Abstract
Laminin-1 is emerging as the key molecule in early embryonic basement membrane assembly. Here we review recent insights into its functions gained from the synergistic application of genetic and structural methods.
Keywords: extracellular matrix; embryo development; mutagenesis; structure determination
Basement membranes (BMs) are cell-associated sheet-like extracellular matrices covering the basal aspect of all epithelia and endothelia and surrounding muscle, fat, and peripheral nerve cells. BMs are essential for tissue formation in all animals. They provide mechanical stability and barriers between different cell types and are critically involved in cell differentiation, survival, and migration. Initially described as a distinct ultrastructure in electron micrographs, BMs are now defined as much by their molecular composition as by their intimate association with cell surfaces (Timpl and Brown, 1996; Erickson and Couchman, 2000; Kalluri, 2003). The major BM proteins and receptors are conserved in Caenorhabditis elegans, Drosophila, and man, although many more isoforms exist in vertebrates (Hutter et al., 2000; Hynes and Zhao, 2000). The first BM protein to be analyzed biochemically was collagen IV, soon followed by the discovery 25 years ago of the major noncollageneous BM glycoprotein, laminin (Chung et al., 1979; Timpl et al., 1979). Today we probably know the identity of most BM proteins (Table I), but many of their activities still remain to be elucidated.
Table I.
Mammalian basement membrane proteins
| Protein (family) | Oligomeric structure, isoforms, etc. | Cellular receptorsa |
|---|---|---|
| Laminin | At least 15 heterotrimers formed from 5α, 3β, and 3γ chains; further diversity created by proteolytic processing and alternative splicing | Integrins (α1β1, α2β1, α3β1, α6β1, α6β4, α7β1); dystroglycan; heparan sulfate proteoglycans; sulfatides; HNK-1 (α1 chain); Lutheran (α5 chain) |
| Collagen IV | At least 3 heterotrimers formed from 6 homologous α chains | Integrins (α1β1, α2β1) |
| Nidogen/entactin | Single chain; 2 isoforms | Integrins (α3β1, αVβ3) |
| Perlecan | Single chain; proteoglycan | Dystroglycan |
| Agrin | Single chain; proteoglycan; biological activities regulated by alternative splicing | Dystroglycan; MuSK/agrin receptor |
| Collagen XV | Homotrimer; proteoglycan | |
| Collagen XVIII | Homotrimer; proteoglycan; alternative splicing | Heparan sulfate proteoglycans |
| Fibulin | 5 isoforms; alternative splicing (fibulin-1); monomers and disulfide-linked dimer (fibulin-2) | Integrins (fibulin-2: αIIbβ3; fibulin-5: αVβ3, αVβ5, α9β1) |
| Osteonectin/SPARC/BM-40 | Single chain; several poorly characterized homologues |
Additional receptors have been described for BM protein fragments (Kalluri, 2003).
This review focuses on structure–function relationships of laminin-1 in BM assembly during development. Sadly, this article was prompted by the recent death of Rupert Timpl, codiscoverer of laminin and pioneer of molecular approaches to BM study. By reviewing what now is a mature field that spans atomic structures to genetic experiments, we wish to pay tribute to a great scientist and good friend.
BM structure and assembly
Scanning electron microscopy of tissues reveals the BM as a delicate lace-like network with numerous connections to the cell surface. A key question has been how this architecture relates to the known shapes and assembly modes of isolated BM proteins. The current model of BM structure postulates two polymeric networks, formed by laminin and collagen IV, respectively (Yurchenco et al., 1992; Timpl and Brown, 1996). The classic laminin-1 is a cross-shaped molecule comprising α1, β1, and γ1 chains (Fig. 1) and is the most important isoform in early development (see below). It spontaneously self-assembles into polygonal lattices in vitro through calcium-dependent interactions between all three short arms. Assembly in vivo is believed to occur by the same mechanism, but additionally requires the long arm of laminin to be tethered to receptors on the cell surface (Colognato and Yurchenco, 2000). Other cross-shaped laminins are likely to assemble by similar mechanisms, but it remains unknown whether or how laminins with truncated short arms polymerize. Like laminin, collagen IV readily forms networks in vitro, through interactions between the COOH-terminal NC1 domains and (limited) association of collagen triple helices (Timpl and Brown, 1996). In contrast to laminin, cellular receptors have not been implicated in collagen IV network assembly in vivo. It is thought that the laminin and collagen IV polymers are intermingled in mature BMs. The remaining BM components, notably nidogen, the proteoglycan perlecan, and collagen XVIII, are believed to be immobilized through a multitude of noncovalent interactions. Rupert Timpl's scientific legacy includes an extensive catalog of these interactions (Timpl and Brown, 1996).
Figure 1.

Schematic drawing of the cross-shaped laminin molecule and selected interaction partners. All proteins are drawn to scale using dimensions obtained from electron micrographs or crystal structures (10-nm scale bar in upper left corner). The cell membrane is represented by a gray bar. Regions of interest are enlarged to show the atomic structure. In the laminin–nidogen complex structure, three laminin residues essential for nidogen binding are marked by red spheres. In the laminin G domain structure, a heparin-binding sequence and a calcium ion involved in binding the α-subunit of DG are marked by red and green spheres, respectively.
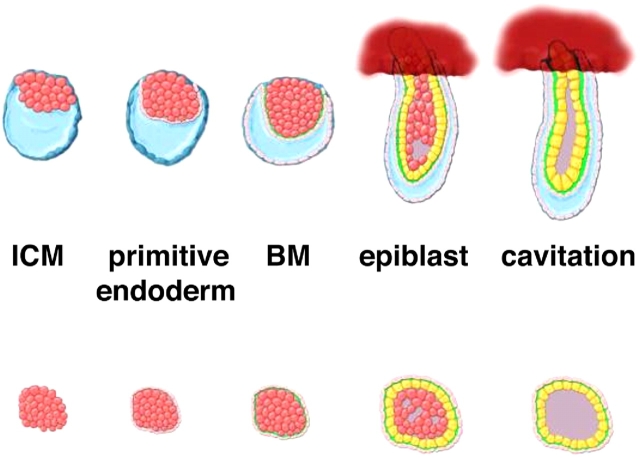
Recent genetic evidence points to a hierarchy in BM formation, with polymerizing laminin-1 acting as a scaffold for the recruitment of other BM components (Li et al., 2003). During the peri-implantation period of mammalian blastocyst development, the first BM to appear in the inner cell mass is deposited beneath the primitive endoderm or hypoblast (Fig. 2). The role of laminin-1 has been elucidated in the past years using embryoid bodies (EBs), which are derived from differentiating mouse embryonic stem cells and provide an in vitro model system that recapitulates cell differentiation in the inner cell mass (ICM) (Coucouvanis and Martin, 1995). Thus, evidence has been provided indicating that the endodermal BM induces primitive ectoderm (epiblast) development of adjacent ICM cells, which leads to programmed cell death of the remaining ICM cells, resulting in formation of the proamniotic cavity (Li et al., 2003).
Figure 2.
Comparison of peri-implantation development in vivo and cell differentiation in EBs in vitro. Key stages of peri-implantation development (top) and EB differentiation (bottom) are shown schematically. During implantation, ICM cells (pink) form an outer layer of primitive endoderm, which deposits a BM (green). The ICM cells adjacent to the BM differentiate into the columnar epiblast epithelium (yellow), while cells residing deeper within the ICM undergo apoptosis, thereby forming the proamniotic cavity. Cell differentiation in EBs closely recapitulates that of the ICM during the peri-implantation period. Hence, EBs are frequently used to delineate the mechanism(s) of gene mutations resulting in peri-implantation lethality.
Whereas ablation of major BM components, such as perlecan, collagen IV, or nidogen, allows normal implantation and development of all three germ layers in vivo (Costell et al., 1999; Murshed et al., 2000; Pöschl et al., 2004), mice lacking the laminin γ1 chain die at the peri-implantation stage (Smyth et al., 1999). Thus, laminin is the only BM component that is critical for BM formation at this stage of development. Because the only laminin α chains detectable at this stage are α1 and α5, and early development does not require the α5 chain (Miner et al., 1998), it follows that laminin-1 is uniquely required for early embryogenesis.
FGF signaling is required for primitive endoderm differentiation and laminin expression (Li et al., 2001). EBs expressing a dominant-negative FGF receptor mutant fail to form BM, epiblast, and cavity. These defects can be partially rescued by the addition of exogeneous laminin, confirming that the primitive endoderm is required for the secretion of laminin, which, in turn, is both necessary and sufficient for subsequent formation of BM, epiblast, and cavity. This interpretation is largely consistent with observations on EBs lacking laminin γ1 expression (Murray and Edgar, 2000, 2001). These EBs form primitive endoderm, which continues to differentiate into visceral and parietal endoderm, but they also fail to deposit a BM and do not develop epiblast and cavity.
Although not necessary for BM formation and stability in early development, the other major BM components, including all other laminin isoforms, nevertheless are essential at later stages. Genetic ablation in mice of the collagen α1(IV) and α2(IV) chains (Pöschl et al., 2004), the laminin α5 chain (Miner et al., 1998), and perlecan (Costell et al., 1999; Arikawa-Hirasawa et al., 1999) results in lethality due to multiple severe defects. Lack of the laminin α2 and α3 chains causes, respectively, severe muscular dystrophy (Kuang et al., 1998) and skin blistering (Ryan et al., 1999), both in knock-out mice and in humans afflicted by hereditary diseases. Finally, mice lacking the laminin α4 chain have defective microvessels (Thyboll et al., 2002). In all cases, the phenotypes are largely consistent with the specific expression pattern of the respective BM component and likely result from both compromised BM integrity and the absence of specific instructive cues.
Laminin–receptor interactions
The identity of the cellular receptor(s) responsible for laminin recruitment to the epiblast surface and subsequent BM assembly is still unknown (Li et al., 2002). Almost all laminin receptors bind to the globular G domain at the COOH terminus of the laminin α chains, which consists of a tandem of five LG domains. The β-sandwich structure of LG domains was recently elucidated, leading to a model of the entire G domain (Hohenester et al., 1999; Tisi et al., 2000). Important features of this model are a clover-leaf arrangement of domains LG1-LG3, a flexible linker, and a COOH-terminal LG domain pair in which LG4 occupies the distal position (Fig. 1). The classic laminin-binding integrins (α3β1, α6β1, α6β4, and α7β1) bind to the LG1–LG3 portion; the COOH terminus of the coiled coil may also be required (Colognato and Yurchenco, 2000). Another laminin receptor, the transmembrane glycoprotein dystroglycan (DG), binds to the LG4–LG5 pair. Finally, binding sites for heparin and heparan sulfate have been mapped to several LG domains, in particular LG4 of the laminin α1 chain (Timpl et al., 2000).
Mice and EBs lacking β1 integrin gene expression resemble those deficient in the laminin γ1 chain (Fässler and Meyer, 1995; Stephens et al., 1995). Mutant mice die at peri-implantation and mutant EBs develop primitive endoderm, but no BM, epiblast, or cavity. However, addition of laminin-1 to β1 integrin-null EBs restores BM formation, showing that β1 integrins are not the critical receptors in primary BM assembly (Li et al., 2002). The phenotype of β1 integrin-null EBs appears to be due to the low level of laminin α1 chain expression by the primitive endoderm (Aumailley et al., 2000; Li et al., 2002). Mice deficient in DG die post-implantation due to impaired development of Reichert's membrane (Williamson et al., 1997), and abnormalities have been described in the BM and cavitation of DG-null EBs (Li et al., 2002).
If neither integrins nor DG are essential, what is the receptor that recruits laminin to nascent BM at the cell surface? The available EB data do not rule out that the functions of β1 integrins and DG are redundant at this stage of development. However, preliminary studies of mice expressing a truncated laminin α1 chain, lacking the LG4–LG5 portion, have shown that these embryos die before gastrulation, i.e., at an earlier stage than the DG-null embryos (Ekblom et al., 2003). This result implies the existence of a critical receptor for the LG4–LG5 portion other than DG or integrins (which bind to the LG1–LG3 region). Indeed, Li et al. (2002) have shown that a heparin-binding sequence in LG4 is required for laminin-mediated BM assembly in laminin γ1-null EBs; this sequence is located at the distal tip of the G domain (Fig. 1). The binding sites for heparin and DG overlap in LG4 of the laminin α1 chain, however, and further experiments with mutant laminins are therefore required to resolve this issue. A recent study of the closely related laminin α2 chain has shown that it is possible to produce mutant laminins that specifically lack only one type of receptor binding site (Wizemann et al., 2003).
Laminin–nidogen interaction
Nidogen was first described as a ubiquitous BM component termed entactin by Carlin et al. (1981). A few years later, a stable laminin–nidogen complex was isolated by Rupert Timpl and colleagues (Dziadek et al., 1985), who subsequently performed a detailed structure–function analysis of nidogen (Fox et al., 1991). Nidogen consists of three globular domains (G1–G3) connected by extended segments (Fig. 1). The G3 domain binds to a single EGF-like domain in the short arm of the laminin γ1 chain (Mayer et al., 1993). A recent crystal structure of a minimal nidogen–laminin complex reveals the β- propeller of the nidogen G3 domain and a small interface of near-perfect complementarity, explaining the very high affinity of the interaction (Takagi et al., 2003). The β-barrel of the G2 domain binds to a single immunoglobulin-like domain of perlecan (Kvansakul et al., 2001). Nidogen also binds to collagen IV, and thus it was proposed that it may act as a cross-linker of the laminin and collagen IV networks in BMs (Timpl and Brown, 1996).
The importance of the laminin–nidogen interaction for epithelial morphogenesis was first demonstrated by antibody perturbation studies in organ culture (Ekblom et al., 1994). Therefore, it was somewhat surprising that mice lacking nidogen-1 displayed only subtle abnormalities (Murshed et al., 2000; Dong et al., 2002). However, it has now been demonstrated that the more recently discovered nidogen-2 can compensate for the lack of nidogen-1 by the appearance of a severe phenotype of mice null for both nidogens (Nischt, R., personal communication).
The laminin–nidogen interaction was addressed directly in an elegant genetic study. Building on previous biochemical mapping studies, Willem et al. (2002) deleted the nidogen-binding LE domain from the laminin γ1 chain. Because this deletion did not affect laminin heterotrimer formation and mutant laminin γ1 chain was present in BMs of heterozygous and homozygous mice, it reasonably was assumed that the mutant laminins retained their ability to polymerize and bind cellular receptors. Nidogen-1, although expressed normally and present in intact form, was not retained in BMs of most tissues, indicating that its interaction with laminins is indeed important for BM localization. Homozygous mice exhibit multiple defects and die at birth due to incomplete maturation of the lungs, as well as impaired kidney and urinary tract development. The lung defects are characterized by a discontinuous BM between alveolar and endothelial cells, as well as by thickening of the connective tissue surrounding the alveoli. Interestingly, however, branching morphogenesis is not affected. Around 90% of mutant embryos lack one or both kidneys; detailed analysis revealed that the renal agenesis is due to a failure of the Wolffian duct (WD) to elongate. Proper growth of the WD is a prerequisite for the outgrowth of the ureteric bud, which in turn induces the metanephric mesenchymalepithelial conversion, resulting in kidney formation. The mechanism by which the laminin–nidogen interaction influences WD growth is unknown, but it is noteworthy that the BM surrounding the tips of the faulty WDs shows subtle discontinuities in mutant embryos.
In addition to the lung and kidney abnormalities, the brain cortex of mutant mice is abnormally laminated and shows multiple ectopias on the surface (Halfter et al., 2002). The brain defects arise from the disruption of the pial BM. This BM covers the brain and serves as the attachment site for radial glia cells, which, in turn, provide a scaffold for neuroblasts migrating toward the pial surface. Because the pial BM is fragile, radial glia cells retract; Cajal-Retzius cells are misplaced or lost; and cortical plate neurons migrate abnormally, either passing through the meninges to form ectopias or terminating their migration prematurely. Similar defects have been observed in mice lacking perlecan (Costell et al., 1999), collagen IV (Pöschl et al., 2004), β1 integrins (Graus-Porta et al., 2001), or DG (Moore et al., 2002). Thus, multiple cell–matrix interactions appear to be essential for the pial BM to resist mechanical forces, as they occur during brain vesicle expansion in development.
The specific deletion of the nidogen binding site on the laminin γ1 chain impressively demonstrates how molecular structure–function analyses can guide genetic studies, leading to much sharper experimental tools compared with the blunt instrument of complete gene knock-outs. Although nidogen binding is the only known activity of this region of the laminin γ1 chain, the remote possibility remains that the observed defects are due to the disruption of other, currently unidentified activities. Such problems could be circumvented by the introduction of suitable point mutations, rather than domain deletions. The pioneering work of Rupert Timpl's laboratory in mapping BM protein interactions at the atomic level should prove to be a treasure chest for such future genetic studies of BM function.
Acknowledgments
This review was written as a tribute to the late Rupert Timpl and we deliberately focused on his contributions to the field. We would like to apologize to all our colleagues whose important work we were unable to mention due to space restrictions. We thank Drs. Ulrike Mayer and David Edgar for helpful comments on the manuscript.
E. Hohenester is a Wellcome Senior Research Fellow.
Abbreviations used in this paper: BM, basement membrane; DG, dystroglycan; EB, embryoid body; ICM, inner cell mass; WD, Wolffian duct.
References
- Arikawa-Hirasawa, E., H. Watanabe, H. Takami, J.R. Hassell, and Y. Yamada. 1999. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23:354–358. [DOI] [PubMed] [Google Scholar]
- Aumailley, M., M. Pesch, L. Tunggal, F. Gaill, and R. Fässler. 2000. Altered synthesis of laminin 1 and absence of basement membrane component deposition in β1 integrin-deficient embryoid bodies. J. Cell Sci. 113:259–268. [DOI] [PubMed] [Google Scholar]
- Carlin, B., R. Jaffe, B. Bender, and A.E. Chung. 1981. Entactin, a novel basal lamina-associated sulfated glycoprotein. J. Biol. Chem. 256:5209–5214. [PubMed] [Google Scholar]
- Chung, A.E., R. Jaffe, I.L. Freeman, J.P. Vergnes, J.E. Braginski, and B. Carlin. 1979. Properties of basement membrane-related glycoprotein synthesized in culture by a mouse embryonal carcinoma-derived cell line. Cell. 16:277–287. [DOI] [PubMed] [Google Scholar]
- Colognato, H., and P.D. Yurchenco. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218:213–234. [DOI] [PubMed] [Google Scholar]
- Costell, M., E. Gustafsson, A. Aszodi, M. Mörgelin, W. Bloch, E. Hunziker, K. Addicks, R. Timpl, and T. Fässler. 1999. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147:1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucouvanis, E., and G.R. Martin. 1995. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 83:279–287. [DOI] [PubMed] [Google Scholar]
- Dong, L., Y. Chen, M. Lewis, J.C. Hsieh, J. Reing, J.R. Chaillet, C.Y. Howell, M. Melhem, S. Inoue, J.R. Kuszak, et al. 2002. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab. Invest. 82:1617–1630. [DOI] [PubMed] [Google Scholar]
- Dziadek, M., M. Paulsson, and R. Timpl. 1985. Identification and interaction repertoire of large forms of the basement membrane protein nidogen. EMBO J. 4:2513–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom, P., M. Ekblom, L. Fecker, G. Klein, H.Y. Zhang, Y. Kadoya, M.L. Chu, U. Mayer, and R. Timpl. 1994. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 120:2003–2014. [DOI] [PubMed] [Google Scholar]
- Ekblom, P., P. Lonai, and J.F. Talts. 2003. Expression and biological role of laminin-1. Matrix Biol. 22:35–47. [DOI] [PubMed] [Google Scholar]
- Erickson, A.C., and J.R. Couchman. 2000. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 48:1291–1306. [DOI] [PubMed] [Google Scholar]
- Fässler, R., and M. Meyer. 1995. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 9:1896–1908. [DOI] [PubMed] [Google Scholar]
- Fox, J.W., U. Mayer, R. Nischt, M. Aumailley, D. Reinhardt, H. Wiedemann, K. Mann, R. Timpl, T. Krieg, J. Engel, and M.L. Chu. 1991. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen IV. EMBO J. 10:3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta, D., S. Blaess, M. Senften, A. Littlewood-Evans, C. Damsky, Z. Huang, P. Prban, R. Klein, J. Schittny, and U. Müller. 2001. β1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 31:1–20. [DOI] [PubMed] [Google Scholar]
- Halfter, W., S. Dong, Y.P. Yip, M. Willem, and U. Mayer. 2002. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22:6029–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester, E., D. Tisi, J.F. Talts, and R. Timpl. 1999. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan and agrin. Mol. Cell. 4:783–792. [DOI] [PubMed] [Google Scholar]
- Hutter, H., B.E. Vogel, J.D. Plenefisch, C.B. Norris, R.B. Proenca, J. Spieth, C. Guo, S. Mastwal, X. Zhu, J. Scheel, and E.M. Hedgecock. 2000. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 287:989–994. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O., and Q. Zhao. 2000. The evolution of cell adhesion. J. Cell Biol. 150:F89–F95. [DOI] [PubMed] [Google Scholar]
- Kalluri, R. 2003. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 3:422–433. [DOI] [PubMed] [Google Scholar]
- Kuang, W., H. Xu, P.H. Vachon, L. Liu, F. Loechel, U.M. Wewer, and E. Engvall. 1998. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J. Clin. Invest. 102:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvansakul, M., M. Hopf, A. Ries, R. Timpl, and E. Hohenester. 2001. Structural basis for the high-affinity interaction of nidogen-1 with immunoglobulin-like domain 3 of perlecan. EMBO J. 20:5342–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., D. Harrison, S. Carbonetto, R. Fässler, N. Smyth, D. Edgar, and P.D. Yurchenco. 2002. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., D. Edgar, R. Fässler, W. Wadsworth, and P.D. Yurchenco. 2003. The role of laminin in cell polarization and tissue organization. Dev. Cell. 4:613–624. [DOI] [PubMed] [Google Scholar]
- Li, X., Y. Chen, S. Scheele, E. Arman, R. Haffner-Krausz, P. Ekblom, and P. Lonai. 2001. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J. Cell Biol. 153:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, U., R. Nischt, E. Pöschl, K. Mann, K. Fukuda, M. Gerl, Y. Yamada, and R. Timpl. 1993. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 12:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J.H., J. Cunningham, and J.R. Sanes. 1998. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol. 143:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S.A., F. Saito, J. Chen, D.E. Michele, M.D. Henry, A. Messing, R.D. Cohn, S.E. Ross-Barta, S. Westra, R.A. Williamson, et al. 2002. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 418:422–425. [DOI] [PubMed] [Google Scholar]
- Murray, P., and D. Edgar. 2000. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 150:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, P., and D. Edgar. 2001. Regulation of the differentiation and behaviour of extra-embryonic endodermal cells by basement membranes. J. Cell Sci. 114:931–939. [DOI] [PubMed] [Google Scholar]
- Murshed, M., N. Smyth, N. Miosge, J. Karolat, T. Krieg, M. Paulsson, and R. Nischt. 2000. The absence of nidogen 1 does not affect murine basement membrane formation. Mol. Cell. Biol. 20:7007–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschl, E., U. Schlötzer-Schrehardt, B. Brachvogel, K. Saito, Y. Ninomiya, and U. Mayer. 2004. Collagen IV is essential for basement membrane stability but dispensable for inititation of its assembly during early development. Development. In press. [DOI] [PubMed] [Google Scholar]
- Ryan, M.C., K. Lee, Y. Miyashita, and W.C. Carter. 1999. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol. 145:1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, N., H.S. Vatansever, P. Murray, M. Meyer, C. Frie, M. Paulsson, and D. Edgar. 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, L.E., A.E. Sutherland, I.V. Klimanskaya, A. Andrieux, J. Meneses, R.A. Pedersen, and C.H. Damsky. 1995. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9:1883–1895. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Y. Yang, J. Liu, J. Wang, and T.A. Springer. 2003. Complex between nidogen and laminin fragments reveals a paradigmatic β-propeller interface. Nature. 424:969–974. [DOI] [PubMed] [Google Scholar]
- Thyboll, J., J. Kortesmaa, R. Cao, R. Soininen, L. Wang, A. Iivanainen, L. Sorokin, M. Risling, Y. Cao, and K. Tryggvason. 2002. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 22:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl, R., and J.C. Brown. 1996. Supramolecular assembly of basement membranes. Bioessays. 18:123–132. [DOI] [PubMed] [Google Scholar]
- Timpl, R., H. Rohde, P.G. Robey, S.I. Rennard, J.M. Foidart, and G.R. Martin. 1979. Laminin - a glycoprotein from basement membrane. J. Biol. Chem. 254:9933–9937. [PubMed] [Google Scholar]
- Timpl, R., D. Tisi, J.F. Talts, Z. Andac, T. Sasaki, and E. Hohenester. 2000. Structure and function of laminin LG modules. Matrix Biol. 19:309–317. [DOI] [PubMed] [Google Scholar]
- Tisi, D., J.F. Talts, R. Timpl, and E. Hohenester. 2000. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 19:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem, M., N. Miosge, W. Halfter, N. Smyth, I. Jannetti, E. Burghart, R. Timpl, and U. Mayer. 2002. Specific ablation of the nidogen-binding site in the laminin γ1 chain interferes with kidney and lung development. Development. 129:2711–2722. [DOI] [PubMed] [Google Scholar]
- Williamson, R.A., M.D. Henry, K.J. Daniels, R.F. Hrstka, J.C. Lee, Y. Sunada, O. Ibraghimov-Beskrovnaya, and K.P. Campbell. 1997. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum. Mol. Genet. 6:831–841. [DOI] [PubMed] [Google Scholar]
- Wizemann, H., J.H.O. Garbe, M.V.K. Friedrich, R. Timpl, T. Sasaki, and E. Hohenester. 2003. Distinct requirements for heparin and α-dystroglycan binding revealed by structure-based mutagenesis of the laminin α2 LG4-LG5 domain pair. J. Mol. Biol. 332:635–642. [DOI] [PubMed] [Google Scholar]
- Yurchenco, P.D., Y.S. Cheng, and H. Colognato. 1992. Laminin forms an independent network in basement mebranes. J. Cell Biol. 117:1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]