Abstract
Growth regulation of epithelial cells is of major concern because most human cancers arise from them. We demonstrated previously a novel signal pathway involving S100C/A11 for high Ca2+-induced growth inhibition of normal human keratinocytes (Sakaguchi, M., M. Miyazaki, M. Takaishi, Y. Sakaguchi, E. Makino, N. Kataoka, H. Yamada, M. Namba, and N.H. Huh. 2003. J. Cell Biol. 163:825–835). This paper addresses a question whether transforming growth factor β (TGFβ) shares the pathway with high Ca2+. On exposure of the cells to TGFβ1, S100C/A11 was phosphorylated, bound to nucleolin, and transferred to the nucleus, resulting in induction of p21WAF1/CIP1 and p15INK4B through activation of Sp1. Protein kinase C α (PKCα) was shown to phosphorylate 10Thr of S100C/A11, which is a critical event for the signal transduction. The TGFβ1-induced growth inhibition was almost completely mitigated when PKCα activity was blocked or when S100C/A11 was functionally sequestered. These results indicate that, in addition to the well-characterized Smad-mediated pathway, the PKCα–S100C/A11-mediated pathway is involved in and essential for the growth inhibition of normal human keratinocytes cells by TGFβ1.
Keywords: calcium; nucleolin; p21WAF1/CIP1; PKC; S100
Introduction
Transforming growth factor β (TGFβ) is a representative growth inhibitor for many kinds of epithelial cells (Moses et al., 1990, 1991). The signal triggered by TGFβ on the cell surface is transduced to the nucleus by a pathway involving Smads (Heldin et al., 1997; Moustakas et al., 2002; Fig. 5). The TGFβ receptor activated by the ligand binding phosphorylates receptor-restricted Smad2 and Smad3, and the activated Smads dimerize with a co-Smad4 and are translocated to nuclei where they exert their growth inhibitory effect by transcriptional activation of Cdk inhibitors, p21WAF1/CIP1 and p15INK4B, and suppression of c-myc (Massague et al., 2000). For the transcriptional regulation, interaction of Smads with a number of different transacting factors, including Sp1 in particular, is needed (Feng et al., 2000). When the intracellular signal transduction for TGFβ is impaired, epithelial cells undergo aberrant growth and eventually acquire malignant phenotypes (Massague et al., 2000; Pasche, 2001). Thus, mutation in the type II TGFβ1 receptor was frequently observed in hereditary nonpolypotic colon cancer (Markowitz et al., 1995). Smad4 was first cloned as a tumor suppressor gene for human pancreatic cancer and was shown to be deleted in 50% of pancreatic cancer cases and 30% of colon cancer cases with high malignancy (Riggins et al., 1997). In addition to the Smad pathway, a number of cytoplasmic protein kinases, including PKC and MAPKs, have been shown to be involved in TGFβ signaling for induction of certain genes (Hanafusa et al., 1999; Ignotz and Honeyman, 2000; Lai and Cheng, 2002; Rosado et al., 2002; Edlund et al., 2003; Yang et al., 2003).
Recently, we found that Ca2+-induced growth inhibition of normal human keratinocytes (NHKs) is mediated by S100C/A11, a Ca2+-binding protein with an EF-hand domain (Sakaguchi et al., 2003; Fig. 5). On exposure of the cells to high Ca2+, cytoplasmic S100C/A11 protein was specifically phosphorylated, bound to nucleolin, and transferred to nuclei. In the nuclei, S100C/A11 liberated Sp1/3 from nucleolin, and the resulting free Sp1/3 transcriptionally activated p21WAF1/CIP1, a negative regulator of cell cycle progression. TGFβ is another representative growth inhibitor for NHK cells. Therefore, we examined whether the S100C/A11 pathway plays any role in TGFβ signaling and which protein kinases are responsible for phosphorylation of S100C/A11.
Results and discussion
TGFβ1 inhibits the growth of NHK cells by inducing p21WAF1/CIP1 via S100C/A11
First, we confirmed the inhibitory action of TGFβ1 on growth of NHK cells (Fig. 1 a). TGFβ1 dose-dependently inhibited incorporation of [3H]thymidine into an insoluble fraction like high Ca2+ (Fig. 1 a), concomitantly inducing p21WAF1/CIP1 mRNA and protein (Fig. 1, b and c). Although protein levels of Sp1 (which is known to play a key role with Smads in inducing p21WAF1/CIP1) remained unchanged (Fig. 1 c), amounts of activated Sp1 that could bind to a GC-rich region of the p21WAF1/CIP1 promoter were time-dependently enhanced by TGFβ1 as demonstrated using the chromatin immunoprecipitation method (Fig. 1 d).
Figure 1.

TGFβ1 inhibits growth of NHKs by inducing p21 WAF1/CIP 1 via S100C/A11. (a) Inhibition of [3H]TdR incorporation in NHK cells treated with Ca2+ (left) or TGFβ1 (right) for 6 h. Standard errors among three wells. (b) Dose-dependent induction of p21WAF1/CIP1 and S100C/A11 mRNA by TGFβ1 (6 h) as assayed by Northern blot analysis. (c) Induction of p21WAF1/CIP1 protein, but not Sp1 protein, by 1 ng/ml of TGFβ1. (d) 1 ng/ml of TGFβ1 enhanced the binding of Sp1 to a GC-rich region in the p21WAF1/CIP1 promoter as determined by chromatin immunoprecipitation. (e) Immunostaining for S100C/A11 of NHK cells treated with Ca2+ or TGFβ1. Bars, 20 μm. (f) Depletion of nucleolin by 0.2 μM of siRNA (48 h before harvest) inhibits the nuclear translocation of S100C/A11 by TGFβ1 (6 h before harvest). Wh, whole cell extract; Cyt, cytoplasmic fraction; Nuc, nuclear fraction. Tubulin and PCNA were used as markers for cytoplasmic and nuclear fractions, respectively. (g) [32P]Phosphate-labeled NHK cells treated with TGFβ1 were immunoprecipitated with anti-S100C/A11 antibody and immunoblotted with indicated antibodies or analyzed by autoradiography. (h) Cells were processed and analyzed as in panels f and g. (i) NHK cells were transfected with various GFP-tagged expression constructs of S100C/A11 harboring mutations at the phosphorylation sites 42 h before harvest. The cells were labeled with [32P]phosphate for 10 h and treated with TGFβ1 for 6 h before harvest and then analyzed as in panel g. Wt, wild type; 10TA, 10Thr was replaced with Ala; 94SA, 94Ser was replaced with Ala; 10/94, 10Thr and 94Ser were replaced with Ala.
Nuclear translocation of S100C/A11, a hallmark of the involvement of the S100C/A11 pathway (Sakaguchi et al., 2003), was observed in NHK cells treated with TGFβ1 even at a low dose of 1.0 ng/ml as was observed in cells exposed to high Ca2+ (Fig. 1 e). The nuclear translocation of S100C/A11 was confirmed by subfractionation of the cells (Fig. 1 f). When nucleolin was depleted with siRNA, the nuclear translocation of S100C/A11 was completely blocked (Fig. 1 f). TGFβ1 induced phosphorylation of S100C/A11 in a time-dependent manner (Fig. 1 g). Nucleolin was coprecipitated with the phosphorylated S100C/A11. In the cells depleted of nucleolin, S100C/A11 remained in the cytoplasm despite the fact that phosphorylation of S100C/A11 by TGFβ1 took place normally (Fig. 1 h). We demonstrated previously that high Ca2+-induced phosphorylation of S100C/A11 at 10Thr and 94Ser in human keratinocytes and that only 10Thr-phosphorylation is relevant to the binding to nucleolin, nuclear translocation, and eventual growth inhibition (Sakaguchi et al., 2003). A transfection experiment using S100C/A11 expression constructs with mutation at either or both phosphorylation sites demonstrated that the same two sites as those phosphorylated by high Ca2+ were phosphorylated by TGFβ1 (Fig. 1 i). These results indicate that the S100C/A11-mediated p21WAF1/CIP1-inducing pathway is activated by TGFβ1 in a manner similar to that by high Ca2+.
PKCα mediates the growth inhibitory signal triggered by TGFβ1 and Ca2+ by phosphorylating S100C/A11 at 10Thr
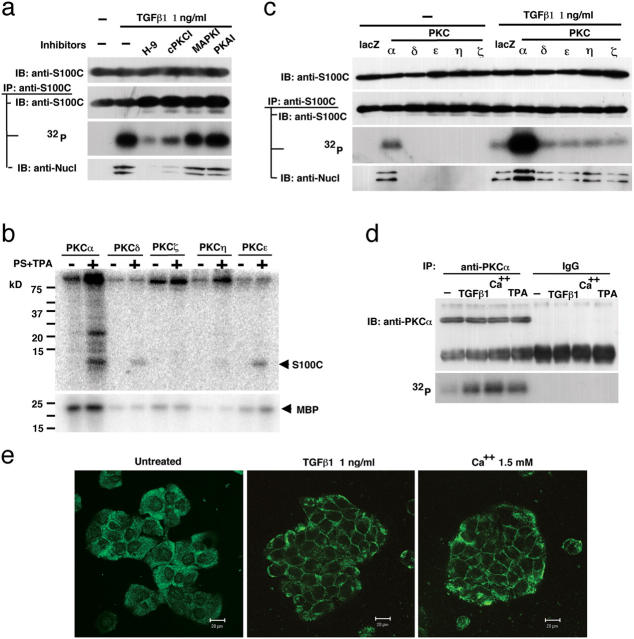
Next, we performed an experiment to determine which protein kinases phosphorylates S100C/A11 in NHK cells exposed to TGFβ1. When the cells were pretreated with various protein kinase inhibitors, the general protein kinase inhibitor H9 and the classical PKC inhibitor cPKCI, but not a MAPK inhibitor (PD98059) or protein kinase A inhibitor, reduced the TGFβ1-induced phosphorylation level of S100C/A11 (Fig. 2 a). Activity to phosphorylate S100C/A11 in vitro in cell extracts was recovered in fractions eluted from an anion-exchange column with 160–180 mM KCl, in which most PKC species were detected by Western blot analysis (unpublished data). These results prompted us to examine possible phosphorylation of S100C/A11 by PKCs. Among the dozen known PKC species, classical PKCα, novel PKCɛ, PKCδ, PKCη, and atypical PKCζ are expressed in skin (Reynolds et al., 1994). When recombinant proteins of these PKCs were incubated with S100C/A11 in vitro, PKCα, PKCδ, and PKCɛ significantly phosphorylated S100C/A11 in an activator-dependent manner (Fig. 2 b). PKCη showed very weak activity and PKCζ did not phosphorylate to any appreciable extent. To examine possible phosphorylation of S100C/A11 by PKCs in cells, PKCα, PKCɛ, PKCδ, PKCη, and PKCζ were expressed in NHK cells using an adenovirus vector. Protein levels of PKCs in the transduced cells were 6.5–19-fold higher than those in untreated cells. S100C/A11 was phosphorylated to an appreciable level only in PKCα-transduced cells in the absence of exogenous stimulation, and the phosphorylation facilitated the association of S100C/A11 to nucleolin (Fig. 2 c). On exposure of the PKC-transduced cells to TGFβ1, S100C/A11 was strongly phosphorylated in the cells transfected with PKCα, but all of the other cells showed responsiveness to TGFβ1 similar to that of the control cells transduced with lacZ. Activation of endogenous PKCα by TGFβ1 and high Ca2+ was demonstrated by detecting autophosphorylation of PKCα (Fig. 2 d) and by translocation of cytosolic PKCα to the plasma membrane (Fig. 2 e). 12-O-tetradecanoylphorbol-13-acetate, an activator of classical and novel PKCs, was used as a positive control.
Figure 2.
Phosphorylation of S100C/A11 by PKCα. (a) NHK cells pretreated (1 h before addition of TGFβ1) with various protein kinase inhibitors (100 μM each) were incubated with TGFβ1 for 6 h and analyzed by immunoblotting or autoradiography with or without immunoprecipitation. H-9, N-(2-aminoethyl)-5-isoquinolinesulfonamide dihydrochloride; cPKCI, myristoylated PKC inhibitor; MAPKI, PD98059; PKAI, cAMP-dependent kinase peptide inhibitor. (b) Recombinant PKCs were incubated with S100C/A11 or myelin basic protein (MBP) in vitro in the presence or absence of activators (PS, phosphatidylserine; TPA, tetradecanoyl phorboracetate). (c) NHK cells were transduced with various PKCs using an adenovirus vector (10 multiplicity of infection, 48 h in advance), treated with 1 ng/ml of TGFβ1 (6 h), and analyzed as in panel a. (d) Autophosphorylation of PKCα in NHK cells treated with 1 ng/ml of TGFβ1, 1.5 mM of Ca2+, or 100 nM of TPA for 1 h. (e) Translocation of PKCα from the cytoplasm to the plasma membrane in NHK cells treated as in panel d. Bars, 20 μm.
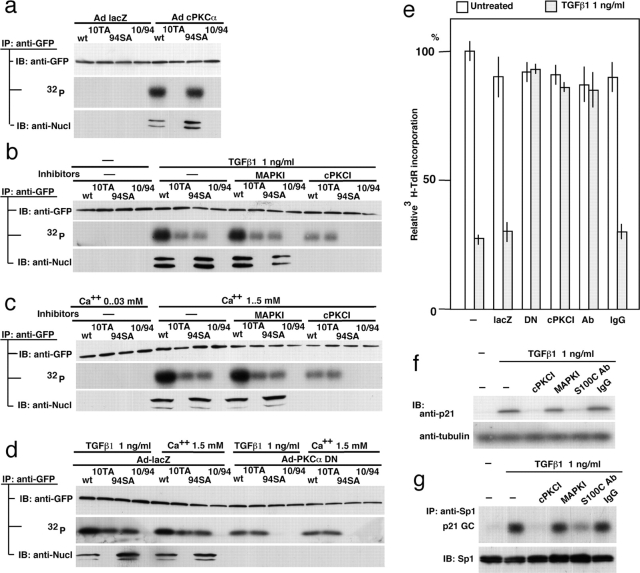
Using the S100C/A11 expression constructs mutated at the phosphorylation sites, exogenous PKCα was shown to phosphorylate 10Thr but not 94Ser (Fig. 3 a). It should be noted that phosphorylation of 10Thr, but not 94Ser, is critical for the binding to nucleolin and eventual nuclear translocation (Sakaguchi et al., 2003). Under the same conditions, exogenous PKCɛ and PKCδ phosphorylated neither 10Thr nor 94Ser (unpublished data). When NHK cells were transfected with the S100C/A11 constructs and exposed to TGFβ1, both 10Thr and 94Ser were phosphorylated (Fig. 1 i and Fig. 3 b). Inhibition of PKCα, the only cPKC expressed in NHK cells, resulted in the blocking of phosphorylation of 10Thr but not 94Ser (Fig. 3 b). Similar results were obtained when the cells were stimulated with 1.5 mM Ca2+ (Fig. 3 c). To confirm the specificity of PKCα further, we transduced a dominant-negative form of PKCα (DN-PKCα) into NHK cells. This DN-PKCα was prepared by replacing 369Lys with Arg and shown to specifically inhibit PKCα activity in cells as well as in vitro (Ohno et al., 1990; Garcia-Bermejo et al., 2002). As shown in Fig. 3 d, phosphorylation of 10Thr in S100C/A11 was specifically and completely blocked by DN-PKCα in NHK cells exposed to TGFβ1 or high Ca2+. Phosphorylation of S100C/A11 by PKCα and autophosphorylation of PKCα induced by TGFβ1 do not need de novo protein synthesis (Figs. S1 and S2, available at http://www.jcb.org/cgi/content/full/jcb.200312041/DC1). These results indicate that PKCα is the sole and specific kinase for 10Thr of S100C/A11 in response to TGFβ1 or high Ca2+ in NHK cells. At present, the precise mechanism of PKCα activation is not clear. However, Ca2+ itself is an activator of cPKCs, and the amount of diacylglycerol, another activator for PKCs, was shown to increase in cells exposed to TGFβ1 (Ignotz and Honeyman, 2000). Chakrabarty et al. (1998) showed that down-regulation of PKCα blocked adhesion response of human colon carcinoma cells to TGFβ1.
Figure 3.
Involvement of PKCα in phosphorylation of S100C/A11 at 10Thr in NHK cells treated with TGFβ1 or Ca2 + and relevance of the S100C/A11 pathway to TGFβ1-induced growth inhibition. (a) NHK cells transduced with various mutant S100C/A11 constructs (Fig. 1 i) in advance were infected with the adenovirus vectors and analyzed as in Fig. 2 c. (b) NHK cells transduced with various mutant S100C/A11 constructs 36 h in advance were treated with 1 ng/ml of TGFβ1 for 6 h before harvest. [32P]Phosphate and the inhibitors were added 4 h and 1 h before the addition of TGFβ1, respectively. The cells were analyzed as in Fig. 2 c. (c) NHK cells were exposed for 1.5 mM Ca2+ for 6 h and analyzed under conditions similar to those in panel b. (d) This experiment was performed under the same conditions as those in panel c except for additional infection with the adenovirus constructs 1 h after transfection of the S100C/A11 constructs. (e) Mitigation of TGFβ1-induced inhibition of DNA synthesis of NHK cells by either inhibiting PKCα or blocking S100C/A11. Adenovirus vectors carrying lacZ (lacZ) or dominant-negative PKCα (DN) were infected at 10 multiplicity of infection cPKCI, 100 μM of myristoylated PKC inhibitor. Anti-S100C/A11 antibody or IgG was introduced into the cells by conjugating with PEI. Standard error among three wells. (f) Under conditions similar to those in panel e, cPKCI and anti-S100C/A11 antibody inhibited the induction of p21WAF1/CIP1 protein by TGFβ1. (g) Amount of Sp1 bound to the p21WAF1/CIP1 promoter was determined by chromatin immunoprecipitation assay (top) using the cells treated under the same conditions as those in panel e. Sp1 protein level remained unchanged (bottom).
Relevance of the S100C/A11-mediated pathway to TGFβ1-induced growth inhibition
We examined whether activation of Sp1 through the S100C/A11-mediated pathway was necessary at all for the growth inhibition induced by TGFβ1. To address this issue, we either inactivated PKCα using DN-PKCα or an inhibitor or functionally depleted S100C/A11 by introducing a specific anti-S100C/A11 antibody into the cells. As shown in Fig. 3 e, TGFβ1 suppressed DNA synthesis to 27% of the control level, and the inhibition of PKCα activity by the dominant-negative construct and cPKC inhibitor resulted in recovery of DNA synthesis to 93% and 86% of the control level, respectively. We demonstrated previously that anti-S100C/A11 antibody blocked phosphorylation and nuclear translocation of S100C/A11 when introduced into cells (Sakaguchi et al., 2003). Under similar conditions, DNA synthesis was recovered to 85% of the control level by the antibody, whereas IgG showed no effect (Fig. 3 e). The mitigation of TGFβ1-induced growth inhibition was dependent on doses of the inhibitor and the antibody (unpublished data). p21WAF1/CIP1 was not induced in the presence of cPKCI or anti-S100C/A11 antibody in TGFβ1-treated cells (Fig. 3 f). Amount of Sp1 bound to a GC-rich region of the p21WAF1/CIP1 promoter was reduced by blocking PKCα or S100C/A11 as demonstrated using the chromatin immunoprecipitation method (Fig. 3 g). These results clearly show that the activation of Sp1 in terms of enhanced binding to the p21WAF1/CIP1 promoter through the S100C/A11-mediated pathway is critically relevant to the inhibition of growth of NHK cells by TGFβ1.
Smad-mediated pathway is also necessary for TGFβ1-induced growth inhibition
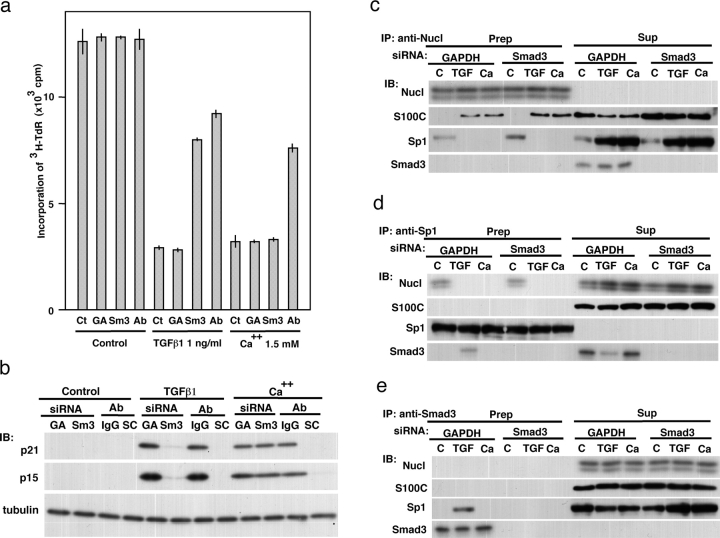
When NHK cells were exposed to siRNA against Smad3, the protein level was efficiently down-regulated within 48 h (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200312041/DC1). Reduced [3H]TdR incorporation by TGFβ1 (23% of the control level) was substantially mitigated to 63% of the control level by the down-regulation of Smad3 (Fig. 4 a). The extent was comparable to that by blocking S100C/A11 with an antibody (73%). This profile of DNA synthesis was correlated well with the extent of induction of p21WAF1/CIP1 and p15INK4B (Fig. 4 b). As expected, high Ca2+-induced growth inhibition does not appear to involve Smad3. These results indicate that the Smad-mediated pathway as well as the S100C/A11-mediated pathway is needed for transduction of the TGFβ1-triggered growth-inhibitory signal. The down-regulation of Smad3 did not affect binding of S100C/A11 to and release of Sp1 from nucleolin (Fig. 4, c and e).
Figure 4.
Involvement of Smad3 in TGFβ1-induced growth inhibition. (a) Mitigation of TGFβ1-induced, but not high Ca2+-induced, growth inhibition of NHK cells by down-regulation of Smad3. The cells were treated with 0.2 μM of siRNA and PEI-conjugated anti-S100C/A11 antibody 48 h and 1 h before, respectively, to the exposure to 1 ng/ml of TGFβ1 or 1.5 mM Ca2+. Ct, control; GA, siRNA against GAPDH; Sm3, siRNA against Smad3; Ab, PEI-conjugated anti-S100C/A11 antibody. Standard error among three wells. (b) NHK cells were treated with various agents under conditions similar to those in panel a and analyzed by Western blotting. SC, antibody against S100C/A11. For other abbreviations, see panel a. (c–e) After treating NHK cells with the various agents under conditions similar to those in panel a, cell lysates (150 μg of protein) were immunoprecipitated with respective antibodies and the precipitates and supernatants that were condensed using acetone were simultaneously analyzed. C, control; TGF, TGFβ1 1 ng/ml; Ca, Ca2+ 1.5 mM.
Signal transduction for TGFβ1- and high Ca2+-induced growth inhibition
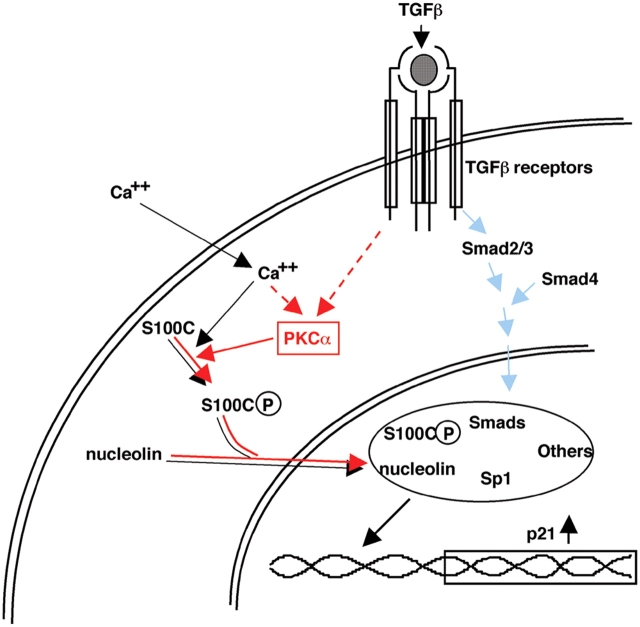
Epidermal keratinocytes are one of the most frequently used cells for studying molecular mechanisms of growth regulation in epithelial cells. High Ca2+ and TGFβ are well-known representative growth inhibitors for NHK cells, but intracellular signaling pathways for the agents remains surprisingly unclear. A number of distinct but mutually interacting pathways may function involving Ca2+-dependent proteins, protein kinases, Smads, and other transacting factors (Heldin et al., 1997; Feng et al., 2000; Santini et al., 2001; Moustakas et al., 2002). This paper, in combination with our previous one (Sakaguchi et al., 2003), clearly illustrates that the S100C/A11-mediated pathway is not only involved in but also essential for the inhibition of growth of NHK cells by high Ca2+ and TGFβ1 (Fig. 5). The evidence presented here unequivocally demonstrates that PKCα is the sole kinase to phosphorylate 10Thr of S100C/A11 in NHK cells in response to TGFβ1 and high Ca2+. Consistent with this, PKCα was recently shown to play a necessary role in mediating Ca2+-induced differentiation of NHK cells (Yang et al., 2003). Furthermore, Tibudan et al. (2002) demonstrated that PKCα was activated during suspension-induced irreversible cell cycle withdrawal in human keratinocytes with concomitant induction of p21WAF1/CIP1.
Figure 5.
Signaling pathways for high Ca 2+ - and TGFβ1-induced growth inhibition in NHK cells. Blue arrows, known from the results of studies by others; black arrows, revealed by our previous paper (Sakaguchi et al., 2003); red arrows, demonstrated in the present experiment.
In recent years, great progress has been made in the understanding of signal transduction for TGFβ at molecular levels, revealing the Smad-mediated pathway in particular. There is increasing concern, however, as to whether the Smad pathway is solely responsible for the entire effect of TGFβ. This paper clearly shows that there is a distinct signaling pathway involving PKCα and S100C/A11 that leads to activation of Sp1, a key partner of activated Smads for inducing p21WAF1/CIP1 (Fig. 5). Sp1 has long been considered to be a ubiquitous transacting factor. It is true in the sense that Sp1 is involved in transcriptional regulation of diverse genes interacting many different proteins, but it may not be true in the sense that it is functionally available whenever needed. Sp1 protein was present at similar levels in NHK cells before and after stimulation with TGFβ1 (Fig. 1 c) but bound to the p21WAF1/CIP1 promoter only after treatment with TGFβ1 (Fig. 1 d and Fig. 3 g). The results of this paper may provide a clue for further study to understand complex functional interactions among critical proteins in nuclei.
Materials and methods
Cells and chemicals
NHK cells (KURABO) were cultured in defined keratinocyte-SFM (GIBCO BRL) with defined keratinocyte-SFM growth supplement (GIBCO BRL). For monitoring DNA synthesis, 1 μCi/ml of tritiated thymidine (American Radiolabeled Chemicals) was added to the cultures 1 h before cell harvest. Subfractionation of cells was performed as described previously (Lindeman et al., 1997). TGFβ1 and H9 (N-[2-aminoethyl]-5-isoquinolinesulfonamide dihydrochloride) were purchased from Sigma-Aldrich and BIOMOL Research Laboratories, Inc., respectively. A myristoylated PKC inhibitor (Eichholtz et al., 1993), cAMP-dependent kinase peptide inhibitor, and PD98059 (2'-amino-3′-methocyflavone; English et al., 1999) were purchased from Promega.
Gel electrophoresis and immunological analyses
Gel electrophoresis, Western blot analysis, and immunoprecipitation were performed under conventional conditions. The antibodies used for Western blot analysis were mouse anti–human nucleolin antibody (Molecular and Biological Laboratories), rabbit anti–human Sp1 antibody (Santa Cruz Biotechnology), mouse anti–human p21WAF1/CIP1 antibody (BD Biosciences), mouse anti–human tubulin antibody (Sigma-Aldrich), mouse anti–human PCNA antibody (BD Biosciences), and mouse anti-GFP antibody (Sigma-Aldrich). Polyclonal rabbit anti–human S100C antibody was prepared in our laboratory (Sakaguchi et al., 2000). For immunoprecipitation, rabbit anti–human S100C antibody, rabbit anti-GFP antibody (Molecular and Biological Laboratories), and rabbit anti–human Sp1 antibody (Upstate Biotechnology) were used.
Plasmid and adenovirus constructs
Plasmid expression constructs prepared as described previously (Sakaguchi et al., 2003) were transfected using Trans IT-keratinocyte transfection reagent (Mirus). Preparation and characteristics of adenovirus constructs of PKCs, and preparation of recombinant PKCs and in vitro kinase assay were described previously (Ohba et al., 1998). Dominant-negative PKCα was designed according to Ohno et al. (1990) and the nature of the construct was confirmed in cells (Garcia-Bermejo et al., 2002).
siRNA
Synthesis of siRNAs against nucleolin and GAPDH was described previously (Sakaguchi et al., 2003). siRNA against Smad3 (5′-aacgtcaacaccaagtgcatc-3′) was produced by in vitro transcription using the Silencer siRNA construction kit (Ambion Inc.). The siRNAs were transfected to logarithmically growing NHK cells using Lipofectamine 2000 (Invitrogen).
Introduction of proteins into cells
Cationic polyethylenimine (PEI)-conjugated S100C antibody was used to introduce antibodies into NHK cells.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed according to the method of Takahashi et al. (2000). In brief, NHK cells were treated with 1% formaldehyde for cross-linking and immunoprecipitated with a rabbit pAb against Sp1 (Santa Cruz Biotechnology). The primers used were those corresponding to positions −328 to −309 and −56 to −37 of the p21WAF1/CIP1 promoter.
Acquisition and processing of images
To acquire images of cells labeled with TRITC or FITC, a laser-scanning microscope (Axioplan 2; objective lens, Plan-Apochromat 63× 1.4 oil DC and Plan-Neofluar 40×/0.75; Carl Zeiss MicroImaging, Inc.) was used.
Online supplemental material
Fig. S1 shows the effect of cycloheximide on phosphorylation of S100C/A11 by TGFβ1. Fig. S2 shows the effect of cycloheximide on autophosphorylation of PKCα by TGFβ1. Fig. S3 shows the down-regulation of Smad3 protein with siRNA. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200312041/DC1.
Acknowledgments
We are indebted to Dr. H. Yamada (Okayama University, Okayama, Japan) for conjugation of anti-S100C antibody with cationic polyethylenimine.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to N. Huh, 14370260), Japan Society for the Promotion of Science (to M. Sakaguchi, 0104310), and Cosmetology Research Promotion Fund (J-03-20).
The online version of this article contains supplemental material.
Abbreviations used in this paper: NHK, normal human keratinocyte; PEI, polyethylenimine; TGFβ, transforming growth factor β.
References
- Chakrabarty, S., S. Rajagopal, and T.L. Moskal. 1998. Protein kinase Calpha controls the adhesion but not the antiproliferative response of human colon carcinoma cells to transforming growth factor beta1: identification of two distinct branches of post-protein kinase Calpha adhesion signal pathway. Lab. Invest. 78:413–421. [PubMed] [Google Scholar]
- Edlund, S., S. Bu, N. Schuster, P. Aspenstrom, R. Heuchel, N.E. Heldin, P. ten Dijke, C.H. Heldin, and M. Landstrom. 2003. Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol. Biol. Cell. 14:529–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz, T., D.B. de Bont, J. de Widt, R.M. Liskamp, and H.L. Ploegh. 1993. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J. Biol. Chem. 268:1982–1986. [PubMed] [Google Scholar]
- English, J., G. Pearson, J. Wilsbacher, J. Swantek, M. Karandikar, S. Xu, and M.H. Cobb. 1999. New insights into the control of MAP kinase pathways. Exp. Cell Res. 253:255–270. [DOI] [PubMed] [Google Scholar]
- Feng, X.H., X. Lin, and R. Derynck. 2000. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. EMBO J. 19:5178–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bermejo, M.L., F.C. Leskow, T. Fujii, Q. Wang, P.M. Blumberg, M. Ohba, T. Kuroki, K.C. Han, J. Lee, V.E. Marquez, and M.G. Kazanietz. 2002. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J. Biol. Chem. 277:645–655. [DOI] [PubMed] [Google Scholar]
- Hanafusa, H., J. Ninomiya-Tsuji, N. Masuyama, M. Nishita, J. Fujisawa, H. Shibuya, K. Matsumoto, and E. Nishida. 1999. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J. Biol. Chem. 274:27161–27167. [DOI] [PubMed] [Google Scholar]
- Heldin, C.H., K. Miyazono, and P. ten Dijke. 1997. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 390:465–471. [DOI] [PubMed] [Google Scholar]
- Ignotz, R.A., and T. Honeyman. 2000. TGF-beta signaling in A549 lung carcinoma cells: lipid second messengers. J. Cell. Biochem. 78:588–594. [DOI] [PubMed] [Google Scholar]
- Lai, C.F., and S.L. Cheng. 2002. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J. Biol. Chem. 277:15514–15522. [DOI] [PubMed] [Google Scholar]
- Lindeman, G.J., S. Gaubatz, D.M. Livingston, and D. Ginsberg. 1997. The subcellular localization of E2F-4 is cell-cycle dependent. Proc. Natl. Acad. Sci. USA. 94:5095–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, S., J. Wang, L. Myeroff, R. Parsons, L. Sun, J. Lutterbaugh, R.S. Fan, E. Zborowska, K.W. Kinzler, B. Vogelstein, et al. 1995. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 268:1336–1338. [DOI] [PubMed] [Google Scholar]
- Massague, J., S.W. Blain, and R.S. Lo. 2000. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 103:295–309. [DOI] [PubMed] [Google Scholar]
- Moses, H.L., E.Y. Yang, and J.A. Pietenpol. 1990. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 63:245–247. [DOI] [PubMed] [Google Scholar]
- Moses, H.L., E.Y. Yang, and J.A. Pietenpol. 1991. Regulation of epithelial proliferation by TGF-beta. Ciba Found Symp. 157:66–74; discussion 75–80. [DOI] [PubMed] [Google Scholar]
- Moustakas, A., K. Pardali, A. Gaal, and C.H. Heldin. 2002. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 82:85–91. [DOI] [PubMed] [Google Scholar]
- Ohba, M., K. Ishino, M. Kashiwagi, S. Kawabe, K. Chida, N.H. Huh, and T. Kuroki. 1998. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol. Cell. Biol. 18:5199–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, S., Y. Konno, Y. Akita, A. Yano, and K. Suzuki. 1990. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J. Biol. Chem. 265:6296–6300. [PubMed] [Google Scholar]
- Pasche, B. 2001. Role of transforming growth factor beta in cancer. J. Cell. Physiol. 186:153–168. [DOI] [PubMed] [Google Scholar]
- Reynolds, N.J., J.J. Baldassare, P.A. Henderson, J.L. Shuler, L.M. Ballas, D.J. Burns, C.R. Moomaw, and G.J. Fisher. 1994. Translocation and downregulation of protein kinase C isoenzymes-alpha and -epsilon by phorbol ester and bryostatin-1 in human keratinocytes and fibroblasts. J. Invest. Dermatol. 103:364–369. [DOI] [PubMed] [Google Scholar]
- Riggins, G.J., K.W. Kinzler, B. Vogelstein, and S. Thiagalingam. 1997. Frequency of Smad gene mutations in human cancers. Cancer Res. 57:2578–2580. [PubMed] [Google Scholar]
- Rosado, E., Z. Schwartz, V.L. Sylvia, D.D. Dean, and B.D. Boyan. 2002. Transforming growth factor-beta1 regulation of growth zone chondrocytes is mediated by multiple interacting pathways. Biochim. Biophys. Acta. 1590:1–15. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, M., M. Miyazaki, Y. Inoue, T. Tsuji, H. Kouchi, T. Tanaka, H. Yamada, and M. Namba. 2000. Relationship between contact inhibition and intranuclear S100C of normal human fibroblasts. J. Cell Biol. 149:1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, M., M. Miyazaki, M. Takaishi, Y. Sakaguchi, E. Makino, N. Kataoka, H. Yamada, M. Namba, and N.H. Huh. 2003. S100C/A11 is a key mediator of Ca2+-induced growth inhibition of human epidermal keratinocytes. J. Cell Biol. 163:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, M.P., C. Talora, T. Seki, L. Bolgan, and G.P. Dotto. 2001. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc. Natl. Acad. Sci. USA. 98:9575–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., J.B. Rayman, and B.D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804–816. [PMC free article] [PubMed] [Google Scholar]
- Tibudan, S.S., Y. Wang, and M.F. Denning. 2002. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J. Invest. Dermatol. 119:1282–1289. [DOI] [PubMed] [Google Scholar]
- Yang, L.C., D.C. Ng, and D.D. Bikle. 2003. Role of protein kinase C alpha in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J. Cell. Physiol. 195:249–259. [DOI] [PubMed] [Google Scholar]