Abstract
It is generally assumed that the functional consequences of stimulation with Ca2+-mobilizing agonists are derived exclusively from the second messenger action of intracellular Ca2+, acting on targets inside the cells. However, during Ca2+ signaling events, Ca2+ moves in and out of the cell, causing changes not only in intracellular Ca2+, but also in local extracellular Ca2+. The fact that numerous cell types possess an extracellular Ca2+ “sensor” raises the question of whether these dynamic changes in external [Ca2+] may serve some sort of messenger function. We found that in intact gastric mucosa, the changes in extracellular [Ca2+] secondary to carbachol-induced increases in intracellular [Ca2+] were sufficient and necessary to elicit alkaline secretion and pepsinogen secretion, independent of intracellular [Ca2+] changes. These findings suggest that extracellular Ca2+ can act as a “third messenger” via Ca2+ sensor(s) to regulate specific subsets of tissue function previously assumed to be under the direct control of intracellular Ca2+.
Keywords: extracellular Ca2+-sensing receptor; polarized epithelial cells; pH microelectrodes; interstitial space; adenylyl cyclase
Introduction
Intracellular Ca2+ signaling events stimulated by specific hormones and neurotransmitters are correlated with enzyme and fluid secretion in many epithelial tissues (Cheek, 1991; Petersen et al., 1994; Tse and Tse, 1999; Kidd and Thorn, 2000; Berridge et al., 2003). It is well established that Ca2+ released from intracellular stores in response to Ca2+-mobilizing agonists is largely extruded from cells through plasma membrane Ca2+ ATPases (PMCAs; Nielsen and Petersen, 1972; Tepikin et al., 1992; Belan et al., 1997; Hofer et al., 1998; Usachev et al., 2002) or in some cells through Na+/Ca2+ exchangers (Sedova and Blatter, 1999). Another important consequence of store emptying is the opening of store-operated channels, ubiquitous entry pathways for Ca2+ (Putney, 1990).
In intact tissues, where the interstitial volume is extremely limited compared with that of the cells, these movements of Ca2+ across the plasma membrane secondary to intracellular signaling events can potentially generate considerable local fluctuations in extracellular [Ca2+]. In recent years, the possibility that these extracellular [Ca2+] changes may be detected by cell surface–expressed Ca2+ sensors, and therefore used to encode information, has gained a limited degree of experimental support (Hofer et al., 2000; De Luisi and Hofer, 2003; Hofer and Brown, 2003).
In a previous work using Ca2+-selective microelectrodes in the intact amphibian gastric mucosa, we directly recorded the profile of agonist-induced changes in extracellular [Ca2+] in the restricted domains near the apical (luminal) and basolateral (serosal) membrane of the gastric oxyntopeptic cells (OCs). Stimulation with carbachol, which mobilizes intracellular Ca2+ stores in the OC, resulted in substantial local increases (as large as 0.5 mM) in extracellular [Ca2+] ([Ca2+]ext) at the luminal face and a comparable depletion at the serosal aspect of the acid-secreting cells (Caroppo et al., 2001). The asymmetry of the changes reflects the polarized distribution of calcium-handling mechanisms in the plasma membrane of the OCs, which in the amphibian gastric gland are the main cell type, secreting both acid and pepsinogen. The decrease in basolateral [Ca2+]ext is likely due to opening of store-operated channels localized predominantly on the basolateral membrane of the OC (Caroppo et al., 2001). The increase in [Ca2+]ext in the restricted space of the gastric gland lumen is the result of activation of PMCA, which is highly expressed at the apical pole of these cells (Caroppo et al., 2001).
Precedent for the polarized distribution of membrane transport pathways linked to Ca2+ signaling events exists in other epithelial cell types, in addition to gastric cells (Ashby and Tepikin, 2002). For example, Ca2+ signals in pancreatic and lacrimal acinar cells are typically initiated at the apical pole, and then can spread toward the basolateral area provided the stimulus is sufficiently large (Kasai and Augustine, 1990; Toescu et al., 1992). In pancreatic acinar cells, Belan et al. (1996) showed that the apical membrane is the main pathway for agonist-induced Ca2+ extrusion. In pancreatic acinar and salivary gland cells, Lee et al. (1997) found high levels of PMCA in the apical membrane. Furthermore, a polarized distribution of capacitative calcium entry pathways has been shown in human renal, bronchial, and colonic epithelial cells, with preferential localization to the basolateral membrane (Gordjani et al., 1997; Kerstan et al., 1999).
In addition to observing a polarized distribution of membrane Ca2+ transport pathways, we found that a cell surface receptor acting as a sensor for extracellular Ca2+, the extracellular Ca2+-sensing receptor (CaR; Brown et al., 1993; Brown and MacLeod, 2001; Hofer and Brown, 2003), was also found only in the apical membrane of the OC, partially colocalized with the PMCA. Because the recorded agonist-induced changes in [Ca2+]ext may be sufficient to modulate CaR, we wondered whether extracellular Ca2+ changes might serve some physiological function in gastric cells. When we examined the effect of mimicking the carbachol-induced [Ca2+]ext fluctuations described in our previous work (Caroppo et al., 2001) on pepsinogen and alkaline secretion, two secretory functions believed to be mediated by intracellular [Ca2+] ([Ca2+]i) in the OC, we found that [Ca2+]ext changes could fully reproduce the secretory responses elicited by carbachol in the intact tissue.
Results
The amphibian gastric mucosa has been used as a model for the study of the function of the gastric mucosa in many laboratories, and its secretory and ion transport properties have been very well characterized (Kasbekar et al., 1965; Forte, 1968; Shoemaker and Sachs, 1972; Silen et al., 1975; Carlisle et al., 1978; Machen and McLennan, 1980; Debellis et al., 1990, 1992, 1998; Supplisson et al., 1991; Ruiz et al., 1993). Taking advantage of the long-lasting anatomical and functional preservation of this model system after isolation, we have established a technique that allows direct access of double-barreled ion-sensitive microelectrodes to the lumen of single gastric glands in the intact isolated gastric mucosa to measure pH or [Ca2+] in the gland lumen (Debellis et al., 1998; Caroppo et al., 2001).
Effect of “physiological” [Ca2+]ext changes
Secretion of pepsinogen, precursor of the proteolytic enzyme pepsin, is stimulated by cholinergic agonists both in mammals (Raufman, 1992) and amphibia (Ruiz et al., 1993), and can be observed independently of acid secretion (Hersey et al., 1983). As shown in Fig. 1, basal pepsinogen secretion increased significantly in response to carbachol, as expected (Hirschowitz, 1967; Helander, 1978). A very similar response was observed after simultaneous elevation (from 1.4 to 2.0 mM) of luminal [Ca2+]ext and decrease (from 1.4 to 1.0 mM) in serosal [Ca2+]ext (“bilateral [Ca2+]ext changes”), a maneuver meant to mimic carbachol-induced extracellular Ca2+ changes. These findings were corroborated by pepsinogen immunostaining experiments. In control tissues, OCs were tightly packed with large, highly fluorescent granules (Fig. 1 a). Exposure to carbachol resulted in granule emptying and reduced pepsinogen staining that was comparable to that induced by physiological bilateral changes in [Ca2+]ext (Fig. 1, b and c). In some cells a residual fluorescence, observed after stimulation with either carbachol or bilateral [Ca2+]ext changes, was detected at the apical pole of the OCs, suggestive of an exocytotic process (Hirschowitz, 1967; Helander, 1978).
Figure 1.
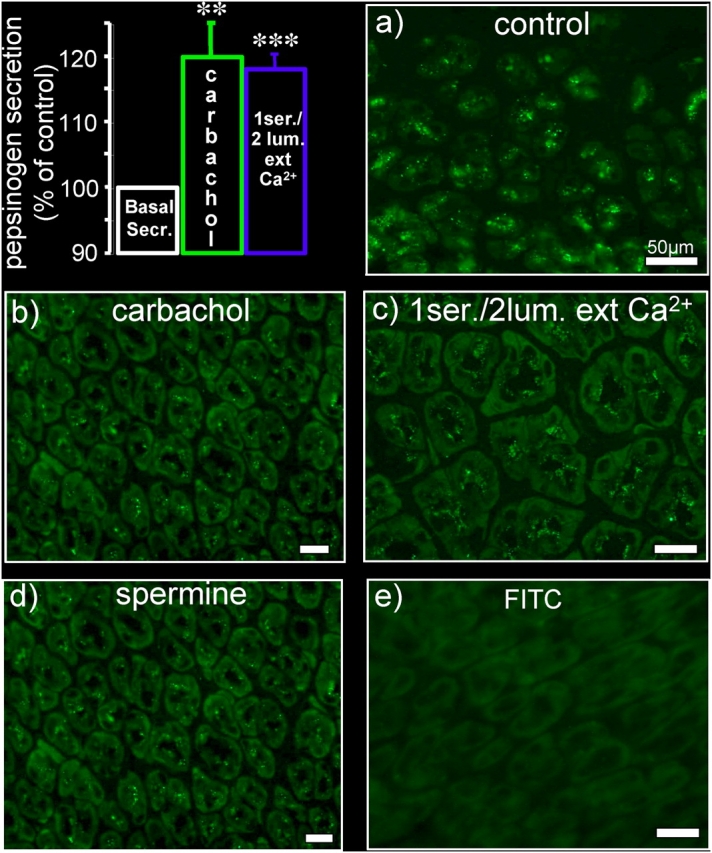
Pepsinogen secretion: effect of carbachol, bilateral changes in [Ca 2+ ] ext , and spermine. Top left: effect of 100 μM serosal carbachol (n = 5) and simultaneous luminal increase (from 1.4 to 2.0 mM) and serosal decrease (from 1.4 to 1.0 mM) in [Ca2+]ext (n = 6) on pepsinogen secretion in the stomach of Rana esculenta. These, as well as all other experiments described in the paper, were performed in the presence of the histamine H2 receptor blocker cimetidine to prevent acid secretion (serosal, 100 μM). Peak pepsinogen release was calculated as percentage of total pepsin activity. All data are expressed as mean ± SEM. **, P < 0.01; ***, P < 0.001. (a–e) Immunofluorescence of pepsinogen in transverse sections of frog stomach. Sections were treated with an affinity-purified mAb against human pepsinogen I and with fluorescein-conjugated goat anti–mouse IgG. (a) Positive pepsinogen staining in control mucosa; the enzyme appears packed in granules abundantly distributed in the cytoplasm. (b–d) Effect of exposure to carbachol, bilateral changes in [Ca2+]ext, or serosal spermine (1 mM). (e) Secondary antibody alone. Bars, 50 μm.
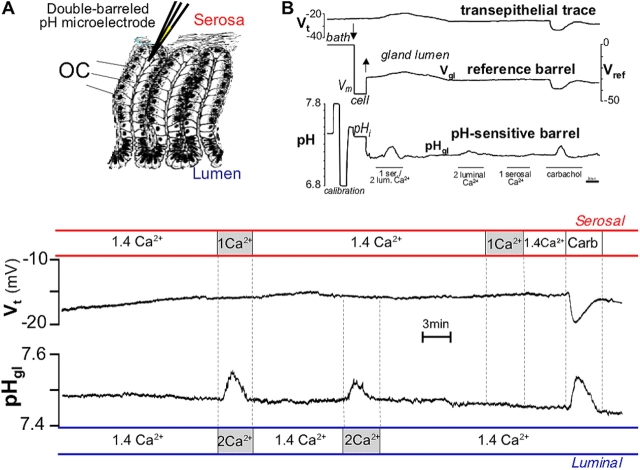
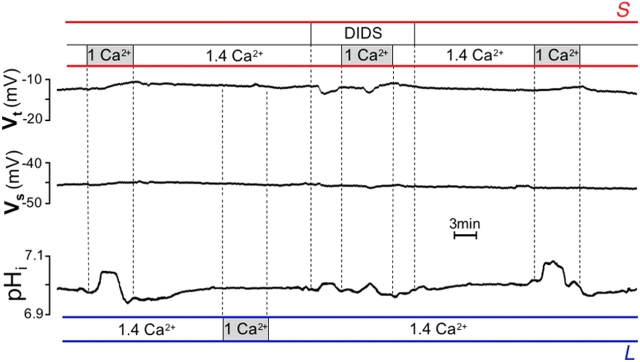
Another phenomenon characteristic of cholinergic stimulation is alkaline secretion, which plays an important protective role in the gastric mucosa (Flemstrom and Isenberg, 2001). Previously, we showed that an alkaline secretory process from the OC can be monitored after cholinergic stimulation, but only during inhibition of acid secretion (Curci et al., 1994; Debellis et al., 1998). We characterized this process in situ using a sensitive electrophysiological technique (Fig. 2, inset) that allows direct, real-time monitoring of intraglandular pH (pHgl; Debellis et al., 1998).
Figure 2.
Alkaline secretion: effect of carbachol and bilateral changes in [Ca 2+ ] ext . (A) Inset: schematic drawing of the technique used to record pH (or Ca2+) in the gland lumen. Double-barreled pH or Ca2+-sensitive microelectrodes were inserted in the gland lumen of a single gastric gland of the intact amphibian gastric mucosa perfused in vitro. (B) Top portion: original trace recording showing the entire experimental protocol. Top trace: transepithelial potential (Vt), luminal surface negative; middle trace: cell membrane potential (Vm) and gland lumen potential (Vgl) as measured by the reference barrel; bottom trace: cell pH (pHi) and pH in the gland lumen (pHgl) as measured by the pH-selective barrel. The microelectrode was calibrated before the puncture by flushing the chamber with Hepes-buffered Ringer's solutions with pH between 6.8 and 7.8. Afterward, the microelectrode was first inserted into a cell (arrow down, Vm and pHi) and then was further advanced into the gland lumen (arrow up) where the potential recording suddenly changed to nearly the same values as those measured transepithelially (Vgl). A similar experiment with longer baselines before and after responses is depicted in the bottom portion in expanded format (as used in all subsequent figures of this paper), with the reference barrel recording (used to ascertain proper positioning of the tip; Debellis et al., 1998) omitted for simplicity. Top bars (red) show changes in the serosal and bottom bars (blue) in the luminal solution. Vt did not change in response to [Ca2+]ext alterations, but it increased after 100 μM carbachol (by −6.3 ± SEM 0.9 mV; P < 0.0001, n = 10); pHgl increased by 0.13 ± 0.01 (P < 0.0001) in response to bilateral changes in [Ca2+]ext, by 0.06 ± 0.01 (P < 0.0001) in response to unilateral increase in luminal [Ca2+]ext, and by 0.11 ± 0.01 pH units (P < 0.0001) in response to carbachol, but did not change in response to unilateral decrease in serosal [Ca2+]ext (n = 10).
We used the same technique in experiments designed to examine alkaline secretion by the OC. Mimicking carbachol-induced fluctuations in [Ca2+]ext also resulted in an increase in pHgl similar to the one observed in response to carbachol itself (Fig. 2; Debellis et al., 1998).
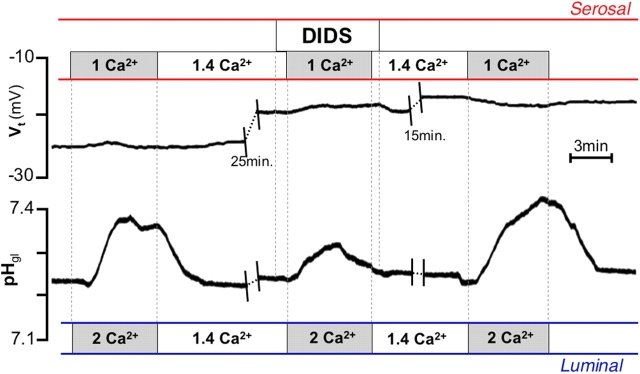
As shown earlier, carbachol-induced luminal alkalinization is sensitive to specific inhibitors of anionic pathways such as the stilbene derivative, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), as it is driven by a basolateral Na+-(HCO3 −)n cotransporter mediating the base uptake (Curci et al., 1994). Fig. 3 shows that the response elicited by bilateral [Ca2+]ext changes was also partially sensitive to DIDS. Noteworthy, the response to bilateral [Ca2+]ext changes was not accompanied by the increase in transepithelial potential (Fig. 2, Vt) typically observed in response to carbachol (Debellis et al., 1998). This change in Vt is caused by the opening of basolateral Ca2+-activated K+ channels (Ueda and Okada, 1989), and indirectly reflects changes in intracellular [Ca2+].
Figure 3.
Effect of DIDS on alkaline secretion elicited by bilateral [Ca2 + ]ext changes. Exposure to 200 μM serosal DIDS resulted in significant reduction of the response to [Ca2+]ext changes (from 0.10 ± 0.02 to 0.04 ± 0.01 pH units; P < 0.01, n = 7).
Although decreasing basolateral [Ca2+]ext alone did not change pHgl, unilateral elevation in luminal [Ca2+]ext was able, by itself, to increase pHgl. (Fig. 2). Interestingly, this effect was significantly smaller than the one observed during bilateral [Ca2+]ext changes. Thus, only luminal elevation of [Ca2+]ext can independently trigger alkaline secretion, but simultaneous decrease in serosal [Ca2+]ext has a significant potentiating effect.
In separate experiments, a higher Ca2+ gradient (0.5 mM serosal and 3 mM luminal) was imposed, but responses were not significantly different from those observed with 1 μM Ca2+ serosal/ 2 μM Ca2+ luminal in the same glands (pHgl increased by 0.108 ± 0.019 and by 0.110 ± 0.020, respectively; n = 4).
Involvement of CaR
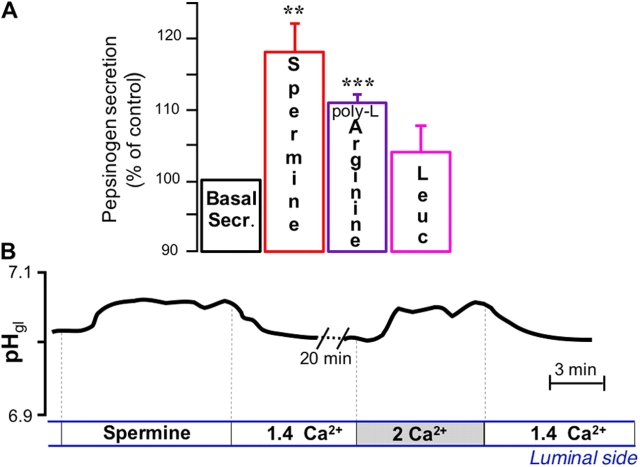
Because we recently provided evidence that OCs possess an extracellular CaR (Brown et al., 1993; Brown and MacLeod, 2001; Hofer and Brown, 2003) located in their apical membrane (Caroppo et al., 2001), we tested whether luminal Ca2+ may act via CaR. Fig. 4 shows that the CaR agonist spermine (Quinn et al., 1997), an endogenous polyamine widely present in the gastrointestinal tract (Fujiwara et al., 1996), could also reproduce the secretory activities induced by carbachol and luminal [Ca2+] elevation, i.e., stimulation of pepsinogen (Fig. 4 A) and alkaline (Fig. 4 B) secretion. The stimulatory effect of spermine on pepsinogen secretion was also visualized in immunohistochemical experiments, revealing a weak granular staining similar to that observed after stimulation with [Ca2+]ext changes or with carbachol (Fig. 1 d). Another potent agonist of CaR, poly-l-arginine, was also able to stimulate pepsinogen secretion, whereas the less potent leucine (Conigrave et al., 2000) did not significantly affect secretion of the proenzyme (Fig. 4 A).
Figure 4.
Involvement of CaR: effect of spermine and amino acids. (A) Effect of 1 mM luminal spermine (n = 8), 1 mM poly-l-arginine (n = 3), and 1 mM leucine (n = 3) on basal pepsinogen secretion. **, P < 0.02; ***, P < 0.001. (B) Effect of luminal spermine and of increase in luminal [Ca2+]ext on pHgl. The increase in pHgl elicited by spermine (0.06 ± 0.01 pH units; P < 0.01, n = 7) was similar to that elicited by increase in luminal [Ca2+]ext. Spermine and high luminal [Ca2+]ext did not affect Vt (not depicted).
Thus, it appears that the two secretory responses evoked by carbachol may be due in part to activation of luminal CaR, after carbachol-induced extrusion of Ca2+ in the microdomain close to the apical pole of the cells. We reasoned that the secretory response to carbachol should be prevented if we were to prevent the luminal increase in free external [Ca2+] using a low affinity Ca2+ buffer, citrate (Hofer et al., 2000; De Luisi and Hofer, 2003).
Luminal citrate alone did not change intracellular pH (pHi) or membrane potential (measured with intracellular double-barreled microelectrodes; pHi 7.27 ± SEM 0.10 before vs. 7.27 ± 0.11 pH units after citrate; Vm −28.4 ± 8.6 mV before vs. −27.0 ± 8.9 mV after; n = 5, respectively), and did not alter resting pHgl (control 7.38 ± 0.05 vs. 7.37 ± 0.04 pH units; n = 6). We also verified that citrate buffer did not impair the ability of glandular cells to secrete acid by monitoring pHgl changes in response to histamine (unpublished data). In separate experiments we also found that citrate buffer did not affect basal secretion of pepsinogen (from 2.26 to 2.27 mg/ml; n = 2).
The actual Ca2+-buffering power of the solution containing citrate was determined in vitro in the range of 1.4–2.0 mM Ca2+ using Ca2+ minielectrodes (Fig. 5 A, inset). Using double-barreled Ca2+-sensitive microelectrodes inserted in the gland lumen, we tested the efficacy of the buffer in preventing carbachol-induced increases in luminal [Ca2+]ext in situ. As shown in Fig. 5 A, carbachol-induced elevation in luminal [Ca2+]ext was abolished in the presence of citrate buffer, whereas notably, the transepithelial hyperpolarization was unaffected, providing additional evidence that the buffer did not alter intracellular calcium signaling.
Figure 5.
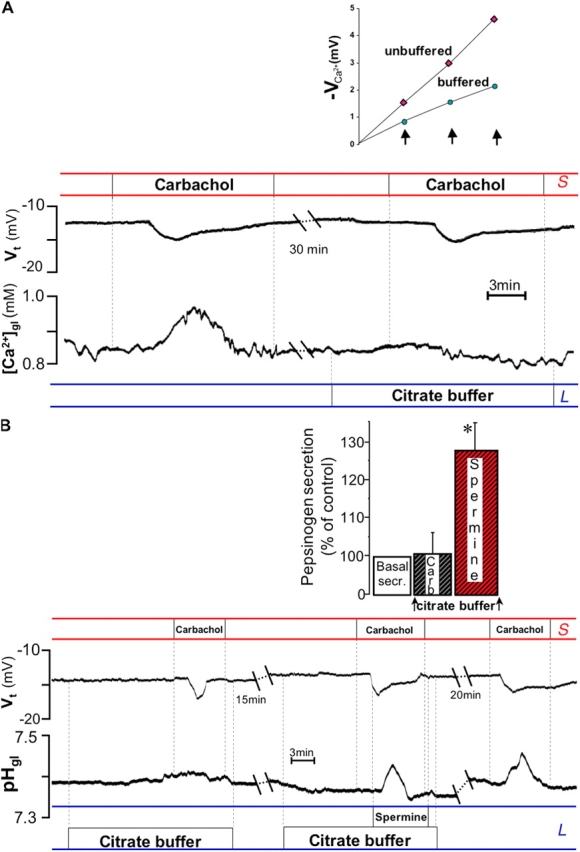
Effect of buffering luminal [Ca 2+ ] ex t increase. (A) Measurement of [Ca2+]ext in the gland lumen with Ca2+-sensitive double-barreled microelectrodes. Inset: Ca2+ buffering power of citrate Ringer's solution. Graph shows effect on potential measured by Ca2+-selective microelectrode (−VCa 2+) on addition of 200-μM aliquots of CaCl2 (arrows). Starting solution contained 1.4 mM added CaCl2; buffering power of citrate solution was at least twice as great as that of control solution at 2 mM Ca2+. Carbachol was not able to elicit an increase in luminal [Ca2+]ext in the presence of the buffer (348.0 ± 70.6 before vs. 23.7 ± 24.0 μM after addition of citrate; P < 0.01, n = 4). (B) Top: in the presence of citrate buffer, basal pepsinogen secretion did not change significantly after carbachol (n = 4), but did increase significantly in response to spermine (*, P < 0.05; n = 5). Bottom: in the presence of citrate buffer, carbachol still increased Vt (by −6.7 ± 1.34, top trace), but was no longer able to stimulate alkaline secretion (by −0.01 ± 0.01, bottom trace). In the same gland, spermine, in the presence of carbachol and citrate, increased pHgl (by 0.060 ± 0.001 pH units; P < 0.01, n = 4). The pHgl response to carbachol was recovered after removal of the buffer.
Fig. 5 B shows that in the presence of luminal citrate (i.e., when carbachol-induced increase in luminal [Ca2+]ext was buffered), carbachol failed to stimulate either pepsinogen or alkaline secretion, whereas spermine was still able to increase alkaline and pepsinogen secretion. Again, the increase in Vt induced by cholinergic stimulation was maintained in the presence of citrate. Alkaline secretion elicited by another Ca2+-mobilizing agonist, pentagastrin (50 μM; 0.07 ± 0.01, n = 3), was similarly blocked in the presence of citrate buffer (0.020 ± 0.001, n = 2). These findings are consistent with a mechanism whereby secretory responses are stimulated by extracellular Ca2+ via the resident CaR located in the luminal membrane of the OC.
The action of [Ca2+]ext is not mediated by [Ca2+]i
Stimulation of CaR has been reported to be associated with [Ca2+]i increases in many cell types, although the receptor is also linked to several other signaling cascades, e.g., inhibition of cAMP production through Giα (Chen et al., 1989; Brown and MacLeod, 2001; Hofer and Brown, 2003). Therefore, we were somewhat surprised that maneuvers designed to stimulate CaR (such as high luminal Ca2+ or spermine) apparently failed to elicit intracellular Ca2+ signals, judging from the lack of Vt responses of the tissue. The experiment in Fig. 6 A supports this view. After prolonged exposure to the sarco-ER ATPase inhibitor 2,5-di-(tert-butyl)hydroquinone (tBHQ), i.e., after store depletion, spermine was still able to elicit a pHgl increase. In addition, no change in pHgl was observed immediately after exposure to tBHQ (i.e., when an increase in [Ca2+]i is generally monitored). Furthermore, when BAPTA-AM was used to buffer intracellular Ca2+ increases, the response to spermine remained unaltered (Fig. 6 B, bottom), whereas the alkalinization induced by carbachol was completely depressed after BAPTA-AM pretreatment in the same glands (Fig. 6 B, top). Likewise, in the presence of BAPTA-AM, spermine was still able to significantly stimulate pepsinogen secretion (from 2.12 ± 022 to 2.85 ± 0.32 mg/ml; n = 5, P < 0.01). Thus, the stimulatory action of spermine on both alkaline and pepsinogen secretion does not appear to be mediated by intracellular [Ca2+].
Figure 6.
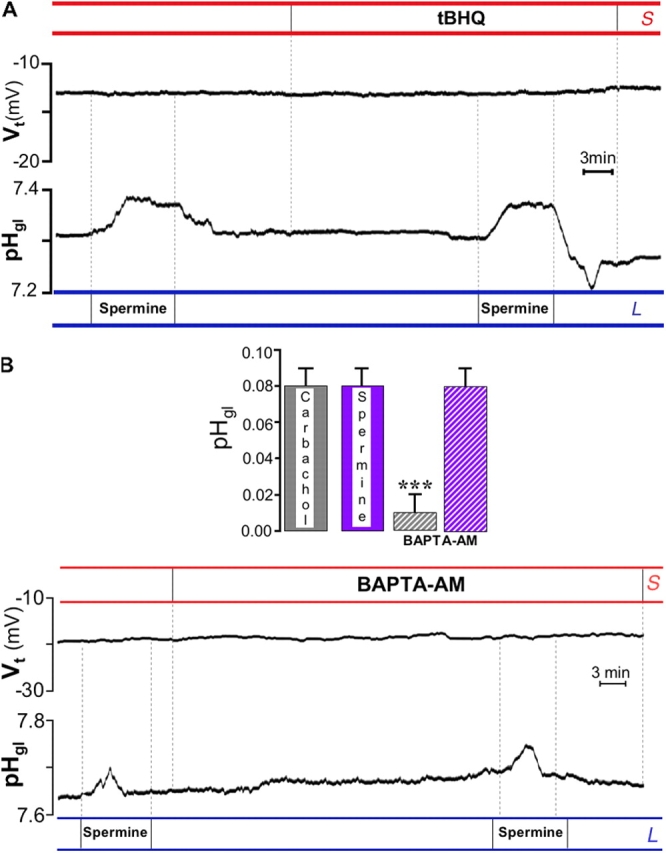
Responses are independent of intracellular Ca 2+ signals. (A) After prolonged (20 min) exposure to the sarco-ER ATPase pump inhibitor tBHQ (15 μM), spermine was still able to stimulate the increase in pHgl (0.070 ± 0.001 pH units before and 0.08 ± 0.01 after tBHQ; n = 4). (B) After pretreatment with 50 μM BAPTA-AM, spermine was still able to alkalinize the gland lumen. In the same glands, the response to carbachol was instead significantly depressed by BAPTA-AM (top, n = 3). ***, P < 0.002.
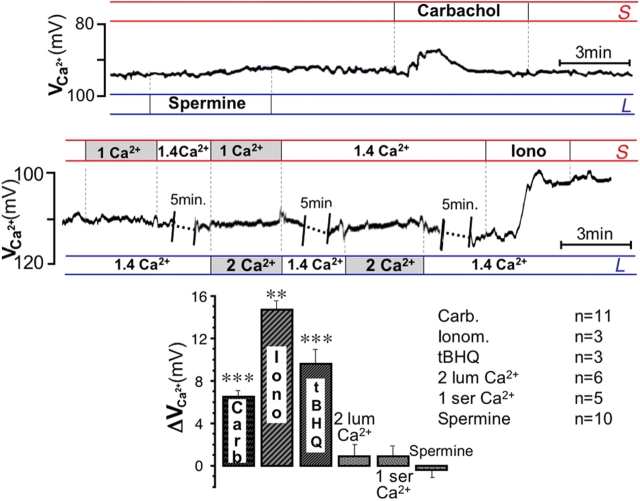
Direct evidence that [Ca2+]i does not increase in the OC in response to stimulation with luminal extracellular Ca2+ or spermine was obtained in experiments where Ca2+-selective microelectrodes were used to measure [Ca2+]i. The latter technique was chosen in alternative to the widely used fluorimetric approach in order to preserve the geometry of the intact, polarized tissue, allowing us to perform all experiments in exactly the same conditions. Fig. 7 shows that although carbachol, ionomycin, and tBHQ were able to elicit significant changes in [Ca2+]i, stimulation with luminal and/or serosal Ca2+ or spermine did not result in measurable changes in [Ca2+]i. Thus, OC may be similar to some other cell types where poor or absent coupling of CaR to intracellular Ca2+ signaling cascades has also been reported (Bruce et al., 1999; Desfleurs et al., 1999; Hofer and Brown, 2003).
Figure 7.
Intracellular Ca 2+ does not change after stimulation with spermine or external Ca 2+ . Changes in OC [Ca2+]i recorded with intracellular double-barreled Ca2+-sensitive microelectrodes. Potentials recorded by the selective barrel are displayed as VCa and have not been converted to free [Ca2+]i; a decrease in potential indicates an increase in [Ca2+]i. Only traces from the selective barrel are shown. Top: response to 2 mM luminal spermine and 100 μM carbachol. Middle: effect of bilateral changes in [Ca2+]ext and 10 μM ionomycin. Bottom: summary of responses. **, P < 0.01; ***, P < 0.001.
Because it appeared that elevation of [Ca2+]i was not necessary to elicit alkaline or pepsinogen secretion, we considered the possibility that CaR agonists might be exerting effects on Giα to inhibit adenylyl cyclase (AC) and reduce basal cellular cyclic AMP levels. We tested this possibility by using an inhibitor of AC, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ 22,536; Harris et al., 1979). As shown in Fig. 8 A, treatment with SQ 22,536 alone resulted in an alkalinization of the gland lumen equivalent to the one elicited by spermine in the same glands. Spermine added on top of SQ 22,536 (i.e., during inhibition of AC) was unable to increase pHgl. Thus, a pharmacological tool known to inhibit cAMP production was able to reproduce the response to spermine, whereas the latter was unable to stimulate alkaline secretion when cAMP levels were already depressed. More direct evidence that high luminal Ca2+ acts via Gi derives from experiments where the Gi inhibitor pertussis toxin (PTX; Locht and Antoine, 1995) was used. As shown in Fig. 8 B, after treatment with PTX, neither spermine nor carbachol were able to elicit alkalinization of the gland lumen, whereas the carbachol-induced increase in Vt remained unaltered. Importantly, direct inhibition of AC with SQ 22,536 was still able to elicit the alkalinization of the gland lumen (Fig. 8 B, top left), indicating that signaling pathways distal to cAMP production were still operative after PTX.
Figure 8.
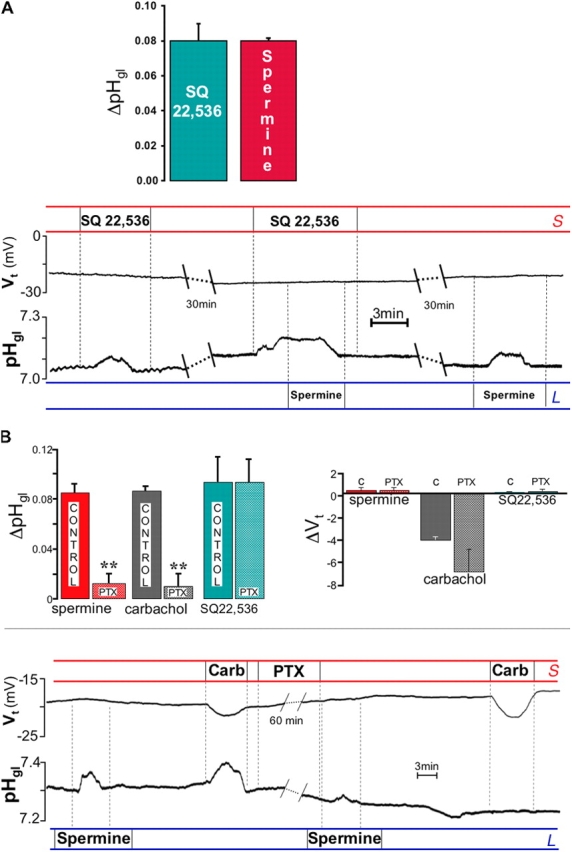
Involvement of the cAMP pathway. (A) The AC inhibitor SQ 22,536 (100 μM) increased pH in the gland lumen, mimicking the response elicited by spermine in the same glands. The responses to SQ 22,536 and spermine were comparable (top, n = 3). Spermine added on top of SQ 22,536 did not elicit any additional alkalinization of the gland lumen (ΔpH = −0.010 ± 0.006 pH units; n = 4). (B) Pretreatment with the Gi inhibitor pertussis toxin (PTX) abolished the increase in pHgl normally elicited by spermine (ΔpH = 0.085 ± 0.006 pH units before and 0.013 ± 0.008 after PTX; n = 4; **, P < 0.01) or carbachol (ΔpH = 0.087 ± 0.003 pH units before and 0.010 ± 0.010 after PTX; n = 3; **, P < 0.01). However, SQ 22,536 was still able to evoke gland lumen alkalinization after PTX treatment (ΔpH = 0.093 ± 0.020 pH units before and 0.093 ± 0.019 after PTX; n = 3). Vt response to carbachol stimulation was not affected by PTX. Top: summary of responses to 2 mM luminal spermine, 100 μM carbachol, and 100 μM SQ 22,536 after a 1-h treatment with 0.25 μg/ml PTX. Left: effect on alkaline secretion; right: effect on Vt. Bottom: trace recording showing responses to luminal spermine, carbachol, and SQ 22,536 after treatment with PTX.
Function of low serosal Ca2+
Although lowering basolateral Ca2+ did not, per se, exert any stimulatory effect on the secretory activities of gastric cells, this maneuver resulted in an amplification of the stimulatory effect of high luminal Ca2+ (Fig. 2). As shown in Fig. 7, the amplifying effect of serosal Ca2+ does not appear to be mediated by intracellular Ca2+. In an attempt to understand the mechanism of action of low serosal Ca2+, we performed experiments using intracellular double-barreled pH microelectrodes. As shown in Fig. 9, lowering serosal Ca2+ resulted in a significant increase in intracellular pH (ΔpHi = 0.13 ± 0.01; n = 6, P < 0.001) that was significantly and reversibly inhibited by DIDS (ΔpHi = 0.04 ± 0.02; n = 6, P < 0.01). Neither Vt nor the serosal membrane potential (Vs) was affected by decreasing serosal Ca2+. The increase in pHi appears to be a specific response to lowering serosal Ca2+ because elevation of luminal Ca2+ did not affect cell pH in the same cells (Fig. 9). In two experiments we also found that increasing serosal Ca2+ to 2 mM did not affect cell pH.
Figure 9.
Effect of lowering serosal [Ca2 + ] on intracellular pH. Decreasing serosal Ca2+ led to an increase in cell pH (pHi) that was reversibly inhibited by serosal application of 200 μM DIDS (from 0.13 ± 0.01 to 0.04 ± 0.02 pH units; P < 0.01, n = 6). This maneuver did not change transepithelial or serosal membrane potentials (Vt and Vs, respectively). High luminal Ca2+did not affect pHi, Vs, or Vt.
Discussion
Here, we show that small, physiological alterations in extracellular [Ca2+] secondary to mobilization of intracellular Ca2+ stores can, by themselves, reproduce two secretory activities of the OC—alkaline and pepsinogen secretion. Buffering carbachol- or gastrin-stimulated changes in [Ca2+]ext using extracellular Ca2+ buffers abolished this secretory activity. It appears that the extracellular Ca2+ sensor CaR was at least partially responsible for mediating these actions because luminal application of other CaR agonists (in addition to Ca2+) was able to stimulate pepsinogen and base secretion; however, these effects were independent of changes in [Ca2+]i in the OC. Results using inhibitors of AC and Gi suggest that alkalinization of the gland lumen in resting tissues is likely related to CaR's interaction with Gi, with subsequent suppression of cAMP production.
It appears that the strategic luminal placement of a Ca2+ sensor, CaR, facing a compartment where [Ca2+]ext increases are a consequence of intracellular Ca2+ signaling events, is not accidental, but rather plays an important functional role in this epithelium. The issue that we address here may possibly be extended to different epithelia and tissues. Changes in extracellular [Ca2+] as a function of cellular activity have in fact been recorded outside of a variety of cells, including submandibular gland (Nielsen and Petersen, 1972), cardiac muscle (Hilgemann and Langer, 1984), neurons (Knox et al., 1996), sterocilia (Yamoah et al., 1998), cells of the zona pellucida (Pepperell et al., 1999), intestine (Mupanomunda et al., 1999), and kidney (Mupanomunda et al., 2000). In addition, extracellular Ca2+ sensors such as CaR appear to be widely expressed in a number of different cell types (for review see Brown and MacLeod, 2001; Hofer and Brown, 2003).
One of the most intriguing findings of this analysis was that the basolateral decrement in [Ca2+]ext had a significant potentiating effect on alkaline secretion. It is unlikely that this effect is mediated by the CaR because our previous immunolocalization experiments showed that the receptor is not present in the OC serosal membrane (Caroppo et al., 2001). Furthermore, our data seem to exclude the involvement of intracellular Ca2+ signaling pathways in the action of serosal Ca2+ (Fig. 7). On the other hand, our findings (Fig. 9) suggest that the potentiating action of basolateral Ca2+ may be the result of an increase in intracellular alkali available for secretion. This appears to be achieved by either activation of the electrogenic Na+(HCO3 −)n cotransporter or inhibition of the Cl−/HCO3 − exchanger, both located basolaterally (Machen and Paradiso, 1987; Paradiso et al., 1987; Debellis et al., 1994; Cox et al., 1996; Seidler et al., 2000). Although both transporters are DIDS sensitive, the fact that the intracellular alkalinization induced by low serosal Ca2+ was not accompanied by cell hyperpolarization suggests serosal Ca2+ may directly interact with the electroneutral exchanger and excludes the involvement of DIDS-sensitive Cl− channels. This would not be the first example of cell surface proteins, other than CaR, directly interacting with extracellular Ca2+. Other examples are the gap junction hemichannel, which begins to open in response to a 200-μM decrease in external [Ca2+] in vitro (Quist et al., 2000), or ion channels that are regulated by small changes in extracellular [Ca2+] like the acid-sensing ion channels ASIC1a and ASIC1b, which belong to a class of proton-gated Na+ channels expressed primarily in sensory neurons (Babini et al., 2002).
Although complete understanding of the mechanisms involved in the action of extracellular Ca2+ still requires further investigation, our data indicate that apical and basolateral Ca2+ appear to operate through different mechanisms. Furthermore, simultaneous “stimulation” by external Ca2+ at the opposite poles of the OC seems to be necessary to obtain the complete final response. This suggests that the optimal secretory response is highly dependent on the preservation of the native geometry of the tissue, allowing for the generation of polarized, asymmetrical Ca2+ signals in the extracellular microenvironment.
Our data show that at least for this epithelial model, secretory responses to Ca2+-mobilizing agonists can be directly regulated by extracellular rather than intracellular [Ca2+] changes. Thus, although the importance of Ca2+ as an intracellular second messenger is incontrovertible, it appears that the amphibian gastric mucosa can also use Ca2+ as an extracellular “third messenger”. This elegant, economical tactic extends the capabilities of a single molecule, Ca2+, by taking advantage of the obligate cycling of Ca2+ between intracellular and extracellular compartments during intracellular signaling events. This strategy may allow intracellular and extracellular Ca2+ to control different subsets of tissue functions.
Materials and methods
Tissue preparation and solutions
Experiments were performed on gastric fundus mucosa of Rana esculenta in accordance with the Italian guidelines for animal experiments. After animals were killed by decapitation, the stomach was isolated and the muscle layer and connective tissue were removed by blunt dissection. The mucosa was mounted horizontally between two halves of a horizontal chamber (aperture 0.2 cm2) with the serosal side facing up. The connective tissue layer was further removed with sharpened watchmaker's forceps under direct microscopic observation in order to expose the gastric glands in a limited area. Both the serosal and mucosal surfaces of the tissue were continuously superfused with oxygenated Ringer's solution at RT. Fast fluid exchange in the chamber was achieved within seconds using a shock-free, remote control, eight-way manifold. Control Ringer's solution had the following composition (mM): 102.4 Na+, 4.0 K+, 1.4 Ca2+, 0.8 Mg2+, 91.4 Cl–, 17.8 HCO3 –, and 11 glucose. All experimental solutions were gassed with 5% CO2/95% O2 and had a pH of 7.36. Citrate buffer solution was prepared as described previously (Hofer et al., 2000; De Luisi and Hofer, 2003); free [Ca2+] was precisely matched in the buffered (+citrate) and unbuffered solutions using a Ca2+-selective minielectrode. All experiments were performed in the presence of 100 μM serosal cimetidine, a histamine H2 receptor blocker used to prevent acid secretion.
Unless otherwise stated, all chemicals were of reagent grade and were purchased from Farmitalia Carlo Erba, Sigma-Aldrich, or Fluka Chemie AG. BAPTA-AM was purchased from Molecular Probes, Inc. Spermine tetrahydrochloride and SQ 22,536 were purchased from Qbiogene.
Electrophysiological measurements
The transepithelial potential difference (Vt) was measured with a high-impedance differential electrometer (model 610C; Keithley) using two flowing-boundary, calomel half-cells filled with 3 M KCl solution, which were connected to each bath solution downstream of the tissue. The serosal bath was connected to ground.
Gastric gland lumen or cells were punctured with double-barreled microelectrodes mounted on a micromanipulator (Leitz) connected to a dual-channel electrometer (model FD-223; World Precision Instruments) and to a strip-chart recorder (Kipp & Zonen). Measurements of pH or [Ca2+] in the lumen of single gastric glands were achieved by first puncturing an OC and, after the basolateral cell membrane had been recorded, gradually advancing the electrode until the tip entered the gland lumen (Fig. 2 A, inset). Correct positioning of microelectrode tip in the gland lumen was established using criteria described in detail elsewhere (Debellis et al., 1998; Caroppo et al., 2001).
Microelectrodes
Double-barreled pH-sensitive microelectrodes were constructed as described previously (Debellis et al., 1998). In brief, two pieces of filament-containing aluminum silicate glass tubing of different diameters (Hilgenberg) were twisted together. Capillaries were then pulled (tip length ∼20 mm) in a PE2 vertical puller (Narishige). The thick channel was silanized in dimethyldichlorosilane vapor. The tip was back-filled with H+ ligand (Hydrogen Ionophore II, Cocktail A; Fluka Chemie AG), and the shaft was filled with a Hepes-buffered Ringer's solution containing (mM): 3.2 KCl, 84.6 NaCl, 3.2 MgCl2, 25 Hepes, and 1.4 CaCl2, pH 7.0.
The reference channel was filled with 3 M KCl, and an Ag–AgCl wire was inserted and sealed in place with wax to prevent leak currents. Average slope and resistance of the electrodes were (mean ± SEM, n = 35) 55.83 ± 0.60 mV/pH unit, 310 ± 30 GΩ (selective channel), and 199 ± 18 MΩ (reference channel). All microelectrodes were calibrated in the chamber before and after each puncture by flushing the chamber with Hepes-buffered Ringer's solutions with pH between 6.8 and 7.8.
For Ca2+-selective microelectrodes, Calcium Ionophore I, Cocktail A was used. Microelectrodes were calibrated in place with a Hepes-buffered Ringer's solution containing different CaCl2 concentrations (0.5, 1.0, and 2.0 mM). Average slope of the electrodes was 28.0 ± 0.4 mV (n = 17) per decade change in [Ca2+]. Slope of microelectrodes was not affected by pH (between 4.0 and 8.4), spermine, citrate buffer, or tBHQ at the concentrations used. Average resistance of microelectrodes was 92.2 ± 23.0 GΩ (selective channel) and 125 ± 9 MΩ (reference channel).
For intracellular [Ca2+] measurements, the shaft of the selective channel was filled with a Hepes-buffered solution containing 0.01 mM CaCl2 (Thomas, 2002). Each microelectrode was tested by monitoring the change in potential in response to a Ca2+ concentration step from 0.14 to 10−7 M; electrodes with changes <100 mV were discarded (average change in potential of electrodes used was 115 ± 2.42 mV, n = 21). Criteria adopted to assess reliability of intracellular measurements are reported elsewhere (Debellis et al., 1992).
Pepsinogen secretion
Fundic mucosa was mounted in a vertical chamber and the luminal solution was removed every 10 min. Pepsinogen secretion was assessed by measuring proteolytic activity (Ruiz et al., 1993), using BSA as substrate and after activation of the proenzyme to pepsin by exposure to 1 N HCl. Absorbance was measured at 750 nm (Spectrophotometer; Varian Inc.). Peak pepsinogen release was calculated as percentage of total pepsin activity.
Immunofluorescence
Thin frozen sections (5-μM thick) of frog stomach were treated as described previously (Caroppo et al., 1997) and incubated for 2 h at RT with an affinity-purified mAb against human pepsinogen I (1:1,000; DPC Biermann). After rinsing in PBS, sections were incubated for 1 h at RT with fluorescein-conjugated goat anti–mouse IgG (1:50; Ancell Corp.). Controls for nonspecific staining were performed by omission of the primary antibody. Fluorescence images were acquired with a microscope (Leica) and a cooled CCD camera (Princeton Instruments).
Data analysis and statistics
All measurements were quantified as mean values ± SEM of n individual measurements. The significance of the observations was evaluated by t test for paired data.
Acknowledgments
We thank Patrizia Leone, Roberta Longo, Annalisa Mira, and Mariangela Satalino for excellent assistance during experiments.
R. Caroppo, L. Debellis, and S. Curci were supported by Cofin, MURST (Rome, I), and by Finanziamenti di Ateneo (Bari, I). A. Gerbino and G. Fisetto were supported by doctoral fellowships awarded jointly from the University of Bari and the European Community (FSE). A.M. Hofer was supported by grants from the Medical Research Service of the Veteran's Administration and by a Harvard Digestive Diseases Center grant.
Abbreviations used in this paper: AC, adenylyl cyclase; CaR, Ca2+-sensing receptor; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; OC, oxyntopeptic cell; PMCA, plasma membrane Ca2+ ATPase; PTX, pertussis toxin; SQ 22,536, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine; tBHQ, 2,5-di-(tert-butyl)hydroquinone.
References
- Ashby, M.C., and A.V. Tepikin. 2002. Polarized calcium and calmodulin signaling in secretory epithelia. Physiol. Rev. 82:701–734. [DOI] [PubMed] [Google Scholar]
- Babini, E., M. Paukert, H.S. Geisler, and S. Grunder. 2002. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J. Biol. Chem. 277:41597–41603. [DOI] [PubMed] [Google Scholar]
- Belan, P., O. Gerasimenko, A. Tepikin, and O. Petersen. 1996. Localization of Ca2+ extrusion sites in pancreatic acinar cells. J. Biol. Chem. 271:7615–7619. [DOI] [PubMed] [Google Scholar]
- Belan, P., O. Gerasimenko, O.H. Petersen, and A.V. Tepikin. 1997. Distribution of Ca2+ extrusion sites on the mouse pancreatic acinar cell surface. Cell Calcium. 22:5–10. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J., M.D. Bootman, and H.L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529. [DOI] [PubMed] [Google Scholar]
- Brown, E.M., and R.J. MacLeod. 2001. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 81:239–297. [DOI] [PubMed] [Google Scholar]
- Brown, E.M., G. Gamba, D. Riccardi, M. Lombardi, R. Butters, O. Kifor, A. Sun, M.A. Hediger, J. Lytton, and S.C. Hebert. 1993. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 366:575–580. [DOI] [PubMed] [Google Scholar]
- Bruce, J.I., X. Yang, C.J. Ferguson, A.C. Elliott, M.C. Steward, R.M. Case, and D. Riccardi. 1999. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J. Biol. Chem. 274:20561–20568. [DOI] [PubMed] [Google Scholar]
- Carlisle, K.S., C.S. Chew, and S.J. Hersey. 1978. Ultrastructural changes and cyclic AMP in frog oxyntic cells. J. Cell Biol. 76:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo, R., L. Debellis, G. Valenti, S. Alper, E. Fromter, and S. Curci. 1997. Is resting state HCO3 − secretion in frog gastric fundus mucosa mediated by apical Cl(−)-HCO3 − exchange? J. Physiol. 499:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo, R., A. Gerbino, L. Debellis, O. Kifor, D.I. Soybel, E.M. Brown, A.M. Hofer, and S. Curci. 2001. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia. EMBO J. 20:6316–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek, T.R. 1991. Calcium signalling and the triggering of secretion in adrenal chromaffin cells. Pharmacol. Ther. 52:173–189. [DOI] [PubMed] [Google Scholar]
- Chen, C.J., J.V. Barnett, D.A. Congo, and E.M. Brown. 1989. Divalent cations suppress 3′,5′-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology. 124:233–239. [DOI] [PubMed] [Google Scholar]
- Conigrave, A.D., S.J. Quinn, and E.M. Brown. 2000. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. USA. 97:4814–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, K.H., T.L. Adair-Kirk, and J.V. Cox. 1996. Variant AE2 anion exchanger transcripts accumulate in multiple cell types in the chicken gastric epithelium. J. Biol. Chem. 271:8895–8902. [DOI] [PubMed] [Google Scholar]
- Curci, S., L. Debellis, R. Caroppo, and E. Fromter. 1994. Model of bicarbonate secretion by resting frog stomach fundus mucosa. I. Transepithelial measurements. Pflugers Arch. 428:648–654. [DOI] [PubMed] [Google Scholar]
- De Luisi, A., and A.M. Hofer. 2003. Evidence that Ca2+ cycling by the plasma membrane Ca2+-ATPase increases the ‘excitability’ of the extracellular Ca2+-sensing receptor. J. Cell Sci. 116:1527–1538. [DOI] [PubMed] [Google Scholar]
- Debellis, L., S. Curci, and E. Fromter. 1990. Effect of histamine on the basolateral K+ conductance of frog stomach oxyntic cells and surface epithelial cells. Am. J. Physiol. 258:G631–G636. [DOI] [PubMed] [Google Scholar]
- Debellis, L., S. Curci, and E. Fromter. 1992. Microelectrode determination of oxyntic cell pH in intact frog gastric mucosa. Effect of histamine. Pflugers Arch. 422:253–259. [DOI] [PubMed] [Google Scholar]
- Debellis, L., C. Iacovelli, E. Fromter, and S. Curci. 1994. Model of bicarbonate secretion by resting frog stomach fundus mucosa. II. Role of the oxyntopeptic cells. Pflugers Arch. 428:655–663. [DOI] [PubMed] [Google Scholar]
- Debellis, L., R. Caroppo, E. Fromter, and S. Curci. 1998. Alkaline secretion by frog gastric glands measured with pH microelectrodes in the gland lumen. J. Physiol. 513:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desfleurs, E., M. Wittner, S. Pajaud, R. Nitschke, R.M. Rajerison, and A. Di Stefano. 1999. The Ca2+-sensing receptor in the rabbit cortical thick ascending limb (CTAL) is functionally not coupled to phospholipase C. Pflugers Arch. 437:716–723. [DOI] [PubMed] [Google Scholar]
- Flemstrom, G., and J.I. Isenberg. 2001. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol. Sci. 16:23–28. [DOI] [PubMed] [Google Scholar]
- Forte, J.G. 1968. The effect of inhibitors of HCl secretion on the unidirectional fluxes of chloride across bullfrog gastric mucosa. Biochim. Biophys Acta. 150:136–145. [DOI] [PubMed] [Google Scholar]
- Fujiwara, K., Y. Masuyama, and T. Kitagawa. 1996. Immunocytochemical localization of polyamines in the gastrointestinal tracts of rats and mice. Histochem. Cell Biol. 106:465–471. [DOI] [PubMed] [Google Scholar]
- Gordjani, N., R. Nitschke, R. Greger, and J. Leipziger. 1997. Capacitative Ca2+ entry (CCE) induced by luminal and basolateral ATP in polarised MDCK-C7 cells is restricted to the basolateral membrane. Cell Calcium. 22:121–128. [DOI] [PubMed] [Google Scholar]
- Harris, D.N., M.M. Asaad, M.B. Phillips, H.J. Goldenberg, and M.J. Antonaccio. 1979. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J. Cyclic Nucleotide Res. 5:125–134. [PubMed] [Google Scholar]
- Helander, H.F. 1978. Quantitative ultrastructural studies on rat gastric zymogen cells under different physiological and experimental conditions. Cell Tissue Res. 189:287–303. [DOI] [PubMed] [Google Scholar]
- Hersey, S.J., M. Miller, D. May, and S.H. Norris. 1983. Lack of interaction between acid and pepsinogen secretion in isolated gastric glands. Am. J. Physiol. 245:G775–G779. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D.W., and G.A. Langer. 1984. Transsarcolemmal calcium movements in arterially perfused rabbit right ventricle measured with extracellular calcium-sensitive dyes. Circ. Res. 54:461–467. [DOI] [PubMed] [Google Scholar]
- Hirschowitz, B.I. 1967. The control of pepsinogen secretion. Ann. N. Y. Acad. Sci. 140:709–723. [DOI] [PubMed] [Google Scholar]
- Hofer, A.M., and E.M. Brown. 2003. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 4:530–538. [DOI] [PubMed] [Google Scholar]
- Hofer, A.M., B. Landolfi, L. Debellis, T. Pozzan, and S. Curci. 1998. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J. 17:1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer, A.M., S. Curci, M.A. Doble, E.M. Brown, and D.I. Soybel. 2000. Intercellular communication mediated by the extracellular calcium-sensing receptor. Nat. Cell Biol. 2:392–398. [DOI] [PubMed] [Google Scholar]
- Kasai, H., and G.J. Augustine. 1990. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 348:735–738. [DOI] [PubMed] [Google Scholar]
- Kasbekar, D.K., R.P. Durbin, and D. Lindley. 1965. An adenosine triphosphatase from frog gastric mucosa. Biochim. Biophys. Acta. 105:472–482. [DOI] [PubMed] [Google Scholar]
- Kerstan, D., J. Thomas, R. Nitschke, and J. Leipziger. 1999. Basolateral store-operated Ca2+-entry in polarized human bronchial and colonic epithelial cells. Cell Calcium. 26:253–260. [DOI] [PubMed] [Google Scholar]
- Kidd, J.F., and P. Thorn. 2000. Intracellular Ca2+ and Cl− channel activation in secretory cells. Annu. Rev. Physiol. 62:493–513. [DOI] [PubMed] [Google Scholar]
- Knox, R.J., E.A. Jonas, L.S. Kao, P.J. Smith, J.A. Connor, and L.K. Kaczmarek. 1996. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J. Physiol. 494:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.G., X. Xu, W. Zeng, J. Diaz, T.H. Kuo, F. Wuytack, L. Racymaekers, and S. Muallem. 1997. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 272:15771–15776. [DOI] [PubMed] [Google Scholar]
- Locht, C., and R. Antoine. 1995. A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit. Biochimie. 77:333–340. [DOI] [PubMed] [Google Scholar]
- Machen, T.E., and W.L. McLennan. 1980. Na+-dependent H+ and Cl− transport in in vitro frog gastric mucosa. Am. J. Physiol. 238:G403–G413. [DOI] [PubMed] [Google Scholar]
- Machen, T.E., and A.M. Paradiso. 1987. Regulation of intracellular pH in the stomach. Annu. Rev. Physiol. 49:19–33. [DOI] [PubMed] [Google Scholar]
- Mupanomunda, M.M., N. Ishioka, and R.D. Bukoski. 1999. Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am. J. Physiol. 276:H1035–H1042. [DOI] [PubMed] [Google Scholar]
- Mupanomunda, M.M., B. Tian, N. Ishioka, and R.D. Bukoski. 2000. Renal interstitial Ca2+. Am. J. Physiol. Renal Physiol. 278:F644–F649. [DOI] [PubMed] [Google Scholar]
- Nielsen, S.P., and O.H. Petersen. 1972. Transport of calcium in the perfused submandibular gland of the cat. J. Physiol. 223:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso, A.M., R.Y. Tsien, J.R. Demarest, and T.E. Machen. 1987. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am. J. Physiol. 253:C30–C36. [DOI] [PubMed] [Google Scholar]
- Pepperell, J.R., K. Kommineni, S. Buradagunta, P.J. Smith, and D.L. Keefe. 1999. Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol. Reprod. 60:1137–1143. [DOI] [PubMed] [Google Scholar]
- Petersen, O.H., C.C. Petersen, and H. Kasai. 1994. Calcium and hormone action. Annu. Rev. Physiol. 56:297–319. [DOI] [PubMed] [Google Scholar]
- Putney, J.W., Jr. 1990. Capacitative calcium entry revisited. Cell Calcium. 11:611–624. [DOI] [PubMed] [Google Scholar]
- Quinn, S.J., C.P. Ye, R. Diaz, O. Kifor, M. Bai, P. Vassilev, and E. Brown. 1997. The Ca2+-sensing receptor: a target for polyamines. Am. J. Physiol. 273:C1315–C1323. [DOI] [PubMed] [Google Scholar]
- Quist, A.P., S.K. Rhee, H. Lin, and R. Lal. 2000. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 148:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman, J.P. 1992. Gastric chief cells: receptors and signal-transduction mechanisms. Gastroenterology. 102:699–710. [DOI] [PubMed] [Google Scholar]
- Ruiz, M.C., A. Acosta, M.J. Abad, and F. Michelangeli. 1993. Nonparallel secretion of pepsinogen and acid by gastric oxyntopeptic cells of the toad (Bufo marinus). Am. J. Physiol. 265:G934–G941. [DOI] [PubMed] [Google Scholar]
- Sedova, M., and L.A. Blatter. 1999. Dynamic regulation of [Ca2+]i by plasma membrane Ca2+-ATPase and Na+/Ca2+ exchange during capacitative Ca2+ entry in bovine vascular endothelial cells. Cell Calcium. 25:333–343. [DOI] [PubMed] [Google Scholar]
- Seidler, U., H. Rossmann, P. Jacob, O. Bachmann, S. Christiani, G. Lamprecht, and M. Gregor. 2000. Expression and function of Na+HCO3 − cotransporters in the gastrointestinal tract. Ann. N.Y. Acad. Sci. 915:1–14. [DOI] [PubMed] [Google Scholar]
- Shoemaker, R.L., and G. Sachs. 1972. Microelctrode studies of Necturus gastric mucosa. Gastric Secretion. Academic Press Inc., New York/London. 147–163.
- Silen, W., T.E. Machen, and J.G. Forte. 1975. Acid-base balance in amphibian gastric mucosa. Am. J. Physiol. 229:721–730. [DOI] [PubMed] [Google Scholar]
- Supplisson, S., D.D. Loo, and G. Sachs. 1991. Diversity of K+ channels in the basolateral membrane of resting Necturus oxyntic cells. J. Membr. Biol. 123:209–221. [DOI] [PubMed] [Google Scholar]
- Tepikin, A.V., S.G. Voronina, D.V. Gallacher, and O.H. Petersen. 1992. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J. Biol. Chem. 267:3569–3572. [PubMed] [Google Scholar]
- Thomas, R.C. 2002. The effects of HCl and CaCl2 injections on intracellular calcium and pH in voltage-clamped snail (Helix aspersa) neurons. J. Gen. Physiol. 120:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu, E.C., A.M. Lawrie, O.H. Petersen, and D.V. Gallacher. 1992. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 11:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, F.W., and A. Tse. 1999. Regulation of exocytosis via release of Ca2+ from intracellular stores. Bioessays. 21:861–865. [DOI] [PubMed] [Google Scholar]
- Ueda, S., and Y. Okada. 1989. Acid secretagogues induce Ca2+ mobilization coupled to K+ conductance activation in rat parietal cells in tissue culture. Biochim. Biophys. Acta. 1012:254–260. [DOI] [PubMed] [Google Scholar]
- Usachev, Y.M., S.J. DeMarco, C. Campbell, E.E. Strehler, and S.A. Thayer. 2002. Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron. 33:113–122. [DOI] [PubMed] [Google Scholar]
- Yamoah, E.N., E.A. Lumpkin, R.A. Dumont, P.J. Smith, A.J. Hudspeth, and P.G. Gillespie. 1998. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 18:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]