Abstract
The endoplasmic reticulum (ER) provides an environment that is highly optimized for oxidative protein folding. Rather than relying on small molecule oxidants like glutathione, it is now clear that disulfide formation is driven by a protein relay involving Ero1, a novel conserved FAD-dependent enzyme, and protein disulfide isomerase (PDI); Ero1 is oxidized by molecular oxygen and in turn acts as a specific oxidant of PDI, which then directly oxidizes disulfide bonds in folding proteins. While providing a robust driving force for disulfide formation, the use of molecular oxygen as the terminal electron acceptor can lead to oxidative stress through the production of reactive oxygen species and oxidized glutathione. How Ero1p distinguishes between the many different PDI-related proteins and how the cell minimizes the effects of oxidative damage from Ero1 remain important open questions.
Proteins that traverse the secretory pathway typically depend on disulfide bonds for their maturation and function. These bonds are often crucial for the stability of a final protein structure, and the mispairing of cysteine residues can prevent proteins from attaining their native conformation and lead to misfolding. Classic experiments by Anfinsen et al. (1961) provided evidence that disulfide formation is a spontaneous process and that the polypeptide itself is sufficient for achieving the native state in vitro. However, compared with other aspects of protein folding, disulfide-linked folding is slow due to its dependence on a redox reaction, which requires an electron acceptor. These considerations hinted that disulfide-linked folding is an assisted process in vivo, which was demonstrated by the discovery of dsbA mutants in Escherichia coli that exhibited compromised disulfide formation (Bardwell et al., 1991).
In eukaryotes, oxidative protein folding occurs in the ER. Studies using the classic substrate ribonuclease A led to the identification of protein disulfide isomerase (PDI), a protein that can rearrange incorrect disulfides as well as catalyze disulfide formation and reduction in vitro (Goldberger et al., 1963). Despite the ability of PDI to enhance the rate of disulfide-linked folding, how the ER disposes of electrons as a result of the oxidative disulfide formation reaction remained unknown. Over the past 40 yr, a number of different factors have been proposed to contribute to maintaining the oxidized environment of the ER, including the preferential secretion of reduced thiols and uptake of oxidized thiols, as well as a variety of different redox enzymes and small molecule oxidants (Ziegler and Poulsen, 1977; Hwang et al., 1992; Carelli et al., 1997; Frand et al., 2000). However, the physiological relevance of these to oxidative folding has been unclear due to a lack of genetic evidence.
A combination of genetic and biochemical studies using the yeast Saccharomyces cerevisiae, and more recently mammalian and plant systems, have begun to reveal the proteins and mechanisms behind this fundamental protein folding process. The conserved, ER-resident protein Ero1p plays an analogous role to the bacterial periplasmic protein DsbB in oxidative folding. Both Ero1p and DsbB specifically oxidize a thioredoxin-like protein (PDI in eukaryotes, DsbA in bacteria) that serves as an intermediary in the transfer of oxidizing equivalents to folding proteins (Bardwell et al., 1993; Frand and Kaiser, 1999; Tu et al., 2000). Molecular oxygen can serve as the terminal electron acceptor for disulfide formation in both prokaryotes and eukaryotes (Bader et al., 1999; Tu and Weissman, 2002). Under anaerobic conditions, the DsbB–DsbA system can support disulfide formation via alternate electron acceptors, such as fumarate (Bader et al., 1999). However, while in bacteria oxidative folding is conveniently coupled to molecular oxygen through the respiratory chain, Ero1p uses a flavin-dependent reaction to pass electrons directly to molecular oxygen (Tu and Weissman, 2002). As a result, Ero1p activity may generate reactive oxygen species (ROS) that could contribute an additional source of cellular oxidative stress, suggesting that its activity must be regulated according to the folding load. Furthermore, the apparent specificity of Ero1p for PDI may allow the numerous homologues of PDI to remain in the reduced state in order to carry out separate redox functions aside from protein oxidation. This review will focus on the mechanism of Ero1p-catalyzed oxidative folding and its broader cell biological ramifications.
The components of the eukaryotic oxidative folding machinery
While it has been appreciated that oxidative folding is dependent on electron acceptors and likely to be catalyzed in vivo, the physiological mechanism governing this process was not known until recently. Genetic screens in yeast identified a conserved, ER membrane–associated protein Ero1p (ER oxidoreductin 1) as an essential component of the oxidative folding machinery (Frand and Kaiser, 1998; Pollard et al., 1998). Mutations in ERO1 lead to sensitivity to the reductant DTT and the accumulation of proteins that normally contain disulfide bonds in a reduced form in the ER (Frand and Kaiser, 1998; Pollard et al., 1998), a phenotype resembling that of bacteria lacking DsbB function (Bardwell et al., 1993; Missiakas et al., 1993). In humans, there are two ERO1 isoforms, hERO1-Lα and hERO1-Lβ (Cabibbo et al., 2000; Pagani et al., 2000), which lack a COOH-terminal tail of ∼127 amino acids required by the yeast protein for membrane association (Pagani et al., 2001). In vivo, membrane association of Ero1p may allow the protein to be retained in the ER and facilitate cotranslational disulfide formation. Ero1p possesses seven conserved cysteine residues that are likely involved in catalyzing electron transfer (Frand and Kaiser, 1998, 2000; Pollard et al., 1998). However, Ero1p has no homology to any redox enzymes or other known proteins. Consistent with a function in folding in the ER, yeast Ero1p and hERO1-Lβ are induced by the unfolded protein response (UPR) (Frand and Kaiser, 1998; Pollard et al., 1998; Pagani et al., 2000). The expression of hERO1-Lα and not hERO1-Lβ is stimulated by hypoxia (Gess et al., 2003).
PDI has long been known to aid the formation of disulfide bonds. It has been shown to catalyze disulfide bond formation and isomerization, as well as reduction, for a wide range of substrates in vitro (Freedman, 1989), but its role in vivo has been less clear. PDI is an essential protein that constitutes ∼2% of the protein in the ER and contains two thioredoxin-like Cys-Gly-His-Cys (CGHC) active sites (Goldberger et al., 1963; Laboissiere et al., 1995). The finding that Cys-Gly-His-Ser (CGHS) active site mutants of PDI result in sensitivity to DTT provided evidence of a role for PDI in the formation of disulfide bonds in vivo (Holst et al., 1997). This mutant PDI cannot function as an oxidant of proteins but can still catalyze the isomerization of protein disulfides. While these observations suggest that the essential role of PDI is to unscramble nonnative disulfide bonds (Laboissiere et al., 1995), the DTT-sensitivity phenotype of this mutant PDI argues that PDI normally plays an important role in catalyzing disulfide formation. Furthermore, in ero1-1 mutants, PDI accumulates in a reduced form, suggesting that Ero1p acts upstream of PDI in a pathway for disulfide formation in the ER (Frand and Kaiser, 1999).
Recently, the sulfhydryl oxidase Erv2p has been implicated as playing a role in oxidative folding in the ER parallel to that of Ero1p (Gerber et al., 2001; Sevier et al., 2001; Gross et al., 2002). Erv2p is a member of the ERV/ALR family of sulfhydryl oxidases (Thorpe et al., 2002), which have been found in a number of subcellular compartments. Although Erv2p can compensate for defects in Ero1p when overexpressed (Sevier et al., 2001) and can oxidize proteins directly in vitro (Gerber et al., 2001), under the conditions examined, deletion of ERV2 at most has a modest effect on growth (Sevier et al., 2001; Tu and Weissman, 2002). These observations established that Ero1p is the major player in protein oxidation and that the physiological role of Erv2p as a sulfhydryl oxidase is limited to a subset of nonessential proteins or growth conditions not yet examined. In yeast, there is an essential homologue of Erv2p (Erv1p) that is localized to the mitochondrial intermembrane space (Lange et al., 2001). The sulfhydryl oxidase activity of Erv1p may be important for proper mitochondrial homeostasis and the assembly of iron-sulfur cluster proteins (Lisowsky, 1994; Lee et al., 2000; Lange et al., 2001) perhaps by catalyzing disulfide bond formation, although the oxidative environment of the mitochondrial intermembrane space is not well characterized. Interestingly, a vaccinia virus protein of this ERV family has been implicated in the formation of disulfides in viral proteins during assembly in the host cytosol (Senkevich et al., 2000).
The Ero1-dependent pathway for the transfer of oxidizing equivalents
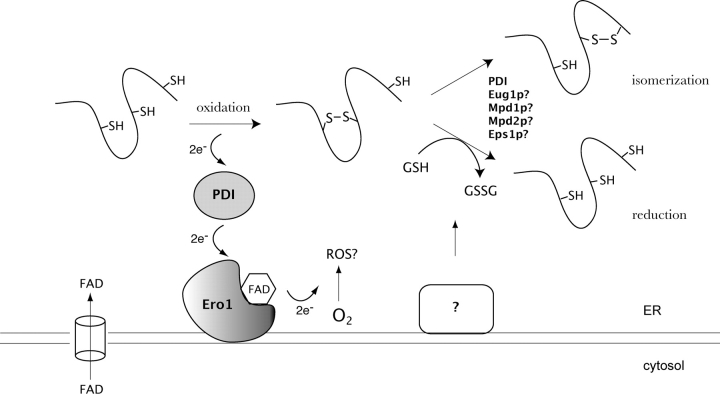
Recently, Ero1p-mediated disulfide formation was reconstituted in vitro using purified Ero1p and PDI as the only protein components (Tu et al., 2000). Even at substoichiometric Ero1p to disulfide concentrations (e.g., 1:340), Ero1p-catalyzed oxidative folding proceeds faster than the PDI-catalyzed oxidative folding of substrates in the presence of an optimal glutathione redox buffer (Tu et al., 2000). Furthermore, this Ero1p-driven reaction can occur independently of glutathione both in vivo and in vitro (Cuozzo and Kaiser, 1999; Tu et al., 2000). A mutant PDI with Cys-Gly-His-Ala (CGHA) active sites acts as a dominant inhibitor of this Ero1p-catalyzed reaction, and a mixed disulfide cross-link can be captured between these two proteins both in vivo and in vitro (Frand and Kaiser, 1999; Tu et al., 2000). These observations demonstrate that Ero1p oxidizes PDI directly through disulfide exchange (Tu et al., 2000). PDI subsequently catalyzes the formation of disulfides in folding proteins. Ero1p does not efficiently act as a direct oxidant of folding substrates and thus relies on PDI to transfer oxidizing equivalents (Tu et al., 2000). Thus, the transfer of oxidizing equivalents occurs via the protein relay: Ero1p to PDI to protein (Fig. 1). This reconstituted system may prove useful for the refolding of recombinant disulfide-containing proteins. Ero1p may also be involved in mediating the retrotranslocation of proteins to the cytosol by oxidizing PDI, changing its conformation, and thus altering its affinity for particular substrates (Tsai and Rapoport, 2002).
Figure 1.
Schematic model of oxidative protein folding in the yeast ER. The formation of disulfide bonds in the ER is driven by Ero1p. FAD-bound Ero1p oxidizes PDI, which then subsequently oxidizes folding proteins directly. FAD-bound Ero1p then passes electrons to molecular oxygen, perhaps resulting in the production of ROS. FAD, which is synthesized in the cytosol, can readily enter the ER lumen and stimulate the activity of Ero1p. Disulfide isomerization and reduction may be performed by some of the four homologues of PDI, Eug1p, Mpd1p, Mpd2p, or Eps1p, in addition to PDI itself. Reduced glutathione (GSH) may also assist in disulfide reduction, resulting in the production of oxidized glutathione (GSSG).
The role of PDI and its homologues in oxidative folding
Despite its ability to catalyze a range of thiol-disulfide reactions, PDI is found predominantly in an oxidized form in vivo (Frand and Kaiser, 1999), implying its main cellular function is to oxidize folding proteins through the Ero1p pathway. Interestingly, there are four homologues of PDI (EUG1, MPD1, MPD2, and EPS1) in yeast and dozens more are found in higher eukaryotes. Preliminary experiments suggest that yeast Ero1p cannot interact with several of these PDI homologues in vitro (unpublished data), and this specificity may extend to the human system as well (Mezghrani et al., 2001). However, a mixed disulfide cross-link between Ero1p and a Cys-Gly-His-Ala (CGHA) active site mutant of Mpd2p has been immunoprecipitated from yeast (Frand and Kaiser, 1999). A mixed disulfide cross-link has also been isolated between hERO1-Lα and ERp44 (Anelli et al., 2002). A complication of these findings is that PDI and its homologues can be trapped as mixed disulfides with proteins they are folding (Molinari and Helenius, 1999), and so the functional relevance of these interactions remains to be explored.
The inability of Ero1p to interact with several PDI variants suggests that it can discriminate between PDI and its homologues. Such discrimination could allow different PDI-related proteins to function as dedicated disulfide isomerases or reductases, which require cysteine residues in their thioredoxin-like active sites to be in the reduced form (Fig. 1). PDI itself probably also contributes to disulfide isomerization and reduction in vivo. In the bacterial system, it has been shown that the role of these PDI-related proteins is dictated by whether they can interact with upstream oxidases or reductases, rather than the redox potential of their active sites. For example, dimerization of the protein DsbC prevents oxidation by DsbB and allows it to function as a dedicated isomerase (Bader et al., 2001). Mutants of DsbC that disrupt dimerization become oxidized by DsbB and can serve the role of the protein oxidant DsbA (Bader et al., 2001). In addition, DsbC is normally maintained in the reduced form by the periplasmic membrane protein DsbD, which transfers the reducing power of cytosolic thioredoxin to DsbC (Rietsch et al., 1997). Some of the eukaryotic PDI homologues may similarly be kept in a reduced form by an upstream reductase. Resolving how and whether these PDI-related proteins interact with Ero1p should lend insight into their cellular roles and the determinants of function.
The role of glutathione in oxidative folding
Glutathione is the major redox buffer in eukaryotic cells (Hwang et al., 1992). The ratio of reduced (GSH) to oxidized (GSSG) glutathione is ∼100:1 in the cytosol (Hwang et al., 1992). This highly reducing environment disfavors disulfide bond formation. However, in the ER where disulfide formation occurs, the ratio of GSH:GSSG is much more oxidizing at ∼3:1 (Hwang et al., 1992). This abundance of GSSG in the secretory pathway was long thought to be the source of oxidizing equivalents for disulfide formation (Hwang et al., 1992). However, Ero1p-catalyzed disulfide formation proceeds independently of glutathione both in vivo and in vitro (Cuozzo and Kaiser, 1999; Tu et al., 2000). What then is the role of glutathione?
Genetic evidence in yeast has demonstrated that glutathione is dispensable for disulfide formation and instead functions as a net reductant in the ER (Cuozzo and Kaiser, 1999). In a screen for suppressors of the temperature sensitivity of the ero1-1 mutant, a deletion of GSH1, which is involved in the biosynthesis of glutathione, was found to strongly suppress the ero1-1 phenotype (Cuozzo and Kaiser, 1999). The interpretation of this observation is that absence of glutathione as a reductant results in less reduction of oxidized PDI and proteins, which allows a compromised ero1-1 oxidation system to support growth. In Δgsh1 strains, the oxidative folding of CPY proceeds with normal kinetics but is highly sensitive to oxidative stresses, consistent with the role of glutathione as a net reductant (Cuozzo and Kaiser, 1999).
What is the basis for the high GSSG content in the ER? Ero1p cannot directly oxidize GSH to GSSG (Tu et al., 2000). However, Ero1p and PDI can drive the oxidation of folding substrates even in the presence of reduced glutathione (GSH). Over time, a gradual production of GSSG resulting from GSH-mediated reduction of disulfides in PDI and folding proteins is observed in vitro (Tu et al., 2000) (Fig. 1). Thus, the abundance of GSSG in the ER is likely a consequence of Ero1p activity, and the GSH:GSSG redox buffer in the ER represents an equilibrium between the consequences of Ero1p-mediated oxidative and glutathione-mediated reductive processes. The kinetic shuttling of oxidizing equivalents by Ero1p and PDI that occurs independently of the bulk redox environment could explain how the ER supports rapid disulfide formation while maintaining the ability to reduce or rearrange incorrect disulfides, perhaps through glutathione and certain PDI homologues.
The source of oxidizing potential for the ER
What then is the source of oxidizing potential for eukaryotic disulfide formation? In bacteria, disulfide formation is coupled to cellular respiration through the oxidation of quinones (Bader et al., 1999). The periplasmic membrane protein DsbB reduces ubiquinone to reoxidize itself after one round of disulfide formation (Bader et al., 1999, 2000). Ubiquinone then becomes reoxidized by the respiratory chain with molecular oxygen serving as the terminal electron acceptor (Bader et al., 1999). A biochemical and genetic approach was used to identify the source of oxidizing equivalents for eukaryotic disulfide formation (Tu et al., 2000; Tu and Weissman, 2002). Using the yeast system, candidate redox molecules were depleted to determine whether they were important for oxidative folding in vivo. Unlike in prokaryotes, oxidative folding in yeast is not dependent on cellular respiration, as it is not affected by the absence of ubiquinone or heme (Tu et al., 2000). However, depletion of riboflavin from yeast results in a striking defect in oxidative folding (Tu et al., 2000). Furthermore, overexpression of FAD1, which converts flavin mononucleotide (FMN) to flavin adenine dinucleotide (FAD), strongly suppresses the temperature sensitivity of the ero1-1 mutant (Tu et al., 2000). These observations strongly suggested that oxidative folding in yeast is dependent on cellular FAD levels.
Further evidence that oxidative folding in eukaryotes is dependent on FAD came from the discovery that purified Ero1p itself is a novel FAD-binding protein (Tu et al., 2000) (Fig. 1). When FAD is added to purified Ero1p and PDI, these components together can support robust oxidative folding in vitro (Tu et al., 2000). Although FAD is crucial for sustained activity of Ero1p, it is not functioning as the terminal electron acceptor. Each FAD-bound Ero1p molecule can support multiple rounds of PDI oxidation, and an excess of free FAD cannot drive Ero1p-catalyzed disulfide formation under anaerobic conditions (Tu and Weissman, 2002). These observations suggested that molecular oxygen rather than FAD is functioning as the terminal electron acceptor (Tu and Weissman, 2002) (Fig. 1). In vitro experiments confirmed that Ero1p-catalyzed disulfide formation is compromised under anaerobic conditions, and that Ero1p directly consumes molecular oxygen during its reaction cycle (Tu and Weissman, 2002). Furthermore, the ero1-1 mutant is completely inviable under anaerobic conditions at normally permissive temperatures (Tu and Weissman, 2002). The efficient use of molecular oxygen as the terminal electron acceptor by FAD-bound Ero1p could explain how the oxidation of millimolar concentrations of PDI (Gilbert, 1990) is achieved despite FAD concentrations in the low micromolar range (Gliszczynska and Koziolowa, 1998). Ero1p likely uses alternate terminal electron acceptors under anaerobic conditions, though the identity of these acceptors remains unknown.
The role of FAD in oxidative folding
The dependency of oxidative folding on FAD defines a novel role for this versatile redox molecule in the ER lumen. Ero1p is the first described flavoprotein localized entirely within the ER lumen, and the existence of a robust transport system that imports FAD into the ER lumen has been suggested (Tu and Weissman, 2002). In yeast, oxidative folding in vivo is highly sensitive to specifically free cellular FAD levels (Tu and Weissman, 2002). This sensitivity could be recapitulated in vitro, as the activity of Ero1p varied significantly with small deviations from physiological FAD concentrations (Tu and Weissman, 2002). Although this sensitivity of Ero1p to free FAD levels is unusual for a flavoprotein, it may provide a means to regulate oxidative folding (see also below). Further work will be required to determine how FAD modulates the activity of Ero1p and whether this is a conserved property of Ero1p.
Oxidative folding as a source of cellular oxidative stress
It seems fitting that molecular oxygen serves as the terminal electron acceptor for disulfide formation since its reduction potential is greater than all other biological oxidants. However, the complete four-electron reduction of molecular oxygen to water is kinetically sluggish, and the reduction intermediates and byproducts such as superoxide and hydrogen peroxide are highly reactive and damaging to macromolecules. In bacteria, this problem is solved by coupling oxidative folding to the respiratory chain (Bader et al., 1999), which consists of a complex series of membrane electron transfer proteins that efficiently reduce molecular oxygen to water. However, since in eukaryotes oxidative folding and respiration are confined to separate organelles (ER versus mitochondria), the Ero1p oxidation system has evolved to function independently of respiration and has adopted the use of flavin-based redox chemistry (Tu et al., 2000). In contrast to quinones, FAD is a relatively weak oxidant due to a low redox potential, but the ability of FAD-bound Ero1p to rapidly pass electrons directly to O2 provides the driving force for eukaryotic disulfide formation (Tu and Weissman, 2002).
The fate of the molecular oxygen consumed by Ero1p remains unclear. A standard two-electron reduction of O2 produces hydrogen peroxide (H2O2). It does not appear that Ero1p is releasing stoichiometric amounts of hydrogen peroxide per disulfide formed, although substoichiometric amounts of hydrogen peroxide can be detected during its catalysis of disulfide formation (Tu and Weissman, 2002). However, on the cellular level, recent studies suggest that uncontrolled Ero1p oxidase activity could be a significant source of oxidative stress. Harding et al. (2003) have recently found that ER stress can lead to the acute production of reactive oxygen species. Stressing the ER in worms lacking the transmembrane kinase PERK, which phosphorylates eIF2α to decrease global translation upon ER stress (Harding et al., 1999), leads to a significant accumulation of peroxides in the cell, and lowering Ero1p function by RNAi largely eliminates this effect (Harding et al., 2003).
These observations indicate that Ero1p could be responsible for a significant proportion of ROS in the cell. A simple calculation indicates the plausibility of this hypothesis. Assuming ∼1/3 of all proteins are secretory proteins, one disulfide must be formed for every ∼500 amino acids translated. If the equivalent of approximately three ATP are consumed per amino acid translated (Stryer, 1995), then one disulfide is formed for every ∼1,500 ATP. ROS from ATP production occurs at 1–2% frequency (Stryer, 1995), and if approximately four to five ATP are produced per molecule of oxygen reduced, then one to two molecules of ROS are expected per 500 ATP produced through respiration. Assuming one molecule of ROS is produced per disulfide formed, Ero1p-mediated oxidation could account for up to ∼25% of cellular ROS produced during protein synthesis, which has been suggested by a recent study to be the major source of cellular energy consumption (Princiotta et al., 2003). ROS production by Ero1p appeared significant but substoichiometric, which may be a limitation of the detection method (Tu and Weissman, 2002). The mechanism and extent of ROS production by Ero1p needs to be explored further. Nonetheless, it is clear that disulfide formation could contribute a significant source of ROS, especially in specialized secretory cells. Many secretory proteins also contain large numbers of disulfides, and their proper formation may require multiple cycles of misoxidation, reduction, and reoxidation. In addition, Ero1p activity is the main source of oxidized glutathione (GSSG) in the cell (Cuozzo and Kaiser, 1999; Tu et al., 2000), which contributes an additional source of oxidative stress.
Thus, Ero1p activity may be a substantial source of oxidative stress, necessitating proper regulation of oxidative folding and the function of reductant systems in the ER. It is likely to be important for a cell to tie protein oxidation to its folding load, since without control of oxidative folding, the ER could become over-oxidized, leading to protein misfolding, the production of reactive oxygen species and oxidized glutathione, and the futile consumption of energy in the form of reducing equivalents. This production of oxidizing equivalents may also need to be controlled in order to facilitate the maintenance of PDI homologues and perhaps a portion of PDI itself in a reduced form, and to minimize the intrinsic toxicity caused by oxidative stress associated with disulfide formation. UPR induction is one mechanism to regulate oxidative folding, but there may be more direct means of regulation. As the Ero1p oxidation system is highly responsive to levels of free FAD in the cell, controlling the levels of free FAD available to Ero1p may be a posttranslational mechanism to regulate oxidative folding according to the cell's needs. Although speculative, it is interesting to note that RIB1, which controls the first step of riboflavin biosynthesis, is a target of the UPR (Travers et al., 2000). Moreover, preliminary experiments indicate that free FAD levels in yeast can vary according to its growth phase and conditions (unpublished data). Alternatively, free FAD levels in the ER could be controlled by a FAD-specific transporter.
Conclusion
In summary, while previous studies of oxidative folding have focused on the bulk redox potential of the ER, it is now evident that eukaryotic disulfide formation proceeds by the kinetic shuttling of oxidizing equivalents to folding substrates by a protein relay. As the Ero1p-driven oxidation machinery is insulated from the bulk redox environment, reduced glutathione and perhaps certain PDI homologues can assist in the isomerization and reduction of incorrect disulfide bonds. Oxidative folding is coupled to the strong reduction potential of molecular oxygen through a FAD-dependent mechanism, but a potential consequence is the production of toxic reactive oxygen species. Indeed, it appears that Ero1p could be a significant contributor to cellular oxidative stress, and suggests that its activity must be carefully adjusted according to the folding load on the ER. Controlling the levels of free FAD available to Ero1p may be a means to regulate oxidative folding according to the cell's nutritional or metabolic state. Future work will reveal how the ER maintains its optimal environment for the multitude of redox processes required for the proper folding of secretory proteins.
B.P. Tu's present address is Department of Biochemistry, UT Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038.
Abbreviations used in this paper: FAD, flavin adenine dinucleotide; PDI, protein disulfide isomerase; ROS, reactive oxygen species; UPR, unfolded protein response.
References
- Anelli, T., M. Alessio, A. Mezghrani, T. Simmen, F. Talamo, A. Bachi, and R. Sitia. 2002. ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J. 21:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinsen, C.B., E. Haber, M. Sela, and F.H. White. 1961. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc. Natl. Acad. Sci. USA. 47:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader, M., W. Muse, D.P. Ballou, C. Gassner, and J.C. Bardwell. 1999. Oxidative protein folding is driven by the electron transport system. Cell. 98:217–227. [DOI] [PubMed] [Google Scholar]
- Bader, M.W., T. Xie, C.A. Yu, and J.C. Bardwell. 2000. Disulfide bonds are generated by quinone reduction. J. Biol. Chem. 275:26082–26088. [DOI] [PubMed] [Google Scholar]
- Bader, M.W., A. Hiniker, J. Regeimbal, D. Goldstone, P.W. Haebel, J. Riemer, P. Metcalf, and J.C. Bardwell. 2001. Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA. EMBO J. 20:1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell, J.C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell. 67:581–589. [DOI] [PubMed] [Google Scholar]
- Bardwell, J.C., J.O. Lee, G. Jander, N. Martin, D. Belin, and J. Beckwith. 1993. A pathway for disulfide bond formation in vivo. Proc. Natl. Acad. Sci. USA. 90:1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabibbo, A., M. Pagani, M. Fabbri, M. Rocchi, M.R. Farmery, N.J. Bulleid, and R. Sitia. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275:4827–4833. [DOI] [PubMed] [Google Scholar]
- Carelli, S., A. Ceriotti, A. Cabibbo, G. Fassina, M. Ruvo, and R. Sitia. 1997. Cysteine and glutathione secretion in response to protein disulfide bond formation in the ER. Science 277:1681–1684. (published erratum appears in Science. 1998. 279:1288–1289) [DOI] [PubMed]
- Cuozzo, J.W., and C.A. Kaiser. 1999. Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1:130–135. [DOI] [PubMed] [Google Scholar]
- Frand, A.R., and C.A. Kaiser. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell. 1:161–170. [DOI] [PubMed] [Google Scholar]
- Frand, A.R., and C.A. Kaiser. 1999. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell. 4:469–477. [DOI] [PubMed] [Google Scholar]
- Frand, A.R., and C.A. Kaiser. 2000. Two pairs of conserved cysteines are required for the oxidative activity of Ero1p in protein disulfide bond formation in the endoplasmic reticulum. Mol. Biol. Cell. 11:2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand, A.R., J.W. Cuozzo, and C.A. Kaiser. 2000. Pathways for protein disulphide bond formation. Trends Cell Biol. 10:203–210. [DOI] [PubMed] [Google Scholar]
- Freedman, R.B. 1989. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 57:1069–1072. [DOI] [PubMed] [Google Scholar]
- Gerber, J., U. Muhlenhoff, G. Hofhaus, R. Lill, and T. Lisowsky. 2001. Yeast ERV2p is the first microsomal FAD-linked sulfhydryl oxidase of the Erv1p/Alrp protein family. J. Biol. Chem. 276:23486–23491. [DOI] [PubMed] [Google Scholar]
- Gess, B., K.H. Hofbauer, R.H. Wenger, C. Lohaus, H.E. Meyer, and A. Kurtz. 2003. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lalpha. Eur. J. Biochem. 270:2228–2235. [DOI] [PubMed] [Google Scholar]
- Gilbert, H.F. 1990. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 63:69–172. [DOI] [PubMed] [Google Scholar]
- Gliszczynska, A., and A. Koziolowa. 1998. Chromatographic determination of flavin derivatives in baker's yeast. J. Chromatogr. A. 822:59–66. [DOI] [PubMed] [Google Scholar]
- Goldberger, R.F., C.J. Epstein, and C.B. Anfinsen. 1963. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J. Biol. Chem. 238:628–635. [PubMed] [Google Scholar]
- Gross, E., C.S. Sevier, A. Vala, C.A. Kaiser, and D. Fass. 2002. A new FAD-binding fold and intersubunit disulfide shuttle in the thiol oxidase Erv2p. Nat. Struct. Biol. 9:61–67. [DOI] [PubMed] [Google Scholar]
- Harding, H.P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397:271–274. [DOI] [PubMed] [Google Scholar]
- Harding, H.P., Y. Zhang, H. Zeng, I. Novoa, P.D. Lu, M. Calfon, N. Sadri, C. Yun, B. Popko, R. Paules, et al. 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 11:619–633. [DOI] [PubMed] [Google Scholar]
- Holst, B., C. Tachibana, and J.R. Winther. 1997. Active site mutations in yeast protein disulfide isomerase cause dithiothreitol sensitivity and a reduced rate of protein folding in the endoplasmic reticulum. J. Cell Biol. 138:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, C., A.J. Sinskey, and H.F. Lodish. 1992. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 257:1496–1502. [DOI] [PubMed] [Google Scholar]
- Laboissiere, M.C., S.L. Sturley, and R.T. Raines. 1995. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J. Biol. Chem. 270:28006–28009. [DOI] [PubMed] [Google Scholar]
- Lange, H., T. Lisowsky, J. Gerber, U. Muhlenhoff, G. Kispal, and R. Lill. 2001. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep. 2:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., G. Hofhaus, and T. Lisowsky. 2000. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett. 477:62–66. [DOI] [PubMed] [Google Scholar]
- Lisowsky, T. 1994. ERV1 is involved in the cell-division cycle and the maintenance of mitochondrial genomes in Saccharomyces cerevisiae. Curr. Genet. 26:15–20. [DOI] [PubMed] [Google Scholar]
- Mezghrani, A., A. Fassio, A. Benham, T. Simmen, I. Braakman, and R. Sitia. 2001. Manipulation of oxidative protein folding and PDI redox state in mammalian cells. EMBO J. 20:6288–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas, D., C. Georgopoulos, and S. Raina. 1993. Identification and characterization of the Escherichia coli gene dsbB, whose product is involved in the formation of disulfide bonds in vivo. Proc. Natl. Acad. Sci. USA. 90:7084–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari, M., and A. Helenius. 1999. Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature. 402:90–93. [DOI] [PubMed] [Google Scholar]
- Pagani, M., M. Fabbri, C. Benedetti, A. Fassio, S. Pilati, N.J. Bulleid, A. Cabibbo, and R. Sitia. 2000. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275:23685–23692. [DOI] [PubMed] [Google Scholar]
- Pagani, M., S. Pilati, G. Bertoli, B. Valsasina, and R. Sitia. 2001. The C-terminal domain of yeast Ero1p mediates membrane localization and is essential for function. FEBS Lett. 508:117–120. [DOI] [PubMed] [Google Scholar]
- Pollard, M.G., K.J. Travers, and J.S. Weissman. 1998. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell. 1:171–182. [DOI] [PubMed] [Google Scholar]
- Princiotta, M.F., D. Finzi, S.B. Qian, J. Gibbs, S. Schuchmann, F. Buttgereit, J.R. Bennink, and J.W. Yewdell. 2003. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 18:343–354. [DOI] [PubMed] [Google Scholar]
- Rietsch, A., P. Bessette, G. Georgiou, and J. Beckwith. 1997. Reduction of the periplasmic disulfide bond isomerase, DsbC, occurs by passage of electrons from cytoplasmic thioredoxin. J. Bacteriol. 179:6602–6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich, T.G., C.L. White, E.V. Koonin, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA. 97:12068–12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier, C.S., J.W. Cuozzo, A. Vala, F. Aslund, and C.A. Kaiser. 2001. A flavoprotein oxidase defines a new endoplasmic reticulum pathway for biosynthetic disulphide bond formation. Nat. Cell Biol. 3:874–882. [DOI] [PubMed] [Google Scholar]
- Stryer, L. 1995. Biochemistry, 4th ed. W.H. Freeman & Co, New York. 1064 pp.
- Thorpe, C., K.L. Hoober, S. Raje, N.M. Glynn, J. Burnside, G.K. Turi, and D.L. Coppock. 2002. Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch. Biochem. Biophys. 405:1–12. [DOI] [PubMed] [Google Scholar]
- Travers, K.J., C.K. Patil, L. Wodicka, D.J. Lockhart, J.S. Weissman, and P. Walter. 2000. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 101:249–258. [DOI] [PubMed] [Google Scholar]
- Tsai, B., and T.A. Rapoport. 2002. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J. Cell Biol. 159:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, B.P., and J.S. Weissman. 2002. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell. 10:983–994. [DOI] [PubMed] [Google Scholar]
- Tu, B.P., S. Ho-Schleyer, K.J. Travers, and J.S. Weissman. 2000. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 290:1571–1574. [DOI] [PubMed] [Google Scholar]
- Ziegler, D.M., and L.L. Poulsen. 1977. Protein disulfide bond synthesis: a possible intracellular mechanism. Trends Biochem. Sci. 2:79–81. [Google Scholar]