Abstract
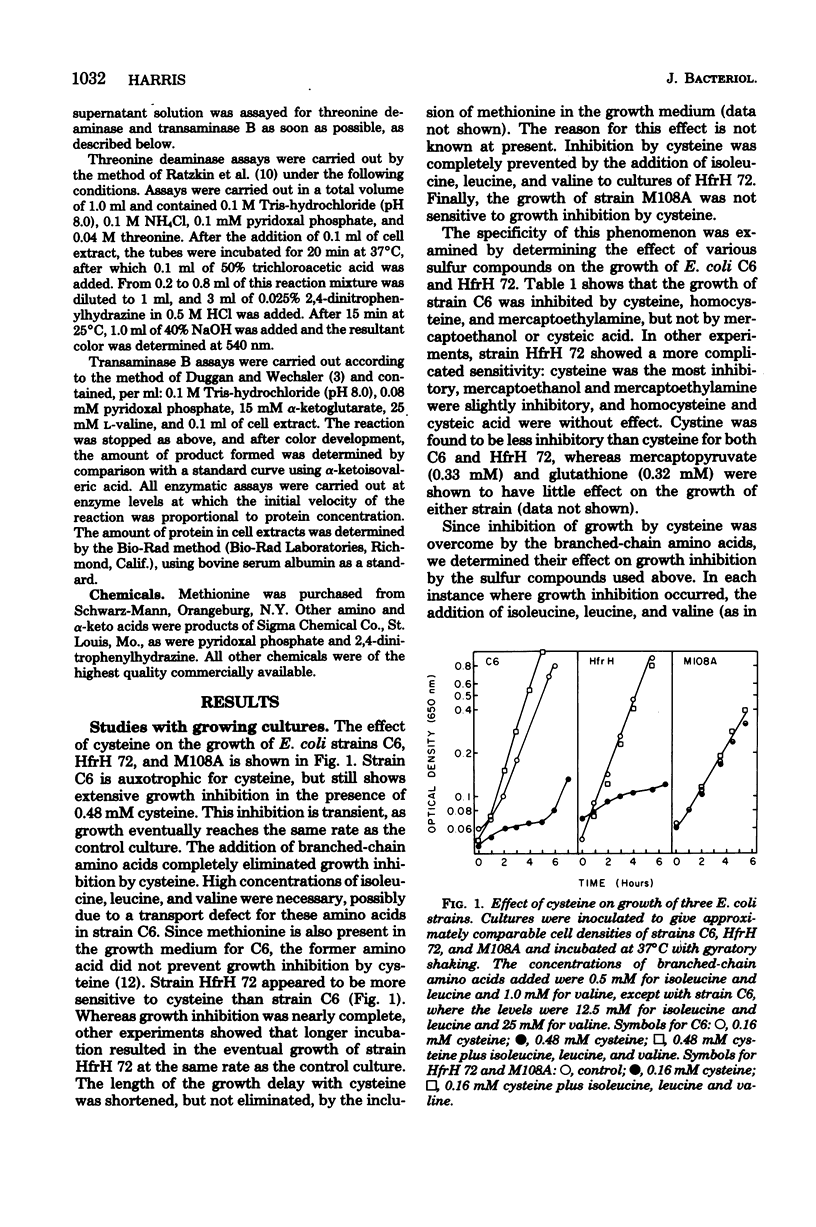
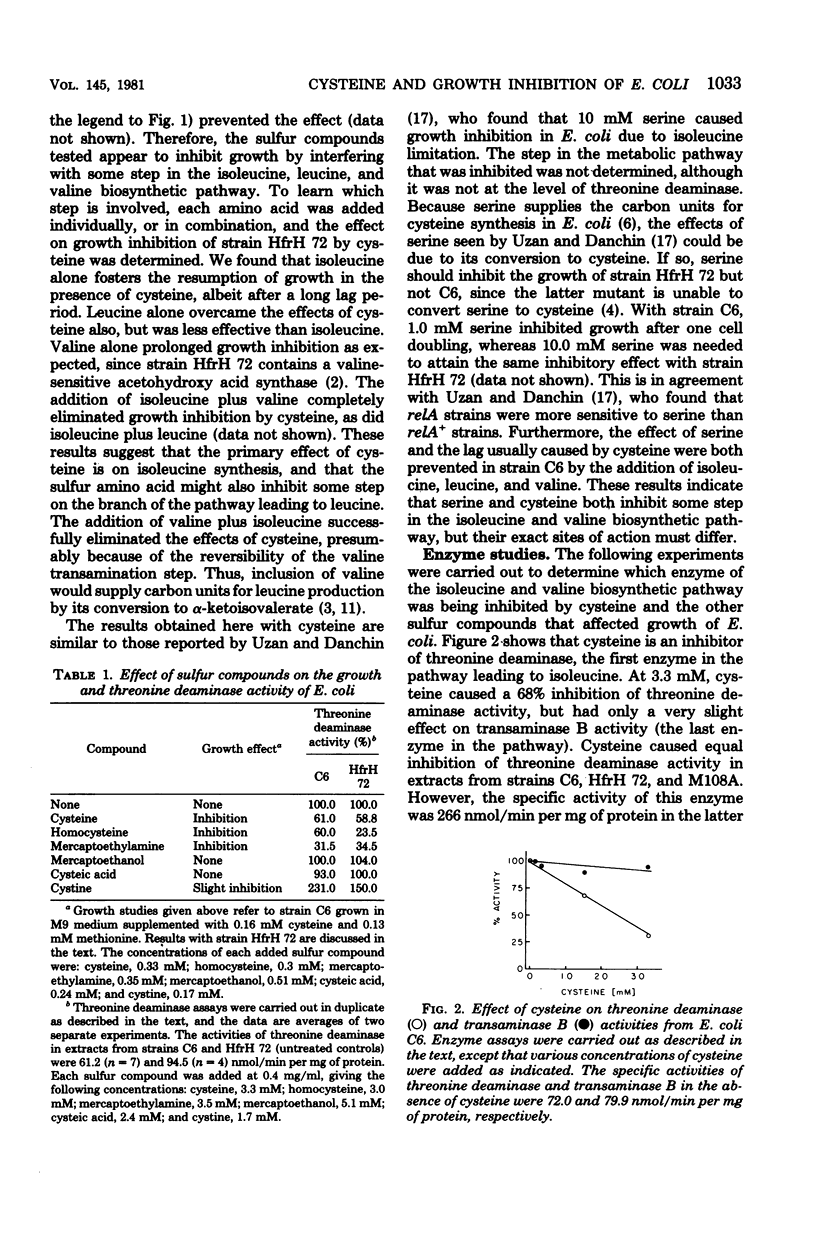
Cysteine has been shown to inhibit growth in Escherichia coli strains C6 and HfrH 72, but not M108A. Growth inhibition was overcome by inclusion of isoleucine, leucine, and valine in the medium. Isoleucine biosynthesis was apparently affected, since addition of this amino acid alone could alter the inhibitory effects of cysteine. Homocysteine, mercaptoethylamine, and mercaptoethanol inhibited growth to varying degrees in some strains, these effects also being prevented by addition of branched-chain amino acids. Cysteine, mercaptoethylamine, and homocysteine were inhibitors of threonine deaminase but not transaminase B, two enzymes of the ilvEDA operon. Cysteine inhibition of threonine deaminase was reversed by threonine, although the pattern of inhibition was mixed. These results suggest a relationship between the growth-inhibitory effects of cysteine and other sulfur compounds and the inhibition of isoleucine synthesis at the level of threonine deaminase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Titchener E. B., Cline A. L. Sulfur-deficient transfer ribonucleic acid in a cysteine-requiring, "relaxed" mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1322–1327. doi: 10.1128/jb.100.3.1322-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S., Anraku Y. Transport of sugars and amino acids in bacteria. IV. Regulation of valine transport activity by valine and cysteine. J Biochem. 1971 Aug;70(2):215–224. doi: 10.1093/oxfordjournals.jbchem.a129633. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M. N., Norton J. S., Peterkofsky A. A requirement for beta-mercaptopyruvate in the in vitro thiolation of transfer ribonucleic acid. Biochemistry. 1967 Mar;6(3):855–860. doi: 10.1021/bi00855a028. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. Interrelationships between amino-acids in the growth of coliform organisms. J Gen Microbiol. 1953 Aug;9(1):37–43. doi: 10.1099/00221287-9-1-37. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Arfin S., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. 18. Induction of acetohydroxy acid isomeroreductase. J Bacteriol. 1972 Oct;112(1):131–141. doi: 10.1128/jb.112.1.131-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VIII. The formation of acetolactate. J Biol Chem. 1958 Nov;233(5):1156–1160. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Threonine deaminases. Adv Enzymol Relat Areas Mol Biol. 1973;37:349–395. doi: 10.1002/9780470122822.ch6. [DOI] [PubMed] [Google Scholar]
- Uzan M., Danchin A. Correlation between the serine sensitivity and the derepressibility of the ilv genes in Escherichia coli relA- mutants. Mol Gen Genet. 1978 Sep 20;165(1):21–30. doi: 10.1007/BF00270372. [DOI] [PubMed] [Google Scholar]



