, and MART1, which are regulated by the MITF transcription factor (Tachibana, 2000; Hornyak et al., 2001; Fang et al., 2002; Du et al., 2003). Because DKK3 had little or no effect on melanocyte proliferation or differentiation compared with DKK1, we focused our further studies on DKK1.
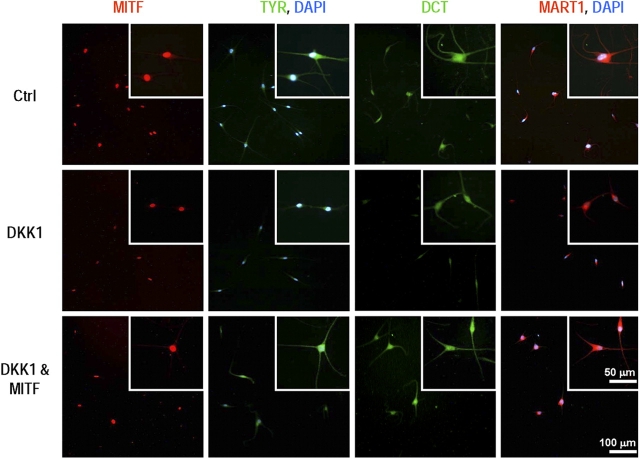
Next, we asked whether or not increasing MITF expression could rescue the suppressed phenotype of melanocytes by transfecting melanocytes with DKK1 with or without MITF. Expression of DKK1 in melanocytes decreased the levels of MITF, TYR, DCT, and MART1 (Fig. 5), and expression of those melanogenic proteins was rescued to control levels by coexpression of MITF in the DKK1-expressing melanocytes.
Figure 5.
MITF rescues DKK1-mediated changes in melanocyte function. Immunohistochemistry of MITF (red), TYR (green), DCT (green), and MART1 (red) expression by control vector–transfected melanocytes (top), DKK1-transfected melanocytes (middle), or DKK1- and MITF-cotransfected melanocytes (bottom); DAPI is shown in blue. Panels for MITF and TYR are from the same field, as are panels for DCT and MART1. Insets show higher magnifications of fields in the larger panels. This experiment was performed four times with similar results.
DKK1 decreases the expression of β-catenin in melanocytes
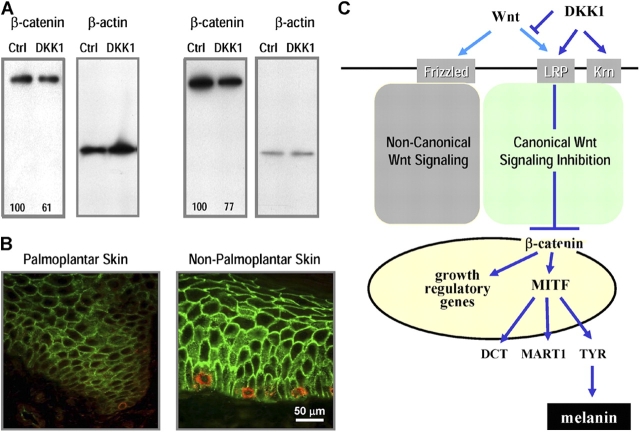
DKK1 has been shown to be an inhibitor of Wnt signaling pathways (Glinka et al., 1998), which also play important roles in determining melanocyte lineages through MITF (Opdecamp et al., 1997; Busca and Ballotti, 2000; Takeda et al., 2000b). Thus, we investigated the expression of a key protein in the canonical Wnt signaling pathway, β-catenin (Kawano and Kypta, 2003). Canonical Wnt signals activate β-catenin expression by inhibiting its degradation via multiple protein complexes, including glycogen synthase kinase-3β, Axin, and APC (Leslie, 2004). The expression of β-catenin in melanocytes cocultured with DKK1-transfected fibroblasts for 5 d was decreased compared with melanocytes cocultured with control-transfected fibroblasts (Fig. 6 A).
Figure 6.
Regulation of cell signaling intermediates by DKK1. (A) Expression of β-catenin was analyzed by Western blot in cell extracts obtained from melanocytes cocultured for 5 d with control- or DKK1-transfected fibroblasts (left) or from melanocytes treated for 3 h with or without 50 ng/ml DKK1 (right). β-actin is shown as a loading control. The numbers below the bands represent their quantitation as a percentage of control, corrected against the β-actin loading control. This experiment was performed four times with melanocytes and fibroblasts derived from different individuals with similar results. (B) Immunohistochemical studies were performed using biopsy specimens of palmoplantar and nonpalmoplantar skin. The expression of β-catenin was examined (stained green), and melanocytes were detected by localization of MART1 (stained red). (C) Scheme illustrating the potential mechanism by which DKK1 decreases melanocyte growth and differentiation.
Examination of signaling pathway intermediates after 5 d of coculture could obviously depend on indirect downstream effects. Therefore, we attempted shorter treatment times to see how early such effects could be seen. In those experiments, melanocytes were treated with 50 ng/ml DKK1 for times ranging from 30 min to 5 d (3 h is shown) and were examined by Western blotting following the protocol described in Tian et al. (2003). DKK1 decreased the level of β-catenin within 3 h, which suggests that DKK1 may have direct effects on that signaling pathway. We examined levels of β-catenin at earlier time points (after 30 min or 1 h of treatment), but no significant differences were noted. Treatment for 2 h gave similar results to 3 h, and treatment at longer times (1 and 3 d) gave results similar to those presented for 5 d.
Finally, immunohistochemical studies were performed using skin tissue specimens obtained from the same subjects to confirm the expression patterns of β-catenin (Fig. 6 B). The expression of β-catenin (green) in palmoplantar skin was lower than that detected in nonpalmoplantar skin; melanocytes are detected by staining for MART1 (red).
Discussion
DKK1 is secreted by fibroblasts in skin on the palms and soles
Among the 10,177 genes examined for expression in adult human palmoplantar fibroblasts and nonpalmoplantar fibroblasts, only 33 (Tables I and II) were significantly different and were thus considered as candidate genes that might regulate melanocyte function in those topographically different types of skin. Our data are consistent with a previous paper in the finding that adult human fibroblasts are diverse as determined by cDNA microarray studies, which suggested that they may regulate topographic differentiation and positional memory (Chang et al., 2002). Some of those fibroblast genes may regulate keratinocyte differentiation, which is implied by the fact that nonpalmoplantar epidermis adopts a palmoplantar phenotype (determined by the expression of keratin 9) through factors secreted from palmoplantar fibroblasts (Yamaguchi et al., 1999; Yamaguchi and Yoshikawa, 2001). In this work, we focused on DKK1 expression in palmoplantar fibroblasts because DKK1 is an inhibitor of Wnt signaling pathways (Glinka et al., 1998), which also play key roles in determining the melanocyte lineage through the regulation of MITF function.
DKK1 is an essential secreted mediator of the vertebrate head organizer because it can induce the formation of ectopic heads in Xenopus laevis in the presence of bone morphogenetic protein inhibitors due to its antagonistic effect on Wnt signaling (Glinka et al., 1998). Numerous studies using X. laevis, zebrafish, and mice support that DKK1 is an inhibitor of the canonical Wnt signaling pathway (Niehrs et al., 1999). Human DKK1 is also highly conserved among vertebrates and can inhibit Wnt-2–induced morphological alterations in NIH3T3 cells by suppressing the Wnt-2–induced increase in uncomplexed β-catenin (Fedi et al., 1999). There are three other members of this novel family of secreted proteins, DKK2, 3, and 4 (Krupnik et al., 1999; Monaghan et al., 1999). Transcripts of DKK1 are found in defined mesodermal lineages including the limb buds, branchial arches, heart, urogenital ridge, tailbud, palate, and additional craniofacial regions from embryonic day 8, whereas transcripts of DKK3 are initially found in the neural-epithelium of the ventral diencephalon on embryonic day 9 and are likely to be restricted in the trunk mesenchyme. mRNAs for DKK2 and DKK3 are detected in numerous adult mouse tissues, whereas prominent expression of DKK1 is found in the eye among adult tissues investigated (Monaghan et al., 1999). So far, expression of DKK1 mRNA has not been found in human adult tissues except human placenta, whereas DKK3 mRNA is found in numerous human adult tissues, especially in heart, brain, and spinal cord (Krupnik et al., 1999). In this work, we focused on human skin and showed a higher expression of DKK1 mRNA in human adult palmoplantar fibroblasts compared with nonpalmoplantar fibroblasts. DKKs may play an important role in epithelial–mesenchymal interactions in adult tissues because Wnts are involved not only in embryogenesis (Reddy et al., 2001) but also in tissue homeostasis (Saitoh et al., 1998) and in carcinogenesis (Taipale and Beachy, 2001).
In this work, we show that DKK1, which is highly expressed by dermal fibroblasts in palmoplantar skin, decreases melanocyte proliferation and function, as judged by the production of melanosomal proteins and melanin, whereas DKK3, which is highly expressed by nonpalmoplantar dermal fibroblasts, does not. These findings suggest that melanocyte migration stops in palmoplantar areas during embryogenesis because of the suppressive effects of DKK1 on melanocytes and that palmoplantar fibroblasts play active roles in regulating and maintaining the homeostasis of topographically different tissues. Our data are consistent with the findings that keratin 14-DKK1 transgenic mice showed no hair follicle development (although keratinocyte differentiation was not affected) and that these mice showed no pigmentation on the trunk because melanocytes do not exist in the inter-follicular epidermis in normal mice (Andl et al., 2002). This finding may also account for the fact that palms and soles are glabrous unlike other sites of the body, even in mice, because of the high expression of DKK1.
DKK1 and 2 are structurally more similar to each other than to DKK3, although all DKKs contain a signal sequence indicating that they are secreted and two characteristic cysteine-rich domains (Krupnik et al., 1999; Monaghan et al., 1999). The transmembrane proteins Kremen1 and 2 are high-affinity DKK1 receptors that functionally cooperate with DKK1 to block Wnt signaling by inducing the rapid endocytosis of the Wnt receptor lipoprotein receptor-related protein 6 complex (Mao et al., 2002) as presented schematically in Fig. 6 C. DKK1 also interacts with lipoprotein receptor-related protein 6 that has a DKK1 binding site besides the Wnt binding sites (Mao et al., 2001; Nusse, 2001). Indeed, DKK1 is the only known secreted antagonist of Wnt signaling that interacts with transmembrane receptors, whereas other inhibitors of Wnt, including Wnt inhibitory factor-1 and secreted frizzled-related protein, directly bind to Wnt to block the signaling pathways (Kawano and Kypta, 2003). These facts suggest that DKK1 has distinct functions among the DKKs, especially DKK1 and 3, and that DKKs can have direct effects on cell activities without interacting with Wnt proteins.
DKK1 inhibits melanocyte growth and differentiation via the inactivation of MITF
Recent works have been paradoxical about the effects of DKK1 on cell proliferation. DKK1 is required for normal mouse limb development by inducing programmed cell death in the interdigital mesenchyme because DKK1 transcripts are expressed in that area at embryonic day 12.5–14.5 (Grotewald et al., 1999; Grotewald and Ruther, 2002a). The effect of DKK1 on programmed cell death is enhanced by UV-induced DNA damage through the activation of p53 (Shou et al., 2002) and c-Jun (Grotewald and Ruther, 2002b). DKK1 knockout mice show polydactyl and syndactyl features at embryonic day 13, suggesting that DKK1 plays a role both in programmed cell death and in cell proliferation via FGF8 activation in response to DKK1 functional ablation (Mukhopadhyay et al., 2001). In contrast, DKK1 is required for reentry into the cell cycle of human adult stem cells from the bone marrow (Gregory et al., 2003).
In this work (summarized in Fig. 6 C), we show that melanocytes respond to DKK1 by suppressing the expression of melanosomal proteins, including TYR, DCT, and MART1, possibly through the decreased expression of MITF, whose consensus binding sites are observed in the promoters of TYR (Hemesath et al., 1994), DCT (Yasumoto et al., 2002), and MART1 (Du et al., 2003). MITF not only regulates differentiation of melanocytes, but also modulates their development, proliferation, and survival (Yasumoto et al., 1998; Tachibana, 2000; McGill et al., 2002). These findings strongly support the decreased melanocyte proliferation and differentiation observed in palmoplantar skin. To further elucidate the mechanisms by which DKK1 decreases melanocyte function, the expression of β-catenin, a key protein in the canonical Wnt signaling pathway, was investigated because, in turn, β-catenin is actively involved in regulating MITF function (Tachibana, 2000; Saito et al., 2002; Yasumoto et al., 2002). DKK1 suppresses the expression of β-catenin, which interacts with the MITF promoter as a coactivator of LEF1/TCF transcription factors (Tachibana, 2000; Widlund et al., 2002; Yasumoto et al., 2002). The finding that DKK1 inhibits β-catenin expression might be sufficient to explain the inhibitory effects of DKK1 on MITF expression because β-catenin enhances MITF activities at the promoter level through the activation of LEF1/TCF (Arias et al., 1999). In turn, this affects melanocyte function because MITF is the key transcriptional regulator of melanocyte growth and differentiation. However, Wnt-5a inhibits the canonical Wnt pathway by promoting the glycogen synthase kinase-3β–independent degradation of β-catenin (Topol et al., 2003). Future studies will be focused on individual signaling proteins involved not only in the canonical Wnt pathway but also in the noncanonical Wnt pathway (Sheldahl et al., 2003).
Concluding remarks
In summary, we show that the density of melanocytes in skin on the palms and soles is five times lower than that found in other sites of the body in adult humans. Coculture with palmoplantar fibroblasts significantly decreased melanocyte function, as measured by effects on proliferation and on the production of melanosomal proteins and melanin. Using cDNA microarray analyses, RT-PCR, and real-time PCR, palmoplantar fibroblasts showed high expression levels of DKK1, whereas nonpalmoplantar fibroblasts showed higher expression levels of DKK3. Transfection studies revealed that DKK1 could indeed decrease melanocyte function, probably through the inactivation of MITF, which can be suppressed by the decreased expression of β-catenin. Thus, our results provide a basis to explain why the palms and soles are generally hypopigmented and why melanocytes stop migrating in palmoplantar areas during human embryogenesis.
Materials and methods
Immunohistochemistry and melanin staining
Skin specimens obtained both from palmoplantar areas (i.e., palm and sole; n = 1 and n = 4, respectively) and from nonpalmoplantar areas (trunk; n = 5) were taken from each of five adult Asian subjects (ages ranged from 31 to 47) during cutaneous surgery. The expression of melanosomal proteins was detected by indirect immunofluorescence using the following as primary antibodies: mouse mAbs, D5 (1:20 dilution; a gift from D.E. Fisher, Dana-Farber Cancer Institute, Boston, MA) specific for human MITF, Ab-3 (1:100 dilution; NeoMarkers) specific for MART 1, and HMB45 (1:100 dilution; DakoCytomation) specific for gp100 (to detect stage II–IV melanosomes). Polyclonal antibodies used were αPEP7h for human TYR (1:1,500 dilution; Virador et al., 2001), αPEP8h for DCT (1:7,500 dilution; Virador et al., 2001), αPEP13h for gp100 (1:4,000 dilution; Virador et al., 2001), and β-catenin (1:50 dilution; Cell Signaling Technology). Bound antibodies were visualized with appropriate secondary antibodies, Alexa Fluor® 488 or 594 goat anti–mouse or anti–rabbit IgG (H+L) (Molecular Probes, Inc.) at 37°C for 30 min at 1:500 dilution with 5% goat serum. Fluorescence was observed and analyzed using a fluorescence microscope (model DMR B/D MLD; Leica), a 3CCD 3-chip color video camera (Dage-MTI), and image software (Scion).
Paraffin-embedded tissues were also processed for the Fontana-Masson silver stain to observe the melanin distribution in skin specimens (Tadokoro et al., 2003).
Melanocyte cultures in 2-well Lab-Tek chamber slides (Nunc) were also processed for indirect immunofluorescence to detect the expression of melanosomal proteins (Virador et al., 2001). Secondary antibodies used were Alexa Fluor® 594 goat anti–mouse IgG (H+L) and Alexa Fluor® 488 goat anti–rabbit IgG (H+L) (Molecular Probes, Inc.). Nuclei were counterstained with DAPI (Vector Laboratories).
Cell cultures and cocultures
Adult human dermal fibroblasts were cultured from palmoplantar and from nonpalmoplantar tissues as detailed in Immunohistochemistry and melanin staining (Yamaguchi et al., 1999), and were used from the third to seventh passage in these experiments.
Neonatal human foreskin melanocytes were cultured as described previously (Swope et al., 1995). Melanocyte cultures were grown in melanocyte growth medium, consisting of Medium 154 and HMGS (Cascade Biologics, Inc.). Melanocytes from the third to fifth passage were used in these experiments.
Cocultures of melanocytes and fibroblasts were performed using the collagen gel model as detailed previously (Yamaguchi et al., 1999). In brief, 106 fibroblasts were embedded in 2 ml of a collagen matrix into the outer culture dish and washed with melanocyte growth medium five times after 24-h incubation in 10% FBS/DME, followed by the placement of 6 × 105 melanocytes seeded onto the insert. All experiments reported were performed using at least four melanocyte lines derived from four different individuals and four palmoplantar and nonpalmoplantar fibroblast lines derived from four different individuals.
To observe the physiological relevance of DKK1 in palmoplantar fibroblasts, we added an excess of the DKK1-neutralizing antibody (at 50 ng/ml; R&D Systems) before the insert with subconfluent melanocytes was placed on the collagen gel embedded with fibroblasts, and then every day for 5 d; then, we measured effects on proliferation and pigmentation. Normal goat IgG (at 50 ng/ml) was used as a control in addition to gels without DKK1-neutralizing antibody. We also compared palmoplantar fibroblast–embedded gels with nonpalmoplantar fibroblast–embedded gels. Those fibroblasts were derived from the same subjects, and the numbers of the embedded fibroblasts were the same measured using a hemocytometer.
Protein extraction and TYR assay
Cultures from quadruplicate 24-mm inserts per group were harvested by brief treatment with 200 μl 0.05% trypsin/0.53 mM EDTA (GIBCO BRL) and were solubilized in 200 μl extraction buffer containing 1% NP-40 (Calbiochem), 0.01% SDS, 0.1 M Tris-HCl, pH 7.2, and protease inhibitor cocktail (Roche). Protein concentrations of the extracts were measured using the BCA protein assay kit (Pierce Chemical Co.). TYR assays were conducted in quadruplicate in 96-well microplates using l-[14C]tyrosine (100 mCi/mmol), as described previously (Yoon et al., 2003). TYR activity is reported as counts per minute per microgram of total protein per hour. Each experiment was repeated at least five times.
Melanin content assay
Melanin content was determined as described previously (Virador et al., 1999). In brief, cell pellets were dissolved in 200 μl 1 N NaOH, and melanin concentrations were quantitated by absorbance at 405 nm in a SpectraMax 250 ELISA reader (Molecular Devices) using a standard curve generated from synthetic melanin (Sigma-Aldrich). Melanin content is expressed as nanogram of melanin per microgram of total protein. Each experiment was repeated at least five times. Pigmentation in cultured human melanocytes was photographed by phase-contrast microscopy.
Cell proliferation assay
The MTT assay (Roche) was conducted according to the manufacturer's instructions (Virador et al., 1999). Each experiment was repeated at least five times. Cell numbers and viability were determined by trypan blue dye exclusion and measured using a hemocytometer in a phase-contrast microscope.
Microarray procedures
Total RNA was prepared from cultured human palmoplantar and from nonpalmoplantar fibroblasts obtained from the same subjects using Isogen RNA extraction reagent (Nippon Gene; Kubo et al., 2002). mRNAs were isolated from the total RNA preparations using oligo(dT) columns and the standard Oligotex (Takara) protocol. The quality of extracted total RNA and mRNA was confirmed with a Bioanalyzer-Bio Sizing (model 2100; Agilent Technologies). A LifeArray chip (Incyte Genomics, Inc.) was used to perform the cDNA microarray procedure. The cDNA from palmoplantar fibroblasts was cyanine 3 labeled by reverse transcription of 200 ng mRNA by a LifeArray probe labeling kit (Incyte Genomics, Inc.), and the cDNA from nonpalmoplantar fibroblasts was cyanine 5 labeled. Two different dye-labeled cDNA probes were hybridized simultaneously with one cDNA chip at 60°C for 6 h using a LifeArray hybridization chamber. Scanning of the two fluorescent intensities of the cDNA chip was performed by a standard two-color microarray scanner (model GenePix 4000A DNA; Axon Instruments, Inc.). Differential gene expression was profiled with GemTools software (Incyte Genomics, Inc.). The experiments were performed twice independently.
RT-PCR and quantitative real-time PCR
To confirm the accuracy of cDNA microarrays, RT-PCR (Lei et al., 2002) and quantitative real-time PCR (Rouzaud et al., 2003) were performed. The oligonucleotide primers for PCR were based on published mRNA sequences and were as follows: human leupaxin sense primer, 5′-AGTTGGATGAGCTCATGGCTCACCTG-3′; leupaxin antisense primer, 5′-CCAGTAGAAAAACTGGTGAAGCAGTCC-3′; human DKK1 sense primer, 5′-TGGCTCTGGGCGCAGCGGGAGCTACC-3′; DKK1 antisense primer, 5′-CGGCAAGACAGACCTTCTCCACAGTAAC-3′; human DKK3 sense primer, 5′-CCATCCATGTGCACCGAGAAATTCAC-3′; DKK3 antisense primer, 5′-TCCCAGCAGTGCAGCGGCGGCAGC-3′; GAPDH sense primer, 5′- GTATGTCGTGGAGTCTACTG-3′; and GAPDH antisense primer, 5′-TACTCCTTGGAGGCCATGTA-3′. After denaturation at 94°C for 2 min, PCR was performed for 34 cycles (30 s at 94°C, 1 min at 58°C, and 1 min at 72°C) for leupaxin, DKK1, and DKK3, and for 20 cycles (30 s at 94°C, 1 min at 58°C, and 1 min at 72°C) for GAPDH. The PCR products for leupaxin, DKK1, DKK3, and GAPDH were 643, 733, 716, and 729 bp, respectively. All amplified products were sequence verified. Control reactions were performed in the absence of reverse transcriptase and were negative. Each experiment was repeated five times independently.
Reactions for quantitative real-time PCR (250 ng cDNA) were performed using the ABI Prism® 7700 Sequence Detection System (Applied Biosystems). SyBr green fluorescence was detected and plotted for each cycle during the 58°C extension phase using Sequence Detection System 1.7 software. Threshold cycles (CT values) for the expression of each gene were calculated using Q-Gene software. The target gene transcripts relative to the housekeeping gene (GAPDH) were quantified by subtracting CT values for the target gene from CT values for the corresponding GAPDH (delta CT values). Comparison of the target transcript levels between palmoplantar fibroblasts and nonpalmoplantar fibroblasts relies on differences between the delta CT values. The values for the target gene obtained from nonpalmoplantar fibroblasts were set as zero, after which the values obtained from palmoplantar fibroblasts were expressed as normalized expression of the target gene to GAPDH using the following formula: If delta CT value from nonpalmoplantar fibroblasts – delta CT value from palmoplantar fibroblasts is >0, then 2delta CT value from nonpalmoplantar fibroblasts – delta CT value from palmoplantar fibroblasts and If delta CT value from nonpalmoplantar fibroblasts – delta CT value from palmoplantar fibroblasts is <0, then −2delta CT value from palmoplantar fibroblasts – delta CT value from nonpalmoplantar fibroblasts. Each group consisted of two samples, and these experiments were repeated three times independently. The values are expressed as means ± SD.
Plasmid construction and transfection studies
Human DKK1 and 3 expression plasmids, pcDNA3.1(−)−DKK1 and pcDNA3.1(−)−DKK3, were constructed as follows. The 819-base pair human DKK1 cDNA and the 1092-base pair human DKK3 cDNA were synthesized by RT-PCR using RNA from cultured palmoplantar fibroblasts and from nonpalmoplantar fibroblasts, respectively. The linear XhoI–BamHI fragment containing the DKK1 cDNA and the XhoI–HindIII fragment containing the DKK3 cDNA were subcloned in pcDNA3.1(−)(Invitrogen), yielding pcDNA3.1(−)−DKK1 and pcDNA3.1(−)−DKK3, respectively. These vectors were confirmed by sequence analyses. The pcDNA3.1 vector alone was used as the control. The human MITF expression plasmid was a gift from S. Shibahara (Tohoku University School of Medicine, Sendai, Japan; Yasumoto et al., 1994).
Transfection was performed either by lipofection for fibroblasts using lipofectamine 2000 (Invitrogen) or by electroporation for melanocytes using the NHEM-Neo Nucleofector™ kit (Amaxa GmBH), according to the manufacturer's instructions. To investigate the effects of DKKs secreted from fibroblasts on human cultured melanocytes, human nonpalmoplantar fibroblasts were seeded at 60% confluency 16 h before transfection in 10% FBS/DME, after which cocultures of melanocytes and transfected fibroblasts were performed using the “gel” model detailed in Cell cultures and cocultures. To investigate the effects of direct transfection on melanocytes, they were electroporated in the Nucleofector™ electroporator (Amaxa GmBH) with the U-20 optimal Nucleofector™ program, after which they were seeded at 80% confluency. The amount of DNA used for transfection and cotransfection studies was 2 μg per 106 cells. After 5 d, transfected cells were harvested for various analyses including immunohistochemistry, TYR activity assay, and Western blotting. The transfection efficiency was determined using the pEGFP-C1 vector (BD Biosciences) and/or a β-Gal staining kit (Invitrogen), and was ∼80% for fibroblasts and ∼70% for melanocytes under these conditions.
ELISA
This assay was performed as previously detailed (Tian et al., 2003), using the anti-DKK1 antibody, recombinant human DKK1, and biotinylated anti-DKK1 antibody obtained from R&D Systems.
Western blotting analysis
Cell extracts (3 μg) were separated on 8–14% gradient SDS polyacrylamide gels (Invitrogen). After electrophoresis, proteins were transferred electrophoretically from the gels to Immobilon-P transfer membranes (Millipore). The filters were incubated in the presence of αPEP7h at 1:2,000, αPEP8h at 1:1,000, Ab-3 at 1:500, C5 (MITF) antibody (Neomarkers) at 1:100, or β-actin (AC-15; Abcam) at 1:3,000 dilution at RT for 1 h and were incubated with HRP-linked anti–rabbit or anti–mouse antibodies (Amersham Biosciences) at 1:1,000 dilution at RT for 1 h. The antigens were detected using an ECL-plus Western blotting detection system (Amersham Biosciences).
Expression of MITF was analyzed using extracts of nuclei prepared by the NE-PER™ nuclear extraction reagent (Pierce Chemical Co.). Blots were quantitated using ScionImage software.
Statistical analysis
t test was used for statistical analyses.
Acknowledgments
We would like to thank Wilfred D. Vieira for assistance with the cell cultures.
This work was supported in part by a grant from the Uehara Memorial Foundation.
Abbreviations used in this paper: DCT, dopachrome tautomerase; DKK, dickkopf; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LEF1/TCF, lymphoid enhancer binding factor 1/T-cell–specific factor; MITF, microphthalmia-associated transcription factor; TYR, tyrosinase.
References
- Andl, T., S. Reddy, T. Gaddapara, and S.E. Millar. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2:643–653. [DOI] [PubMed] [Google Scholar]
- Arias, A.M. 2001. Epithelial mesenchymal interactions in cancer and development. Cell. 105:425–431. [DOI] [PubMed] [Google Scholar]
- Arias, A.M., A.M. Brown, and K. Brennan. 1999. Wnt signalling: pathway or network? Curr. Opin. Genet. Dev. 9:447–454. [DOI] [PubMed] [Google Scholar]
- Bordin, S., R.C. Page, and A.S. Narayanan. 1984. Heterogeneity of normal human diploid fibroblasts: isolation and characterization of one phenotype. Science. 223:171–173. [DOI] [PubMed] [Google Scholar]
- Busca, R., and R. Ballotti. 2000. Cyclic AMP: a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 13:60–69. [DOI] [PubMed] [Google Scholar]
- Chang, H.Y., J.T. Chi, S. Dut, C. Bondre, M. van de Rijn, D. Botstein, and P.O. Brown. 2002. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA. 99:12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky, R.I., R.T. Moon, and D.W. Raible. 2000. a. Environmental signals and cell fate specification in premigratory neural crest. Bioessays. 22:708–716. [DOI] [PubMed] [Google Scholar]
- Dorsky, R.I., D.W. Raible, and R.T. Moon. 2000. b. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 14:158–162. [PMC free article] [PubMed] [Google Scholar]
- Du, J., A.J. Miller, H.R. Widlund, M.A. Horstmann, S. Ramaswamy, and D.E. Fisher. 2003. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol. 163:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger, A., A. Stockinger, B. Schaffhauser, H. Beug, and R. Foisner. 2000. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of β-catenin and upregulation of β-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J. Cell Biol. 148:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, C.A., and M.V. Reedy. 1998. Neural crest development: the interplay between morphogenesis and cell differentiation. Curr. Top. Dev. Biol. 40:177–209. [DOI] [PubMed] [Google Scholar]
- Fang, D., Y. Tsuji, and V. Setaluri. 2002. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 30:3096–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi, P., A. Bafico, A.N. Soria, W.H. Burgess, T. Miki, D.P. Bottaro, M.H. Kraus, and S.A. Aaronson. 1999. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J. Biol. Chem. 274:19465–19472. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro, M.I., C. Marcelle, and M. Bronner-Fraser. 2002. Ectodermal Wnt function as a neural crest inducer. Science. 297:848–851. [DOI] [PubMed] [Google Scholar]
- Glinka, A., W. Wu, H. Delius, A.P. Monaghan, C. Blumenstock, and C. Niehrs. 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 391:357–362. [DOI] [PubMed] [Google Scholar]
- Gregory, C.A., H. Singh, A.S. Perry, and D.J. Prockop. 2003. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J. Biol. Chem. 278:28067–28078. [DOI] [PubMed] [Google Scholar]
- Grotewald, L., and U. Ruther. 2002. a. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int. J. Dev. Biol. 46:943–947. [PubMed] [Google Scholar]
- Grotewald, L., and U. Ruther. 2002. b. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 21:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewald, L., T. Theil, and U. Ruther. 1999. Expression pattern of DKK-1 during mouse limb development. Mech. Dev. 89:151–153. [DOI] [PubMed] [Google Scholar]
- Hemesath, T.J., E. Steingrimsson, G. McGill, M.J. Hansen, J. Vaught, C.A. Hodgkinson, H. Arnheiter, N.G. Copeland, N.A. Jenkins, and D.E. Fisher. 1994. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 8:2770–2780. [DOI] [PubMed] [Google Scholar]
- Hornyak, T.J., D.J. Hayes, L.-Y. Chiu, and E.B. Ziff. 2001. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech. Dev. 101:47–59. [DOI] [PubMed] [Google Scholar]
- Kawano, Y., and R. Kypta. 2003. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116:2627–2634. [DOI] [PubMed] [Google Scholar]
- Knapp, A.C., W.W. Franke, H. Heid, M. Hatzfeld, J.L. Jorcano, and R. Moll. 1986. Cytokeratin No. 9, an epidermal type I keratin characteristic of a special program of keratinocyte differentiation displaying body site specificity. J. Cell Biol. 103:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumas, L., A.E. King, H.O. Critchley, R.W. Kelly, and R.P. Phipps. 2001. Fibroblast heterogeneity: existence of functionally distinct Thy 1+ and Thy 1− human female reproductive tract fibroblasts. Am. J. Pathol. 159:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnik, V.E., J.D. Sharp, C. Jiang, K. Robison, T.W. Chickering, L. Amaravadi, D.E. Brown, D. Guyot, G. Mays, K. Leiby, et al. 1999. Functional and structural diversity of the human Dickkopf gene family. Gene. 238:301–313. [DOI] [PubMed] [Google Scholar]
- Kubo, T., T. Yamashita, A. Yamaguchi, K. Hosokawa, and M. Tohyama. 2002. Analysis of genes induced in peripheral nerves after axotomy using cDNA microarrays. J. Neurochem. 82:1129–1136. [DOI] [PubMed] [Google Scholar]
- Lei, T.C., V. Virador, K. Yasumoto, W.D. Vieira, K. Toyofuku, and V.J. Hearing. 2002. Stimulation of melanoblast pigmentation induced by 8-methoxypsoralen: the involvement of microphthalmia-associated transcription factor, the protein kinase A signal pathway and proteasome-mediated degradation. J. Invest. Dermatol. 119:1341–1349. [DOI] [PubMed] [Google Scholar]
- Leslie, M. 2004. DATABASE: Heads or Tails. Science. 303:1589. [Google Scholar]
- Mao, B., W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, and C. Niehrs. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf protein. Nature. 411:321–325. [DOI] [PubMed] [Google Scholar]
- Mao, B., W. Wu, G. Davidson, J. Marhold, M. Li, B.M. Mechler, H. Delius, D. Hoppe, P. Stannek, C. Walter, et al. 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 417:664–667. [DOI] [PubMed] [Google Scholar]
- McGill, G.G., M.A. Horstmann, H.R. Widlund, J. Du, G. Motyckova, E. Nishimura, Y.L. Lin, S. Ramaswamy, W. Avery, H.F. Ding, et al. 2002. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 109:707–718. [DOI] [PubMed] [Google Scholar]
- Monaghan, A.P., P. Kioschis, W. Wu, A. Zuniga, D. Bock, A. Poustka, H. Delius, and C. Niehrs. 1999. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech. Dev. 87:45–56. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, M., S. Shtrom, C. Rodriguez-Esteban, L. Chen, T. Tsukui, L. Gomer, D.W. Dorwand, A. Glinka, A. Grinberg, S.P. Huang, et al. 2001. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell. 1:423–434. [DOI] [PubMed] [Google Scholar]
- Niehrs, C., O. Kazanskaya, W. Wu, and A. Glinka. 1999. Dickkopf1 and the Spemann-Mangold head organizer. Int. J. Dev. Biol. 45:237–240. [PubMed] [Google Scholar]
- Nusse, R. 2001. Developmental biology. Making head or tail of Dickkopf. Nature. 411:255–256. [DOI] [PubMed] [Google Scholar]
- Opdecamp, K., A. Nakayama, M.T.T. Nguyen, C.A. Hodgkinson, W.J. Pavan, and H. Arnheiter. 1997. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development. 124:2377–2386. [DOI] [PubMed] [Google Scholar]
- Reddy, S., T. Andl, O. Bagasra, M.M. Lu, D.J. Epstein, E.E. Morrisey, and S.E. Millar. 2001. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107:69–82. [DOI] [PubMed] [Google Scholar]
- Rouzaud, F., J.P. Annereau, J.C. Valencia, E.G. Costin, and V.J. Hearing. 2003. Distinct regulation of melanocortin 1 receptor expression at the mRNA and protein levels by its natural agonist and antagonist. FASEB J. 17:2154–2156. [DOI] [PubMed] [Google Scholar]
- Saito, H., K. Yasumoto, K. Takeda, K. Takahashi, A. Fukuzaki, S. Orikasa, and S. Shibahara. 2002. Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with lymphoid-enhancing factor 1. J. Biol. Chem. 277:28787–28794. [DOI] [PubMed] [Google Scholar]
- Saitoh, A., L.A. Hansen, J.C. Vogel, and M.C. Udey. 1998. Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp. Cell Res. 243:150–160. [DOI] [PubMed] [Google Scholar]
- Sheldahl, L.C., D.C. Slusarski, P. Pandur, J.R. Miller, M. Kuhl, and R.T. Moon. 2003. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embros. J. Cell Biol. 161:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou, J., F. Ali-Osman, A.S. Multani, S. Pathak, P. Fedi, and K.S. Srivenugopal. 2002. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 21:878–889. [DOI] [PubMed] [Google Scholar]
- Spritz, R.A. 1997. Piebaldism, Waardenburg syndrom, and related disorders of melanocyte development. Semin. Cutan. Med. Surg. 16:15–23. [DOI] [PubMed] [Google Scholar]
- Swope, V.B., E.E. Medrano, D. Smalara, and Z.A. Abdel-Malek. 1995. Long-term proliferation of human melanocytes is supported by the physiologic mitogens α-melanotropin, endothelin-1, and basic fibroblast growth factor. Exp. Cell Biol. 217:453–459. [DOI] [PubMed] [Google Scholar]
- Syrris, P., K. Heathcote, R. Carrozzo, K. Devriendt, N. Elcioglu, C. Garrett, M. McEntagart, and N.D. Carter. 2002. Human piebaldism: six novel mutations of the proto-oncogene KIT. Hum. Mutat. 20:234. [DOI] [PubMed] [Google Scholar]
- Szabo, G. 1954. The number of melanocytes in human epidermis. BMJ. 1:1016–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabowski, A., N. Maas-Szabowski, S. Andrecht, A. Kolbus, M. Schorpp-Kistner, N.E. Fusenig, and P. Angel. 2000. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 103:745–755. [DOI] [PubMed] [Google Scholar]
- Tachibana, M. 2000. MITF: a stream flowing for pigment cells. Pigment Cell Res. 13:230–240. [DOI] [PubMed] [Google Scholar]
- Tadokoro, T., N. Kobayashi, B.Z. Zmudzka, S. Ito, K. Wakamatsu, Y. Yamaguchi, K.S. Korossy, J.Z. Beer, and V.J. Hearing. 2003. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 17:1177–1179. [DOI] [PubMed] [Google Scholar]
- Taipale, J., and P.A. Beachy. 2001. The Hedgehog and Wnt signalling pathways in cancer. Nature. 411:349–354. [DOI] [PubMed] [Google Scholar]
- Takeda, K., C. Takemoto, I. Kobayashi, A. Watanabe, Y. Nobukuni, D.E. Fisher, and M. Tachibana. 2000. a. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum. Mol. Genet. 9:125–132. [DOI] [PubMed] [Google Scholar]
- Takeda, K., K. Yasumoto, R. Takada, S. Takada, K. Watanabe, T. Udono, H. Saito, K. Takahashi, and S. Shibahara. 2000. b. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 275:14013–14016. [DOI] [PubMed] [Google Scholar]
- Tian, E., F. Zhan, R. Walker, E. Rasmussen, Y. Ma, B. Barlogie, and J.D. Shaughnessy. 2003. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349:2483–2494. [DOI] [PubMed] [Google Scholar]
- Topol, L., X. Jiang, H. Choi, L. Garrett-Beal, P.J. Carolan, and Y. Yang. 2003. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3–independent β-catenin degradation. J. Cell Biol. 162:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor, P., L. Ariza-McNaugton, and R. Krumlauf. 2002. Role of the isthmus and FGF in resolving the paradox of neural crest plasticity and prepatterning. Science. 295:1288–1291. [DOI] [PubMed] [Google Scholar]
- Virador, V., N. Kobayashi, J. Matsunaga, and V.J. Hearing. 1999. A standardized protocol for assessing regulators of pigmentation. Anal. Biochem. 270:207–219. [DOI] [PubMed] [Google Scholar]
- Virador, V., N. Matsunaga, J. Matsunaga, J. Valencia, R.J. Oldham, K. Kameyama, G.L. Peck, V.J. Ferrans, W.D. Vieira, Z.A. Abdel-Malek, and V.J. Hearing. 2001. Production of melanocyte-specific antibodies to human melanosomal proteins: expression patterns in normal human skin and in cutaneous pigmented lesions. Pigment Cell Res. 14:289–297. [DOI] [PubMed] [Google Scholar]
- Widlund, H.R., M.A. Horstmann, E.R. Price, J. Cui, S.L. Lessnick, M. Wu, X. He, and D.E. Fisher. 2002. β-Catenin–induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J. Cell Biol. 158:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S.L., A. Rydstrom, T. Trimborn, K. Willert, R. Nusse, T.M. Jessell, and T. Edlund. 2001. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 411:325–330. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., and K. Yoshikawa. 2001. Cutaneous wound healing: an update. J. Dermatol. 28:521–534. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., S. Itami, M. Tarutani, K. Hosokawa, H. Miura, and K. Yoshikawa. 1999. Regulation of keratin 9 in nonpalmoplantar keratinocytes by palmoplantar fibroblasts through epithelial-mesenchymal interactions. J. Invest. Dermatol. 112:483–488. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., S. Crane, L. Zhou, S.M. Ochoa, and V. Falanga. 2000. Lack of co-ordinate expression of the alpha1(I) and alpha1(III) procollagen genes in fibroblast clonal cultures. Br. J. Dermatol. 143:1149–1153. [DOI] [PubMed] [Google Scholar]
- Yasumoto, K., K. Yokoyama, K. Shibata, Y. Tomita, and S. Shibahara. 1994. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol. Cell. Biol. 14:8058–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto, K., S. Amae, T. Udono, N. Fuse, K. Takeda, and S. Shibahara. 1998. A big gene linked to small eyes encodes multiple Mitf isoforms: many promoters make light work. Pigment Cell Res. 11:329–336. [DOI] [PubMed] [Google Scholar]
- Yasumoto, K., K. Takeda, H. Saito, K. Watanabe, K. Takahashi, and S. Shibahara. 2002. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 21:2703–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, T.J., T.C. Lei, Y. Yamaguchi, J. Batzer, R. Wolber, and V.J. Hearing. 2003. Reconstituted 3-dimensional human skin as a novel in vitro model for studies of pigmentation. Anal. Biochem. 318:260–269. [DOI] [PubMed] [Google Scholar]