Abstract
TCF and SOX proteins belong to the high mobility group box transcription factor family. Whereas TCFs, the transcriptional effectors of the Wnt pathway, have been widely implicated in the development, homeostasis and disease of the intestine epithelium, little is known about the function of the SOX proteins in this tissue. Here, we identified SOX9 in a SOX expression screening in the mouse fetal intestine. We report that the SOX9 protein is expressed in the intestinal epithelium in a pattern characteristic of Wnt targets. We provide in vitro and in vivo evidence that a bipartite β-catenin/TCF4 transcription factor, the effector of the Wnt signaling pathway, is required for SOX9 expression in epithelial cells. Finally, in colon epithelium-derived cells, SOX9 transcriptionally represses the CDX2 and MUC2 genes, normally expressed in the mature villus cells of the intestinal epithelium, and may therefore contribute to the Wnt-dependent maintenance of a progenitor cell phenotype.
Keywords: SOX9; Wnt; CDX2; intestinal epithelium; differentiation
Introduction
Proliferation, differentiation, and migration of cells must be coordinately regulated to maintain the integrity of the continuously renewing intestine epithelium. This epithelium consists of a proliferative compartment, the crypt of Lieberkühn, and a differentiated, functional compartment, consisting of the villus in the small intestine and the luminal surface in the colon. Multipotent stem cells, located near the bottom of the crypts, generate new cells which migrate to the villus while differentiating into enterocyte, goblet, and enteroendocrine cells. In the small intestine, a fourth cell type, the Paneth cells, migrates downward and settles at the bottom of the crypts as terminally differentiated cells (Potten and Moeffler, 1990; Stappenbeck et al., 1998).
Extracellular signals, including Wnt molecules, are required for this organization of the intestine epithelium. Upon binding of a secreted Wnt molecule to its corresponding Frizzled receptor, the canonical Wnt pathway is activated, resulting in the stabilization and accumulation of β-catenin in the nucleus. Interaction with β-catenin activates the TCF/LEF transcription factors, resulting in transcription of target genes (Brantjes et al., 2002). In the intestine, the Wnt signaling pathway has been implicated in the regulation of the proliferation/differentiation balance (van de Wetering et al., 2002). Consistent with this, no proliferation can be detected, and all epithelial cells appear differentiated, in the intestine of mice null for TCF4, the main Wnt pathway transcription factor in the intestinal epithelium (Korinek et al., 1998). The Wnt pathway also regulates the expression of the Eph/Ephrin surface molecules, responsible for the ordered positioning of epithelial cells along the crypt-villus axis (Batlle et al., 2002). In addition, Wnt signals control the differentiation of the secretory cell lineage of the epithelium, because overexpression of the Wnt-pathway inhibitor Dickkopf1 blocks differentiation of the Paneth, goblet, and enteroendocrine cell types (Pinto et al., 2003). Finally, mutations in components of the Wnt pathway, including the tumor suppressor APC and the multifunctional β-catenin protein, are found in the vast majority of colon cancers (Morin et al., 1997). Such mutations result in stabilization of β-catenin, which then continuously interacts with TCF4, leading to constitutive activation of target genes (Korinek et al., 1997). Moreover, targeted mutations of APC or β-catenin are sufficient to initiate tumorigenesis in the mouse (Fodde et al., 1994; Shibata et al., 1997; Harada et al., 1999), highlighting the importance of the Wnt pathway in the development of cancer.
The diversity of cellular processes involving Wnt signals suggests branching of the pathway to permit specific cell responses. However, evidence for such branch-determining factors, each mediating part of the Wnt response downstream of the TCF/LEF transcription factors, is presently lacking.
The TCF/LEF transcription factors share a common high mobility group DNA binding domain with the SOX transcription factors. Similarly to TCFs, SOX proteins have been widely implicated in the establishment of cell multipotency (Avilion et al., 2003; Kim et al., 2003), cell commitment (Mori-Akiyama et al., 2003; Stolt et al., 2003), and differentiation (Kamachi et al., 2000; Stolt et al., 2002). Here, we screened the intestine epithelium for expressed SOX genes to investigate the possibility of a functional link with the related Tcf factors. This unexpectedly led to the identification of SOX9.
SOX9 function is best known for its essential roles in chondrogenesis (Foster et al., 1994; Wagner et al., 1994; Wright et al., 1995; Bi et al., 1999, 2001) and the development of the male gonad (Foster et al., 1994; Wagner et al., 1994; Kent et al., 1996; Südbeck et al., 1996). SOX9 is also involved in the development of the neural crest (Spokony et al., 2002) and of the spinal cord glial cells (Stolt et al., 2003). Strikingly, SOX9 controls the transcription of target genes which are specific for each process: in chondrogenesis, it regulates the cartilage specific genes Col2a1 (Bell et al., 1997; Lefebvre et al., 1997; Bi et al., 1999), Col11a2 (Bridgewater et al., 1998), Aggrecan (Sekiya et al., 2000), and campomelic dysplasia (CD)–RAP (Xie et al., 1999). During male gonad development, it controls the expression of the anti-Müllerrian hormone gene (de Santa Barbara et al., 1998). In the neural crest, the expression of the transcription factor Slug depends on SOX9 (Spokony et al., 2002), whereas SOX9 target genes remain to be identified in the spinal cord.
Here, we report the expression of SOX9 in an additional structure, the intestinal epithelium. We show that this SOX9 expression depends on the activity of the Wnt pathway and that SOX9 is involved in repression of differentiation genes, including the homeobox gene CDX-2 and the MUC-2 gene, encoding the main intestinal mucin, a major constituent of the mucous gel covering the digestive tract surface.
Results
Screening for SOX genes expressed in the mouse intestine
RT-PCR was performed with mouse neonate intestine, using degenerate oligonucleotide primers (Cremazy et al., 1998). The amplified products were cloned and sequenced, leading to the identification of SOX3, SOX4, SOX5, SOX7, SOX9, and SOX18. Northern blot analysis revealed that SOX9 mRNA was strongly expressed not only during development, but also in the adult intestine, and represented the prominent gene among the identified set of expressed SOX genes (unpublished data). In consequence, further studies were focused on SOX9.
SOX9 is expressed in the proliferative compartment of the intestinal epithelium
In the mouse intestine, the SOX9 protein is robustly expressed from the duodenum to the distal colon (Fig. 1 a). SOX9 expression is restricted to the nuclei of the immature cells constituting the proliferative compartment of the epithelium (Fig. 1, b, d, and f) and almost perfectly overlaps with that of Ki-67, a proliferation marker (Fig. 1, c, e, and g). In the fetal intestine, crypts are not developed and the proliferative cells are found in the intervillus region (Fig. 1 c), whereas in the adult intestine, proliferation is restricted to the lower half of the crypts of Lieberkühn (Fig. 1, e and g). In addition to the proliferative compartment, SOX9 expression was observed in the nuclei of the Paneth cells, located at the bottom of the crypts of Lieberkühn of the adult small intestine (Fig. 1 d). As these cells are postmitotic and fully differentiated, they do not express the Ki-67 antigen (Fig. 1 e). In addition to the SOX9 nuclear staining, a weak cytoplasmic staining is visible in the same sections. As the subcellular localization of SOX9 is regulated in some structures, such as the gonad (Smith and Koopman, 2004), we tested whether the intestinal epithelium cytoplasmic staining was specific, using Western blot analysis of nuclear and cytoplasmic subcellular fractions of mouse intestine epithelial cells and of human cultured HT29-16E cells. This revealed that SOX9 is present only in the nuclear fraction (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200311021/DC1), thus demonstrating that the faint cytoplasmic signals obtained with the anti-SOX9 antibody throughout the intestine epithelium (Fig. 1, d and f) is nonspecific.
Figure 1.
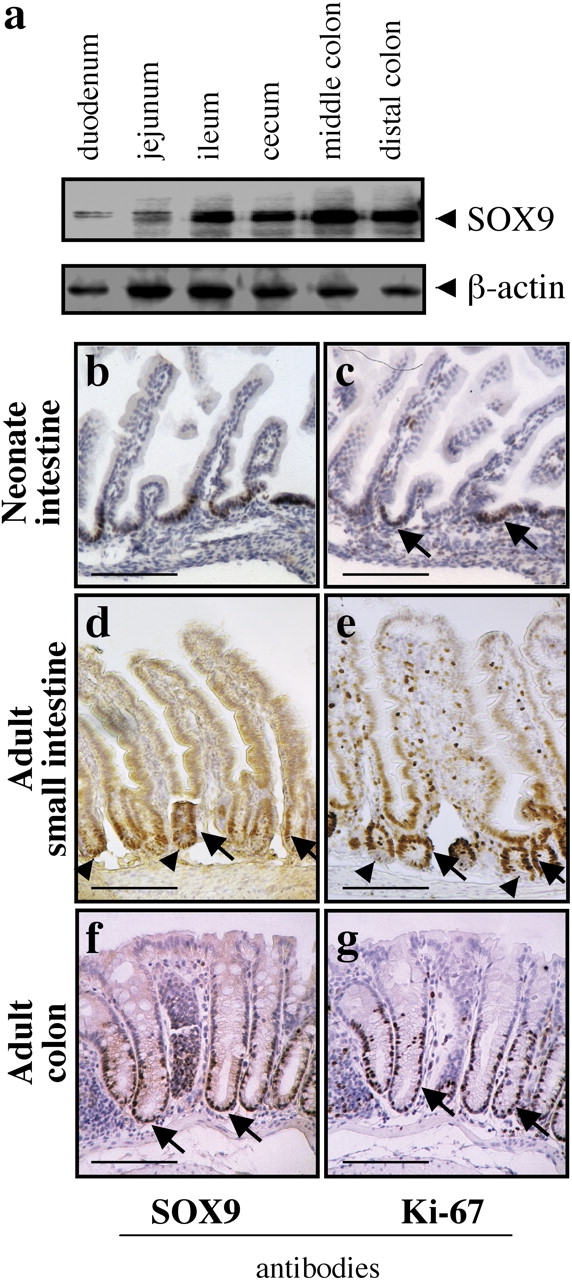
SOX9 is expressed in the proliferative compartment of the epithelium. (a) Western blot with mouse tissue samples representative of the overall length of the intestine (5 μg protein/lane). Antibodies used are indicated. (b) Immunohistochemistry with neonate and adult intestine samples. In the neonate mouse, the architecture of the intestine is not complete, crypts are not formed and the proliferative, Ki-67 positive, compartment is restricted to the intervillus region (top). In the adult small intestine, crypts are invaginated between the villi in the underlying mesenchyme (middle). In the adult colon, crypts contain more cells, villi are absent and the differentiated compartment constitutes the flat luminal surface of the epithelium. Cells constituting the proliferative compartment are positive for both SOX9 and Ki-67 (arrows). Arrowheads point at Paneth cells, positive for SOX9 and negative for Ki-67. Bars: (b–e) 50 μm; (f and g) 40 μm.
The SOX9 expression pattern strikingly matches the domain of the epithelial cells stimulated by Wnt molecules, where nuclear β-catenin can interact with TCF4 to activate transcription (van de Wetering et al., 2002). Although the Wnt pathway controls multiple aspects of intestine epithelium physiology, little is known about downstream transcription factors. This prompted us to test whether the SOX9 transcription factor is regulated by the Wnt pathway and constitutes one of its downstream effectors.
SOX9 expression in human colon carcinomas
As SOX9 is expressed in cells in which the Wnt signaling pathway is active, we anticipated strong SOX9 expression in cancer cells where there is constitutive activation of the Wnt pathway. We first tested SOX9 expression in human colon cancer–derived cell lines containing activating mutations of components of the Wnt pathway, which result in constitutive expression of β-catenin/TCF4 target genes (Korinek et al., 1997). In all the colon cancer cell lines we tested (DLD-1, LS174T, SW480, TC-7, and HT29), SOX9 mRNA (not depicted) and protein were strongly expressed (Fig. 2 a). In contrast, SOX9 expression was hardly detectable in HEK293 cells, a nonintestinal epithelial cell line.
Figure 2.
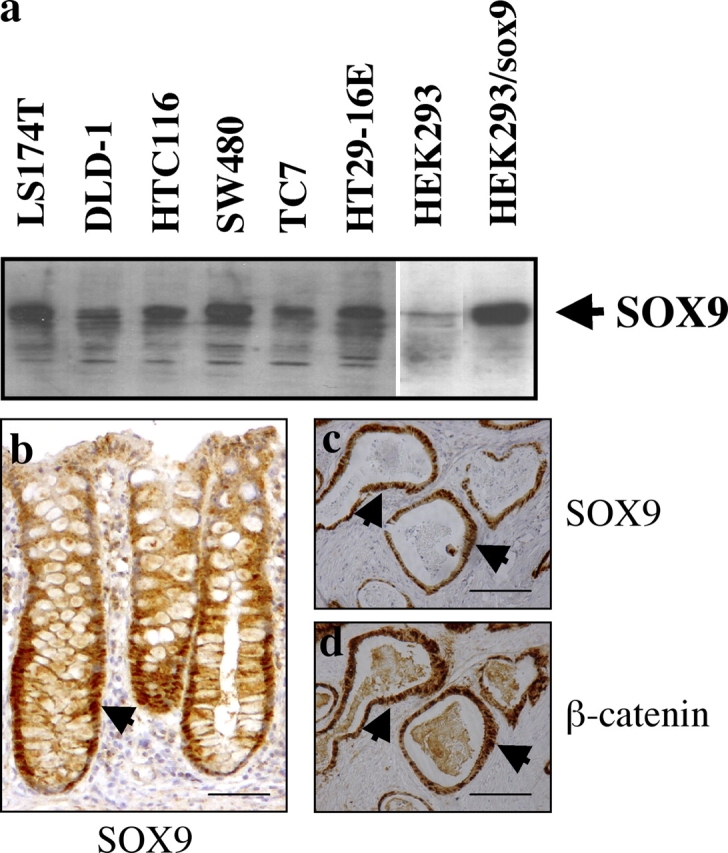
SOX9 is expressed in various colon carcinoma cell lines. (a) Western blot with cell extracts from a panel of human colon carcinoma cell lines (1 μg/lane) and the nonintestinal epithelial cell line HEK293. Endogenous SOX9 protein was detected in all colon carcinoma–derived cell lines but not in HEK293. HEK293 cells, transiently transfected with SOX9, were used as a positive control. (b–d). Immunohistochemistry with sections of human colon. (b) SOX9 staining in healthy colon epithelium; (c) SOX9 staining in adenocarcinomatous colon; (d) β-catenin staining in adenocarcinomatous colon. Arrows point at cells expressing the indicated proteins. Bars: (b) 50 μm; (c and d) 80 μm.
In the healthy human colon epithelium, the expression of SOX9 is restricted to the proliferative, lower half of the crypt (Fig. 2 b). Analysis of sections from human colorectal adenocarcinomas revealed that SOX9 is strongly expressed in specific regions of the lesion (Fig. 2 c). We then asked whether this pattern corresponded to cells where there is an active β-catenin–TCF4 complex, driving aberrant transcription of target genes. Such cells can be identified because they contain cytoplasmic and nuclear β-catenin (Korinek et al., 1997). When we stained serial sections for β-catenin, it was clear that the same structures that strongly expressed SOX9 also contain cytoplasmic and nuclear β-catenin (Fig. 2, c and d). This result again correlates SOX9 expression with activity of the β-catenin–TCF4 complex and suggests that SOX9 expression might be driven by this complex.
SOX9 expression is controlled by the Wnt pathway in human colon carcinoma cells
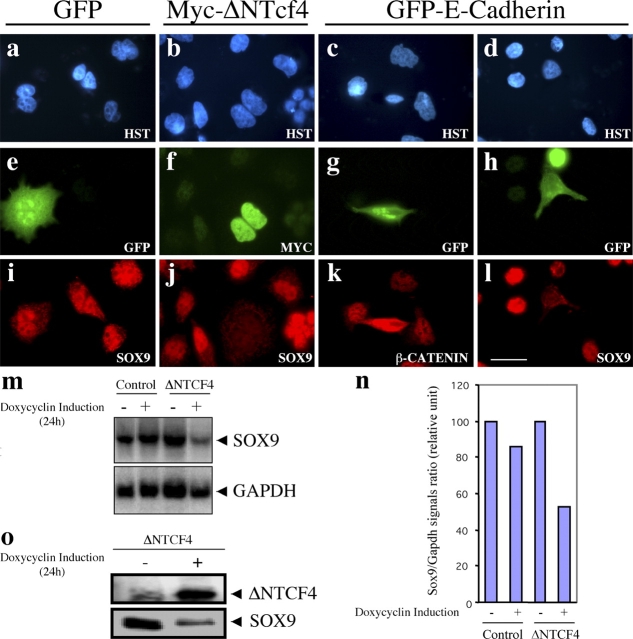
To test this hypothesis further, we overexpressed a dominant negative TCF4 mutant (ΔNTCF4) in the LS174T human colon carcinoma cell line. This NH2-terminally deleted mutant lacks the β-catenin-interaction domain and has been shown to interfere with the activity of the endogenous, constitutively active, β-catenin–TCF4 complex present in these cells (van de Wetering et al., 2002). Cells were transfected with either the ΔNTCF4 or a mock GFP expression construct and the endogenous SOX9 protein expression was monitored by immunofluorescence. As expected, overexpression of GFP did not affect the SOX9 nuclear staining in transfected LS174T cells, compared with adjacent nontransfected cells (Fig. 3, a, e, and i). In contrast, overexpression of ΔNTCF4 always resulted in undetectable endogenous nuclear SOX9. A typical result in shown in Fig. 3 (b, f, and j). This indicates that the β-catenin–TCF4 complex activity is required for SOX9 expression.
Figure 3.
The Wnt–β-catenin–Tcf pathway is required for SOX9 expression in colon epithelial cells. (a–l) Immunofluorescence in transiently transfected LS174T colon carcinoma cells, fixed 36 h after transfection. (a–d) Hoechst nuclear staining. (e) GFP. (f) Detection of exogenous Myc-tagged ΔNTCF4 in transfected cells with an anti-Myc antibody. (g and h) GFP shows the perinuclear accumulation of the GFP–cyt-E-cadherin fusion in transfected cells. (i) Identical SOX9 staining in GFP-transfected and nontransfected cells. (j and l) SOX9 staining is absent in cells transfected with the Wnt pathway interfering constructs ΔNTCF4 and GFP–cyt-E-cadherin. (k) β-Catenin is efficiently sequestered by GFP–cyt-E-cadherin, as shown by their identical distribution in transfected cells. The type of staining is indicated on each panel. (m) Northern blot. SOX9 mRNA detection in LS174T colon carcinoma cells stably transfected with an inducible ΔNTCF4 construct. Time of doxycyclin induction is indicated for both the control and the ΔNTCF4 expressing cell line. A GAPDH probe was used as a loading control. (n) Phosphorimager quantification of the Northern blot signals. (o) Western blot. Detection of the SOX9 protein in the ΔNTCF4 stably transfected LS174T cells with or without doxycycline induction. Bar, 15 μm.
To rule out the possibility that the overexpressed ΔNTCF4 protein might interfere with DNA binding of non-TCF proteins, we overexpressed the cytoplasmic domain of E-cadherin (cyt-E-cadherin), which has been reported to sequester signaling-competent β-catenin, thus abrogating the β-catenin–TCF4 transcriptional activity (Gottardi et al., 2001; Simcha et al., 2001). As expected, overexpression of a GFP–cyt-E-cadherin construct in LS174T cells resulted in a strong decrease of nuclear β-catenin staining, which then colocalized with the GFP–cyt-E-cadherin fusion in a perinuclear pattern (Fig. 3, c, g, and k). Accordingly, SOX9 expression was abolished in the nucleus of all the GFP–cyt-E-cadherin transfected cells, as exemplified in Fig. 3 (d, h, and l). Hence, an active β-catenin–TCF4 complex is required for expression of the SOX9 protein in colon carcinoma cells.
We found that this regulation of SOX9 occurs at the RNA level using LS174T human colon carcinoma cells stably transfected with an inducible ΔNTCF4 construct (van de Wetering et al., 2002). As noted above, LS174T cells express high levels of endogenous SOX9 mRNA and protein. This mRNA expression level was strongly reduced after expression of ΔNTCF4 (Fig. 3 m). Phosphorimager quantification of SOX9 hybridization signals, after normalization with the GAPDH hybridization signals, indicated that SOX9 expression is down-regulated by ∼50% (Fig. 3 n). The expression of the SOX9 protein was also significantly decreased after induction of the expression of the ΔNTCF4 construct (Fig. 3 o). The quantitative difference in the SOX9 repression, using transient versus stable transfection of Wnt-interfering constructs, likely reflects the levels of expression of these constructs and their dominant negative mode of action.
Repression of SOX9 occurs rapidly upon interference with the β-catenin–TCF4 activity
We wanted to determine whether the repression of SOX9 is an early consequence of the arrest of Wnt signaling, or if it is a more long-term, indirect consequence of the global alteration of cell physiology. LS174T cells were transfected with either GFP or GFP–cyt-E-cadherin in order to interfere with Wnt signaling, and the SOX9 protein was visualized by immunofluorescence after 0, 8, 16, 24, 32, 40, and 48 h. Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200311021/DC1, shows that as soon as the GFP–cyt-E-cadherin construct became detectable (8 h), it resulted in a decrease of SOX9 expression in most of the transfected cells. After 16 h, all the cells expressing GFP–cyt-E-cadherin had hardly detectable nuclear SOX9. In contrast, transfection of GFP alone did not alter the SOX9 staining, even after 48 h. Thus, interference with the activity of the β-catenin–TCF4 complex results in a rapid decrease of the expressed SOX9 protein. We conclude that SOX9 is a physiological target of Wnt signaling.
Nuclear SOX9 expression is lacking in the intestine epithelium of TCF4 null mice
The Wnt–β-catenin–TCF4 pathway is necessary for the maintenance of a functional crypt-villus axis in the mouse intestinal epithelium (Pinto et al., 2003). The gene knockout of its transcriptional effector, TCF4, leads to the loss of the proliferative compartment of the epithelium. As a consequence, TCF4 null mice die perinatally (Korinek et al., 1998). As the Wnt signaling pathway is essential for SOX9 expression in cultured cells, we expected that SOX9 expression would be disrupted in the intestinal epithelium of TCF4 null mice. Indeed, when we examined the intestine of neonate TCF4 knockout mice, the SOX9 protein was undetectable in the nucleus of epithelial cells, in contrast to TCF4 heterozygous littermates where SOX9 was correctly expressed in the proliferative, Ki-67 positive, intervillus region of the intestine epithelium (Fig. 4). Therefore, TCF4-mediated Wnt signaling is required for appropriate SOX9 expression in the mouse intestine epithelium.
Figure 4.
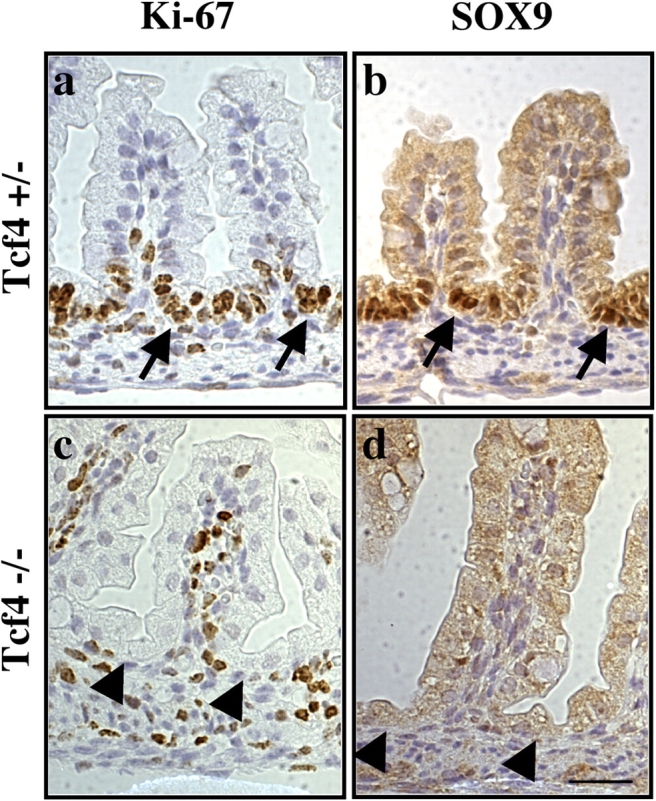
Absence of the SOX9 protein in the proliferative compartment of TCF4-deficient neonate mouse. (a and b) TCF4 heterozygous mice are healthy and fertile. The actively proliferating intervillus cells are Ki-67 positive and express abundant nuclear SOX9 protein (arrows). (c and d) In TCF4 null mice, no Ki-67 positive proliferating cells can be found in the intervillus region (arrowheads), and SOX9 expression is completely abrogated. Bar, 5 μm.
SOX9 represses CDX2 and MUC2, two genes associated with differentiation
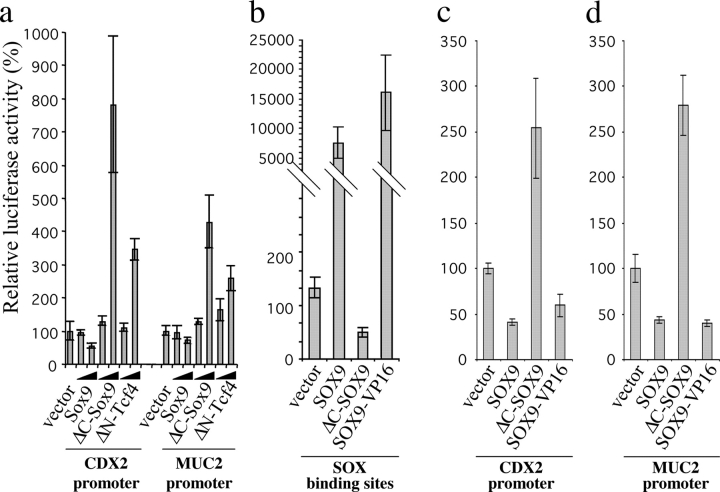
As SOX9 encodes a transcription factor, and its expression is regulated by the Wnt–β-catenin–TCF4 pathway, we hypothesized that it might mediate all or part of the Wnt response in intestine epithelial cells. To test this, we transfected human LS174T cells, which contain an endogenous constitutively active β-catenin–TCF4 complex, and therefore endogenous SOX9, with wild-type, antisense, or dominant-negative SOX9 (ΔC-SOX9) constructs and GFP. For these experiments, cells were cotransfected with the plasmid pMACS-Kk, which allowed efficient selection of transfected cells. We then screened by RT-PCR a panel of previously reported Wnt–β-catenin–TCF4-regulated genes (van de Wetering et al., 2002). This led to the identification of both SOX9-dependent and SOX9-independent Wnt target genes (Fig. 5), indicating branching of the Wnt response, which might explain how the Wnt pathway can exert a wide spectrum of cellular effects. CDX1/-2 genes, both homologues of the Drosophila homeobox Caudal gene (Duprey et al., 1988; Suh et al., 1994), exemplified such branching. In the intestinal epithelium, the CDX1 transcription factor is mostly expressed in the crypts whereas CDX2 is mostly active on the villi (Subramanian et al., 1998; Silberg et al., 2000). Both genes are thought to be important for the antero-posterior patterning of the intestinal epithelium and in defining patterns of proliferation and differentiation along the crypt-villus axis (Silberg et al., 2000). CDX1/-2 are, respectively, positively and negatively regulated by the Wnt pathway. Indeed, coculture experiments, as well as loss of CDX1 expression in the gut of TCF4-deficient mice, led to the conclusion that CDX1 is a target of the Wnt–β-catenin–TCF4 pathway (Lickert et al., 2000; Ikeya and Takada, 2001). On the other hand, APC up-regulates CDX2 (da Costa et al., 1999), although the signaling pathway downstream of APC has not yet been elucidated. In our experiment, CDX1 expression was not altered by gain/loss of SOX9 function in LS174T cells, in contrast with CDX2 expression which was down-regulated when SOX9 was overexpressed. Conversely, CDX2 expression increased when we overexpressed the antisense or the ΔC-SOX9 constructs (Fig. 5). We then monitored CDX2 promoter activity using a human CDX2 promoter-luciferase reporter construct, cotransfected together with the various SOX9 constructs or with the ΔNTCF4 construct. Again, SOX9 overexpression resulted in a decrease of the promoter activity, whereas interfering with the endogenous SOX9 function, either directly with ΔC-SOX9 or SOX9 antisense constructs, or indirectly with the ΔNTCF4 construct, strongly increased CDX2 promoter activity (Fig. 6 a). We conclude that SOX9 is involved in the regulation of CDX2 but not CDX1, which is consistent with previous reports showing that CDX1 is a direct transcriptional target of the β-catenin–TCF4 complex (Lickert et al., 2000). c-Myc has also been described as a direct β-catenin–TCF4 target (He et al., 1998), but its expression level is not altered neither by SOX9 gain/loss of function (Fig. 5). Several SOX9-dependent genes are down-regulated in cells overexpressing SOX9 and up-regulated in cells where SOX9 function is challenged. In addition to CDX2 and its target gene MUC2 (Yamamoto et al., 2003; Figs. 5 and 6 a), there was slight but reproducible SOX9-dependent down-regulation of genes encoding the differentiation markers Fabp-i and Galectin-4 (not depicted). This experiment indicates that SOX9, which is expressed in the crypt proliferative compartment of the epithelium, represses genes expressed in the villus compartment, encoding markers of differentiated cells. Indeed, the constitutive Wnt–β-catenin–TCF4-mediated repression of these genes is abrogated when SOX9 function is disrupted, using either antisense or dominant-negative constructs. Hence, SOX9 function might contribute to the Wnt-dependent maintenance of an undifferentiated progenitor phenotype in the intestinal epithelium by repressing differentiation genes such as CDX2 and MUC2. TCF4 gene expression, in turn, was not modulated by SOX9 (Fig. 5).
Figure 5.
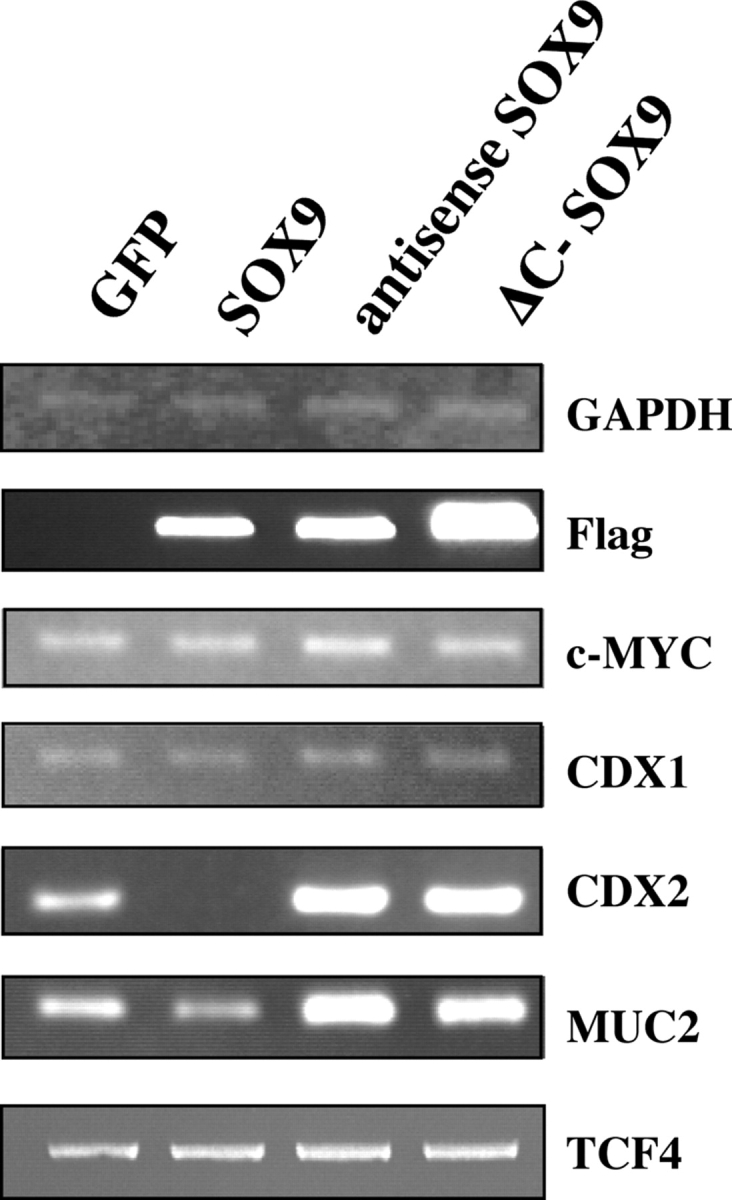
SOX9 represses intestine epithelium differentiation genes. LS174T cells were transiently transfected with GFP, Flag-SOX9 fusion, Flag-SOX9 antisense, or Flag-COOH–truncated SOX9 (ΔC-Sox9) constructs, and the expression of the indicated putative target genes was monitored by RT-PCR. The primers used are listed in Table S1.
Figure 6.
SOX9 negatively regulates the promoters of the CDX2 and MUC2 genes. LS174T cells were cotransfected, in triplicate, with the indicated expression constructs (10 and 100 ng) together with CDX2-luciferase, MUC2-luciferase and SOX-luciferase reporters (500 ng) and relative luciferase activities were measured 36 h after transfection. The reporter activity in mock-transfected cells was arbitrarily set to 100. (a) CDX2 and MUC2 promoters regulation by SOX9; (b–d) SOX9-VP16 recapitulates the transcription regulation properties of the wild-type SOX9 on SOX-luciferase (b), CDX2-luciferase (c), and MUC2-luciferase constructs (d). The histograms represent mean values of triplicate experiments and SDs are shown with error bars.
To confirm these data, we constructed a cell line derived from the human colon adenocarcinoma cell line HT29-16E, in which we stably introduced a doxycycline-inducible flag-SOX9 construct. Doxycycline induction of Flag-SOX9 caused the decline of endogenous CDX2 and MUC2 gene expression (unpublished data), confirming the data obtained above in transiently transfected LS174T cells. To further address the role of SOX9 in a living animal context, we induced in vivo tumor formation by grafting HT29-16E-SOX9 cells subcutaneously in nude mice (Fig. 7). In nondoxycycline-treated mice (n = 3), the resulting tumors, grown over 11 wk, were anatomically heterogeneous, with mucinous tubular structures (Fig. 7 a) together with poorly differentiated and poorly organized structures (Fig. 7 d). In these tumors, we failed to detect Flag-SOX9. Nuclear CDX2 as well as MUC2 production were found in the tubular structures, whereas these proteins were hardly detected in the poorly organized structures (Fig. 7, b, c, e, and f). Identical pictures were obtained after MUC2 antibody staining and alcian blue staining of mucins (unpublished data). In animals induced with doxycycline for 10 wk (n = 3), the secreting tubular structures were not readily found (Fig. 7, a, g, and j). Flag-SOX9 was detected in some areas of the tumor, and in these areas, there was no expression of CDX2 nor of MUC2 (Fig. 7, g–l). Weak CDX2 staining could, however, occasionally be observed in doxycycline-treated mice but only in structures where the SOX9 transgene expression could not be detected. We conclude from these data that SOX9 inhibits CDX2 and MUC2 protein expression both in intestinal adenocarcinoma cells cultures in vitro and in living animals grafted with tumor cells.
Figure 7.
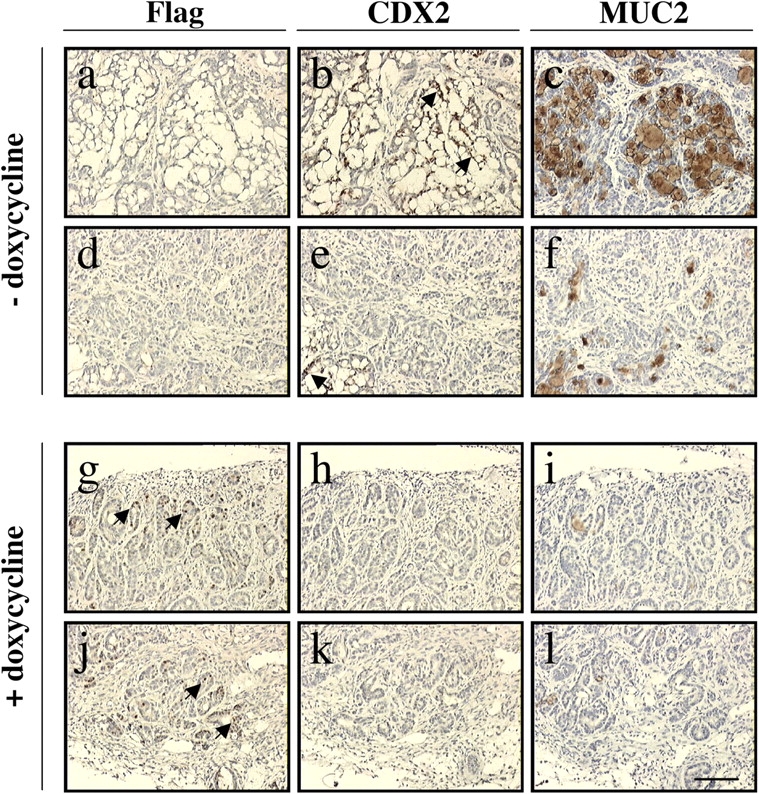
SOX9 represses CDX2 and MUC2 in xenograft-derived tumors. Sections from tumors resulting from xenografts of HT29-16E-SOX9 cells, stained with antibodies against the Flag tag, the CDX2 and MUC2 proteins. Antibodies used for staining are indicated. The expression of the Flag-SOX9 construct is detected uniquely in the nucleus of tumors from doxycycline-fed mice. Bar, 130 μm.
SOX9-mediated repression of CDX2 and MUC2 involves intermediate activation of a repressor protein
Although SOX9 is known as a transcriptional activator, it represses the expression of the target genes identified in this work. In consequence, SOX9 may either act as a repressor in the intestinal epithelium, or activate transcription of an intermediate transcription repressor. To discriminate between these two possibilities, we analyzed the luciferase activity driven by the CDX2 and MUC2 promoters in LS174T cells, transfected with wild-type SOX9, ΔC-SOX9, and a dominant-activating SOX9 protein in which the transcription activation domain of SOX9 has been replaced with that of the VP-16 viral transactivator, making this chimeric protein a forced transcription activator. Fig. 6 (b–d) shows that the SOX9-VP16 fusion protein mimics all the aspects of SOX9 transcription regulation properties that we analyzed: it strongly activates transcription through a synthetic enhancer containing multimerized SOX binding sites and represses the promoters of the CDX2 and MUC2 differentiation genes to a similar extent as the wild-type SOX9. This implies that the SOX9-mediated repression of CDX2 and MUC2 involves prior transcriptional activation of an as-yet unidentified repressor gene.
Discussion
SOX9 was first described as the gene responsible, when heterozygously mutated, for the skeletal malformation syndrome, CD, which is sometimes associated with XY sex reversal (Foster et al., 1994; Wagner et al., 1994). Of note, no intestinal epithelium defect has been reported to date in CD patients, indicating that SOX9 haploinsufficiency may not be critical in this tissue. More recently, SOX9 was found to be essential for the development of the neural crests and the central nervous system (Xie et al., 1999; Spokony et al., 2002; Mori-Akiyama et al., 2003). Here, we report that SOX9 is also expressed in the intestinal epithelium. In this highly organized structure, the SOX9 protein is only expressed in the lower third of the crypts of Lieberkühn, in both the small intestine and colon. However, whereas the lower part of the colonic crypts constitute the proliferative compartment of the epithelium, the situation is different in the small intestine. Paneth cells, which occupy the bottom of the small intestine crypts, beneath the cells constituting the proliferative compartment, are postmitotic, fully differentiated cells (Cheng, 1974). Thus, SOX9 is expressed in proliferative cells, which probably include the stem cells, as well as in the terminally differentiated Paneth cells. This suggests that SOX9 expression is not restricted either to a single cell type or to the proliferation state. Rather, it corresponds to a location, the bottom of the crypt, which is also considered to be the cellular “niche” of the epithelium, where cells contain nuclear β-catenin in response to Wnt signaling (Batlle et al., 2002; van de Wetering et al., 2002).
The nature of the β-catenin–TCF4-dependent regulation of SOX9
In addition to the similar pattern of SOX9 and nuclear β-catenin expression along the crypt-villus axis, we demonstrate that SOX9 expression in the intestinal epithelium depends on the canonical Wnt–β-catenin–TCF pathway. In cultured human colon carcinoma cell lines, SOX9 expression was strictly dependent on the activity of the TCF4–β-catenin complex. In vivo, SOX9 expression could no longer be detected in the intestinal epithelium of TCF4-null mice, whereas it was strongly expressed in human colon carcinomas, which contain a constitutive β-catenin–TCF4 complex. Given the striking overlap between SOX9 expressing cells and Wnt-stimulated cells on the one hand, and the experimental evidence that SOX9 expression depends on TCF4–β-catenin transcriptional activity on the other, we conclude that SOX9 constitutes a physiological target of the Wnt–β-catenin–TCF4 pathway in the intestinal epithelium. This regulation of SOX9 expression by the Wnt pathway might or might not depend on a direct interaction of TCF with the SOX9 promoter. In an attempt to clarify this, we cloned a human genomic DNA fragment, spanning 2.6 kb upstream of the SOX9 transcription start site, into a luciferase reporter plasmid. This fragment did not contain a canonical TCF binding sequence (ATCAAAGG), but contained several noncanonical TCF binding sites, such as described, for instance, in the Siamois (Brannon et al., 1997) and Cyclin D1 (Tetsu and McCormick, 1999) promoters. However, the basal luciferase activity driven by this 2.6-kb SOX9 promoter fragment in LS174T colon carcinoma cells was not modulated by cotransfected ΔNTCF4 (unpublished data). We conclude that our construct does not contain functional TCF binding sites, which still does not exclude the possibility that TCF transcription factors might directly regulate the SOX9 promoter. Indeed, although this promoter has been poorly characterized, scattered regulatory enhancer elements have been detected up to 1 megabase upstream of the human SOX9 transcriptional unit (Wunderle et al., 1998; Bishop et al., 2000). We performed in silico screening of the genomic region situated from 200 kb upstream to 100 kb downstream of the SOX9 transcriptional unit for putative canonical TCF binding sites. Although several putative sites were identified, the closest mapped at 15 kb upstream of SOX9 (unpublished data). This means that a direct regulation of SOX9 by the Wnt effector TCF4 is possible but that the formal identification of a Wnt responsive element in the SOX9 promoter region would require a long range in vivo analysis of the SOX9 promoter in transgenic animals.
In an alternative model, the Wnt regulation of SOX9 might involve one or several intermediate transcription factors, such as CDX-1 or c-MYC, which have already been described as direct Wnt targets (He et al., 1998; Lickert et al., 2000) and are not regulated by SOX9 in our RT-PCR experiment.
Although the direct/indirect status of the Wnt regulation of SOX9 remains unclear, we showed that down-regulation of the SOX9 protein occurs rapidly after interference with the Wnt pathway activity, i.e., as soon as the expression of the interfering protein factor can be detected. This indicates that SOX9 down-regulation does not result from a indirect, long-term modification of cell physiology initiated by the arrest of Wnt signaling, but represents a direct or early physiological response to this arrest.
Interestingly, overexpressing the SOX9 protein in 293 epithelial cells resulted in a strong decrease of the β-catenin–TCF activity (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200311021/DC1), suggesting that SOX9 might also mediate a negative feedback loop on the β-catenin–TCF activity to fine tune the level of transcriptional activity of the Wnt pathway.
Wnt-regulated SOX9 function outside of the intestine epithelium?
A study of the transcriptional response of NCCIT human embryonal carcinoma cells to the Wnt-3a signal identified SOX9 as one of the genes which was up-regulated upon Wnt-3a signaling (Willert et al., 2002). Such Wnt-regulation of SOX9 thus might also exist in additional structures where SOX9 expression has been reported. For instance, in chondrogenesis, Wnt-4 signals through the β-catenin–TCF-LEF pathway to accelerate chondrogenesis (Hartmann and Tabin, 2000), a process in which SOX9 function is essential (Bi et al., 1999; Akiyama et al., 2002). Moreover, in the developing mouse limb buds, the expression patterns of SOX9 and of the Wnt-antagonist Dickkopf are mutually exclusive (Grotewold and Ruther, 2002). Wnt signals are also involved in the development of the neural crests (Yanfeng et al., 2003), where SOX9 has also recently been found to be required (Spokony et al., 2002; Cheung and Briscoe, 2003; Mori-Akiyama et al., 2003). It will be interesting to determine whether Wnt signals are involved in the regulation of SOX9 in such structures.
The SOX9 transcriptional activator represses differentiation genes
Our small-scale screening of Wnt target genes for possible regulation by SOX9 identified both SOX9-regulated and SOX9-independent Wnt target genes. This indicates that the cellular response to Wnt signaling branches at the level of downstream transcription factors and that SOX9 determines one of these branches, probably involved in the maintenance of undifferentiated cells.
Strikingly, this experiment only revealed genes expressed in the differentiated cells of the epithelium, the expression of which is repressed by SOX9 expression. For instance, SOX9 represses the expression of the CDX2 transcription factor, known to be mostly active in villus cells (Silberg et al., 2000; Rings et al., 2001) and to promote cell differentiation by activating transcription of genes encoding typical differentiation markers, including MUC2, sucrase-isomaltase, and lactase (Lorentz et al., 1997; Yamamoto et al., 2003). To date, SOX9 has generally been described as a transcriptional activator (Südbeck et al., 1996; Bell et al., 1997; Lefebvre et al., 1997; Ng et al., 1997; de Santa Barbara et al., 1998; Liu et al., 2000; Sekiya et al., 2000; Liu et al., 2001; Panda et al., 2001), although it was recently found to mediate a dual transcriptional effect on the COL2A1 gene, depending on the promoter elements involved (Kypriotou et al., 2003). Interestingly, recent studies in the central nervous system showed that SOX1, SOX2, and SOX3 act as repressors of postmitotic neuronal markers. In this case, SOX1–3 inhibit neurogenesis by activating transcription and, conversely, repression of their as yet unknown target genes facilitates neuronal differentiation (Bylund et al., 2003). Here, we show that a forced activator version of SOX9 (SOX9-VP16) mimics the properties of wild-type SOX9, which suggests an analogous situation, where the intermediate activation of a repressor transcription factor mediates the repression of differentiation genes.
Because one reported role of the Wnt pathway is the maintenance of an undifferentiated cell phenotype (van de Wetering et al., 2002), our results suggest that SOX9 might be involved in mediating part of this function. Furthermore, SOX10, closely related to SOX9, is involved in the maintenance of neural crest stem cells multipotency and inhibition of neural differentiation (Kim et al., 2003), SOX2 maintains precursor cells of the mouse blastocyst in a multipotent state (Avilion et al., 2003), and SOX1–3 counteract the activity of proneural proteins to keep neural cells undifferentiated (Bylund et al., 2003). Thus, contribution to the maintenance of cells in an undifferentiated state might be a property shared by several SOX transcription factors, and our finding that SOX9 is regulated by the Wnt pathway, which is also known to inhibit differentiation, might also concern other SOX genes.
Possible involvement of SOX9 in cancer
We found that SOX9 negatively regulates the CDX2 tumor suppressor gene, which is frequently very weakly expressed in colon cancers (Ee et al., 1995; Mallo et al., 1997), especially in poorly differentiated lesions (Hinoi et al., 2001). Mice heterozygous for the CDX2 gene are hypersensitive to sporadic, chemically induced, colon carcinogenesis (Bonhomme et al., 2003), and this was confirmed in a mouse model of familial adenomatous polyposis (Aoki et al., 2003). MUC2 deficiency also leads to spontaneous intestinal tumors (Velcich et al., 2002). Conversely, restoration of CDX2 expression in human colon carcinoma cells suppressed proliferation and soft agar growth (Hinoi et al., 2003). Here, we find that overexpressing a dominant-negative version of SOX9 results in increased CDX2 expression in cultured colon carcinoma cells. This suggests that strong SOX9 expression in tumors which contain a constitutive activation of Wnt signaling may contribute to cancer progression and/or determination of the level of tumor differentiation.
In summary, we report that SOX9 is expressed throughout the intestinal epithelium under the control of the Wnt pathway and we propose that SOX9 function contributes to the Wnt-dependent maintenance of undifferentiated cells in healthy and tumor epithelial cells. The function of SOX9 in Paneth cells, where the Wnt pathway is also active, remains to be determined. Our finding might have implications for other structures where SOX9 function is essential, such as sex determination, chondrogenesis, neural crest, or nervous system development.
Materials and methods
DNA constructs
NH2-terminally flagged wild-type and COOH-terminally truncated SOX9-pcDNA expression constructs have been described previously (Südbeck et al., 1996; de Santa Barbara et al., 1998). The antisense SOX9 expression construct was generated by cloning the BamHI–EcoRI fragment containing the SOX9 ORF from SOX9-pcDNA into the same restriction sites of pIRESneo (CLONTECH Laboratories, Inc.) The GFP-E-cadherin construct was a gift from A. Blangy and C. Gauthier (CNRS FRE2593, Montpellier, France). NH2-truncated TCF4 (ΔN-TCF4 dominant negative) has been described previously (van de Wetering et al., 2002) and was provided by B. Vogelstein (Howard Hughes Medical Institute, Baltimore, MD). The SOX9-VP16 (Kamachi et al., 1999) was provided by H. Kondoh (Institute for Molecular and Cellular Biology, Osaka University, Osaka, Japan), and the MUC-2-luciferase reporter (Yamamoto et al., 2003) was a gift from Y. Yuasa (Tokyo Medical and Dental University, Tokyo, Japan). The CDX-2 promoter reporter constructs were published previously (Lorentz et al., 1999).
Cell culture and transfections
All cell lines were grown in RPMI medium supplemented with 10% FBS (Life Technologies). Stably transfected LS174T cell lines with inducible ΔNTCF4 have been described previously (van de Wetering et al., 2002). HT29-16E human colorectal carcinoma cells were given by C. Laboisse (INSERM U539, Nantes, France). The T-RexTM system (Invitrogen) was used for the generation of stable SOX9-inducible HT29-16E cells. In brief, tetracycline-inducible HT29-16E cells were obtained by stable transfection of the regulatory plasmid pcDNA6/TR. NH2-terminally flagged SOX9 was then inserted in pcDNA4/TO and stably transfected in the tetracycline-inducible HT29-16E cells.
SOX9 mRNA and protein expression was analyzed 36 h after doxycycline induction. For transient transfections, the LS174T colon carcinoma cell line was transfected using Fugene 6 (Roche), according to the instructions of the supplier, and cells were lysed for analysis 36 h after transfection. Cells transfected for RT-PCR purpose were cotransfected with the pMACS-Kk plasmid kit (Miltenyi Biotec), encoding the mouse MHC class I molecule H-2Kk as a selection marker. Dual luciferase kit (Promega) was used for luciferase measurement and normalization.
RNA purification and RT-PCR
Before RT-PCR experiments, transfected cells harboring the H-2Kk surface molecule were selected using the MACSelect H-2KK transfected cells selection kit (Miltenyi Biotec). Standard procedures were used for RNA purification and reverse transcription. Each PCR amplification was repeated several times with different numbers of cycles to ensure that the obtained amplified products reflected the original DNA concentrations. The primers used are listed in Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200311021/DC1.
Xenografts
Cells from the HT29-16E cell line, stably transfected with the doxycycline-inducible Flag-SOX9 construct, were resuspended in DME medium containing penicillin-streptomycin at 200 U/ml (Life Technologies). Six nude mice were injected subcutaneously with 107 cells each in 50 μl of culture medium. From day 6 after cell injection until tumor removal at week 11, 200 μg/ml doxycycline was administered in drinking water to three mice, whereas the other three mice received doxycycline-free water.
Immunohistochemistry, immunofluorescence, Northern and Western blotting
Experiments were performed according to standard procedures. Samples of human colon tumors were provided by C. Marty-Double (CHU, Nîmes, France). For immunohistochemistry, Envision + (DakoCytomation) was used as a secondary reagent, stainings were developed with DAB (brown precipitate), and hematoxylin counterstain was used. The TCF4 K.O. mouse has been described previously (Korinek et al., 1998). For immunofluorescence experiments, nuclei were stained with Hoechst (H33258; Sigma-Aldrich). For Western blotting, an equal amount of protein, measured by the Bradford assay, was loaded on each lane of the gel.
Antibodies
The SOX9 antibody has been described previously (de Santa Barbara et al., 1998). The MUC2 antibody (1:1,000) was provided by I. van Seuningen (INSERM U560, Lille, France). β-Actin A5441 and Flag (1:500) were purchased from Sigma-Aldrich; Ki-67 was purchased from Novocastra (1:150); β-catenin was purchased from BD Transduction Laboratories (1:50); CDX2 was purchased from Biogenex (1:500); and Myc tag 9E10 was purchased from Santa Cruz Biotechnology, Inc. (1:100).
Image acquisition and manipulation
Immunofluorescence images were acquired at RT using a microscope (model DMR; Leica), 40 × 1.0 Pl Fluotar or 63 × 1.32 PL Apo lenses (Leica), and a camera (model C5985; Hamamatsu). Images were acquired and manipulated with the Adobe Photoshop software. Fluorochromes were Alexa 488 and 568 dyes (Molecular Probes).
Immunohistochemistry images were acquired at RT using an Axiophot microscope (Carl Zeiss MicroImaging, Inc.), 10 × 0.3 Plan Neofluor or 40 × 1.0 Plan Apochromat lenses (Carl Zeiss MicroImaging, Inc.) and a camera (model DXM1200; Nikon). Images were acquired and manipulated respectively with the Nikon ACT-1 and Adobe Photoshop softwares.
Online supplemental material
Fig. S1 shows that the SOX9 protein is expressed only in the nucleus of cultured colon carcinoma cells or of intestine epithelial cells. Fig. S2 contains a kinetic analysis of the repression of SOX9 expression after interference with the Wnt pathway activity. Fig. S3 shows that the SOX9 protein acts as a negative feedback loop to inhibit the Wnt pathway activity in cultured colon carcinoma cells. Table S1 is a set of oligonucleotide primers used for RT-PCR. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200311021/DC1.
Acknowledgments
The authors wish to thank B. Vogelstein, Y. Yuasa, H. Kondoh, C. Laboisse, A. Blangy, and C. Gauthier for reagents; I. van Seuningen for anti-Muc2 antibody; E. Batlle for helpful advice; and D. Fischer and J.-M. Brondello for critical reading of the manuscript. P. Jay and P. Berta thank the team members for support and assistance. We also thank E. Martin for technical support.
This work was supported by the CNRS, the INSERM, and French Cancer Research Association grants (ARC 4293 and ARC 3286) as well as a Ligue against Cancer grant to P. Jay.
The online version of this article contains supplemental material.
Abbreviations used in this paper: CD, campomelic dysplasia; cyt-E-cadherin, cytoplasmic domain of E-cadherin.
References
- Akiyama, H., M.C. Chaboissier, J.F. Martin, A. Schedl, and B. de Crombrugghe. 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16:2813–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, K., Y. Tamai, S. Horiike, M. Oshima, and M.M. Taketo. 2003. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Δ716 Cdx2+/− compound mutant mice. Nat. Genet. 35:323–330. [DOI] [PubMed] [Google Scholar]
- Avilion, A.A., S.K. Nicolis, L.H. Pevny, L. Perez, N. Vivian, and R. Lovell-Badge. 2003. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle, E., J.T. Henderson, H. Beghtel, M.M.W. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell. 111:251–263. [DOI] [PubMed] [Google Scholar]
- Bell, D.M., K.K.H. Leung, S.C. Wheatley, L.J. Ng, S. Zhou, K.W. Ling, M.H. Sham, P. Koopman, P.P.L. Tam, and K.S.E. Cheah. 1997. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16:174–178. [DOI] [PubMed] [Google Scholar]
- Bi, W., J.M. Deng, Z. Zhang, R.R. Behringer, and B. de Crombrugghe. 1999. Sox9 is required for cartilage formation. Nat. Genet. 22:85–89. [DOI] [PubMed] [Google Scholar]
- Bi, W., W. Huang, D.J. Whitworth, J.M. Deng, Z. Zhang, R.R. Behringer, and B. de Combrugghe. 2001. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA. 98:6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, C.E., D.J. Whitworth, Y. Qin, A.I. Agoulnik, W.R. Harrison, R.R. Behringer, and P.A. Overbeek. 2000. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat. Genet. 26:490–494. [DOI] [PubMed] [Google Scholar]
- Bonhomme, C., I. Duluc, E. Martin, K. Chawengsaksophak, M.-P. Chenard, M. Kedinger, F. Beck, J.N. Freund, and C. Domon-Dell. 2003. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon, in addition to the homeotic role during gut development. Gut. 52:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon, M., M. Gomperts, L. Sumoy, R.T. Moon, and D. Kimelman. 1997. A β-catenin/xTcf3 complex binds to the siamois promoter to regulate dorsal axis specification in xenopus. Genes Dev. 11:2359–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantjes, H., N. Barker, J. van Es, and H. Clevers. 2002. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 383:255–261. [DOI] [PubMed] [Google Scholar]
- Bridgewater, L.C., V. Lefebvre, and B. de Crombrugghe. 1998. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J. Biol. Chem. 273:14998–15006. [DOI] [PubMed] [Google Scholar]
- Bylund, M., E. Andersson, B.G. Novitch, and J. Muhr. 2003. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 6:1162–1168. [DOI] [PubMed] [Google Scholar]
- Cheng, H. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II. Mucous cells. Am. J. Anat. 141:481–491. [DOI] [PubMed] [Google Scholar]
- Cheung, M., and J. Briscoe. 2003. Neural crest development is regulated by the transcription factor Sox9. Development. 130:5681–5693. [DOI] [PubMed] [Google Scholar]
- Cremazy, F., S. Soullier, P. Berta, and P. Jay. 1998. Further complexity of the human SOX gene family revealed by the combined use of highly degenerate primers and nested PCR. FEBS Lett. 438:311–314. [DOI] [PubMed] [Google Scholar]
- da Costa, L.T., T.C. He, J. Yu, A.B. Sparks, P.J. Morin, K. Polyak, S. Laken, B. Vogelstein, and K.W. Kinzler. 1999. CDX2 is mutated in a colorectal cancer with normal APC/beta-catenin signaling. Oncogene. 18:5010–5014. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara, P., N. Bonneaud, B. Boizet, M. Desclozeaux, B. Moniot, P. Sudbeck, G. Scherer, F. Poulat, and P. Berta. 1998. Direct interaction of SRY-related protein Sox9 and steroidogenic factor 1 regulates transcription of the human anti-mullerian hormone gene. Mol. Cell. Biol. 18:6653–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprey, P., K. Chowdhury, G.R. Dressler, R. Balling, D. Simon, J.L. Guenet, and P. Gruss. 1988. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 2:1647–1654. [DOI] [PubMed] [Google Scholar]
- Ee, H., T. Erler, P.S. Bhathal, G.P. Young, and R.J. James. 1995. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am. J. Pathol. 147:586–592. [PMC free article] [PubMed] [Google Scholar]
- Fodde, R., W. Edelmann, K. Yang, C. van Leeuwen, C. Carlson, B. Renault, C. Breukel, E. Alt, M. Lipkin, and P.M. Khan. 1994. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl. Acad. Sci. USA. 91:8969–8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J.M., M.A. Dominguez-Steglich, S. Guioli, C. Kwok, P.A. Weller, M. Stevanovic, J. Weissenbach, S. Mansour, I.D. Young, P.N. Goodfellow, et al. 1994. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 372:525–530. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., E. Wong, and B.M. Gumbiner. 2001. E-Cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J. Cell Biol. 153:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold, L., and U. Ruther. 2002. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int. J. Dev. Biol. 46:943–947. [PubMed] [Google Scholar]
- Harada, N., Y. Tamai, T. Ishikawa, B. Sauer, K. Tabaku, M. Oshima, and M. Taketo. 1999. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 18:5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, C., and C.J. Tabin. 2000. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 127:3141–3159. [DOI] [PubMed] [Google Scholar]
- He, T.-C., A.B. Sparks, C. Rago, H. Hermeking, L. Zawel, L.T. da Costa, P.J. Morin, B. Vogelstein, and K.W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science. 281:1509–1512. [DOI] [PubMed] [Google Scholar]
- Hinoi, T., M. Tani, P.C. Lucas, K. Caca, R.L. Dunn, E. Macri, M. Loda, H.D. Appelman, K.R. Cho, and E.R. Fearon. 2001. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am. J. Pathol. 159:2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi, T., M. Loda, and E.R. Fearon. 2003. Silencing of CDX2 expression in colon cancer via a dominant repression pathway. J. Biol. Chem. 278:44608–44616. [DOI] [PubMed] [Google Scholar]
- Ikeya, M., and S. Takada. 2001. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech. Dev. 103:27–33. [DOI] [PubMed] [Google Scholar]
- Kamachi, Y., K.S. Cheah, and H. Kondoh. 1999. Mechanism of regulatory target selection by the SOX high-mobility-group domain proteins as revealed by comparison of SOX1/2/3 and SOX9. Mol. Cell. Biol. 19:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi, Y., I. Uchikawa, and H. Kondoh. 2000. Pairing SOX off with partners in the regulation of embryonic development. Trends Genet. 16:182–187. [DOI] [PubMed] [Google Scholar]
- Kent, J., S.C. Wheatley, J.E. Andrews, A.H. Sinclair, and P. Koopman. 1996. A male specific role for SOX9 in vertebrate sex determination. Development. 122:2813–2822. [DOI] [PubMed] [Google Scholar]
- Kim, J., L. Lo, E. Dormand, and D.J. Anderson. 2003. SOX10 Maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 38:17–31. [DOI] [PubMed] [Google Scholar]
- Korinek, V., N. Barker, P.J. Morin, D. van Wichen, R. de Weger, K.W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 275:1784–1787. [DOI] [PubMed] [Google Scholar]
- Korinek, V., N. Barker, P. Moerer, E. van Donselaar, G. Huls, P.J. Peters, and H. Clevers. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19:379–383. [DOI] [PubMed] [Google Scholar]
- Kypriotou, M., M. Fossard-Demoor, C. Chadjichristos, C. Ghayor, B. de Crombrugghe, J.P. Pujol, and P. Galera. 2003. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 22:119–129. [DOI] [PubMed] [Google Scholar]
- Lefebvre, V., W. Huang, V.R. Harley, P.N. Goodfellow, and B. de Crombrugghe. 1997. SOX9 is a potent activator of the chondrocyte-specific enhancer of the proa1(II) collagen gene. Mol. Cell. Biol. 17:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert, H., C. Domon, G. Huls, C. Wehrle, I. Duluc, H. Clevers, B.I. Meyer, J.-N. Freund, and R. Kemler. 2000. Wnt/β-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 127:3805–3813. [DOI] [PubMed] [Google Scholar]
- Liu, S., R. Guo, and L.D. Quarles. 2001. Cloning and characterization of the proximal murine Phex promoter. Endocrinology. 142:3987–3995. [DOI] [PubMed] [Google Scholar]
- Liu, Y., H. Li, K. Tanaka, N. Tsumaki, and Y. Yamada. 2000. Identification of an enhancer sequence within the first intron required for cartilage-specific transcription of the α2(XI) collagen gene. J. Biol. Chem. 275:12712–12718. [DOI] [PubMed] [Google Scholar]
- Lorentz, O., I. Duluc, A. De Arcangelis, P. Simon-Assmann, M. Kedinger, and J.-N. Freund. 1997. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J. Cell Biol. 139:1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz, O., A. Cadoret, I. Duluc, J. Capeau, C. Gespach, G. Cherqui, and J.-N. Freund. 1999. Downregulation of the colon tumour-suppressor homeobox gene Cdx-2 by oncogenic ras. Oncogene. 18:87–92. [DOI] [PubMed] [Google Scholar]
- Mallo, G.V., H. Rechreche, J.-M. Frigerio, D. Rocha, A. Zweibaum, M. Lacasa, B.R. Jordan, N.J. Dusetti, J.-C. Dagorn, and J.L. Iovanna. 1997. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and CDX2 homeobox, down-regulation of Cdx1 and Cdx2 expression during colorectal carcinogenesis. Int. J. Cancer. 74:35–44. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama, Y., H. Akiyama, D.H. Rowitch, and B. De Crombrugghe. 2003. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA. 100:9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, P.J., A.B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K.W. Kinzler. 1997. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 275:1787–1790. [DOI] [PubMed] [Google Scholar]
- Ng, L.-J., S. Wheatley, G.E.O. Muscat, J. Conway-Campbell, J. Bowles, E. Wright, D.M. Bell, P.P.L. Tam, K.S.E. Cheah, and P. Koopman. 1997. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 183:108–121. [DOI] [PubMed] [Google Scholar]
- Panda, D.K., D. Miao, V. Lefebvre, G.N. Hendy, and D. Golzman. 2001. The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrogenic CFK2 cells. J. Biol. Chem. 276:41229–41236. [DOI] [PubMed] [Google Scholar]
- Pinto, D., A. Gregorieff, H. Begthel, and H. Clevers. 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17:1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten, C.S., and M. Moeffler. 1990. Stem cells: attributes, cycles, pitfalls and uncertainties lessons for and from the crypt. Development. 110:1001–1020. [DOI] [PubMed] [Google Scholar]
- Rings, E.H.H.M., F. Boudreau, J.K. Taylor, J. Moffett, E.R. Suh, and P.G. Traber. 2001. Phsphorylation of the serine 60 residue within the Cdx2 activation domain mediates its transactivation capacity. Gastroenterology. 121:1437–1450. [DOI] [PubMed] [Google Scholar]
- Sekiya, I., K. Tsuji, P. Koopman, H. Watanabe, Y. Yamada, K. Shinomiya, A. Nifuji, and M. Noda. 2000. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 275:10738–10744. [DOI] [PubMed] [Google Scholar]
- Shibata, H., K. Toyama, H. Shioya, M. Ito, M. Hirota, S. Hasegawa, H. Matsumoto, H. Takano, T. Akiyama, K. Toyoshima, et al. 1997. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 278:120–123. [DOI] [PubMed] [Google Scholar]
- Silberg, D.G., G.P. Swain, E.R. Suh, and P.G. Traber. 2000. Cdx1 and Cdx2 expression during intestinal development. Gastroenterology. 119:961–971. [DOI] [PubMed] [Google Scholar]
- Simcha, I., C. Kirkpatrick, E. Sadot, M. Shtutman, G. Polevoy, B. Geiger, M. Peifer, and A. Ben-Ze'ev. 2001. Cadherin sequences that inhibit beta-catenin signaling: a study in yeast and mammalian cells. Mol. Biol. Cell. 12:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.M., and P.A. Koopman. 2004. The ins and outs of transcriptional control: nucleocytoplasmic shuttling in development and disease. Trends Genet. 20:4–8. [DOI] [PubMed] [Google Scholar]
- Spokony, R.F., Y. Aoki, N. Saint-Germain, E. Magner-Fink, and J.P. Saint-Jeannet. 2002. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development. 129:421–432. [DOI] [PubMed] [Google Scholar]
- Stappenbeck, T.S., M.H. Wong, J.R. Saam, I.U. Mysorekar, and J.I. Gordon. 1998. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr. Opin. Cell Biol. 10:702–709. [DOI] [PubMed] [Google Scholar]
- Stolt, C.C., S. Rehberg, M. Ader, P. Lommes, D. Riethmacher, M. Schachner, U. Bartsch, and M. Wegner. 2002. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt, C.C., P. Lommes, E. Sock, M.C. Chaboissier, A. Schedl, and M. Wegner. 2003. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 17:1677–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, V., B. Meyer, and G.S. Evans. 1998. The murine Cdx1 gene product localises to the proliferative compartment in the developing and regenerating intestinal epithelium. Differentiation. 64:11–18. [DOI] [PubMed] [Google Scholar]
- Südbeck, P., L. Schmitz, P.A. Baeuerle, and G. Scherer. 1996. Sex reversal by loss of the C-terminal transactivation domain of human SOX9. Nat. Genet. 13:230–232. [DOI] [PubMed] [Google Scholar]
- Suh, E., L. Chen, J. Taylor, and P.G. Traber. 1994. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol. Cell. Biol. 14:7340–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu, O., and F. McCormick. 1999. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 398:422–426. [DOI] [PubMed] [Google Scholar]
- van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A.P. Haramis, et al. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 111:241–250. [DOI] [PubMed] [Google Scholar]
- Velcich, A., W.C. Yang, J. Heyer, A. Fragale, C. Nicholas, S. Viani, R. Kucherlapati, M. Lipkin, K. Yang, and L. Augenlicht. 2002. Colorectal cancer in mice genetically deficient in the Mucin Muc2. Science. 295:1726–1729. [DOI] [PubMed] [Google Scholar]
- Wagner, T., J. Wirth, J. Meyer, B. Zabel, M. Held, J. Zimmer, J. Pasantes, F.D. Bricarelli, J. Keutel, E. Hustert, et al. 1994. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 79:1111–1120. [DOI] [PubMed] [Google Scholar]
- Willert, J., M. Epping, J.R. Pollack, P.O. Brown, and R. Nusse. 2002. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E., M.R. Hargrave, J. Christiansen, L. Cooper, J. Kun, T. Evans, U. Gandadharan, A. Greenfield, and P. Koopman. 1995. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 9:15–20. [DOI] [PubMed] [Google Scholar]
- Wunderle, V.M., R. Critcher, N. Hastie, P.N. Goodfellow, and A. Schedl. 1998. Deletion of long-range regulatory elements upstream of SOX9 causes campomelic dysplasia. Proc. Natl. Acad. Sci. USA. 95:10649–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W.F., X. Zhang, S. Sakano, V. Lefebvre, and L.J. Sandell. 1999. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J. Bone Miner. Res. 14:757–763. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H., Y.Q. Bai, and Y. Yuasa. 2003. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem. Biophys. Res. Commun. 300:813–818. [DOI] [PubMed] [Google Scholar]
- Yanfeng, W., J.P. Saint-Jeannet, and P.S. Klein. 2003. Wnt-frizzled signaling in the induction and differentiation of the neural crest. Bioessays. 25:317–325. [DOI] [PubMed] [Google Scholar]