Abstract
The Golgi apparatus has long been suggested to be important for directing secretion to specific sites on the plasma membrane in response to extracellular signaling events. However, the mechanisms by which signaling events are coordinated with Golgi apparatus function remain poorly understood. Here, we identify a scaffolding function for the Golgi matrix protein GM130 that sheds light on how such signaling events may be regulated. We show that the mammalian Ste20 kinases YSK1 and MST4 target to the Golgi apparatus via the Golgi matrix protein GM130. In addition, GM130 binding activates these kinases by promoting autophosphorylation of a conserved threonine within the T-loop. Interference with YSK1 function perturbs perinuclear Golgi organization, cell migration, and invasion into type I collagen. A biochemical screen identifies 14-3-3ζ as a specific substrate for YSK1 that localizes to the Golgi apparatus, and potentially links YSK1 signaling at the Golgi apparatus with protein transport events, cell adhesion, and polarity complexes important for cell migration.
Keywords: Ste20 kinases; cell migration; polarity; collagen invasion; scaffold
Introduction
Recent evidence suggests that the Golgi apparatus, in addition to its function in protein secretion, may be the site of signaling events important for diverse cellular processes (for review see Ferri and Kroemer, 2001; Bivona and Philips, 2003; Rios and Bornens, 2003). Thus, it has been proposed that the Golgi apparatus may act as a signaling platform, sensing and integrating signals from growth factor signaling pathways, and thereby participating in the regulation of downstream events (Bivona and Philips, 2003). This raises the question of which cellular processes require coordination of signaling at the cell surface and the various functions associated with the Golgi apparatus. The position of the Golgi apparatus within the cell responds to extracellular signals, rapidly reorienting in cells at experimental wound edges, migrating and polarizing cells, and in activated cytotoxic T lymphocytes toward the site of killing (Kupfer et al., 1982; Kupfer and Dennert, 1984). These processes involve a complex series of signaling events coordinated with reorientation of the microtubule cytoskeleton and changes in actin dynamics dependent on dynein, Rho family GTPases, and the Arp2/3 complex (Ridley et al., 2003), and it is possible that membrane traffic and signaling via the Golgi apparatus are significant in this context. However, to date it is unknown how this integration between Golgi apparatus–associated signaling molecules and Golgi apparatus function could be achieved. Parallels between the organizational functions of Golgi matrix proteins and signaling scaffolds can be drawn (Barr and Short, 2003; Gillingham and Munro, 2003), and it is possible that Golgi matrix proteins might organize and thereby control signaling complexes on Golgi membranes. This would also provide a means for coordinating signaling events with Golgi apparatus integrity and function.
The Ste20 family of serine/threonine protein kinases is implicated in a variety of signaling pathways including those involved in the control of cell migration and polarity (Dan et al., 2001). In mammals, over 30 Ste20 kinases exist classified into two subgroups. These are the p21-activated kinases and the germinal center kinases (Dan et al., 2001), and it is this latter group that is of interest here. Germinal center kinases possess an NH2-terminal kinase domain and a COOH-terminal regulatory domain and can be further subdivided into eight groups based on sequence homologies (Dan et al., 2001). Subgroups II and III contain the mammalian Ste20 (MST) kinases, MST1 (Creasy et al., 1996), MST2 (Creasy and Chernoff, 1995), MST3 (Schinkmann and Blenis, 1997), MST4 (Lin et al., 2001; Qian et al., 2001), and yeast Sps1/Ste20-related kinase 1 (YSK1; Pombo et al., 1996; Osada et al., 1997). MST1 and MST2 are cleaved during apoptosis by caspase-3 and translocate into the nucleus (Lee et al., 2001; Ura et al., 2001) where they function in proapoptotic signaling (De Souza et al., 2002; Lin et al., 2002). Less is known about the other MST kinases. YSK1, also known as Ste20/oxidant stress response kinase 1, is weakly activated by reactive oxygen intermediates but not by any other environmental stresses, or by growth factors (Pombo et al., 1996). However, this kinase does not participate in any of the known MAPK pathways (Pombo et al., 1996; Osada et al., 1997) and a physiological function for YSK1 remains unknown. Like YSK1, MST4 overexpression fails to activate the JNK and p38 MAPK pathways, although it promotes anchorage-independent growth and tumor formation, and has been implicated in prostate cancer progression (Qian et al., 2001; Sung et al., 2003). Here, we investigate the MST family kinases YSK1 and MST4 and uncover a signaling function linked with the Golgi matrix protein GM130 that may play a role in the control of cell migration and polarization.
Results
YSK1 and MST4 localize to the Golgi apparatus
Antibodies raised against the recombinant human YSK1 kinase domain detected a protein of the expected size in total HeLa cell extract, rat liver cytosolic extract, and purified rat liver Golgi membranes (Fig. 1 A). This reaction was competed by preincubation of the antibody with the purified antigen before Western blotting (Fig. 1 A). Due to the high degree of sequence similarity between the MST family kinases these antibodies were further tested on lysates of cells transfected with GFP-tagged MST3, MST4, and YSK1. This revealed that polyclonal antibodies raised to YSK1 also react to a lesser extent with MST4, but not with MST3. An antibody raised to a unique sequence in the first 19 aa of YSK1 recognized only YSK1 (Fig. 1 A). When used in immunofluorescence studies of HeLa cells these antibodies gave a cytosolic and Golgi apparatus staining pattern most similar to the cis-Golgi protein GM130 and the medial-Golgi protein GRASP55, and adjacent to but not overlapping with the trans-Golgi marker golgin97 (Fig. 1 B). A similar staining pattern was observed in two other human cell lines, MelJuSo and HS68 (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200310061/DC1; unpublished data). Endogenous YSK1/MST4 are therefore Golgi proteins based on their localization in human cell lines and presence in purified Golgi membrane fractions.
Figure 1.
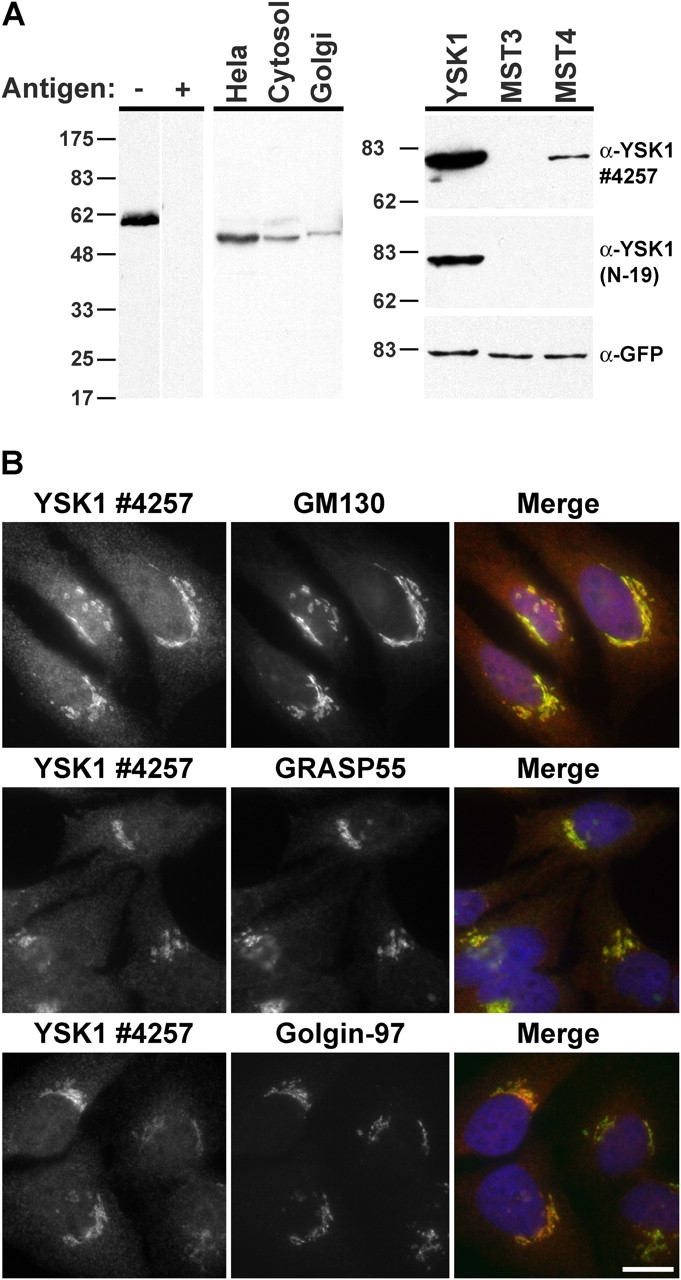
YSK1 localizes to the Golgi apparatus. (A) Samples containing 25 μg of protein from insect cells expressing His6-tagged human YSK1 (up shifted due to the presence of the tag), HeLa cells, rat liver cytosol, or 10 μg of rat liver Golgi membranes were analyzed by Western blotting with 1 μg/ml of affinity-purified rabbit antibody 4257 to human YSK1. For antibody competition, 10 μg/ml of the antigen was preincubated with the antibody for 1 h before use. Samples containing 20 μg of protein from HeLa cells transfected with constructs for GFP-tagged YSK1, MST3, and MST4 for 18 h were blotted with 1 μg/ml of affinity-purified rabbit antibody 4257 to YSK1, a peptide antibody raised against the unique first 19 aa of YSK1 (N19), or a sheep antibody to GFP. (B) HeLa cells were costained with rabbit antibodies to YSK1 and sheep antibodies to GM130, GRASP55, or golgin97. Bar, 10 μm.
YSK1 and MST4 interact with the Golgi matrix protein GM130
To identify interaction partners for these kinases that could explain their localization to the Golgi apparatus, a yeast two-hybrid screen of 2.2 million clones from a human testis cDNA library was performed. Only two clones interacted specifically with YSK1, and both contained the full-length ORF for the human Golgi matrix protein GM130 (Fig. 2 A). A directed two-hybrid analysis was then used to map the interaction sites for YSK1 on rat GM130 (Fig. 2 A). The YSK1 binding site of GM130 maps to amino acids 75–271, a region predicted to adopt a coiled-coil structure. This is discrete from the binding site for the vesicle docking protein p115 that lies in the first 75 aa (Fig. 2 A), and the COOH-terminal region containing the binding sites for GRASP65 and Rab GTPases (Barr et al., 1998). Binding assays using purified recombinant proteins confirmed that GM13075-271 bound directly to YSK1 (Fig. 2 B), whereas the NH2-terminal domain of another Golgi matrix protein, golgin45, and GST did not (Fig. 2 B). Furthermore, YSK1 failed to localize to the Golgi apparatus in cells depleted of GM130 using small interfering RNA (siRNA) duplexes (Fig. 2 C). Therefore, YSK1 binds to a specific domain of the Golgi matrix protein GM130 and shows GM130-dependent localization in cells, thus providing a mechanism for its localization to the Golgi apparatus.
Figure 2.
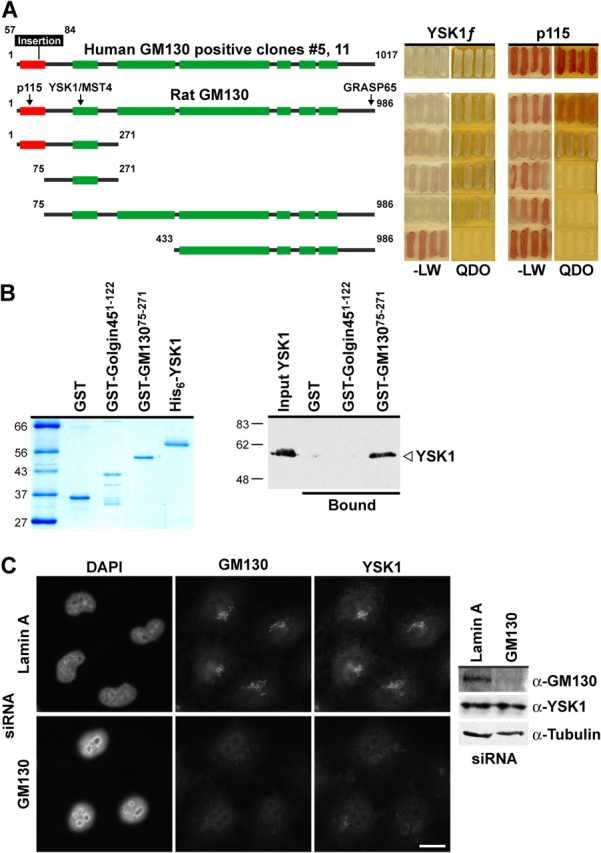
Identification of the Golgi protein GM130 as a binding partner for YSK1. (A) Bait constructs comprising the full-length human GM130 splice variant rescued from the library screen, full-length rat GM130 or the indicated deletion constructs of rat GM130 were tested against full-length human YSK1 for the ability to grow on selective medium (QDO), compared with nonselective medium (−LW) in the yeast two-hybrid system. Lighter colony color on QDO is an indicator of a strong signal. Boxed regions indicate the p115 binding site (red) and predicted coiled-coil domains (green). (B) His-tagged YSK1 was incubated with GST-tagged fragments of rat GM130, golgin45, or GST and recovered on glutathione-agarose. Recovered complexes were analyzed by Western blotting. (C) HeLa cells depleted for lamin A or GM130 for 67 h using siRNA were stained and Western blotted for GM130, YSK1, and α-tubulin. Bar, 10 μm.
Other MST kinases were then tested for interaction with GM130 and localization to the Golgi apparatus. A directed two-hybrid analysis revealed that MST4 interacted with full-length GM130 and the first 271 aa of GM130, but failed to interact with the empty vector control or golgin45 (Fig. 3 A). MST3 showed no interactions with any of the proteins tested (Fig. 3 A). Binding assays confirmed that MST4 bound directly to GM13075-271 but not to the NH2-terminal domain of golgin45 (Fig. 3 B). Consistent with these results MST4 targeted to the Golgi apparatus in HeLa cells (Fig. 3 C), whereas MST3 showed a cytoplasmic distribution (Fig. 3 D). YSK1 displays significant homology with MST3 and MST4, to a high degree within the kinase domain and GM130 binding region and to a lesser but significant extent in the COOH terminus (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200310061/DC1). MST4 like YSK1 may target to the Golgi apparatus via an interaction with GM130, and therefore have a common regulatory mechanism. Interestingly, MST3 although highly homologous to YSK1 and MST4 does not localize to the Golgi apparatus or bind to GM130, suggesting it has different regulatory partners.
Figure 3.
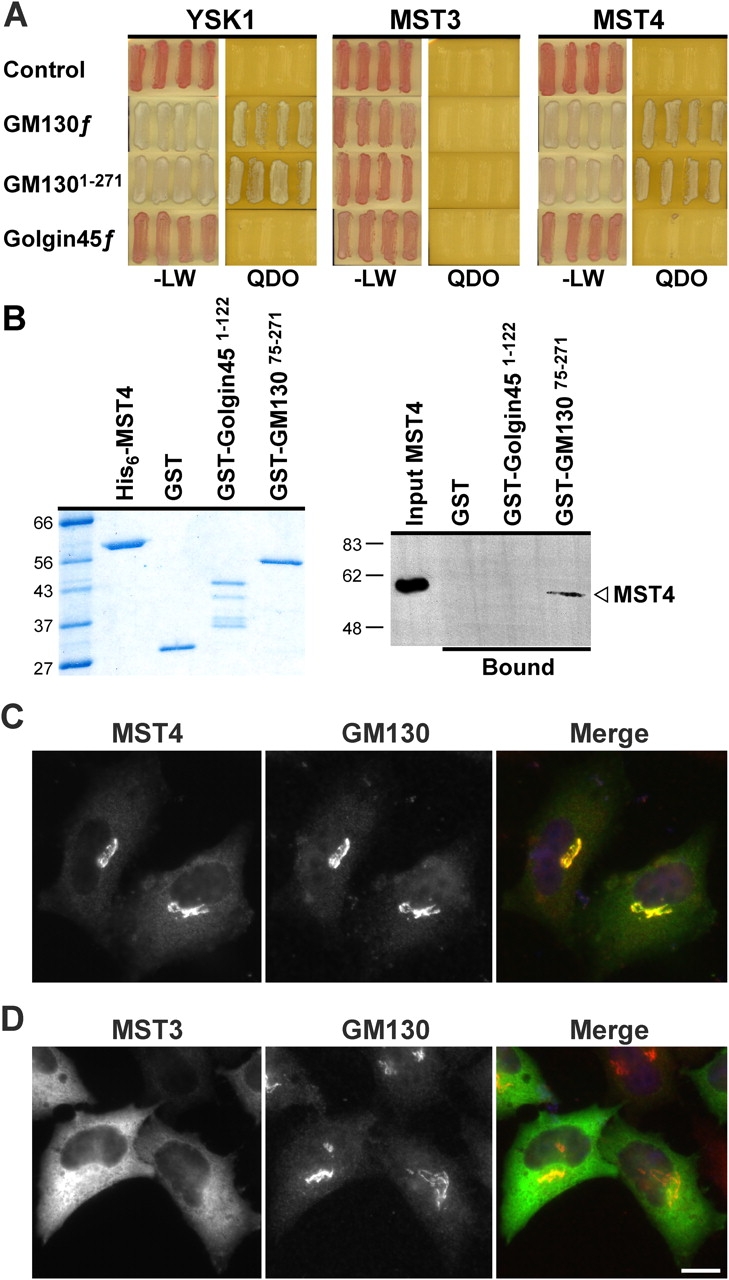
The YSK1-related kinase MST4 is Golgi apparatus localized. (A) Full-length YSK1, MST3, and MST4 were tested against empty vector (control), full-length GM130, GM1301-271, and golgin45 for the ability to grow on selective medium (QDO), compared with nonselective medium (−LW) in the yeast two-hybrid system. Lighter colony color on QDO is an indicator of a strong signal. (B) His-tagged MST4 was incubated with GST-tagged fragments of rat GM130, golgin45, or GST and recovered on glutathione-agarose. Recovered complexes were analyzed by Western blotting. (C and D) HeLa cells transfected with myc-tagged MST3 and MST4 constructs for 18 h were costained with the 9E10 mAb to the myc epitope (green) and a sheep antibody to GM130 (red). Bar, 10 μm.
YSK1 shows activity-dependent targeting to the Golgi apparatus
To investigate the targeting of YSK1 to the Golgi apparatus further, the features of YSK1 needed for its interaction with GM130 were mapped. Amino acids 20–302, comprising the kinase domain and a short region of charged amino acids distal to it, was the smallest fragment tested that interacted with GM130 (Fig. 4 A). The NH2- and COOH-terminal sequences before amino acid 20 and after amino acid 302, respectively, were not essential for the interaction with GM130 (Fig. 4 A). These constructs were then tested for their ability to target to the Golgi apparatus in transfection assays. Full-length YSK1 and, to a lesser extent, the first 302 aa comprising the GM130 binding region were Golgi apparatus localized (Fig. 4 B). Decreased Golgi apparatus targeting of the first 302 aa of YSK1 compared with the wild-type protein may be explained by the observation that the COOH terminus is important for YSK1 homodimerization (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200310061/DC1). Two kinase activity-deficient mutants YSK1K49R and YSK1D158A, the kinase domain alone, and a COOH-terminal construct lacking the kinase domain were unable to target to the Golgi apparatus (Fig. 4 B). Therefore, the targeting of YSK1 to the Golgi apparatus requires an NH2-terminal region from amino acids 20 to 302 that binds to GM130, and includes the kinase domain. Kinase activity is also required for Golgi apparatus targeting and stable GM130 binding in vivo because kinase-dead mutants do not localize to the Golgi apparatus.
Figure 4.
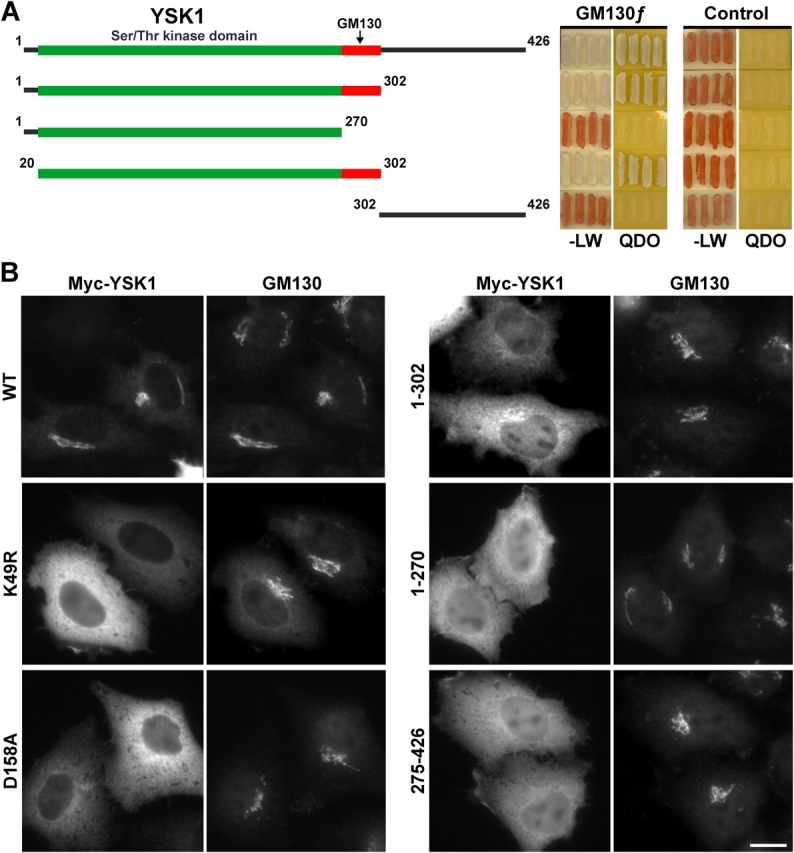
YSK1 shows activity-dependent targeting to the Golgi apparatus. (A) Bait constructs comprising full-length YSK1, or the indicated deletion constructs were tested against full-length rat GM130 or an empty bait plasmid (control) for the ability to grow on selective medium (QDO), compared with nonselective medium (−LW) in the yeast two-hybrid system. Lighter colony color on QDO is an indicator of a strong interaction. Boxed regions indicate the canonical serine/threonine kinase domain (green), and the extension to this necessary for binding to GM130 (red). (B) HeLa cells transfected with myc-tagged YSK1 constructs for 18 h were costained with the 9E10 mAb to the myc epitope and a sheep antibody to GM130. Bar, 10 μm.
GM130 is an activator of YSK1 and MST4
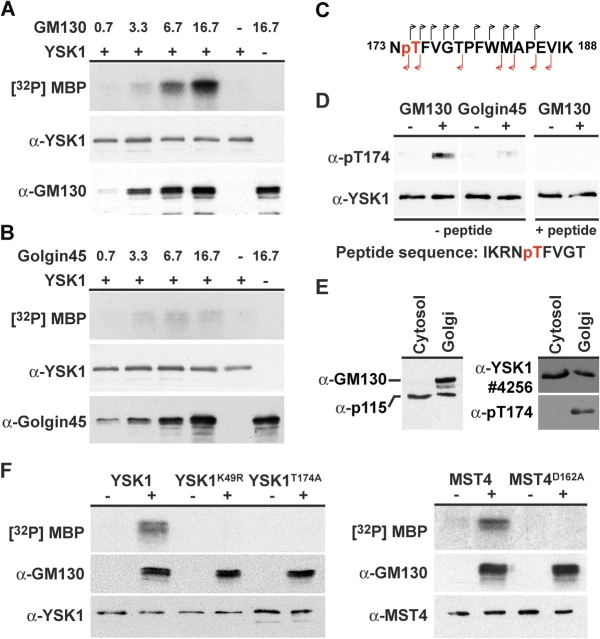
Because GM130 appears to function as a Golgi apparatus localized scaffold protein for YSK1, the relationship between YSK1 kinase activity and GM130 binding was investigated. A preliminary analysis of recombinant YSK1 purified from insect cells found that the pure kinase showed no activity toward the Golgi proteins GM130, GRASP65, and p115. From a variety of model substrates tested, only myelin basic protein (MBP) was phosphorylated (unpublished data), and this was therefore used for subsequent experiments. Titration of the GM130 NH2-terminal domain into YSK1 kinase assays up to a 20-fold molar excess resulted in a 25-fold stimulation of YSK1 activity above the basal level of activity (Fig. 5 A). Under the same conditions, the NH2-terminal domain of golgin45 maximally activated YSK1 3.5-fold, whereas the protein storage buffer did not stimulate YSK1 (Fig. 5 B). Analysis of activated YSK1 revealed phosphorylation of threonine 174, a modification absent from the nonactivated kinase (Fig. 5 C and Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200310061/DC1). This observation could be confirmed with pT174, an antibody directed against phosphorylated threonine 174, which detected YSK1 only after activation by GM130 (Fig. 5 D, − peptide), and was competed by the corresponding phosphopeptide (Fig. 5 D, + peptide). Using the pT174 antibody, phosphorylated YSK1 could only be detected in Golgi membranes but not cytosol, even though like p115, another GM130 partner, a pool of YSK1 was present in cytosol (Fig. 5 E). This supports the model that a pool of activated YSK1 exists at the Golgi apparatus.
Figure 5.
Binding to the NH2-terminal domain of GM130 triggers autophosphorylation and activation of YSK1. (A) Purified YSK1, 0.8 pmoles, was incubated in the absence or presence of 0.7, 3.3, 6.7, and 16.7 pmoles of His-tagged GM13075-271 or (B) His-tagged golgin451-122 at 37°C for 30 min. After this preincubation, samples were analyzed for kinase activity toward the model substrate MBP using γ-[32P]ATP, and by Western blotting for YSK1 and GM130 or golgin45 to control for gel loading. (C) Fragmentation of the phosphorylated NTFVGTPFWMAPEVIK peptide (Fig. S4) by tandem mass spectrometry gives daughter ions derived from the NH2 and COOH termini of the peptide indicating that threonine 174 (pT), shown in red, is the phosphorylated residue. (D) Activated and mock-activated YSK1 was Western blotted with affinity-purified antibody N19 to YSK1 and the pT174 antibody to the phosphorylated T-loop sequence IKRNpTFVGT, in the presence and absence of 5 μg of blocking peptide. (E) 25 μg of rat liver cytosol and 10 μg of Golgi membranes were Western blotted with antibodies to GM130, p115, YSK1 4256, and pT174 to phosphorylated YSK1. (F) Purified YSK1, YSK1K49R, YSK1T174A, MST4, and MST4D162A, 0.8 pmoles, were incubated in the absence or presence of 16.7 pmoles of His-tagged GM13075-271 at 37°C for 30 min. After this preincubation, samples were analyzed for kinase activity toward the model substrate MBP using γ-[32P]ATP, and by Western blotting for YSK1 MST4 and GM130 to control for gel loading.
Phosphorylation at the equivalent position of the T-loop in many other kinases is an important determinant for their activation (Russo et al., 1996). Mutation of the T-loop threonine to alanine to give YSK1T174A resulted in a form of the kinase that showed no activity toward MBP in the presence or absence of GM130, and was comparable to the kinase-dead ATP binding site mutant YSK1K49R (Fig. 5 F). Similar results to those obtained for YSK1 were also found with MST4. MST4 is specifically activated by binding to GM130 and is autophosphorylated as a consequence on threonine 178 (Fig. 5 F; unpublished data). Therefore, activation of YSK1 and MST4 involves autophosphorylation at threonine 174 (178 in MST4), possibly as a consequence of dimerization stabilized by binding to GM130 at the Golgi apparatus.
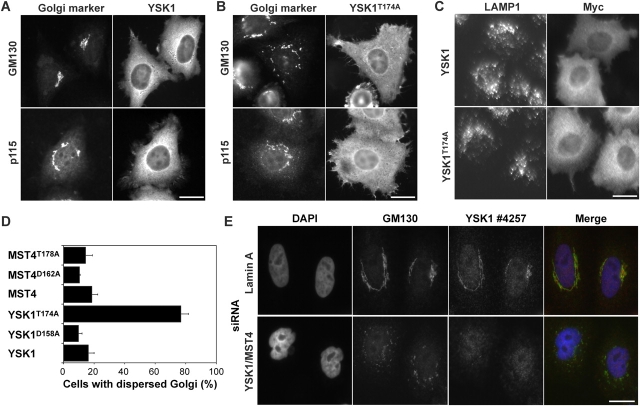
Dominant-negative YSK1T174A perturbs Golgi apparatus localization
In addition to its function as part of the Golgi apparatus localized tethering complex required for vesicle docking and stacking of Golgi cisternae, GM130 has been proposed to form a structural landmark important for establishment of a polarized Golgi structure (Pfeffer, 2001). One aspect of polarized Golgi structure is the cis- to trans-polarity of the stacked cisternae, and the other is its asymmetric distribution within the cell. Association of the Ste20 family kinases YSK1 and MST4 with GM130 suggest this complex might act as a landmark in a transduction event important for signaling Golgi apparatus integrity and position within the cell, and controlling Golgi apparatus function. The effects of expressing wild-type YSK1, and YSK1T174A, which should behave as a dominant-negative mutant form of YSK1 unable to be activated by GM130, were then compared (Fig. 6, A and B). At high expression levels, YSK1 saturated the available binding sites at the Golgi apparatus and accumulated in the cytoplasm without causing any obvious change in the two Golgi markers GM130 and p115 (Fig. 6 A). Expression of YSK1T174A resulted in the dispersal of the perinuclear ribbon-like Golgi apparatus pattern of GM130 and p115 typical of HeLa cells (Fig. 6 B). This effect was specific to the Golgi apparatus because the perinuclear late-endosomal and lysosomal compartments defined by LAMP1 showed no obvious differences in YSK1 and YSK1T174A expressing cells (Fig. 6 C). Golgi apparatus dispersal was observed in >75% of cells expressing YSKT174 but not with the dominant-negative MST4T178A or other YSK1 and MST4 constructs (Fig. 6 D). Preliminary investigation revealed that transport of the transmembrane glycoprotein of vesicular stomatitis virus was not compromised in YSK1T174A expressing cells, indicating they do not have a general defect in secretion (unpublished data). To obtain supporting evidence for a function of endogenous MST kinases at the Golgi apparatus in HeLa cells, depletion of YSK1 and MST4 was performed using siRNA and the cells stained for the Golgi marker GM130. In cells depleted of YSK1 and MST4 the Golgi apparatus was dispersed into the cell periphery, whereas in control cells depleted for lamin A the typical perinuclear Golgi apparatus morphology was preserved (Fig. 6 E). Interfering with YSK1 and MST4 function, therefore, disturbs the ordered localization of the Golgi apparatus in the perinuclear region.
Figure 6.
YSK1/MST4 are required for Golgi apparatus localization in the perinuclear region. (A) HeLa cells were transfected with expression constructs for the wild-type myc-tagged (A) YSK1 or (B) YSK1T174A for 44 h and then stained with mouse antibodies to the myc epitope and sheep antibodies to either GM130 or p115. (C) HeLa cells were transfected with expression constructs for the wild-type myc-tagged YSK1 or YSK1T174A for 44 h and then stained with rabbit antibodies to the myc epitope and mouse antibodies to LAMP1. (D) Golgi apparatus dispersal was scored after 48 h in HeLa cells transfected with YSK1 and MST constructs indicated in the legend. Mean values are plotted (n = 3) with at least 300 cells counted per experiment for each condition. (E) HeLa cells were transfected with siRNA duplexes for YSK1 and MST4 for 112 h then stained with antibodies to GM130 (green) and YSK1/MST4 (red), and with DAPI for DNA (blue). Exposure times of 1 s were used for all images. Bars, 10 μm.
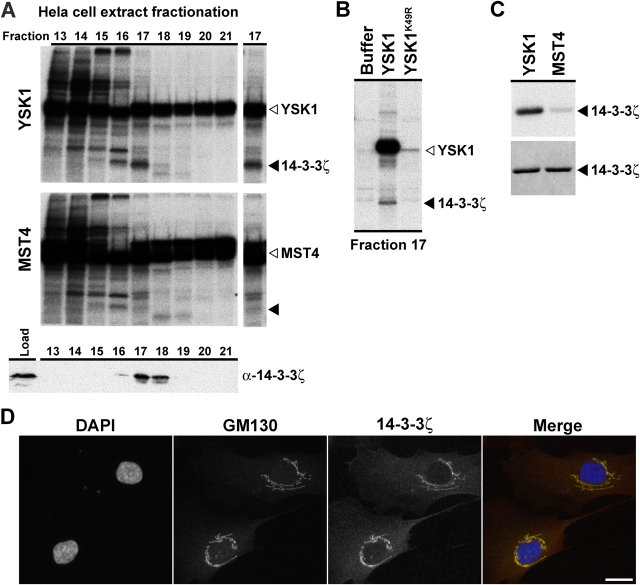
A biochemical screen for YSK1 targets identifies 14-3-3ζ
One explanation for the differences seen with the dominant-negative forms of YSK1 and MST4 is that they have different substrates. To test this hypothesis, a biochemical screening approach for the kinase substrate tracking and elucidation (KESTREL) was used (Knebel et al., 2001). Application of this method to HeLa cell extracts fractionated by size exclusion chromatography revealed a number of phosphorylation events (Fig. 7 A). Strong autophosphorylation of both kinases hindered the identification of substrates in the 50–60-kD region. Significantly, a protein of apparent molecular mass 32 kD in fraction 17 was phosphorylated in YSK1 treated samples (Fig. 7 A), but was not in MST4, kinase inactive YSK1K49R or buffer-treated samples (Fig. 7, A and B). Further analysis using mass spectrometry and Western blotting revealed that this phosphoprotein corresponds to 14-3-3ζ phosphorylated at Serine 58 (Fig. 7 A; unpublished data). Confirming these observations, recombinant 14-3-3ζ was phosphorylated by YSK1 but not MST4 (Fig. 7 C). Furthermore, an mAb against 14-3-3ζ (Leffers et al., 1993) stained the Golgi apparatus and showed considerable overlap with the YSK1 activator GM130 (Fig. 7 D). Therefore, 14-3-3ζ fulfills the criteria of a specific target for YSK1 at the Golgi apparatus.
Figure 7.
A biochemical screen for YSK1 targets identifies 14-3-3ζ. (A) A modified KESTREL approach was used to identify substrates for YSK1 and MST4 as described in the Materials and methods. Phosphorylations with YSK1 and MST4 of fractions 13–21 from a fractionation of HeLa S3 cell extract by gel filtration on Superose-6 together with a Western blot for 14-3-3ζ are shown. Fraction 17 is shown as a cut out to the right, with the position of 14-3-3ζ indicated by a closed arrowhead and the respective kinases indicated by open arrowheads. (B) Superose-6 column fraction 17 was treated with buffer, YSK1, or kinase-dead YSK1K49R using the KESTREL method, and analyzed by SDS-PAGE and autoradiography. Closed and open arrowheads indicate phosphorylation of 14-3-3ζ and YSK1 autophosphorylation, respectively. (C) Kinase assays were performed for 60 min at 37°C in KESTREL assay buffer with 2 μg of recombinant His-tagged 14-3-3ζ, and 500 ng of preactivated YSK1 or MST4. An autoradiograph of 14-3-3ζ phosphorylations by YSK1 and MST4 is shown in the top panel, and the corresponding region of a Coomassie blue stained gel in the bottom panel. Closed arrowheads indicate 14-3-3ζ. (D) HS68 cells were costained with a sheep antibody to GM130 and the mouse mAb 22-II-D8 to 14-3-3ζ. Bar, 10 μm.
Dominant-negative YSK1T174A abolishes collagen invasion and cell migration
It has recently been reported that in mammalian cells there is a link between the oncogenic Ras-signaling pathway and cell invasion induced by the actin severing protein gelsolin (De Corte et al., 2002). Together with other recent findings that Ras-signaling also occurs at the surface of the Golgi apparatus (Chiu et al., 2002; Bivona et al., 2003), these observations hint that YSK1 and MST4 may signal some aspect of cell migration involving the Golgi apparatus. The effects of expressing wild-type and various mutant forms of YSK1 and MST4 on gelsolin-induced invasion were therefore explored, using an established assay (Braecke et al., 2001). Coexpression of GFP, YSK1, and the kinase-dead YSK1K49R mutant had no significant effect on gelsolin-induced invasion (Fig. 8 A), whereas coexpression of dominant-negative YSK1T174A abrogated collagen invasion. If 14-3-3ζ is the key YSK1 substrate mediating these effects, then expressing 14-3-3ζS58A should give the same result as dominant-negative YSK1T174A, whereas 14-3-3ζS58D should oppose YSK1T174A, and this was indeed the case (Fig. 8 B). Gelsolin-induced invasion was abrogated by 14-3-3ζS58A, but not by other forms of 14-3-3ζ, whereas 14-3-3ζS58A triggered collagen invasion in the absence of gelsolin, and this was not overcome by the expression of YSK1T174A (Fig. 8 B). Therefore, 14-3-3ζ may act downstream of both gelsolin and YSK1 in collagen invasion, and be the key substrate mediating the effects of YSK1.
Figure 8.
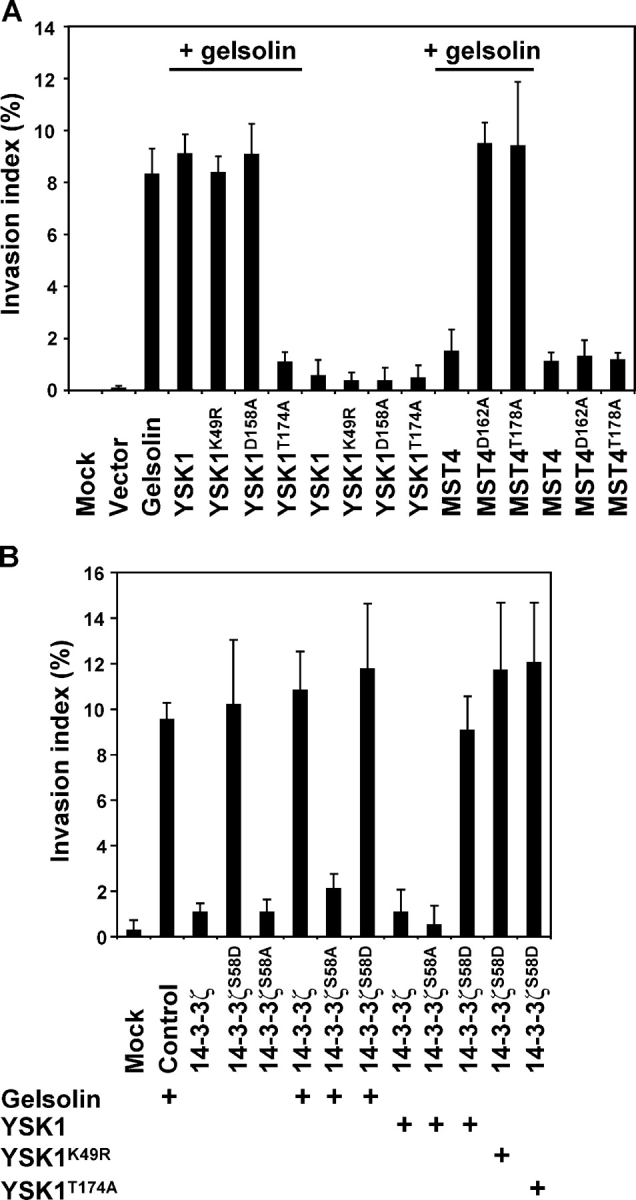
Cell migration and invasion is modulated by YSK1 and MST4. (A) Cotransfection of YSK1T174A but not other forms of YSK1 abrogates gelsolin-induced invasion of collagen type I by HEK293T cells. HEK293T cells were left untransfected, or cotransfected with plasmids encoding gelsolin and constructs for wild-type YSK1 and MST4, or point mutants of these kinases, as indicated in the figure (n = 3). (B) Effects of 14-3-3ζ phosphorylation mutants on invasion into collagen type I. HEK293T cells were left untransfected, or cotransfected with plasmids encoding wild-type and point mutant forms of 14-3-3ζ, gelsolin, YSK1, YSK1K49R, and YSK1T174A, as indicated in the figure (n = 3). Collagen invasion assays were performed and quantitated as described in the Materials and methods. The invasive index is the percentage of cells invading the collagen gel over the total number of cells.
To analyze the effects of YSK1 on the polarization of the Golgi apparatus and centrosome a second assay for cell migration was used, in which confluent monolayers of cells are wounded and then cells along the wound edge microinjected with the constructs of interest (Etienne-Manneville and Hall, 2001). Consistent with the collagen invasion defect YSK1T174A expressing cells were unable to migrate into the wound in this assay (Fig. 9 A). Furthermore, cells expressing YSK1T174A failed to show polarization of the Golgi apparatus and centrosome in the direction of migration toward the wound edge (Fig. 9 B). Interestingly, YSK1T174A expression, although causing dispersal of the Golgi apparatus in some cells consistent with the phenotype seen in HeLa cells (Fig. 6 B), resulted in the displacement of essentially intact Golgi apparatus away from the perinuclear region in others (Fig. 9 C, arrowhead). Expression of wild-type YSK1 had little effect on either cell migration or Golgi apparatus and centrosome polarization (Fig. 9, A–D). Signaling via YSK1 appears to be required for cell migration and invasion into collagen because dominant-negative YSK1T174A blocks these processes. Intriguingly, the wild-type MST4 kinase abrogated gelsolin-induced invasion into collagen, whereas kinase-dead MST4D162A and dominant-negative MST4T178A had no effect (Fig. 8 A), suggesting that MST4 opposes the signaling pathway leading to invasion into collagen. Therefore, YSK1 and MST4 may function in a signaling pathway at the surface of Golgi membranes required for cell migration and polarization.
Figure 9.
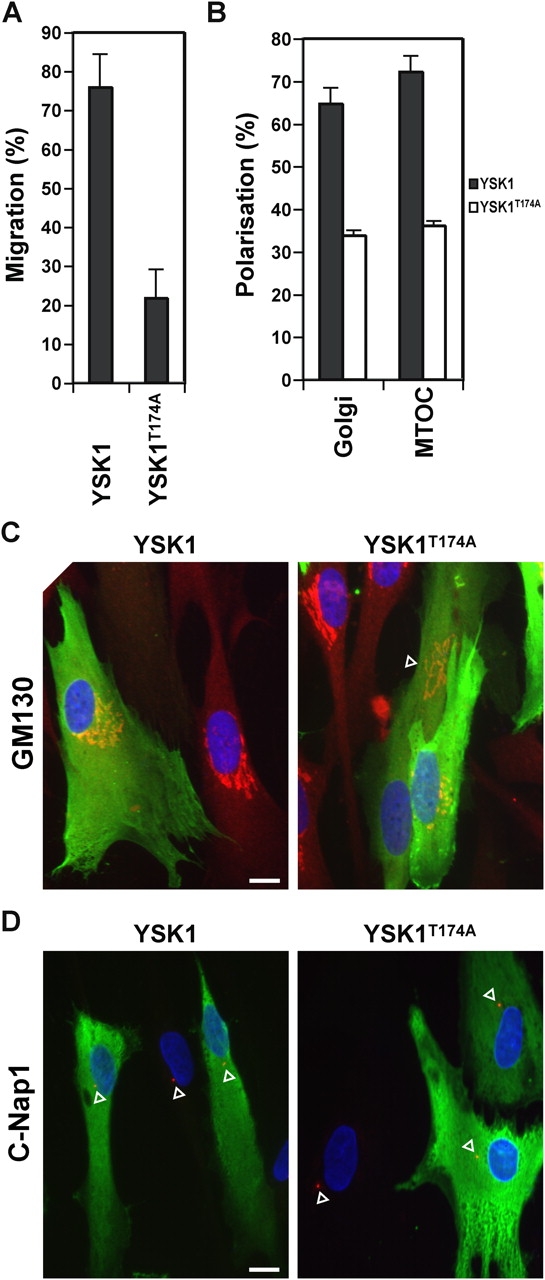
Golgi apparatus and centrosome polarization is inhibited by YSK1T174A. (A and B) Cells were microinjected with plasmids encoding myc-tagged YSK1 and YSK1T174A, fixed after 16 h, and stained with antibodies to GM130 or c-Nap1, and 9E10 monoclonal to the myc epitope. Bar graphs show the percentage of YSK1 or YSK1T174A expressing cells migrating at the wound edge or into the wound (n = 12), and the percentage of cells with Golgi apparatus and centrosomes (MTOC) polarized toward the wound edge (n = 4). (C and D) Images of cells expressing YSK1 or YSK1T174A (green) and stained for GM130 or c-Nap1 (red) are shown. The position of the wound corresponds to the bottom of the figure. Arrowheads indicate the position of the Golgi apparatus and centrosome. Bars, 10 μm.
Discussion
GM130: a scaffold and activator for MST kinases
We have investigated the MST family of Ste20 kinases and identified two members, YSK1 and MST4, as Golgi apparatus–associated kinases. These two kinases bind to the Golgi matrix protein GM130 and thereby target to the Golgi apparatus, whereas a third and the most closely related family member MST3 is not associated with the Golgi apparatus and fails to bind to GM130. A similar mechanism has been observed for the MAPK ERK1 localized on the surface of endosomes, which involves a small scaffolding protein termed p14 (Wunderlich et al., 2001; Teis et al., 2002). There is a second consequence of the interaction of YSK1 and MST4 with GM130. These kinases, when purified from insect cells, show only low levels of activity toward the model substrate MBP, and incubation with GM130 causes a >25-fold increase in their activity. Analysis of activated YSK1 and MST4 reveals that they become autophosphorylated on T-loop residue threonine 174 (T178 in MST4) as a consequence of incubation with GM130, and that mutation of this residue in YSK1 abolishes kinase activity. Supporting evidence for the view that this is a key regulatory modification comes from studies on MST1 that identified phosphorylation of the equivalent residue in the T-loop as an activating event (Glantschnig et al., 2002). Other studies on MST1 have shown that the region COOH-terminal to the kinase domain also contributes to MST1 regulation, possibly via an autoinhibitory mechanism because truncation or cleavage of this domain during apoptosis results in kinase activation (Creasy et al., 1996; Graves et al., 1998; Ura et al., 2001). We have found that YSK1 and MST4 associate with GM130 via the kinase domain and a small region immediately COOH-terminal to it (Fig. 4 A). Together, with the fact that GM130 is a dimer, this suggests that two molecules of YSK1 or MST4 may associate with a single molecule of GM130 and autophosphorylate in trans, thus providing a mechanism for the activation process and suggesting that Golgi apparatus–associated pools of these kinases are active.
Functions for Golgi apparatus–associated signaling pathways
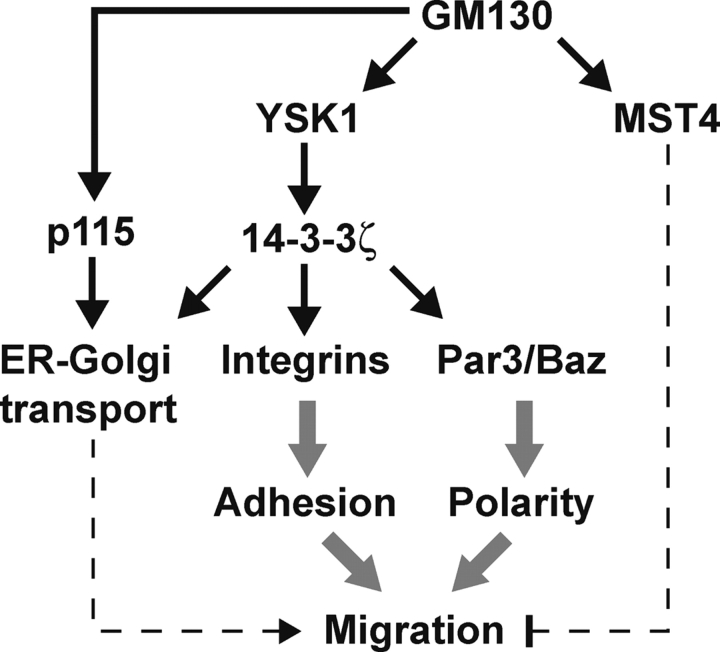
Directed cell motility is a highly complex process involving many discrete events such as polarization of the cytoskeleton, signal transduction events, and regulation of cell adhesion complexes (Ridley et al., 2003). In particular, cytoskeletal rearrangements and the secretion of proteins and lipids to specific subdomains of the plasma membrane accompany changes in cell polarity, and Golgi apparatus–localized kinases such as YSK1 and MST4 are in an ideal location to regulate these processes by modulating Golgi apparatus function. The association with GM130, and high degree of similarity between, YSK1 and MST4 might indicate that they have similar functions and downstream effectors. However, this appears not to be the case. Dominant-negative YSK1 but not MST4 causes dispersal of the Golgi apparatus and blocks cell migration, whereas wild-type MST4 but not YSK1 blocks cell migration without having any visible effects on the Golgi apparatus. One possibility is that competition for GM130 controls the relative levels of YSK1 and MST4 activity, although how this would be achieved is unclear at present. Together with their differential effects on cell migration they would therefore be predicted to act on different downstream pathways. The identification of 14-3-3ζ as a specific Golgi apparatus localized substrate for YSK1 but not MST4 supports this idea, and gives some clues as to how YSK1 signaling may control Golgi apparatus function and cell migration. The 14-3-3 proteins are dimeric adaptors typically binding to phosphorylated acceptor sites on their targets, thereby regulating a wide variety of cellular processes (Tzivion et al., 2001). Furthermore, 14-3-3 proteins are themselves regulated by phosphorylation and dimerization (Tzivion et al., 2001). In the context of this work, three particular functions reported for these proteins may be of relevance (depicted schematically in Fig. 10). First, 14-3-3ζ binds to phosphorylated Raf in the Ras-signaling pathway and stimulates its activity (Fantl et al., 1994; Freed et al., 1994). Because a pool of activated Ras is generated at the Golgi apparatus in response to growth factor receptor activation, it is possible that YSK1 via 14-3-3ζ can modulate this pathway. Second, 14-3-3 proteins have been found associated with the cytoplasmic domains of specific integrin complexes (Han et al., 2001; Bialkowska et al., 2003; Santoro et al., 2003), and with Par3/Baz, one of a number of proteins important for control of cell polarity and cell asymmetry during development (Benton et al., 2002; Hurd et al., 2003). Overexpression of 14-3-3 proteins blocks cell migration, and this could be exerted via integrins and their function in cell adhesion (Han et al., 2001; Santoro et al., 2003). An obvious way for YSK1 to control cell migration would therefore be via the 14-3-3ζ–dependent modulation of cell adhesion. Finally, 14-3-3 proteins have been reported to act in the quality control pathway regulating assembly and transport of multimeric membrane protein complexes from the ER to the Golgi apparatus (O'Kelly et al., 2002; Yuan et al., 2003). This provides another point of control for YSK1 that may be relevant for regulation of Golgi apparatus function. Exactly which if any of these potential mechanisms is relevant for YSK1 will require further characterization of 14-3-3ζ binding partners.
Figure 10.
MST kinases: linking Golgi apparatus function with cell migration? Together with the vesicle tethering factor p115, GM130 is part of a landmark complex on the Golgi apparatus important for protein transport and Golgi structure. Binding to GM130 activates YSK1 and MST4. Activated YSK1 phosphorylates 14-3-3ζ and potentially other downstream targets needed for normal cell migration, whereas MST4 acts via an uncharacterized pathway. Known targets of 14-3-3ζ important for regulating cell migration and polarity are depicted.
Until recently, the range of signaling events occurring at the Golgi apparatus was unsuspected, and now includes specific aspects of Ras and growth factor signaling (Chiu et al., 2002; Bivona et al., 2003), apoptotic signaling (Lane et al., 2002), and evidence that the Golgi apparatus acts as a sensor controlling mitotic entry (Sutterlin et al., 2002). To these we now add signaling events important for cell migration.
Materials and methods
Reagents and antibodies
Reagents were obtained from Sigma-Aldrich unless specified otherwise. Chromatography reagents and γ-[32P]ATP (3,000 Ci/mmol and 10 mCi/ml) were obtained from Amersham Biosciences. Antisera specific to YSK1 was raised in rabbits 4256 and 4257 immunized with recombinant human YSK1 by Biogenes, and then affinity purified over a column of YSK1 coupled to Affigel-15 (Bio-Rad Laboratories). Peptide antibodies to YSK1 (SC-6865, N19) and MST4 (3822) were purchased from Santa Cruz Biotechnology, Inc. and New England Biolabs, Inc., respectively. Mouse monoclonal anti-LAMP1/CD107a was purchased from Becton Dickinson. Antibodies specific to the phosphorylated YSK1/MST4 activation loop peptide CIKRNpTFVGT were produced by Abcam Ltd., and then isolated from a protein-A purified IgG fraction of the serum over the same peptide immobilized on Sulfolink resin according to the manufacturer's instructions (Pierce Chemical Co.). E. Nigg (Max-Planck-Institute of Biochemistry) and J. Celis (Institute of Cancer Biology, Copenhagen, Denmark) provided antibodies to the centrosome marker c-Nap1 and 14-3-3ζ, respectively. Other antibodies used in this experiment have been described and characterized previously (Barr et al., 1997; Shorter et al., 1999; Short et al., 2001).
Molecular biology and two-hybrid screening
Full-length human YSK1, MST3, MST4, and 14-3-3ζ were amplified from human testis cDNA (Becton Dickinson) using the pfu polymerase (Stratagene) and cloned in pCRII-TOPO (Invitrogen). Point mutants were constructed using the Quickchange mutagenesis protocol (Stratagene). DNA oligonucleotides were purchased from Thermo-Hybaid and QIAGEN. All constructs were confirmed by DNA sequencing (Medigenomix). Mammalian expression constructs were made in pcDNA3.1+ (Invitrogen) and pEGFP-C2 (CLONTECH Laboratories, Inc.). For baculovirus expression, pVL1393 or the pAcSG2 vector (Becton Dickinson) modified to include the hexahistidine-tag from pQE32 (QIAGEN) was used. Baculoviruses were produced and proteins expressed in Sf9 cells according the manufacturer's protocols (Becton Dickinson). Bacterial expression was performed using the His-GST expression vector pGAT2 and the His-tag expression vector pQE32 (QIAGEN). Inserts encoding GM13075-271, golgin451-122, and 14-3-3ζ were inserted into pGAT2 or pQE32 and proteins expressed in BL21(DE3) or JM109 cells, respectively. Proteins were purified over nickel-NTA agarose (QIAGEN). Insect cell expressed kinases were desalted in MEB (50 mM Tris-HCl, pH 7.3, 50 mM KCl, 10 mM MgCl2, 20 mM β-glycerophosphate, 15 mM EGTA), and other proteins were dialysed into PBS and aliquots snap frozen in liquid nitrogen for storage at −80°C. For two-hybrid screening, a system described previously was used (James et al., 1996). The entire coding region of the human YSK1 cDNA was inserted into the two-hybrid bait vector pFBT9 (a version of pGBT9 [CLONTECH Laboratories, Inc.] modified to encode kanamycin resistance), and this plasmid transformed into the reporter strain PJ69-4A. A human testis cDNA library (CLONTECH Laboratories, Inc.) was transformed into this bait strain and plated on synthetic media lacking leucine, tryptophan, histidine, and adenine with 2% (wt/vol) glucose as the carbon source (QDO). Library plasmids were rescued using the ampicillin resistance marker and retransformed into PJ69-4A together with either pFBT9 or the YSK1 bait plasmid on synthetic medium lacking leucine and tryptophan (−LW), and five independent colonies streaked onto QDO. Those showing strong growth on QDO after 2 d at 30°C were taken as positive clones and the inserts were sequenced. Light colony color is indicative of a strong signal, whereas dark colony color indicates a weaker signal.
GM130 binding assays
1 μg of recombinant His-tagged YSK1 or MST4 expressed in baculovirus-infected Sf9 cells was incubated with 5 μg of either GST-tagged GM13075-271, golgin451-122, or GST alone for 1 h at 4°C in HNTM buffer (50 mM Hepes-KOH, pH 7.2, 200 mM NaCl, 0.5% [vol/vol] Triton X-100, 5 mM MgCl2) in the presence of 15 μl of glutathione-sepharose and 100 μM ATP in a total volume of 300 μl. Beads were washed in 3× 1 ml HNTM and bound protein was eluted directly in SDS-PAGE sample buffer. Samples were Western blotted and probed with either goat anti-YSK1 N19 (Santa Cruz Biotechnology, Inc.) or affinity-purified rabbit anti-YSK1/MST4. Loading controls were analyzed by SDS-PAGE and Coomassie brilliant blue staining.
Kinase assays and substrate identification
For activation experiments with YSK1 or MST4, and comparisons of different wild-type and mutant kinases, 0.8 pmoles of kinase in 7 μl MEB+ (MEB containing 1 mM DTT and 2 mM ATP) were mixed with 5 μl PBS containing the amount of His-tagged GM13075-271 or golgin451-122 indicated in the figures and incubated for 30 min at 37°C. To this was added 8 μl MEB containing 1.5 μg of MBP and 0.1 μl γ-[32P]ATP. After incubation for 60 min at 37°C, reactions were analyzed by SDS-PAGE and autoradiography. For mass spectrometry, 40 pmoles of kinase in 20 μl MEB+ were either mock activated or activated for 2 h at 37°C, 10 μl of reducing sample buffer was added and the reaction mixtures were heated for 5 min at 95°C followed by SDS-PAGE. The corresponding bands were excised and processed for mass spectrometry as described previously (Shevchenko et al., 1996).
Potential substrates for YSK1 and MST4 were screened for using a modified KESTREL protocol (Knebel et al., 2001). HeLa S3 cells were grown in suspension using 1 liter of spinner flask at 37°C and 5% CO2 in DME containing 10% FCS (Invitrogen). Cell pellets were lysed on ice for 30 min in an equal volume of lysis buffer (40 mM Tris-HCl, pH 7.0, 1% [vol/vol] Triton X-100, 2.5 mM EDTA, 15 mM DTT) containing a protease inhibitor cocktail (Roche Diagnostics). The lysate was centrifuged at 112,000 g for 30 min at 4°C. The clarified extract (typically 20 mg/ml) was aliquoted, snap frozen in liquid nitrogen, and stored at −80°C. Before further use extracts were thawed, desalted on Biogel P6-DG (Bio-Rad Laboratories) into KESTREL buffer (40 mM Tris-HCl, pH 7.0, 0.1% [vol/vol] β-mercaptoethanol, 0.1 mM EGTA, 0.1% [vol/vol] Triton X-100), and incubated at 30°C for 20 min to allow dephosphorylation. For the fractionation shown, 10 mg of dephosphorylated cell extract was separated on a Superose-6 HR10/30 column equilibrated in KESTREL buffer containing 140 mM KCl. 1-ml fractions were collected throughout and each one subjected to a kinase assay. For kinase assays to identify substrate proteins, 10 μl of the dephosphorylated extract or column fractions were adjusted to 10 mM MgCl2, 100 μM ATP, 5 μCi γ-[32P]ATP, 2 μg YSK1 or MST4, and incubated at 37°C for 10 min. Assays were analyzed by SDS-PAGE and autoradiography. To identify phosphorylated proteins the autoradiograph was overlaid on to a Coomassie blue stained gel and the corresponding region excised and processed for mass spectrometry (Shevchenko et al., 1996).
Cell culture, RNA interference, and microscopy
HeLa cells were cultured at 37°C and 5% CO2 in DME containing 10% FCS. Cells plated on glass coverslips were transfected 24–36 h after plating using Fugene-6 (Roche), and left to grow for 18–24 h before fixation and processing for immunofluorescence microscopy at RT. RNA interference was performed on HeLa cells transfected using oligofectamine (Invitrogen) with duplex RNA (Dharmacon Research Inc.) for 24 h, coverslips were placed in fresh growth medium for a further 24–112 h, and then the cells were processed for fluorescence microscopy (Elbashir et al., 2001). YSK1 and MST4 were targeted with the sequences AACACATTCGTGGGCACCCCC and AATGGAATACCTGGGCGGTGG, GM130 with the sequence AACCCTGAGACAACCACTTCT, and the lamin-A control was described previously (Elbashir et al., 2001). Cells were fixed for 20 min in 3% (wt/vol) PFA, quenched for 10 min with 50 mM ammonium chloride, and permeabilized with 0.1% (vol/vol) Triton X-100 for 5 min. All solutions were made in PBS, and antibody staining was performed for 60 min using a 1,000-fold dilution of antiserum or purified antibody at a final concentration of 1 μg/ml. Coverslips were mounted in 10% (wt/vol) Moviol 4-88, 1 μg/ml DAPI, 25% (wt/vol) glycerol in PBS. Images were collected using an Axioskop-2 with a 63× Plan Apochromat oil immersion objective of NA 1.4, except for wounding assays, which were imaged with a 40× Plan Neofluar objective of NA 0.75, standard filter sets (Carl Zeiss MicroImaging, Inc.), a 1,300 by 1,030 pixel-cooled CCD camera (model CCD-1300-Y; Princeton Instruments, Inc.) and Metavue software (Visitron Systems). Images were cropped in Adobe Photoshop 7.0, sized, and placed in figures using Adobe Illustrator 10.0 (Adobe Systems Inc.).
Collagen invasion assays
HEK293T cells were grown in DME supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg streptomycin (Invitrogen), and transfected with calcium phosphate. Invasion into collagen type I was performed as described previously (Braecke et al., 2001). Six-well plates were filled with 1.25 ml of neutralized type I collagen (0.09% [wt/vol]; Upstate Biotechnology Inc.) and incubated for at least 1 h at 37°C to allow gelification. The cells were harvested using Moscona buffer and Trypsin/EDTA and seeded on top of the collagen gel. The cultures were incubated for 24 h at 37°C. The depth of migration inside the gel was measured using a phase-contrast microscope controlled by a computer program. Invasive and superficial cells were counted in 12 fields of 0.157 mm2. The invasive index is the percentage of cells invading the gel over the total number of cells.
Wounding assays
HS68 cells were grown at 37°C and 5% CO2 in DME containing 10% FCS on glass coverslips until a confluent monolayer was obtained, typically 3– 4 d after seeding. Wounds in the monolayers were created by scraping a 200 μl tip across the coverslips. Cells along the front of the wound edge were injected (300 hPa injection pressure, 0.2 s, 60 hPa holding pressure) with 200 ng/μl of plasmid DNA using a Femtojet microinjection system (Eppendorf AG) mounted on an Axiovert 25 with 20× LD A-Plan objective of NA 0.30 (Carl Zeiss MicroImaging, Inc.). After injection, the cells were grown for 16 h under normal growth conditions and stained with the appropriate antibodies. The orientations of the centrosome and Golgi apparatus were assessed according to a published method (Etienne-Manneville and Hall, 2001).
Online supplemental material
Fig. S1 illustrates how YSK1/MST4 localize to the Golgi apparatus in HS68 cells. Fig. S2 shows a sequence comparison of YSK1 with MST3 and MST4; conserved features and mutations are also marked. In Fig. S3, a two-hybrid analysis reveals that YSK1 forms homodimers via the COOH terminus but cannot heterodimerize with MST4. In Fig. S4, mass spectrometry of activated and nonactivated YSK1 identifies the T-loop as one site of autophosphorylation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200310061/DC1.
Acknowledgments
We thank Ulrike Grüneberg and Thomas Mayer for useful discussions and comments on the manuscript.
The Max-Planck Society generously supports research in the group of F.A. Barr. V. De Corte is a Postdoctoral Fellow of the Fund for Scientific Research-Flanders (Belgium). J. Gettemans and V. De Corte greatly appreciate support from Marc Mareel and Joël Vandekerckhove, and acknowledge the support of the Belgian Federation against Cancer and Fortis Bank Verzekeringen.
C. Preisinger, B. Short, and V. De Corte contributed equally to this paper.
The online version of this article contains supplemental material.
Abbreviations used in this paper: MBP, myelin basic protein; MST, mammalian Ste20; siRNA, small interfering RNA; YSK1, yeast Sps1/Ste20-related kinase 1.
References
- Barr, F.A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405–413. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., N. Nakamura, and G. Warren. 1998. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17:3258–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton, R., I.M. Palacios, and D. St Johnston. 2002. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev. Cell. 3:659–671. [DOI] [PubMed] [Google Scholar]
- Bialkowska, K., Y. Zaffran, S.C. Meyer, and J.E. Fox. 2003. 14-3-3 zeta mediates integrin-induced activation of Cdc42 and Rac. Platelet glycoprotein Ib-IX regulates integrin-induced signaling by sequestering 14-3-3 zeta. J. Biol. Chem. 278:33342–33350. [DOI] [PubMed] [Google Scholar]
- Bivona, T.G., and M.R. Philips. 2003. Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol. 15:136–142. [DOI] [PubMed] [Google Scholar]
- Bivona, T.G., I. Perez De Castro, I.M. Ahearn, T.M. Grana, V.K. Chiu, P.J. Lockyer, P.J. Cullen, A. Pellicer, A.D. Cox, and M.R. Philips. 2003. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 424:694–698. [DOI] [PubMed] [Google Scholar]
- Braecke, M.E., T. Boterberg, E.A. Bruyneel, and M.M. Mareel. 2001. Collagen invasion assay. Metastasis Research Protocols. Vol. 58. S.A. Brooks and U. Schumacher, editors. Humana Press, Totowa, NJ. 81–89. [DOI] [PubMed]
- Chiu, V.K., T. Bivona, A. Hach, J.B. Sajous, J. Silletti, H. Wiener, R.L. Johnson, II, A.D. Cox, and M.R. Philips. 2002. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4:343–350. [DOI] [PubMed] [Google Scholar]
- Creasy, C.L., and J. Chernoff. 1995. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene. 167:303–306. [DOI] [PubMed] [Google Scholar]
- Creasy, C.L., D.M. Ambrose, and J. Chernoff. 1996. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J. Biol. Chem. 271:21049–21053. [DOI] [PubMed] [Google Scholar]
- Dan, I., N.M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220–230. [DOI] [PubMed] [Google Scholar]
- De Corte, V., E. Bruyneel, C. Boucherie, M. Mareel, J. Vandekerckhove, and J. Gettemans. 2002. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J. 21:6781–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, P.M., H. Kankaanranta, A. Michael, P.J. Barnes, M.A. Giembycz, and M.A. Lindsay. 2002. Caspase-catalyzed cleavage and activation of Mst1 correlates with eosinophil but not neutrophil apoptosis. Blood. 99:3432–3438. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2001. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 106:489–498. [DOI] [PubMed] [Google Scholar]
- Fantl, W.J., A.J. Muslin, A. Kikuchi, J.A. Martin, A.M. MacNicol, R.W. Gross, and L.T. Williams. 1994. Activation of Raf-1 by 14-3-3 proteins. Nature. 371:612–614. [DOI] [PubMed] [Google Scholar]
- Ferri, K.F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255–E263. [DOI] [PubMed] [Google Scholar]
- Freed, E., M. Symons, S.G. Macdonald, F. McCormick, and R. Ruggieri. 1994. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 265:1713–1716. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., and S. Munro. 2003. Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta. 1641:71–85. [DOI] [PubMed] [Google Scholar]
- Glantschnig, H., G.A. Rodan, and A.A. Reszka. 2002. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J. Biol. Chem. 277:42987–42996. [DOI] [PubMed] [Google Scholar]
- Graves, J.D., Y. Gotoh, K.E. Draves, D. Ambrose, D.K. Han, M. Wright, J. Chernoff, E.A. Clark, and E.G. Krebs. 1998. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17:2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D.C., L.G. Rodriguez, and J.L. Guan. 2001. Identification of a novel interaction between integrin beta1 and 14-3-3beta. Oncogene. 20:346–357. [DOI] [PubMed] [Google Scholar]
- Hurd, T.W., S. Fan, C.J. Liu, H.K. Kweon, K. Hakansson, and B. Margolis. 2003. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 13:2082–2090. [DOI] [PubMed] [Google Scholar]
- James, P., J. Halladay, and E.A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel, A., N. Morrice, and P. Cohen. 2001. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 20:4360–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer, A., and G. Dennert. 1984. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J. Immunol. 133:2762–2766. [PubMed] [Google Scholar]
- Kupfer, A., D. Louvard, and S.J. Singer. 1982. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl. Acad. Sci. USA. 79:2603–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, J.D., J. Lucocq, J. Pryde, F.A. Barr, P.G. Woodman, V.J. Allan, and M. Lowe. 2002. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J. Cell Biol. 156:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.K., T. Ohyama, N. Yajima, S. Tsubuki, and S. Yonehara. 2001. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276:19276–19285. [DOI] [PubMed] [Google Scholar]
- Leffers, H., P. Madsen, H.H. Rasmussen, B. Honore, A.H. Andersen, E. Walbum, J. Vandekerckhove, and J.E. Celis. 1993. Molecular cloning and expression of the transformation sensitive epithelial marker stratifin. A member of a protein family that has been involved in the protein kinase C signalling pathway. J. Mol. Biol. 231:982–998. [DOI] [PubMed] [Google Scholar]
- Lin, J.L., H.C. Chen, H.I. Fang, D. Robinson, H.J. Kung, and H.M. Shih. 2001. MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene. 20:6559–6569. [DOI] [PubMed] [Google Scholar]
- Lin, Y., A. Khokhlatchev, D. Figeys, and J. Avruch. 2002. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J. Biol. Chem. 277:47991–48001. [DOI] [PubMed] [Google Scholar]
- O'Kelly, I., M.H. Butler, N. Zilberberg, and S.A. Goldstein. 2002. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 111:577–588. [DOI] [PubMed] [Google Scholar]
- Osada, S., M. Izawa, R. Saito, K. Mizuno, A. Suzuki, S. Hirai, and S. Ohno. 1997. YSK1, a novel mammalian protein kinase structurally related to Ste20 and SPS1, but is not involved in the known MAPK pathways. Oncogene. 14:2047–2057. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. 2001. Constructing a Golgi complex. J. Cell Biol. 155:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo, C.M., J.V. Bonventre, A. Molnar, J. Kyriakis, and T. Force. 1996. Activation of a human Ste20-like kinase by oxidant stress defines a novel stress response pathway. EMBO J. 15:4537–4546. [PMC free article] [PubMed] [Google Scholar]
- Qian, Z., C. Lin, R. Espinosa, M. LeBeau, and M.R. Rosner. 2001. Cloning and characterization of MST4, a novel Ste20-like kinase. J. Biol. Chem. 276:22439–22445. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, J.T. Parsons, and A.R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- Rios, R.M., and M. Bornens. 2003. The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 15:60–66. [DOI] [PubMed] [Google Scholar]
- Russo, A.A., P.D. Jeffrey, and N.P. Pavletich. 1996. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat. Struct. Biol. 3:696–700. [DOI] [PubMed] [Google Scholar]
- Santoro, M.M., G. Gaudino, and P.C. Marchisio. 2003. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell. 5:257–271. [DOI] [PubMed] [Google Scholar]
- Schinkmann, K., and J. Blenis. 1997. Cloning and characterization of a human STE20-like protein kinase with unusual cofactor requirements. J. Biol. Chem. 272:28695–28703. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850–858. [DOI] [PubMed] [Google Scholar]
- Short, B., C. Preisinger, R. Korner, R. Kopajtich, O. Byron, and F.A. Barr. 2001. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol. 155:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., R. Watson, M.E. Giannakou, M. Clarke, G. Warren, and F.A. Barr. 1999. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 18:4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, V., W. Luo, D. Qian, I. Lee, B. Jallal, and M. Gishizky. 2003. The Ste20 kinase MST4 plays a role in prostate cancer progression. Cancer Res. 63:3356–3363. [PubMed] [Google Scholar]
- Sutterlin, C., P. Hsu, A. Mallabiabarrena, and V. Malhotra. 2002. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 109:359–369. [DOI] [PubMed] [Google Scholar]
- Teis, D., W. Wunderlich, and L.A. Huber. 2002. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell. 3:803–814. [DOI] [PubMed] [Google Scholar]
- Tzivion, G., Y.H. Shen, and J. Zhu. 2001. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene. 20:6331–6338. [DOI] [PubMed] [Google Scholar]
- Ura, S., N. Masuyama, J.D. Graves, and Y. Gotoh. 2001. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc. Natl. Acad. Sci. USA. 98:10148–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich, W., I. Fialka, D. Teis, A. Alpi, A. Pfeifer, R.G. Parton, F. Lottspeich, and L.A. Huber. 2001. A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J. Cell Biol. 152:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H., K. Michelsen, and B. Schwappach. 2003. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr. Biol. 13:638–646. [DOI] [PubMed] [Google Scholar]