Abstract
Both in Drosophila and vertebrate epithelial cells, the establishment of apicobasal polarity requires the apically localized, membrane-associated Par-3–Par-6–aPKC protein complex. In Drosophila, this complex colocalizes with the Crumbs–Stardust (Sdt)–Pals1-associated TJ protein (Patj) complex. Genetic and molecular analyses suggest a functional relationship between them. We show, by overexpression of a kinase-dead Drosophila atypical PKC (DaPKC), the requirement for the kinase activity of DaPKC to maintain the position of apical determinants and to restrict the localization of basolateral ones. We demonstrate a novel physical interaction between the apical complexes, via direct binding of DaPKC to both Crb and Patj, and identify Crumbs as a phosphorylation target of DaPKC. This phosphorylation of Crumbs is functionally significant. Thus, a nonphosphorylatable Crumbs protein behaves in vivo as a dominant negative. Moreover, the phenotypic effect of overexpressing wild-type Crumbs is suppressed by reducing DaPKC activity. These results provide a mechanistic framework for the functional interaction between the Par-3–Par-6–aPKC and Crumbs–Sdt–Patj complexes based in the posttranslational modification of Crb by DaPKC.
Keywords: DaPKC; Crumbs; Drosophila; epithelial cell polarity; signal transduction
Introduction
Epithelial cells are polarized along their apicobasal axis. Their plasma membranes are subdivided into apical and basolateral domains that are separated by specialized junctional structures, such as tight junctions (TJ) and the zonula adherens (ZA; for reviews see Drubin and Nelson, 1996; Müller, 2000; Tepass et al., 2001; Roh and Margolis, 2003). Formation and stabilization of these junctions are paramount for the maintenance of epithelial polarity, which, in turn, is required for proper epithelial cell physiology, cell motility, asymmetric division, and intercellular signaling (for review see Tepass et al., 2001). Hence, the functional analysis of the factors that establish and/or maintain epithelial polarity is a major issue in developmental biology.
The mechanisms that underlie the establishment and maintenance of epithelial polarity have been extensively studied in Drosophila. Thus, three membrane-associated multiprotein complexes, the Bazooka (Baz), Crumbs, and Discs large (Dlg) complexes, are required to organize apicobasal polarity in the epithelial cells of the Drosophila embryo (for reviews see Müller, 2000; Ohno, 2001; Tepass et al., 2001; Knust and Bossinger, 2002; Henrique and Schweisguth, 2003; Roh and Margolis, 2003). The Baz complex is formed by the PSD-95/Dlg/ZO-1 domain containing proteins Baz and DPar-6, and the Drosophila atypical PKC (DaPKC), a Ser/Thr kinase (Kuchinke et al., 1998; Wodarz et al., 2000; Petronczki and Knoblich, 2001). This complex, which is localized at the marginal zone of the apico-lateral cell membrane, above the ZA, is formed stepwise during early embryogenesis. Thus, at blastoderm stage, Baz and DaPKC are found at the apical membrane domain (Wodarz et al., 2000; Bilder et al., 2003). Slightly later, at the onset of gastrulation, DPar-6 is incorporated to the Baz–DaPKC complex, where it binds directly to both Baz and DaPKC (Petronczki and Knoblich, 2001). Embryos deficient in Baz or DPar-6 cannot properly assemble the ZA and lack several apical markers of the cell membrane (Müller and Wieschaus, 1996; Petronczki and Knoblich, 2001). Similarly, epithelial cells of DaPKC mutant imaginal discs display disrupted apicobasal polarity (Rolls et al., 2003). Thus, each of the components of the Baz complex is essential to establish epithelial polarity.
The Crumbs complex, which is assembled at gastrulation, is also localized at the marginal zone. It consists of the transmembrane (TM) protein Crumbs (Crb) and the cytoplasmic PDZ-containing proteins Stardust (Sdt) and Pals1-associated TJ protein (Patj; formerly known as Discs lost; Tepass et al., 1990; Bhat et al., 1999; Bachmann et al., 2001; Hong et al., 2001; Pielage et al., 2003). Crb contains 30 epidermal growth factor–like and four laminin A G-domain–like repeats in its extracellular region and a short intracellular domain (Tepass et al., 1990). Expression of this intracellular domain partially normalizes mutant crb embryos (Wodarz et al., 1995; Klebes and Knust, 2000). This suggests that Crb exerts its function, at least in part, by protein–protein interactions mediated by this domain. The intracellular domain has been subdivided into proximal juxtamembrane and COOH-terminal subdomains. The latter contains the binding site for Sdt (Bachmann et al., 2001; Hong et al., 2001; Roh et al., 2002), which on its turn binds to Patj (Roh et al., 2002). The juxtamembrane domain is required for Crb to form a complex with DMoesin and β-heavy spectrin and thus mediates the interaction of the Crb complex with the apical spectrin cytoskeleton (Medina et al., 2002). Genetic studies have shown that the Crb complex is, similarly to the Baz complex, indispensable for the establishment of epithelial apicobasal polarity and stabilization of the ZA (Knust et al., 1993; Grawe et al., 1996; Müller and Wieschaus, 1996; Tepass, 1996; Bachmann et al., 2001; Hong et al., 2001).
The third complex, formed by the PDZ proteins Dlg (Woods and Bryant, 1991) and Scribble (Scrib; Bilder and Perrimon, 2000), and the Myosin type II binding protein Lethal giant larvae (Lgl; Mechler et al., 1985), is found at the basolateral domain of the cell membrane, basal to the ZA. These proteins are required to restrict the Baz and Crb complexes to the apical cell membrane and for correct positioning of the ZA (Bilder et al., 2000, 2003; Bilder and Perrimon, 2000; Tanentzapf and Tepass, 2003).
The three polarity complexes are evolutionarily conserved (for reviews see Ohno, 2001; Roh and Margolis, 2003; Macara, 2004). Thus, in mammalian epithelial cells, the homologues of DaPKC, Baz, and DPar-6, namely aPKC, Par-3/atypical PKC isotype-specific interacting protein (ASIP), and Par-6, and the corresponding homologues of Crb, Sdt, and Patj, that is, Crb3, Pals1 (protein associated with Lin seven 1), and Patj, respectively, form two complexes that are localized at the TJ and regulate the assembly of these junctions. The basolateral proteins Scrib, mDlg, and mLgl also play important roles in epithelial cell polarity (Izumi et al., 1998; Gao et al., 2002; Hirose et al., 2002; Lemmers et al., 2002; Roh et al., 2002, 2003; Suzuki et al., 2001, 2002; Hurd et al., 2003; Straight et al., 2004).
In Drosophila, the Baz and Crb complexes not only colocalize in the apicolateral region of the cytocortex (Hong et al., 2001; Bilder et al., 2003), but they interact physically by means of DPar-6 and Patj (Nam and Choi, 2003). Furthermore, absence of either complex similarly disrupts epithelial polarity (for review see Tepass et al., 2001), which suggests a functional relationship between them. Here, we report a novel physical connection between the Baz and Crb complexes mediated through direct binding of DaPKC to both Crb and Patj. We find that the kinase activity of DaPKC is necessary to maintain the apical localization of the components of the Crb complex and to prevent the apical localization of basolateral markers. Furthermore, Crb is a phosphorylation target of DaPKC. These results provide a molecular mechanism for the functional interaction between the Crb and Baz complexes based on DaPKC-dependent phosphorylation of Crb.
Results
DaPKC kinase activity is required for epithelial polarity in the Drosophila embryo
To gain insight into the role of DaPKC in epithelial cells, we first examined whether or not its kinase activity is required for epithelial polarity, as it is the case in cultured mammalian epithelial cells. In these cells, overexpression of a kinase-defective aPKC disrupts the assembly of the TJ (Suzuki et al., 2001, 2002). We interfered with DaPKC signaling in vivo by overexpressing a form of DaPKC that was targeted to the cell membrane (by addition of the CAAX sequence), and that harbored a mutation (K293W) in the ATP binding site (DaPKC CAAXDN; see Materials and methods). Similar modifications in Xenopus aPKCλ converted it into a kinase-inactive protein that acted as a dominant negative (DN; Berra et al., 1993). We examined the subcellular distribution of apical (Fig. 1, A, D, and G, DaPKC, Crb, and Patj) and basolateral (Fig. 1 J, Scrib) markers in embryos overexpressing DaPKC CAAXDN using the Gal4/UAS system (Brand and Perrimon, 1993) with a maternal Gal4 line (Fig. 1 B). These embryos showed abnormal localization of both types of markers. Thus, Crb and Patj became undetectable in many cells (Fig. 1, E and H), whereas basolateral Scrib was often mislocalized to the apical cell membrane (Fig. 1 L). Importantly, the overexpression of wild-type membrane targeted DaPKC (Fig. 1 C), which should mimic constitutive activation of DaPKC, had the opposite effects. Namely, Crb (Fig. 1 F), Patj (Fig. 1 I), and Baz (not depicted) were found redistributed to the whole cell contour. Conversely, accumulation of the basolateral marker Neurotactin (Nrt; Hortsch et al., 1990), was significantly reduced (Fig. 1, K and M). Overexpression of nonmembrane targeted DaPKC did not modify these markers (not depicted), which indicated that membrane localization was essential for DaPKC to affect cell polarity. Together, these results suggest that DaPKC kinase activity is essential and instructive for proper localization of apical and basolateral proteins and therefore for establishing and/or maintaining cell polarity.
Figure 1.

The kinase activity of DaPKC is required for epithelial cell polarity. In wild-type embryos (A, D, G, J, and K), DaPKC (A), Crb (D), and Patj (G) are localized at the apicolateral cell membrane, whereas Scrib (J) and Nrt (K) mark the basolateral membrane domain. (B, E, H, and L) In embryos with maternal VP16V67-Gal4 driven overexpression of UAS-DaPKC CAAXDN (B), Crb (E), and Patj (H) fail to localize to the cell membrane, concomitant with apical mislocalization of the basolateral protein Scrib (L, asterisks). (C, F, I, and M) Overexpression of membrane targeted wild-type DaPKC with maternal VP16V67-Gal4, shown in C, causes the redistribution of Crb (F) and Patj (I) to the whole cell contour, and interferes with the localization of Nrt (M). B, E, C, and F show the localization of DaPKC (purple) or Crb (green) in the same embryo. Bars, 10 μm. A–C, E, F, H, I, and M, stage 13 embryos; D, G, J–L, stage 11 embryos. Embryonic stages are according to Campos-Ortega and Hartenstein (1985). Apical is up in all confocal images.
The Baz and Crb apical complexes directly interact
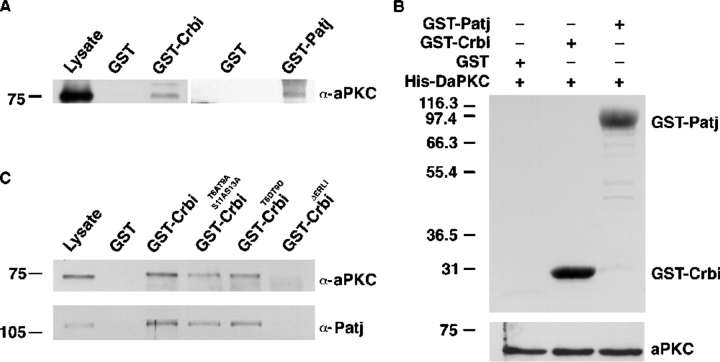
The colocalization of the Baz and Crb complexes (Hong et al., 2001; Bilder et al., 2003) suggested that components of these two complexes might physically interact. Indeed, in GST pull-down assays, the intracellular domain of Crumbs (Crbi) was able to precipitate DaPKC and Baz from embryonic extracts (Fig. 2 A and not depicted). Similarly, Patj was able to pull down DaPKC (Fig. 2 A). To identify directly interacting proteins, we performed binding assays with bacterially purified His-tagged DaPKC and GST-Crbi and GST-Patj fusion proteins. As shown in Fig. 2 B, DaPKC was able to bind directly both GST-Crbi and GST-Patj.
Figure 2.
Physical interaction between the apical complexes. (A) Drosophila embryonic extracts were incubated with GST or with the indicated GST-fusion proteins (GST-Crb wild-type intracellular domain, GST-Crbi, and GST-Patj) bound to a glutathione matrix. Immunoblotting of bound proteins, resolved by SDS-PAGE, with anti-aPKC antibody, indicates the interaction of a protein complex containing DaPKC with Crbi and Patj. First lane (Lysate) shows a 13% of the extract used in the assay. (B) Direct binding of DaPKC to Crbi and Patj. His-tagged DaPKC was bound to Ni-NTA-agarose and incubated with purified GST, GST-Crbi, and GST-Patj. Bound proteins were revealed by immunoblotting with anti-GST antibody. Bottom panel is a loading control. Half the amount of His-tagged DaPKC used in the experiment was run in a parallel gel and revealed with anti-Xpress epitope antibody. (C) Pull-down experiments of embryonic extracts were performed as in A with GST or with the indicated GST-fusion proteins: GST-Crbi, GST-CrbiT6AT9AS11AS13A, GST-CrbiT6DT9D, and GST-CrbiΔERLI. Bound proteins were resolved by SDS-PAGE and immunoblotted with anti-aPKC and anti-Patj antibodies. First lane (Lysate) shows a 10% of the extract used in the assay. Molecular weight markers are shown on the left.
To investigate further if Patj or Sdt bound to Crb are required in vivo for the interaction between the two apical complexes, we performed pull-down assays with CrbiΔERLI, a truncated form of Crbi devoid of the four COOH-terminal amino acids (ERLI) which constitute the Sdt binding domain (Bachmann et al., 2001; Hong et al., 2001). As expected, CrbiΔERLI was unable to pull down Patj (Fig. 2 C). Interestingly, it was also unable to pull down DaPKC (Fig. 2 C). These results suggest that although Crb on its own is able to bind DaPKC, in vivo Sdt and/or Patj may also intervene in the interaction between the two apical complexes.
Crb is a phosphorylation target of DaPKC
Because the kinase activity of DaPKC was required for the apical localization of the Crb complex and DaPKC directly binds Crb and Patj, we analyzed if these proteins were phosphorylation substrates of DaPKC. We found that in an in vitro assay recombinant aPKCζ, the human homologue of DaPKC, phosphorylated a GST-Crbi fusion protein, either with or without the ERLI domain (Fig. 3 A, arrow). Interestingly, Patj, which was not phosphorylated by aPKCζ, strongly decreased Crb phosphorylation when present in the reaction mixture (Fig. 3 A). Note however that Patj was not a general inhibitor of aPKCζ, as it did not block aPKCζ autophosphorylation (Fig. 3 A, asterisk) or phosphorylation of another substrate, the myelin basic protein (not depicted).
Figure 3.
Crb is a phosphorylation target of DaPKC. (A) In vitro kinase assay. The indicated GST-fusion proteins were subjected to aPKCζ phosphorylation in vitro and resolved by SDS-PAGE followed by autoradiography. Arrow points to phosphorylated Crbi. Asterisk marks autophosphorylated aPKC. Patj (position indicated by arrowhead) is not phosphorylated. Note that the presence of Patj largely prevents Crbi phosphorylation. Position of molecular weight markers is indicated on the left. Immunoblotting with anti-aPKC is shown in the bottom left panel. (Right) Staining with Coomassie blue of the total protein loaded. (B) Alignment of the amino acid sequences of the cytoplasmic domains of Drosophila (DmCrbi), mouse Crb3 (MmCrbi3), human Crb3 (HCrbi3), and C. elegans Crb1 (CeCrbi1). The conserved Thr and Ser residues (T6, T9, S11, and S13) are indicated with asterisks. All of these amino acids were mutated to A in the CrbiT6AT9AS11AS13A protein. T6 and T9 were mutated to D in DmCrbiT6DT9D and to A in DmCrbiT6AT9A. (C) Mutagenesis analysis of the phosphorylation sites of Crbi. aPKC-dependent in vitro phosphorylation was performed with the indicated fusion proteins. aPKC is unable to phosphorylate the mutated Crbi devoid of its four Ser/Thr residues (top left, CrbiT6AT9AS11AS13A). Phosphorylation is restored only after reversion in the mutated Crbi protein of Ala6 or Ala9 to Thr (top middle, named ABT6 and ABT9) Mutated CrbiT6AT9A is not phosphorylated by aPKC (top right). Total proteins, stained with Coomassie blue, are shown in the bottom panels as loading control.
The intracellular domain of Crb contains four Ser/Thr residues (T6, T9, S11, and S13) in an evolutionarily conserved region (Fig. 3 B), which are putative targets for DaPKC phosphorylation in vivo. To analyze the possibility of a functional regulation of Crb by phosphorylation, first we replaced with Ala all the putatively phosphorylatable sites of Crbi, either individually or all together (CrbiT6AT9AS11AS13A) and performed in vitro kinase assays using the mutated GST-Crbi fusion proteins as substrates. Mutation of the four putative phosphorylatable residues in Crbi abolished its phosphorylation by DaPKC (Fig. 3 C, left), although interestingly, CrbiT6AT9AS11AS13A was still able to interact with DaPKC, Patj, and DMoesin in pull-down assays (Fig. 2 C and not depicted). Individual point mutations did not allow unambiguous identification of the phosphorylation site(s) (not depicted). To clarify this point, wild-type amino acids were added back to the Crbi protein mutated in the four Ser/Thr residues and the corresponding Crbi proteins were assayed for aPKCζ-dependent phosphorylation. This experiment identified T6 and T9 as substrates of aPKCζ (Fig. 3 C, middle). Accordingly, the double mutant CrbiT6AT9A was no longer phosphorylated by aPKCζ (Fig. 3 C, right).
In vivo activity of Crb is modulated by its state of phosphorylation
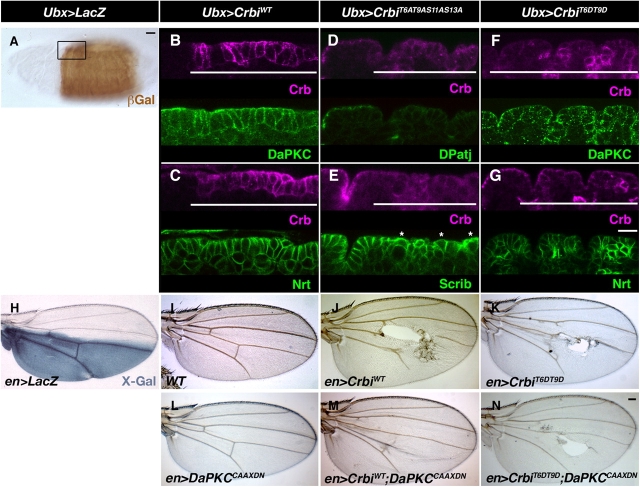
To examine the functional relevance of Crb phosphorylation, we compared the in vivo activity of wild-type and nonphosphorylatable Crb proteins. Wild-type and mutated Crb intracellular domains, either CrbiT6AT9AS11AS13A or CrbiT6AT9A, were fused to Crb signal peptide (SP) and TM domains (see Materials and methods). These proteins were overexpressed in embryos under the control of the Ubx-Gal4 driver line. Use of this driver enables direct comparison, in a single embryo, of the wild-type nonexpressing and the Crb-overexpressing domains (Fig. 4 A). As described previously (Wodarz et al., 1995; Klebes and Knust, 2000), overexpression of membrane-tethered Crbi conferred partial apical character to the whole cell membrane, as shown by the distribution of DaPKC and Patj around the whole cell contour, coincident with the ectopic distribution of Crb (Fig. 4 B and not depicted). However, localization of the basolateral markers Nrt and Scrib was not affected (Fig. 4 C and not depicted). In contrast, in embryos overexpressing nonphosphorylatable Crbi constructs (either UAS-Crbi T6AT9AS11AS13A or UAS-Crbi T6AT9A), both the endogenous and overexpressed Crb as well as Patj and DaPKC failed to associate with the membrane and were found diffusely in the cytoplasm (Fig. 4, D and E; not depicted). In addition, the basolateral marker Scrib appeared mislocalized in the apical membrane (Fig. 4 E). Thus, overexpression of a nonphosphorylatable Crbi had the opposite effect than overexpression of wild-type Crbi and resembles the polarity phenotype of strong crb mutants, where Patj and DaPKC are lost from the plasma membrane (Bhat et al., 1999; Klebes and Knust, 2000; Bilder et al., 2003). This suggested that the nonphosphorylatable Crbi acted as a DN. Indeed, nonphosphorylatable Crbi was still able to pull down DaPKC, Patj, and DMoesin from embryonic extracts (Fig. 2 C and not depicted) and thus it may compete with the endogenous Crb for its interacting partners.
Figure 4.
Regulation of Crbi activity by DaPKC-dependent phosphorylation. Different forms of Crbi were overexpressed in embryos (B–G, Ubx-Gal4 line) and in the wing disc (J–N, en-Gal4 line). (A) Embryos expressing UAS-LacZ under the control of Ubx-Gal4 line were stained with anti–β-galactosidase antibody to reveal the domain of expression of the Ubx-Gal4 line. Similar regions to the one marked in A are shown in B–G. In these panels, the extent of the Ubx-Gal4 expressing domain is indicated by the white bar. (B–G) Embryos of the indicated genotype were stained with an antibody raised against the intracellular domain of Crb to reveal the distribution of both the endogenous and overexpressed Crb (purple) and costained (green) for DaPKC (B and F), Nrt (C and G), Patj (D), and Scrib (E). (B and C) Embryos expressing wild-type Crbi showed extensive relocalization of DaPKC to the whole cell contour in the cells overexpressing Crb (B). The localization of the basolateral marker Nrt was not affected (C). (D and E) On the contrary, overexpression of Crbi T6AT9AS11AS13A was associated with loss from the apical membrane of Crb and Patj (D) and with ectopic apical localization of Scrib (E, asterisks). (F and G) Overexpression of Crbi T6DT9D caused, similarly to Crbi, relocalization of DaPKC to the whole cell cortex (F) without affecting Nrt distribution (G). (H) en-gal4/UAS-LacZ wing stained with X-gal to show (blue) the posterior compartment of the wing. (I–N) Wild-type (I) and mutant wings expressing the indicated UAS transgenes driven by en-Gal4 (J–N). Overexpression of UAS-Crbi WT (J) or UAS-Crbi T6DT9D (K) causes strong cuticular defects. Overexpression of UAS-DaPKC CAAXDN does not affect wing cuticle deposition (L) but its coexpression suppresses the dominant phenotype of UAS-Crbi WT (M) but not that of UAS-Crbi T6DT9D (N). Bars: (A) 20 μm; (G, small white bar) 10 μm; (N) 10 μm.
To further analyze the role of Crb phosphorylation, T6 and T9 were mutated to Aspartate to mimic a constitutively phosphorylated state. This protein, CrbiT6DT9D, was still able to pull down DaPKC, Patj, and DMoesin (Fig. 2 C and not depicted) and its ectopic overexpression caused redistribution of the apical proteins DaPKC and Patj to most of the cytocortex (Fig. 4 F and not depicted). Furthermore, and similarly to Crbi, it did not affect the localization of the basolateral marker Nrt (Fig. 4 G). These results support that CrbiT6DT9D mimics wild-type Crbi.
As a further functional test, we analyzed the consequences of overexpressing the different forms of Crb in the imaginal disc epithelia using the en-Gal4 line. This line drives expression of UAS transgenes in the posterior compartment of the wing disc, which gives rise to the posterior wing (Fig. 4 H). Overexpression of Crbiwt caused severe tissue disorganization in the wing, an effect that has been attributed to apicalization of the wing disc cells (Fig. 4 J; Bilder et al., 2003; Tanentzapf and Tepass, 2003). Although the overexpression of nonphosphorylatable CrbiT6AT9AS11AS13A generally lead to embryonic lethality, in a few cases, surviving flies were obtained and displayed only mild defects in the posterior crossvein (not depicted). In contrast, overexpression of CrbiT6DT9D in the imaginal discs disrupted the wing epithelium in a way similar to that caused by Crbiwt (Fig. 4 K). This supports the results obtained in the embryo.
The above experiments indicate that the phosphorylation of Crb by DaPKC is important for its function. Hence, reducing the DaPKC activity should decrease the effect of the overexpression of Crbiwt. This was the case. Expression of UAS-DaPKC CAAXDN largely normalized the effect caused by overexpression of wild-type Crbi (Fig. 4, L and M). As expected, the effect of overexpressing CrbiT6DT9D was less sensitive to the decrease of DaPKC activity. Thus, whereas 100% of flies overexpressing simultaneously UAS-Crbi wt and UAS-DaPKC CAAXDN showed essentially normal wings (Fig. 4 M), ∼40% of the flies with concomitant expression of UAS-Crbi T6DT9D and UAS-DaPKC CAAXDN still displayed strong cuticular defects (Fig. 4 N).
Discussion
In the Drosophila embryo, several regulators of epithelial polarity are sequentially associated, in the form of protein complexes, with the apicolateral (Crb and Baz complexes) and basolateral (Dlg complex) domains of the plasma membrane. The concerted activities of these protein complexes establish and maintain apicobasal polarity. Thus, the Baz and Crb complexes are indispensable for the formation of the ZA and the establishment of the apical domain. The basolateral proteins Scrib, Lgl, and Dlg restrict the localization of the apical complexes, whereas the Crb complex appears to counteract the activity of these proteins (Bilder and Perrimon, 2000; Bilder et al., 2000, 2003; Tanentzapf and Tepass, 2003). Recently, another basolateral protein, the Par-1 kinase has been involved in epithelial polarity. Par-1 inhibits formation of the Baz complex at the basolateral domain by blocking the oligomerization of Baz and its binding to DaPKC (Benton and Johnston, 2003). In addition to these regulatory interactions, ours and previous data indicate a functional reciprocal interaction between the two apical complexes. Thus, DaPKC is required and instructive for membrane localization of Crb and Patj (this work). Conversely, the Crb complex is required to maintain the Baz complex at the apical membrane, as shown by the mislocalization of Baz and DaPKC in sdt or crb embryos (Bachmann et al., 2001; Hong et al., 2001; Bilder et al., 2003). This functional interaction is supported by the synergism between sdt and baz mutations (Müller and Wieschaus, 1996). Thus, the similar mutant phenotypes associated with the absence of either of the apical complexes (Tepass et al., 2001) may then be accounted for by their functional relationship. Here, we show that physical interaction between the apical complexes results in DaPKC-dependent phosphorylation of Crb, which is a requisite for the proper activity of Crb in the control of apicobasal polarity.
Requirement of aPKC kinase activity in epithelial polarity
We have addressed the question of whether the kinase activity of DaPKC is required for epithelial polarity and have found that this is indeed the case. Thus, diminishing the activity of DaPKC by overexpressing a DN, kinase-dead DaPKCCAAXDN, causes the loss of membrane associated Crb and Patj concomitant with an apical expansion of basolateral markers. Conversely, membrane tethered wild-type DaPKC, located all around the cell cortex, targets apical proteins (Crb, Patj, and Baz) to the basolateral domain. These polarity defects are not due to nonspecific effects of DaPKCCAAXDN because its overexpression strongly normalized the cuticular malformations associated with the overexpression of UAS-DaPKC CAAXWT (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200311031/DC1).
In mammalian epithelial cells in culture, overexpression of kinase-inactive aPKC disrupts the establishment of epithelial polarity (Suzuki et al., 2001). Our results fully agree with these data. We have also found that overexpression in Drosophila embryos of Xenopus kinase-dead aPKCλ causes the same effects than overexpression of kinase-dead DaPKC (unpublished data). aPKC kinase activity is also needed to establish epithelial polarity in zebra fish. Thus, the zebra fish mutant Heart and soul encodes an aPKCλ mutated in a domain critical for the activation of its kinase function and this results in a defective ZA (Horne-Badovinac et al., 2001). All these results show that the kinase activity of aPKC is necessary to establish epithelial polarity in different animal phila and poses the question as to the identification of the phosphorylation targets of DaPKC in epithelial cells.
Crb is a phosphorylation target of DaPKC in epithelial cells
The identified targets of aPKC belong to two groups: basolateral proteins, negatively regulated by aPKC (Lgl, both in vertebrate and invertebrate cells [Betschinger et al., 2003; Plant et al., 2003; Yamanaka et al., 2003] and mammalian Par-1 [Hurov et al., 2004]), and apical proteins, namely Par-3 (Hirose et al., 2002) and Crb (this work), which require such phosphorylation for their proper activity.
Our results strongly suggest that interaction of DaPKC with Crb and the subsequent phosphorylation of the latter are most relevant for epithelial polarity. This conclusion is based in several experimental observations. First, aPKC phosphorylates Crb in vitro at its intracellular domain. Second, this phosphorylation appears to be essential in vivo because mutation of the putatively phosphorylatable Thr/Ser of the Crb intracellular domain into Ala inactivates Crb. Nonphosphorylatable Crbi still binds DaPKC, Patj, and DMoesin. Thus, it should act as a DN form competing with wild-type Crb and we find that this is indeed the case. Third, mutation of the putatively phosphorylatable residues of Crbi into negatively charged Asp maintains its biological function. This suggests that the DN character of the CrbiT6AT9A is not due to a mere change of T6 and T9 to Ala but to the conversion of Crbi into a nonphosphorylatable protein. Fourth, the phenotypic effects of overexpressing Crbiwt were suppressed by a reduction of DaPKC kinase activity, supporting that DaPKC is required for Crb activity. CrbiT6DT9D was less sensitive to a reduction in DaPKC activity than CrbiWT. However, there was still a partial recovery of the cuticular malformations caused by overexpression of CrbiT6DT9D when DaPKC activity was compromised. This suggested that conversion of both Thr into Asp did not render Crb into a completely constitutively active protein. Note also that CrbiT6DT9D still retains Ser11 and Ser13. Although our in vitro experiments do not point at these residues as DaPKC-dependent phosphorylation sites, we cannot totally exclude their functionality in vivo, which could be modified by reduced DaPKC activity. Other alternative, but not mutually excluding explanations, may be that the partial rescue of CrbiT6DT9D by DAPKCCAAXDN results from the interference of DAPKCCAAXDN with the endogenous Crb and/or may be caused by the functional antagonism between Crb and the basolateral proteins that colocalize on the whole cytocortex in these experimental conditions.
Together, these results indicate that DaPKC-dependent phosphorylation of Crb is essential for its wild-type activity. Because the putatively aPKC-dependent phosphorylatable residues of Crb are conserved in mammalian homologues, one of the main functions of aPKC in epithelial cells may be to maintain apicobasal polarity through posttranslational modification of Crb. Note, however, that the genetic control of epithelial polarity may be different in C. elegans, because the putatively phosphorylatable residues are absent from C. elegans Crb-1 and inactivation of crb-1 by dsRNAi did not disturb formation of the C. elegans apical junction (Bossinger et al., 2001).
Additionally, and although it has not been demonstrated, it is conceivable that DaPKC-dependent phosphorylation of Baz may be required for apicobasal polarity in Drosophila, as it is the case in cultured mammalian epithelial cells where phosphorylation of the mammalian homologue of Baz, ASIP/Par-3, by PKC-3 is indispensable for the formation of the TJ (Hirose et al., 2002).
aPKC plays a dual role in the control of epithelial polarity. In addition to its requirement for the proper activity of the apical proteins, aPKC negatively regulates the activity of the basolateral proteins. Thus, in mammalian epithelial cells aPKC-dependent phosphorylation of Lgl and Par-1b prevents the association of these proteins with the plasma membrane (Yamanaka et al., 2003; Hurov et al., 2004). In the apical side of the Drosophila neuroblasts, DaPKC counteracts the activity of Lgl releasing it from its association with the cell membrane (Betschinger et al., 2003). A similar antagonistic effect between DaPKC and Lgl and Dlg appears to take place in the imaginal discs (Rolls et al., 2003). In agreement with these observations, we have found that the kinase activity of DaPKC is required to exclude Scrib from the apical region of the embryonic epithelial cells. This function of DaPKC would be independent of its role in Crb activation because ectopic basolateral localization of Crb on its own did not affect the localization of basolateral markers (Fig. 4 C). Although ectopic accumulation of Crb is associated with relocalization of endogenous DaPKC to the whole cell contour, conceivably that amount of DaPKC is insufficient to affect the localization of basolateral markers.
A molecular mechanism for the functional interaction between the apical complexes
It has been proposed that the Baz, Crb, and Dlg complexes act sequentially to direct the maturation of epithelial cell polarity, the Baz complex being the first to be assembled and the most critical apical regulator (Bilder et al., 2003). Our findings provide a mechanistic framework for the regulation of the Crb complex by the Baz complex. The functional interaction between the Drosophila apical complexes relies in physical contact between some of their components. Thus, DPar-6 and Patj directly interact in vitro (Nam and Choi, 2003). Now we find that DaPKC directly binds to Crb and Patj. Although in vitro DaPKC interacts with and is able to phosphorylate a Crbi protein devoid of the COOH-terminal ERLI domain (Fig. 3 A), pull-down experiments of embryonic extracts show that this domain is necessary for the interaction between the Baz and Crb complexes. This suggests that in vivo the interaction may be reinforced by proteins that, directly or indirectly, are bound to the ERLI domain. Sdt is a likely candidate because it binds to this domain of Crb (Bachmann et al., 2001; Hong et al., 2001; Roh et al., 2002) and its mammalian homologue Pals-1 binds Par-6 (Hurd et al., 2003). Additionally, human Crb-3 and Par-6 interact (Lemmers et al., 2004), but it is not yet known whether this also occurs in Drosophila.
The interaction between the apical complexes results in DaPKC-dependent phosphorylation and activation of Crb. Although our results show that the phosphorylation state of Crb does not affect its interaction with several known binding partners, we speculate that such phosphorylation may be necessary for Crb to adopt a correct conformation and/or to interact with proteins required for Crb stabilization at the apical domain. Moreover, the finding that Patj inhibits the DaPKC-dependent phosphorylation of Crb, suggests the existence of alternative and excluding complexes. During early embryonic stages, Patj and DaPKC may directly interact, although such interaction would not affect the kinase activity of DaPKC (in fact, DaPKC kinase activity is required for the apical localization of Patj at blastoderm stage; unpublished data). At gastrulation, when the Crb complex accumulates at the plasma membrane, we hypothesize that a greater affinity of Patj for Sdt (bound to Crb) than for DaPKC would release Patj from its interaction with DaPKC and allow the phosphorylation of Crb. Note that the Baz complex may perform additional roles in relation to the Crb complex. Thus, previous data suggested the existence of Crb-dependent and -independent mechanisms for the localization of Patj and Sdt (Tanentzapf et al., 2000; Nam and Choi, 2003). The interaction between components of the apical complexes, as stated by Nam and Choi (2003), would account for these results.
In summary, the present results stress the intricacy of the relationships between the evolutionarily conserved proteins that establish and maintain epithelial polarity. There is a feedback mechanism between the Baz and Crb complexes. Thus, phosphorylation of Crb by DaPKC is a requisite for the ulterior Crb complex-dependent maintenance of the apical localization of the Baz complex. The wealth of data demonstrating the physical and functional interactions between both complexes allows them to be considered as part of a unique apical determinant complex, whose components are added stepwise. Additionally, there is a functional antagonism among the apical and the basolateral domain determining proteins (Bilder et al., 2003, Tanentzapf and Tepass, 2003; this work). Clearly, identification of additional targets of DaPKC and comprehensive molecular and genetic analyses of the functional interactions among the different complexes will allow further insight into the establishment and maintenance of epithelial polarity.
Materials and methods
Fly strains
Stocks used were: wild type (Oregon R). Transgenic lines expressing wild-type DaPKC (UAS-DaPKC), membrane targeted wild-type DaPKC (UAS-DaPKC CAAXWT), membrane-targeted kinase-dead DaPKC (UAS-DaPKC CAAXDN), membrane targeted kinase-dead Xenopus aPKCλ (UAS-XaPKCλCAAXDN) and wild-type and mutated membrane bound Crb-intra (UAS-Crbi) were generated by subcloning the corresponding cDNAs (Berra et al., 1993; see Site directed mutagenesis) in the pUAST vector (Brand and Perrimon, 1993). The resulting pUAST-plasmids were used to transform w 1118 embryos (Ashburner, 1989) using 0.3 mg/ml of pUChsΔ2-3 as the source of transposase. Several lines were established and analyzed for each transgene.
Mis-expression studies
Mis-expression experiments were performed using the Gal4/UAS system (Brand and Perrimon, 1993) and en-Gal4, Ubx-Gal4 (Calleja et al., 1996; a gift from M. Calleja, CBMSO, Madrid, Spain) and the maternal Gal4 line MatVP16V67-Gal4 (generated by D. St. Johnston, University of Cambridge, Cambridge, UK). Embryos expressing UAS-DaPKC, UAS-DaPKC CAAXWT, UAS-DaPKCCAAXDN, or UAS-XaPKCλCAAXDN driven by the MatVP16V67-Gal4 line were collected after 6–16 h at 18 or 25°C, fixed, and stained. Embryos expressing UAS-Crbi, UAS-Crbi T6DT9D, UAS-Crbi T6AT9A, and UAS-Crbi T6AT9AS11AS13A driven by Ubx-Gal4 were collected for 16 h at 18 or 25°C. To assay the effect of a reduction of DaPKC signaling on the activity of Crbi, either wild-type or mutated, en-Gal4 females were mated with UAS-DaPKC CAAXDN/If; UAS-Crbi/MKRS males. This cross scheme allows unambiguous identification of en-Gal4/UAS-DaPKC CAAXDN; en-Gal4; UAS-Crbi and en-Gal4/UAS-DaPKC CAAXDN; UAS-Crbi flies in the progeny. The progeny of the above mentioned cross was maintained at 18°C during embryonic development and then transferred to 25°C for subsequent development in order to minimize deleterious effects associated to the expression of Crb constructs at the embryonic stages. The effect of concomitant expression of UAS-DaPKC CAAXWT and UAS-DaPKC CAAXDN was examined in the progeny of the cross of en-Gal4 females with UAS-DaPKC CAAXDN/If; UAS-DaPKC CAAXWT/MKRS males.
Immunohistochemistry
Embryos were fixed as in Tepass et al. (1990) and stained with rabbit anti-Patj and rat anti–Crb-intra (Bhat et al., 1999; a gift from H. Bellen, Baylor College of Medicine, Houston, TX), mouse Mab cq4 anti-Crb (DSHB), rabbit anti-Baz (a gift from A. Wodarz, Heinrich-Heine-Universität Düsseldorf, Dusseldorf, Germany), rabbit anti-aPKCζ C20 (Santa Cruz Biotechnology, Inc.), mouse Mab BP106 anti-Nrt (Hortsch et al., 1990), rabbit anti-Scrib (a gift from C. Doe, University of Oregon, Eugene, OR), rabbit anti–β-galactosidase (Cappel) and mouse anti–β-galactosidase (Promega). FITC and rhodamine red-X secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Embryos were mounted in Vectashield (Vector Laboratories). Staining of en-Gal4/UAS-lacZ wings with X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was performed as described in Calleja et al. (1996).
Confocal images were acquired in a MicroRadiance 2000 microscope (Bio-Rad Laboratories). Images of Fig. 1 were collected with a 63×/1.4 oil Plan-Apochromat objective and magnified 2×. Images of Fig. 4 were collected with 40×/1.3 oil Plan-Neofluar objective and magnified 2.4×. The acquisition software was Lasersharp 2000 BioRad 5.0. Wing images shown in Fig. 4 and Fig. S1 were obtained in an Axiophot microscope (Carl Zeiss MicroImaging, Inc.) with 2.5×/0.075 Plan-Neofluar objective, optovar 2×, and acquired with a camera (model DFC300; Leica) using Fire Cam software (Leica). Images were imported into Adobe Photoshop 7.0 for cropping and contrast adjustment before being assembled into figures using the same software.
Protein binding and in vitro phosphorylation assays
GST-fusion proteins were obtained from subclones in pGEX-3X (Amersham Biosciences) of full-length Patj (Bhat et al., 1999), a gift from H. Bellen, and Crb wild-type or mutated intracellular domain (see Site directed mutagenesis) and CrbiΔERLI (Hong et al., 2001), a gift from Y.N Jan (University of San Francisco, San Francisco, CA). To perform pull-down experiments, protein extracts were prepared from 0-16h Drosophila embryos by homogenizing them in lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EGTA; 2 mM EDTA, 1% Triton X-100 containing protease inhibitors). Debris were removed by centrifugation at 12,000 g for 15 min at 4°C. 300 μg of Drosophila protein extract were incubated for 16 h at 4°C with 30 μg of GST or of the different GST-fusion proteins bound to glutathione beads as described in Sanz et al. (1999). Bound proteins were separated by SDS-PAGE and immunoblotted with anti-aPKCζ C20, anti-Baz (a gift from A. Wodarz), anti-DMoesin (from D. Kiehart, Duke University, Durham, NC) or anti-Patj antibodies. For in vitro binding assays, His-tagged DaPKC was obtained from a subclone in a pRSET (Invitrogen) that also tagged the expressed protein with the Xpress epitope. 100 μg of the different purified GST-fusion proteins were mixed with 40 μl of His-tagged DaPKC immobilized in Ni-NTA-agarose and incubated over night in lysis buffer at 4°C. Bound proteins were separated by SDS-PAGE and immunoblotted with anti-GST (Santa Cruz Biotechnology, Inc.). Control of His-tagged DaPKC loading was performed by immunoblotting with anti-Xpress epitope antibody (Invitrogen). In vitro kinase assay were performed as in (Leitges et al., 2001) using 30 ng of baculovirus-expressed recombinant human aPKCζ (Calbiochem) and 3 μg of the indicated GST-fusion proteins or of myelin basic protein. This phosphorylation depended on aPKC activity because it was inhibited by previous denaturation of the kinase. Note that although a human aPKC was used, the high similarity between human aPKC and DaPKC (79% of identity and 90% of similarity in the kinase domain) makes it unlikely the possibility of obtaining different results with DaPKC instead of human aPKC.
Site directed mutagenesis
Mutagenesis were performed by the site directed Quick-Change system (Stratagene). Full length DaPKC cDNA, isolated from a cDNA Drosophila λZAP library (a gift from P. Hurban, Paradigm Genetics, Research Triangle Park, NC), was used as a template to incorporate to DaPKC the CAAX motif with the following primers: CAAX-forward: 5′-ATATGGTGCACGGATCGTTT-3′ and CAAX-reverse: 5′-GCGAATTCTCAGGACAGCACACATTTCGAGCTCATGCAGCCAGGACCACTCTCATCGGGTGGGTTCA-GTTTGACGCAATCCTCCAGAGACATCAGCAAGG-3′.
The DaPKC CAAX clone was used to generate a DaPKC CAAX kinase inactive (K293W) with the following primers: DN forward: 5′-CGCATCTACGCCATGTGGGTGATCAAGAAGG-3′ and DN reverse: 5′-CCTTCTTGATCACCCACATGGCGTAGATGCG-3′.
Generation of the UAS-Crb i construct was essentially as described previously (Wodarz et al., 1995). crb cDNA (a gift from A. Wodarz) was used as a template to amplify the SP and TM domains using the following pairs of primers: CRB SP fwd: 5′-GCGAATTCTAGCCAAAAGCAACCC-3′ and CRB SP rev: 5′-GCAGATCTGGAGCCATTAAAGTACG-3′; and CRB TM fwd: 5′-GCAGATCTTCGACCACAGACATTG-3′ and CRB TM rev: 5′-CGTCTAGAAGGTGTATATGGGCGG-3′.
PCR products were subcloned in the pGEMT-easy (Promega). pBluescript or pGEX-3X subclones containing the wild-type Crbi sequence (Wodarz et al., 1995), were used as templates to obtain CrbiT6A with the following primers: 5′-CAGGAACAAGCGAGCAGCGAGGGGCACC-3′ and 5′-GTGCCCCTCGCTGCTCGCTTGTTCCTGG-3′. Crbi T6A was used as a template to generate CrbiT6A S13A with the following primers: 5′-CCTATAGCCCGGCCGCGCAAGAGTACTGC-3′ and 5′-GCAGTACTCTTGCGCGGCCGGGCTATAGG-3′. Crbi T6A was used as a template to generate CrbiT6A T9A with the following primers: 5′-CAGCAGGGGCGCGTATAGCCCGAGC-3′ and 5′-GCTCGGGCTATACGCGCCCCTCGCTG-3′. Crbi T6A S13A was used as a template to obtain CrbiT6A T9A S11A S13A with the following primers: 5′-GCGAGGGGCGCGTATGCCCCGGCCGCG-3′ and 5′-CGCGGCCGGGGCATACGCGCCCCTCGC-3′. T6, T9, S11, and S13 were added back to CrbiT6A T9A S11A S13A using the following primers, respectively: ABT6: 5′-CAGGAACAAGCGAGCAACCAGGGGCGCG-3′ and 5′-GCGCCCCTGGTTGCTCGCTTGTTCCTG-3′; ABT9: 5′-GCAGCGAGGGGCACCTATGCCCCGG-3′ and 5′-CCGGGGCATAGGTGCCCCTCGCTGC-3′; ABS11: 5′-GGGGCGCGTATAGCCCGGCCGCGC-3′ and 5′-GCGCGGcCGGGCTATACGCGCCCC-3′; and ABS13: 5′-CCTATGCCCCGAGCGCGCAAGAGTACTGC-3′ and 5′-GCAGTACTCTTGCGCGctCGGGgcATAGG-3′.
pBluescript or pGEX-3X subclones containing the wild-type Crbi sequence (Wodarz et al., 1995), were used as templates to obtain CrbiT6D with the following primers: 5′-CCAGGAACAAGCGAGCAGACAGGGGCACC-3′ and 5′-GGTGCCCCTGTCTGCTCGCTTGTTCCTGG-3′. Crbi T6D was used as a template to obtain CrbiT6DT9D with the following primers: 5′-GCAGACAGGGGCGACTATAGCCCGAGCG-3′ and 5′-CGCTCGGGCTATAGTCGCCCCTGTCTGC-3′. All mutations were confirmed by sequencing. Wild-type and mutated Crbi domains were ligated with the SP and TM domains and subcloned in pUAST.
Online supplemental material
An overexpression assay was used to exclude the possibility of nonspecific effects of DN-DaPKC expression. Fig. S1 shows that the polarity defects, found in wings and associated with the overexpression of DaPKCCAAXWT are normalized by overexpression of UAS-DaPKC CAAXDN. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200311031/DC1.
Acknowledgments
We are grateful to J. Modolell, N. Azpizau, J.F. de Celis, J. Culí, M.J. García-García, M. Ruiz-Gómez, E. Sánchez-Herrero, and colleagues of J. Modolell's laboratory for constructive criticisms on the manuscript; and to H. Bellen, M. Calleja, C. Doe, P. Hurban, Y.N. Jan, A. Wodarz, and the Bloomington Stock Center for materials and stocks. Postdoctoral fellowship from the Comunidad Autónoma de Madrid (CAM) to S. Sotillos is acknowledged.
This work was supported by grants from CAM (08.5/0030/2000) to S. Campuzano; Dirección General de Investigación Científica y Técnica (PB98-0682) and MCYT (BMC2002-00411) to J. Modolell; SAF1999-0053, 2FD97-1429, and SAF2000-0175 from MCYT, 08.1/0060/2000 from CAM, and “II Ayuda de la Fundación Juan March a la Investigación básica” to J. Moscat. An institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa is acknowledged.
Abbreviations used in this paper: ASIP, atypical PKC isotype-specific interacting protein; Baz, Bazooka; Crbi, intracellular domain of Crumbs; DaPKC, Drosophila atypical PKC; Dlg, Discs large; DN, dominant negative; Lgl, Lethal giant larvae; Nrt, Neurotactin; Patj, Pals1-associated TJ protein; Scrib, Scribble; Sdt, Stardust; SP, signal peptide; TJ, tight junctions; TM, transmembrane; ZA, zonula adherens.
References
- Ashburner, M. 1989. Drosophila. Transformation. A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1017–1063.
- Bachmann, A., M. Schneider, B. Theilenberg, F. Grawe, and E. Knust. 2001. Drosophila stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. [DOI] [PubMed] [Google Scholar]
- Benton, R., and D.S. Johnston. 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 115:691–704. [DOI] [PubMed] [Google Scholar]
- Berra, E., M.T. Diaz-Meco, I. Dominguez, M.M. Municio, L. Sanz, J. Lozano, R.S. Chapkin, and J. Moscat. 1993. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 74:555–563. [DOI] [PubMed] [Google Scholar]
- Betschinger, J., K. Mechtler, and J.A. Knoblich. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 422:326–330. [DOI] [PubMed] [Google Scholar]
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. [DOI] [PubMed] [Google Scholar]
- Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. [DOI] [PubMed] [Google Scholar]
- Bilder, D., M. Li, and N. Perrimon. 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 289:113–116. [DOI] [PubMed] [Google Scholar]
- Bilder, D., M. Schober, and N. Perrimon. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58. [DOI] [PubMed] [Google Scholar]
- Bossinger, O., A. Klebes, C. Segbert, C. Theres, and E. Knust. 2001. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230:29–42. [DOI] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Calleja, M., E. Moreno, S. Pelaz, and G. Morata. 1996. Visualization of gene expression in living adult Drosophila. Science. 274:252–255. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J.A., and V. Hartenstein. 1985. The embryonic development of Drosophila melanogaster. Springer-Verlag, Berlin Heidelberg, Germany. 227 pp.
- Drubin, D.G., and W.J. Nelson. 1996. Origins of cell polarity. Cell. 84:335–344. [DOI] [PubMed] [Google Scholar]
- Gao, L., G. Joberty, and I.G. Macara. 2002. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 12:221–225. [DOI] [PubMed] [Google Scholar]
- Grawe, F., A. Wodarz, B. Lee, E. Knust, and H. Skaer. 1996. The Drosophila genes crumbs and stardust are involved in biogenesis of adherens junctions. Development. 122:951–959. [DOI] [PubMed] [Google Scholar]
- Henrique, D., and F. Schweisguth. 2003. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr. Opin. Genet. Dev. 13:341–350. [DOI] [PubMed] [Google Scholar]
- Hirose, T., Y. Izumi, Y. Nagashima, Y. Tamai-Nagai, H. Kurihara, T. Sakai, Y. Suzuki, T. Yamanaka, A. Suzuki, K. Mizuno, and S. Ohno. 2002. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J. Cell Sci. 115:2485–2495. [DOI] [PubMed] [Google Scholar]
- Hong, Y., B. Stronach, N. Perrimon, L.Y. Jan, and N.J. Jan. 2001. Drosophila stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 414:634–638. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac, S., D. Lin, S. Waldron, M. Schwarz, G. Mbamalu, T. Pawson, Y. Jan, D.Y. Stainier, and S. Abdelilah-Seyfried. 2001. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr. Biol. 11:1492–1502. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., N.H. Patel, A.J. Bieber, Z.R. Traquina, and C.S. Goodman. 1990. Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development. 110:1327–1340. [DOI] [PubMed] [Google Scholar]
- Hurd, T.W., L. Gao, M.H. Roh, I.G. Macara, and B. Margolis. 2003. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 5:137–142. [DOI] [PubMed] [Google Scholar]
- Hurov, J.B., J.L. Watkins, and H. Piwinica-Worms. 2004. atypical PKC phosphorylates Par-1 kinases to regulate localization and activity. Curr. Biol. 14:736–741. [DOI] [PubMed] [Google Scholar]
- Izumi, Y., T. Hirose, Y. Tamai, S. Hirai, Y. Nagashima, T. Fujimoto, Y. Tabuse, K.J. Kemphues, and S. Ohno. 1998. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebes, A., and E. Knust. 2000. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 10:76–85. [DOI] [PubMed] [Google Scholar]
- Knust, E., and O. Bossinger. 2002. Composition and formation of intercellular junctions in epithelial cells. Science. 298:1955–1959. [DOI] [PubMed] [Google Scholar]
- Knust, E., U. Tepass, and A. Wodarz. 1993. crumbs and stardust, two genes of Drosophila required for the development of epithelial polarity. Dev Suppl. 261–268. [PubMed]
- Kuchinke, U., F. Grawe, and E. Knust. 1998. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 8:1357–1365. [DOI] [PubMed] [Google Scholar]
- Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J.F. Garcia, F. Camacho, M.T. Diaz-Meco, P.D. Rennert, and J. Moscat. 2001. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaβ pathway. Mol. Cell. 8:771–780. [DOI] [PubMed] [Google Scholar]
- Lemmers, C., E. Medina, M.H. Delgrossi, D. Michel, J.P. Arsanto, and A. Le Bivic. 2002. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J. Biol. Chem. 277:25408–25415. [DOI] [PubMed] [Google Scholar]
- Lemmers, C., D. Michel, L. Lane-Guermonprez, M.H. Delgrossi, E. Medina, J.P. Arsanto, and A. Le Bivic. 2004. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell. 15:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara, I.G. 2004. Parsing the polarity code. Nat. Rev. Mol. Cell Biol. 5:220–231. [DOI] [PubMed] [Google Scholar]
- Mechler, B.M., W. McGinnis, and W.J. Gehring. 1985. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, E., J. Williams, E. Klipfell, D. Zarnescu, G. Thomas, and A. Le Bivic. 2002. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J. Cell Biol. 158:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, H.A., and E. Wieschaus. 1996. armadillo, bazooka and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, H.J. 2000. Genetic control of epithelial cell polarity: Lessons from Drosophila. Dev. Dyn. 218:52–67. [DOI] [PubMed] [Google Scholar]
- Nam, S.C., and K.W. Choi. 2003. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 130:4363–4372. [DOI] [PubMed] [Google Scholar]
- Ohno, S. 2001. Intercellular junctions and cellular polarity: the PAR–aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13:641–648. [DOI] [PubMed] [Google Scholar]
- Petronczki, M., and J.A. Knoblich. 2001. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 3:43–49. [DOI] [PubMed] [Google Scholar]
- Pielage, J., T. Stork, I. Bunse, and C. Klambt. 2003. The Drosophila cell survival gene discs lost encodes a cytoplasmic Codanin-1-like protein, not a homolog of tight junction PDZ protein Patj. Dev. Cell. 5:841–851. [DOI] [PubMed] [Google Scholar]
- Plant, P.J., J.P. Fawcett, D.C. Lin, A.D. Holdorf, K. Binns, S. Kulkarni, and T. Pawson. 2003. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 5:301–308. [DOI] [PubMed] [Google Scholar]
- Roh, M.H., and B. Margolis. 2003. Composition and function of PDZ protein complexes during cell polarization. Am. J. Physiol. Renal Physiol. 285:F377–F387. [DOI] [PubMed] [Google Scholar]
- Roh, M.H., O. Makarova, C.-J. Liu, K. Shin, S. Lee, S. Laurinec, M. Goyal, R. Wiggins, and B. Margolis. 2002. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 157:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh, M.H., S. Fan, C.J. Liu, and B. Margolis. 2003. The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J. Cell Sci. 116:2895–2906. [DOI] [PubMed] [Google Scholar]
- Rolls, M.M., R. Albertson, H.P. Shih, C.Y. Lee, and C.Q. Doe. 2003. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 163:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, L., P. Sanchez, M.J. Lallena, M.T. Diaz-Meco, and J. Moscat. 1999. The interaction of p62 with RIP links the atypical PKCs to NF-kappaβ activation. EMBO J. 18:3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight, S.W., K. Shin, V.C. Fogg, S. Fan, C.J. Liu, M. Roh, and B. Margolis. 2004. Loss of PALS1 expression leads to Tight Junction and polarity defects. Mol. Biol. Cell. 15:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A., T. Yamanaka, T. Hirose, N. Manabe, K. Mizuno, M. Shimizu, K. Akimoto, Y. Izumi, T. Ohnishi, and S. Ohno. 2001. Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 152:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, A., C. Ishiyama, K. Hashiba, M. Shimizu, K. Ebnet, and S. Ohno. 2002. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 115:3565–3573. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., and U. Tepass. 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5:46–52. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., C. Smith, J. McGlade, and U. Tepass. 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass, U. 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177:217–225. [DOI] [PubMed] [Google Scholar]
- Tepass, U., C. Theres, and E. Knust. 1990. crumbs encodes an EFG-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799. [DOI] [PubMed] [Google Scholar]
- Tepass, U., G. Tanentzapf, R. Ward, and R. Fehon. 2001. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35:747–784. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., U. Hinz, M. Engelbert, and E. Knust. 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia in Drosophila. Cell. 82:67–76. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., A. Ramrath, A. Grimm, and E. Knust. 2000. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelial neuroblasts. J. Cell Biol. 150:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, D.F., and P.J. Bryant. 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 66:451–464. [DOI] [PubMed] [Google Scholar]
- Yamanaka, T., Y. Horikoshi, Y. Sugiyama, C. Ishiyama, A. Suzuki, T. Hirose, A. Iwamatsu, A. Shinohara, and S. Ohno. 2003. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13:734–743. [DOI] [PubMed] [Google Scholar]