Abstract
Stratified squamous epithelia express the αvβ5 integrin, but in squamous cell carcinomas (SCCs) there is down-regulation of αvβ5 and up-regulation of αvβ6. To investigate the significance of this finding, we transduced an αv-negative human SCC line with retroviral vectors encoding αv integrins. αvβ5-expressing cells underwent suspension-induced apoptosis (anoikis), whereas αv-negative cells and cells expressing αvβ6 did not. Resistance to anoikis correlated with PKB/Akt activation in suspension, but not with changes in PTEN or p110α PI3 kinase levels. Anoikis was induced in parental and αvβ6-expressing cells by inhibiting PI3 kinase. Conversely, activation of Akt or inhibition of caspases in αvβ5-expressing cells suppressed anoikis. Caspase inhibition resulted in increased phosphoAkt, placing caspase activation upstream of decreased Akt activation. Anoikis required the cytoplasmic domain of β5 and was independent of the death receptor pathway. These results suggest that down-regulation of αvβ5 through up-regulation of αvβ6 may protect SCCs from anoikis by activating an Akt survival signal.
Keywords: keratinocyte; apoptosis; differentiation; Akt; extracellular matrix
Introduction
Extracellular matrix receptors of the integrin family regulate the growth, differentiation, and tissue assembly of keratinocytes in stratified squamous epithelia such as the epidermis and the lining of the oral cavity (van der Flier and Sonnenberg, 2001; Watt, 2002). In normal stratified squamous epithelia, integrin expression is largely confined to the basal, proliferative layer of cells attached to the underlying basement membrane; expression is lost as cells move through the suprabasal layers and undergo terminal differentiation. The most abundant integrins in stratified squamous epithelia are α2β1 (collagen receptor), α3β1 (laminin receptor), and α6β4 (laminin receptor; Watt, 2002). Other integrins, such as αvβ5 (vitronectin receptor), are expressed at lower levels.
Integrin expression is frequently perturbed in squamous cell carcinomas (SCCs), which are tumors of stratified squamous epithelia (Mercurio and Rabinovitz, 2001; Watt, 2002). One such change is an up-regulation of αvβ6. In normal epidermis, αv forms a heterodimer exclusively with β5 (Pasqualini et al., 1993). In hyperproliferative stratified squamous epithelia, both αvβ5 and αvβ6 are expressed (Breuss et al., 1995; Haapasalmi et al., 1996). In SCCs, αvβ6 is up-regulated, and this is often correlated with a down-regulation of αvβ5 expression (Jones et al., 1997; Regezi et al., 2002). The down-regulation of αvβ5 in association with expression of αvβ6 probably reflects a hierarchy in the preference of αv to heterodimerize with different β subunits (Koistinen and Heino, 2002).
There are several ways in which up-regulation of the αvβ6 integrin can affect keratinocyte behavior. αvβ6 binds the latency-associated peptide derived from latent TGFβ and activates TGFβ (Munger et al., 1999). Expression of αvβ6 increases epithelial cell motility and invasion (Thomas et al., 2001b; Ramos et al., 2002), stimulates cell proliferation (Ahmed et al., 2002), and inhibits fibronectin matrix assembly (Ramos et al., 2002). The effects on growth and invasion may reflect the abilities of αvβ6 to activate Erk2 MAPK (Ahmed et al., 2002) and to up-regulate or activate matrix metalloproteinases (Agrez et al., 1999; Thomas et al., 2001a; Ramos et al., 2002).
We have previously described a cell line that provides an excellent model system for studying the significance of different αv integrins in SCCs. The H357 cell line is derived from a human SCC of the tongue and lacks expression of αv integrins (Sugiyama et al., 1993). When the αv subunit is transfected into H357 cells and αv-positive clones selected by multiple rounds of FACS®, αv is expressed on the cell surface as αvβ5. Expression of αvβ5 is correlated with inhibition of anchorage-independent growth and induction of terminal differentiation in H357 cells (Jones et al., 1996). In the present experiments, we have uncovered a previously unrecognized function of αvβ5 in promoting suspension-induced apoptosis, known as anoikis (Frisch and Francis, 1994), and show that when αvβ5 is replaced at the cell surface with αvβ6, anoikis is prevented. These observations suggest that up-regulation of αvβ6 may confer a survival advantage on cells in SCCs.
Results
Transduction of H357 cells with αv integrin subunit results in surface expression of functional αvβ5
In previous experiments, the αv integrin subunit was introduced into H357 cells by transfection followed by multiple rounds of FACS® and clonal selection (Jones et al., 1996). To avoid such intense selection pressure, we transduced H357 cells with a retroviral vector encoding αv (Levy et al., 1998). As controls, H357 cells were transduced with the empty retroviral vector or with a vector encoding the α4 integrin subunit, which is not expressed in normal stratified squamous epithelia or SCCs (Watt, 2002).
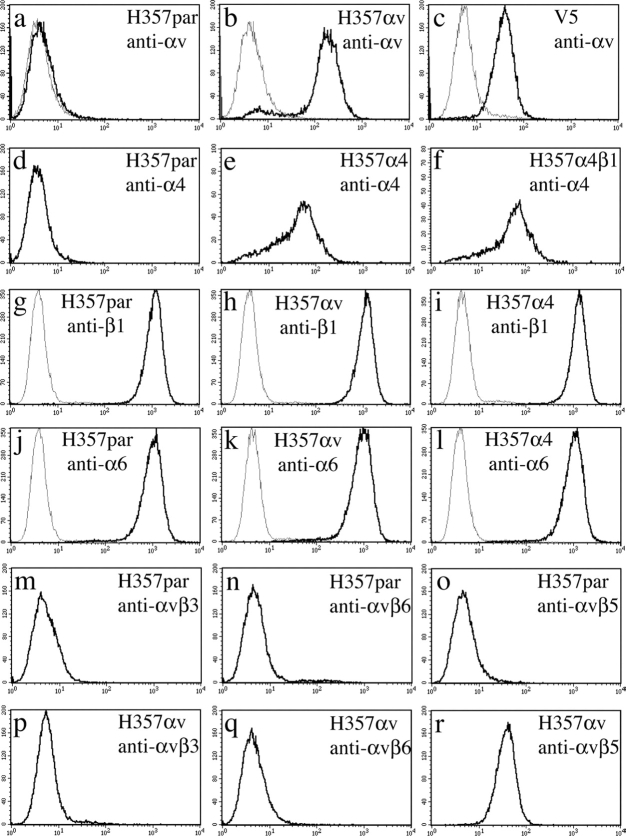
Cell surface expression of αv and α4 was examined by flow cytometry (Fig. 1). As reported previously (Sugiyama et al., 1993), parental H357 cells did not express detectable surface levels of αv (Fig. 1 a). Transduction with the αv retroviral vector resulted in higher cell surface levels of the integrin subunit (Fig. 1 b) than achieved previously by transfection and clonal selection (Fig. 1 c). As expected, parental cells did not express the α4β1 integrin (Fig. 1 d). The level of cell surface expression of the α4 subunit after retroviral transduction (Fig. 1, e and f) was the same whether or not the cells were also transduced with the chick β1 subunit (Fig. 1 f), indicating that the endogenous pool of β1 subunits was not limiting. Introduction of the αv or α4 integrin subunits had no effect on cell surface levels of any of the endogenous β1 integrins (Fig. 1, g–i, and not depicted) nor on levels of α6β4 (Fig. 1, j–l).
Figure 1.
Expression of αvβ5 and α4β1 integrins in H357 cells. (a–c) αv expression of H357 retrovirally infected cells (H357αv) compared with parental cells (H357par) and a clone of αv transfected cells, V5, previously described by Jones et al. (1996). (d–f) Infection of H357 cells with α4 alone (H357α4) or in combination with the chick β1 integrin subunit (H357α4β1). (g–l) Comparison of endogenous β1 (g–i) or α6 (j–l) integrin expression in H357par, H357αv, or H357α4. (m–r) H357par and H357αv were labeled with the heterodimer-specific αv antibodies shown. Gray lines, second antibody only; black lines, primary and second antibodies.
When H357 cells are transfected with αv and clonally selected the integrin is expressed as a heterodimer with the β5 subunit (Jones et al., 1996). To examine heterodimer formation in H357 cells transduced with the αv retrovirus, cells were labeled with heterodimer-specific antibodies and analyzed by flow cytometry (Fig. 1, m–r) using parental H357 cells as negative controls (Fig. 1, m–o). There was no detectable labeling of αv-transduced cells with antibodies to αvβ3 (Fig. 1 p) or αvβ6 (Fig. 1 q). However, αvβ5 was readily detected (Fig. 1 r). We conclude that when αv is introduced into H357 cells, whether by transfection and clonal selection (Jones et al., 1996) or by retroviral infection (Fig. 1), it is expressed as the αvβ5 heterodimer.
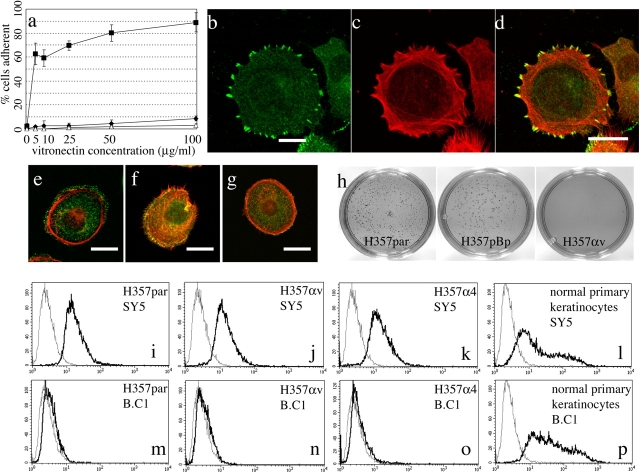
To examine whether or not the αvβ5 heterodimer expressed by retrovirally transduced H357 cells was functional, adhesion assays on vitronectin were performed. Up to 90% of H357 cells expressing αvβ5 adhered to vitronectin, compared with <5% of the parental cells or H357 cells transduced with the α4 integrin subunit (Fig. 2 a). H357 cells transduced with the αv integrin subunit spread rapidly on vitronectin, and within 20 min αvβ5 was detected in focal adhesions (Fig. 2, b–d). αvβ5 did not localize to focal adhesions in cells plated on fibronectin (Fig. 2 e). Conversely, β1 integrins localized to focal adhesions when cells were plated on fibronectin (Fig. 2 f) but not on vitronectin (Fig. 2 g), suggesting that αvβ1 heterodimers did not form.
Figure 2.
Effects of αvβ5 on cell adhesion, differentiation, and growth in suspension. (a) Adhesion to vitronectin. Diamonds, parental H357 cells; triangles, H357 cells expressing α4β1; squares, H357 cells expressing αvβ5. Data are means and SDs of triplicate samples. (b–g) H357 cells expressing αvβ5 plated on 20 μg/ml vitronectin (b–d and g) or fibronectin (e and f) for 30 min, double labeled with phalloidin (red fluorescence) and antibodies to αvβ5 (b–d), αv (e), or β1 (f and g; green fluorescence). Panel d is the merged image of b and c. Bars, 2 μm. (h) Colony formation in soft agar. 35-mm dishes of parental H357 (H357par), H357 expressing empty retroviral vector (H357pBp), and cells expressing αvβ5 (H357αv). (i–p) Flow cytometric analysis of transglutaminase (B.C1) and involucrin (SY5) expression in H357par, H357αv, or H357α4, and normal primary keratinocytes. Gray lines, second antibody only; black lines, primary and secondary antibodies.
αvβ5 expression inhibits anchorage-independent growth without inducing terminal differentiation of H357 cells
Clones of H357 cells transfected with αv were unable to form colonies in soft agar (Jones et al., 1996). To examine if retroviral transduction with αv had the same effect on a polyclonal population, cells were suspended in soft agar for 3 wk, after which time the number of colonies was scored. Cells transduced with αv showed a marked reduction in colony formation compared with parental cells and cells transduced with the empty vector (Fig. 2 h and Table I).
Table I. Percentage of colony-forming efficiency (CFE) of H357 cells in soft agar.
| Percentage of CFE |
H357 parental | H357 expressing empty vector |
H357 expressing αvβ5 |
|---|---|---|---|
| Experiment 1 | 25.4 ± 3.0 | 20.3 ± 2.2 | 0.2 ± 0.1 |
| Experiment 2 | 18.7 ± 1.2 | 16.2 ± 1.4 | 0.1 ± 0.1 |
| Experiment 3 | 25.2 ± 2.8 | 24.6 ± 1.6 | 0.0 ± 0.1 |
Three separate experiments are shown. Data are means and SDs of triplicate dishes.
In clones of H357 cells transfected with αv, suppression of anchorage-independent growth correlates with an increased ability to undergo terminal differentiation, as measured by expression of involucrin (Jones et al., 1996). Terminal differentiation of retrovirally transduced H357 cells was examined by Western blotting, immunofluorescence microscopy, and flow cytometry with antibodies to involucrin and two additional markers of terminal differentiation, transglutaminase 1 and cornifin (Fig. 2 and not depicted). Neither transduction with αv nor with α4 increased the proportion of terminally differentiated cells (Fig. 2, i–k and m–o). As reported previously (Jones et al., 1996), H357 cells had a much lower proportion of differentiated cells (<0.5%) than primary human keratinocytes (Fig. 2, l and p). Whereas suspension induces terminal differentiation of primary keratinocytes, this was not the case for H357 cells, irrespective of whether or not they expressed αv (Jones et al., 1996; unpublished data).
αvβ5 induces anoikis of H357 cells
Next, we investigated whether or not expression of αvβ5 induced anoikis (Frisch and Francis, 1994). We determined the proportion of cells with a sub-G1 DNA content, as a measure of the number of apoptotic cells (Frisch, 1999b). We compared parental H357 cells, cells transduced with the empty retroviral vector, cells transduced with αv, and cells transduced with α4.
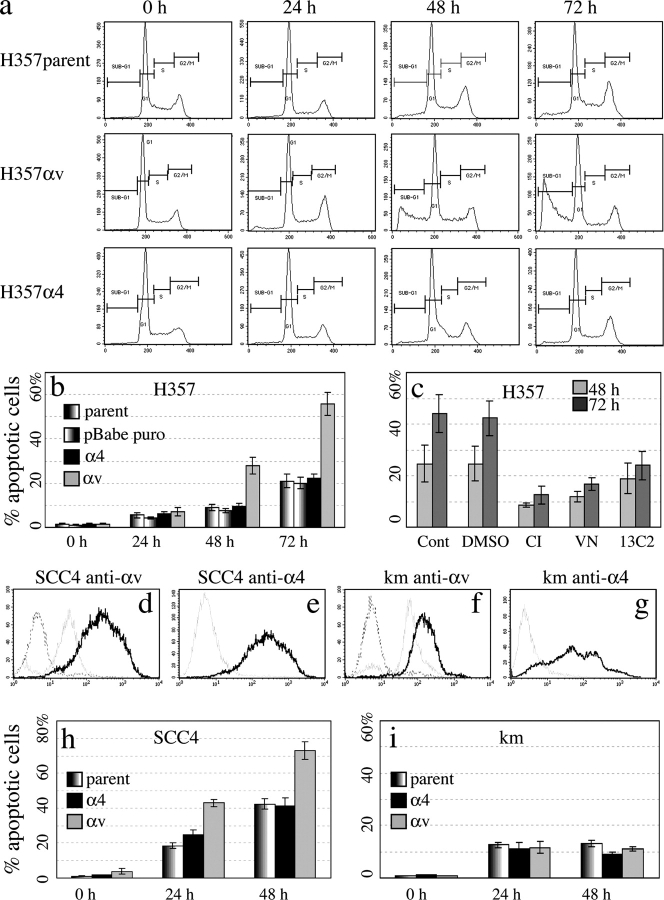
Typical flow cytometry profiles are shown in Fig. 3 a, and data pooled from six experiments are shown in Fig. 3 b. The proportion of cells with a sub-G1 DNA content was <3% in freshly harvested cells. In parental cells and cells transduced with α4 or empty vector, the proportion increased to almost 10% by 48 h and 20% by 72 h in suspension. Cells transduced with αv had a similar level of apoptosis to controls after 24 h in suspension, but by 48 h the proportion of apoptotic cells had increased to almost 30%, and to over 50% by 72 h. The increased apoptosis of H357 cells transduced with αv compared with controls was statistically significant (P = 0.008 at 72 h; t test).
Figure 3.
Expression of αvβ5 in SCC cells induces anoikis. (a) DNA content of H357 cells (parental or transduced with αv or α4) after 0, 24, 48, or 72 h in suspension. Regions corresponding to sub-G1, G1, S, and G2+M are indicated. (b) Percentage of anoikis was determined as the percentage of cells with sub-G1 DNA content. SEM of six experiments are shown, comparing parental H357 cells to those expressing empty vector (pBabe puro), α4, or αv. (c) H357 cells transduced with αv suspended in medium alone (Cont) or supplemented DMSO, z-VAD-fmk (CI) at 100 μM, 100 μg/ml vitronectin (VN), or 100 μg/ml 13C2 anti-αv. Means and SDs of three experiments are shown. (d–g) Flow cytometry profiles of SCC4 and primary human keratinocytes (strain km) labeled with secondary antibody alone (dashed lines) or antibodies to αv or α4 integrins. Gray lines, parental cells; black lines, cells transduced with αv (d and f) or α4 (e and g). (h and i) Percentage of anoikis (sub-G1 DNA) of parental SCC4 cells and primary keratinocytes (km) or cells transduced with αv or α4 integrin subunits. SEM of three experiments are shown.
The results were confirmed using two other methods to quantitate apoptosis, TUNEL (unpublished data) and microscopic evaluation of nuclear morphology in cells labeled with Hoechst 33258. The percentage of cells with condensed, fragmented nuclei and the SEM of three separate experiments (each counting 200 cells) were as follows. At time 0, 1.7 ± 0.1% of H357 cells expressing α4 were apoptotic, and this increased to 14.6 ± 1.7% after 72 h in suspension. At time 0, 1.5 ± 0.1% H357 cells expressing αv were apoptotic, a level that was similar to cells expressing α4. However, after 72 h in suspension this level had risen to 64.2 ± 3.2%, fourfold higher than in α4-expressing cells.
Anoikis is inhibited by integrin ligation of suspended cells or by treatment with caspase inhibitors (Frisch, 1999b). Therefore, we examined the effects of these treatments on H357 cells expressing αvβ5 (Fig. 3 c). The cells were held in suspension for 48 or 72 h in medium alone (control) or containing DMSO (caspase inhibitor solvent), the pan caspase inhibitor z-VAD–fmk (100 μM), vitronectin (100 μg/ml; αv ligand), or the αv function-blocking antibody 13C2 (100 μg/ml; Davies et al., 1989). At both time points, z-VAD-fmk and vitronectin substantially reduced the proportion of apoptotic cells. 13C2 also had an inhibitory effect, albeit slightly smaller.
We conclude that unligated αvβ5 expression in H357 cells leads to an increase in suspension-induced apoptosis and that this is the likely mechanism by which anchorage-independent growth is inhibited.
Effect of overexpressing αv in cells with normal endogenous levels of αvβ5
To investigate whether or not αvβ5-induced anoikis was specific to H357 cells or a more general phenomenon, we transduced a second SCC line, SCC4, and primary human keratinocytes with the αv or α4 retroviral vectors (Fig. 3, d–g). SCC4 has a similar level of endogenous αv integrins to primary keratinocytes (Fig. 3, d and f; Levy et al., 2000), but is heterozygous for an activating β1 integrin mutation (Evans et al., 2003). The levels of αv expression achieved by retroviral transduction were higher than the endogenous levels (Fig. 3, d and f) and equivalent to that of αv-transduced H357 cells (Fig. 1 b).
Fig. 3 h shows pooled data from three experiments in which the proportion of apoptotic SCC4 cells was compared immediately after detachment from the culture dish (0 h) or after suspension for 24 or 48 h. At both 24 and 48 h, the proportion of apoptotic cells was significantly higher in cells transduced with αv than in parental SCC4 or SCC4 transduced with the α4 retroviral vector (P < 0.05).
It has previously been reported that when primary human keratinocytes are placed in suspension they undergo terminal differentiation as opposed to anoikis (Gandarillas et al., 1999). Consistent with those observations, the proportion of primary keratinocytes in suspension with a sub-G1 DNA content did not exceed 15%, even after 48 h (Fig. 3 i). The proportion of apoptotic cells was not significantly different when parental keratinocytes were compared with keratinocytes transduced with α4 or αv retroviral vectors (Fig. 3 i). The proportion of cells that underwent terminal differentiation was also unaffected by overexpression of αv or α4 (unpublished data).
We conclude that in the SCC lines examined elevated expression of αv stimulated anoikis independent of whether or not the parental cells expressed αv. However, overexpression of αv in primary keratinocytes was not sufficient to induce anoikis.
Role of PI3 kinase activation in anoikis
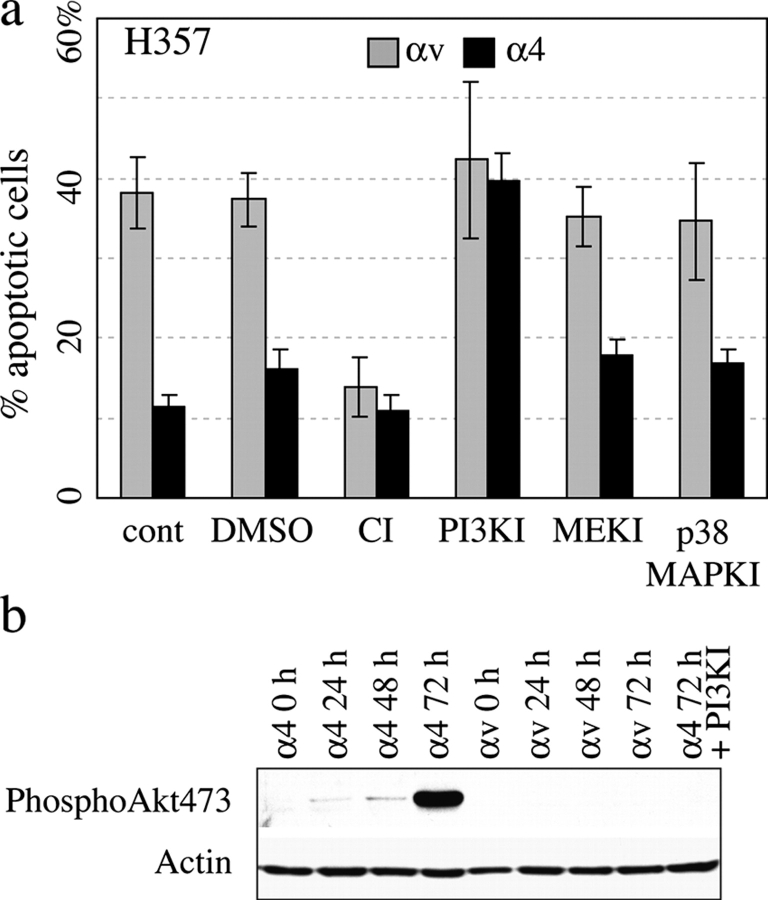
Signaling pathways known to protect cells from anoikis include FAK activation leading to Erk MAPK phosphorylation and PI3 kinase activation resulting in activation of PKB/Akt (Frisch et al., 1996). There is conflicting evidence regarding c-Jun kinase and p38 MAPK (Frisch et al., 1996; Khwaja and Downward, 1997). To examine the role of these pathways in anoikis of H357 cells, we added the PI3 kinase inhibitor LY294002, the MEK1/2 inhibitor UO126, and the p38MAPK inhibitor SB203580 to H357 cells transduced with αv or α4 and measured the proportion of apoptotic cells after 72 h in suspension (Fig. 4 a). z-VAD-fmk was added as a positive control to block anoikis (Fig. 4 a). None of the inhibitors tested had any effect on the proportion of αvβ5-expressing cells that underwent apoptosis. However, the PI3 kinase inhibitor increased apoptosis of α4-expressing cells to the level of cells expressing αvβ5 (Fig. 4 a).
Figure 4.
Protection from anoikis correlates with Akt activation. (a) Anoikis of H357 cells transduced with αv or α4 integrin after 72 h in suspension. cont, medium alone; DMSO, solvent control; CI, 100 μM z-VAD-fmk; PI3KI, 50 μM LY294002; MEKI, 10 μM UO126; p38MAPKI, 10 μM SB203580. Data are SEM of three experiments. (b) Western blot of H357 cells expressing α4 or αv held in suspension for the number of hours shown. (right hand lane) H357α4 suspended in the presence of LY 294002 for 72 h. Blot was probed with an antibody to phosphoAkt 473 or actin (loading control).
Activation of PI3 kinase leads to recruitment of Akt to the plasma membrane, where it is activated by phosphorylation on threonine 308 and serine 473 (Toker and Newton, 2000). To test if parental H357 cells in suspension had an active PKB/Akt signaling pathway that was overcome by expression of αvβ5, Western blots of proteins from suspended cells were probed with an antibody to the serine 473–phosphorylated (and hence activated) form of Akt (Fig. 4 b). In parental H357 cells, cells transduced with pBabe puro, or cells transduced with α4, activation of Akt was detected within 24 h of suspension and became maximal at 72 h (Fig. 4 b and not depicted). However, in cells expressing αvβ5, Akt phosphorylation was not detected (Fig. 4 b). Treatment of α4-expressing cells with the PI3 kinase inhibitor that induced them to undergo anoikis (Fig. 4 a) blocked Akt activation (Fig. 4 b).
The proapoptotic effect of αvβ5 is not shared with αvβ6 and is mediated by the β5 cytoplasmic domain
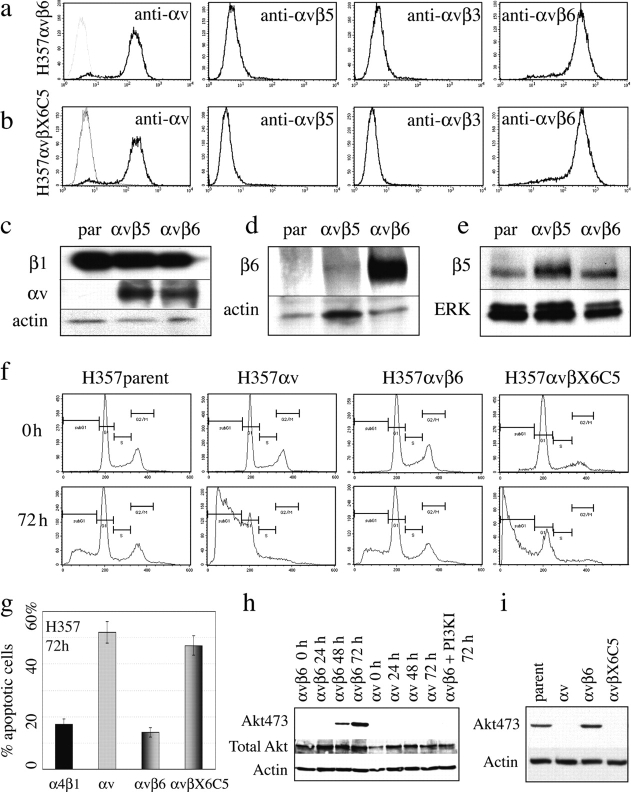
Because up-regulation of αvβ6 is a feature of many SCCs, we investigated whether or not this integrin, like αvβ5, promoted anoikis. H357 cells transduced with the αv retroviral vector were infected with a second vector that encoded the β6 integrin subunit. Introduction of β6 had no effect on cell surface levels of αv (Fig. 5 a; compare Fig. 1 b). However, cells no longer expressed surface αvβ5 (detected with P1F6 antibody); instead all of the αv on the cell surface was now in a heterodimer with the β6 subunit (detected with 10D5 antibody; Fig. 5 a).
Figure 5.
The proapoptotic effects of αvβ5 are mediated by the β5 cytoplasmic domain. (a and b) Flow cytometry of H357 cells expressing αvβ6 (a) or αvβX6C5 (b), using antibodies specific for the αv heterodimers indicated. (c–e) Western blots of parental H357 cells (par), H357 cells transduced with αv alone (αvβ5) or αv and β6 (αvβ6) probed with antibodies to the integrin subunits shown. Loading controls were actin or Erk MAPK. (f) DNA content of cells suspended for the times indicated. Shown are H357par and cells transduced with αv, αvβ6, or αvβX6C5. (g) SEM of percentage of anoikis in three separate experiments. (h and i) Western blot probed with antibodies to phosphoAkt473, total Akt, or actin. (h) Cells were suspended for the times shown. (right hand lane) Cells suspended with Ly294002 (50 μM). (i) Cells suspended for 72 h.
The relative levels of integrins in H357 cells were also compared by Western blotting (Fig. 5, c–e). As expected, there was no difference in β1 integrin levels between parental H357 cells and cells expressing αvβ5 or αvβ6 (Fig. 5 c). The αv subunit was not detected in parental cells and was expressed at similar levels in cells transduced with αv (αvβ5-expressing cells) or with αv and β6 (Fig. 5 c). There was no detectable β6 protein in parental H357 cells; β6 was weakly detectable in cells transduced with αv alone and was strongly expressed in cells transduced with both αv and β6 (Fig. 5 d). β5 protein was detectable in all three cell populations, but was more abundant in cells transduced with αv than parental cells or cells transduced with αv and β6 (Fig. 5 e). The β5 protein band in Western blots of parental and αvβ6 cells was less diffuse and migrated slightly faster than in cells transduced with αv, which is consistent with intracellular accumulation and incomplete glycosylation (Fig. 5 e).
In contrast to cells expressing αvβ5, cells expressing αvβ6 did not undergo anoikis and had the same sub-G1 DNA profiles as parental H357 cells (Fig. 5 f) and cells transduced with the α4 integrin subunit (Fig. 5 g). Whereas cells expressing αvβ5 did not activate Akt in suspension, cells expressing αvβ6 did activate Akt (Fig. 5 h) and the kinetics were similar to parental cells and cells expressing α4 (Fig. 4 b and not depicted). Akt activation in αvβ6-expressing cells was blocked by treatment with the PI3 kinase inhibitor LY294002 (Fig. 5 h).
To investigate whether or not the cytoplasmic domain of the β5 subunit conferred sensitivity to anoikis, we constructed a chimeric integrin subunit, consisting of the β6 extracellular and transmembrane domains and the β5 cytoplasmic domain (βX6C5). H357 cells were doubly infected with αv and βX6C5 retroviral vectors, and cells were examined by flow cytometry with an αvβ6-specific antibody (Fig. 5 b). Cells transduced with βX6C5 alone did not express the chimera on the cell surface, confirming that it only heterodimerized with αv (unpublished data).
Cells were placed in suspension for 72 h, and the proportion of apoptotic cells was compared in parental H357 cells and cells expressing αvβ5, αvβ6, or αvβX6C5. The results of one experiment are shown in Fig. 5 f, and data pooled from three separate experiments are shown in Fig. 5 g. H357 cells expressing αvβX6C5 were as susceptible to anoikis as cells expressing αvβ5. We conclude that the cytoplasmic domain of β5 mediates the proapoptotic effect of αvβ5.
The ability of H357 cells to avoid anoikis again correlated with their ability to activate Akt in suspension (Fig. 5 i). Parental cells and cells expressing αvβ6 activated Akt, whereas cells transduced with αv (expressed on the cell surface as αvβ5) or αvβX6C5 did not.
Activation of Akt can overcome the proapoptotic effect of αvβ5
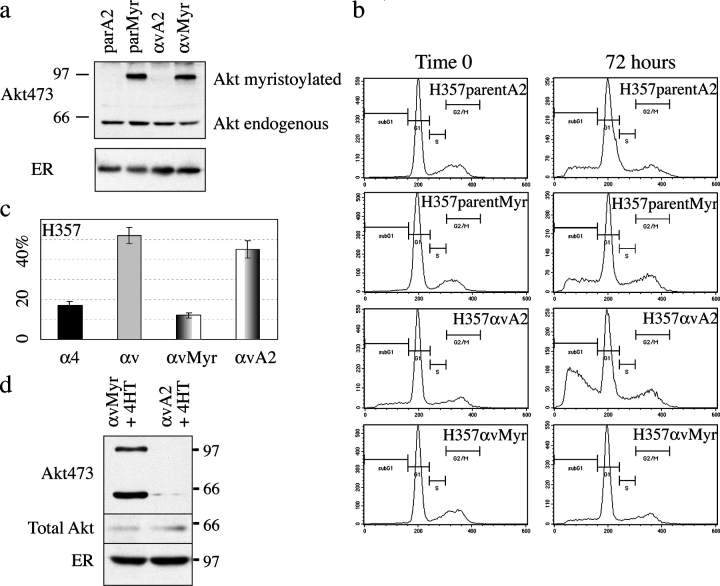
If αvβ5 expression triggers anoikis by preventing activation of Akt, then constitutive activation of Akt should allow αvβ5-expressing cells to survive in suspension. Two Akt constructs were introduced into H357 cells. The first construct, M+Akt:ER*, has a myristoylation targeting sequence fused to the NH2 terminus of a constitutively active form of Akt lacking the PH domain (myrAkt del4-129). At the COOH terminus is a modified form of the hormone-binding domain of the mouse estrogen receptor (ER) that binds 4-hydroxytamoxifen (OHT) but is refractory to estrogen. In response to OHT, M+Akt:ER* is rapidly recruited to the plasma membrane where it is activated by local PI3 kinases. The second construct, A2M+Akt:ER*, acts as an inactive control because the myristoylated glycine at the second amino acid position has been converted to alanine to eliminate the membrane-targeting function (Kohn et al., 1998). M+Akt:ER* and A2M+ Akt:ER* were expressed in retroviral vectors and introduced into parental and αv-expressing H357 cells. Adherent cultures were treated with OHT for 1 h, lysed, and examined by Western blotting. An antibody to the mutant ER detected expression of the Akt constructs at equal levels in all cell populations (Fig. 6 a). The level of endogenous serine 473–phosphorylated Akt was also the same in all cells (Fig. 6 a). However, only cells expressing M+Akt:ER* had a second band detected by anti-phosphoAkt, which migrated at 97 kD, the size of the AktER fusion protein (Fig. 6 a). H357 parental cells and cells expressing αv or α4 were transduced with the active or inactive Akt constructs and placed in suspension for 72 h in the presence of OHT (Fig. 6, b and c). Parental H357 cells and α4-expressing cells had a low level of anoikis that was not influenced by Akt activation. H357 cells expressing αvβ5 and the inactive Akt construct underwent anoikis, and the proportion of apoptotic cells was equivalent to that seen in cells expressing αvβ5 alone (Fig. 6 c). H357 cells expressing active Akt were protected from anoikis and had the same low level of apoptotic cells as H357 parental cells and cells expressing α4 (Fig. 6, b and c). The conclusions from the flow cytometry experiments were confirmed by evaluating nuclear morphology of cells stained with Hoechst 33258 (unpublished data). The level of serine 473–phosphorylated Akt was compared in αv-expressing H357 cells transduced with M+Akt:ER* or A2Akt:ER* after 72 h in suspension (Fig. 6 d). Cells expressing the inactive construct had no activation of AktER and either low or no phosphorylation of endogenous Akt. In contrast, both the myristoylated construct and endogenous Akt were activated in cells expressing M+Akt:ER*.
Figure 6.
Akt activation protects against anoikis. (a) Western blot of adherent H357 cells transduced with M+Akt:ER* or A2Akt:ER* alone (parMyr and parA2, respectively) or in combination with αv integrin (αvMyr and αvA2, respectively) and treated with OHT (1 μM) for 1 h. (top) The antibody to phospho-Akt detects endogenous phospho-Akt in all cells (bottom band), and phosphorylated M+Akt:ER in cells expressing that construct. (bottom) Probed with anti-ER. (b and c) Anoikis of H357 cells transduced with M+Akt:ER* (Myr) or A2Akt:ER* (A2) alone (parent) or in combination with αv or α4 integrins. Cells were suspended for 72 h in the presence of OHT. (c) SEM of three experiments. (d) Western blot of H357αvMyr or αvA2 suspended for 72 h with 0.1 μM OHT, probed with anti-phosphoAkt473 (top), total Akt (middle), and ER (bottom). (top panel) Anti-phosphoAkt recognizes two bands, corresponding to endogenous Akt (bottom band) and to phosphorylated M+Akt:ER (top band).
αvβ5-induced anoikis of H357 cells is not mediated by activation of the death receptor pathway
Apoptosis can be triggered by intrinsic or extrinsic pathways. The intrinsic pathway involves alterations in mitochondrial homeostasis and activation of the apoptosome; the extrinsic pathway occurs through ligand binding of death domain surface receptors such as Fas (Grossmann, 2002). Experiments on MDCK cells, derived from a simple epithelium, have shown that anoikis is induced by the extrinsic pathway, involving death receptors and their associated adaptor molecules (Frisch, 1999a; Rytömaa et al., 1999). MDCK cell anoikis can be blocked by a dominant-negative (dn) FADD (FAS associated death domain protein) mutant (dNFADD), which cannot activate caspase 8. Caspase 8 can also be stimulated by a mitochondrially driven feedback mechanism (intrinsic pathway) involving caspase 9 (Rytömaa et al., 1999).
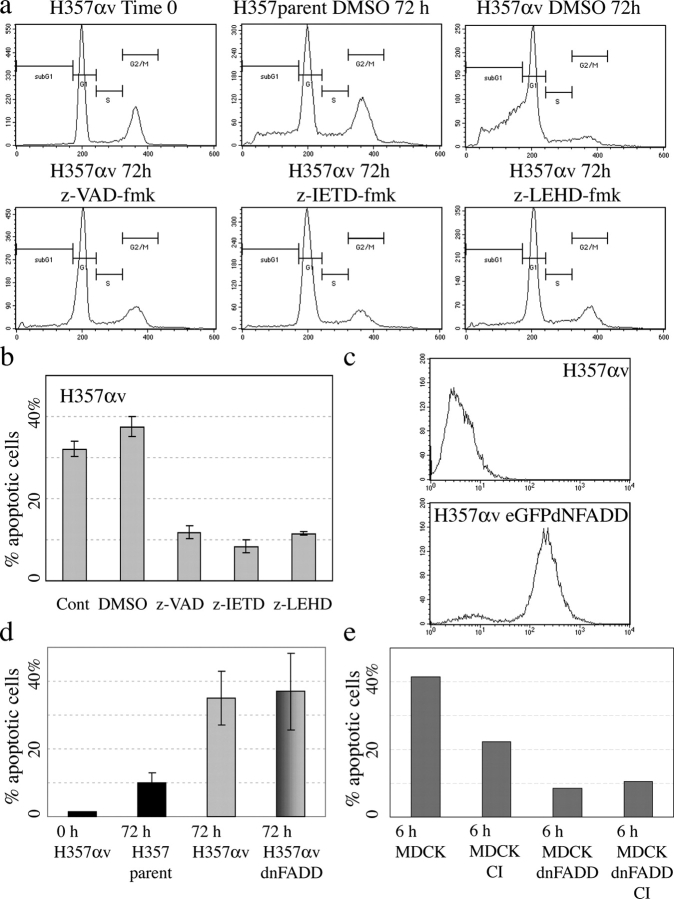
Suspension experiments were performed in the presence of the general caspase inhibitor z-VAD-fmk (Fig. 4 a and Fig. 7 a), the caspase 8 specific inhibitor z-IETD-fmk, and the caspase 9 inhibitor z-LEHD-fmk (Fig. 7 a). All three drugs inhibited apoptosis of αvβ5-expressing cells to the same extent (Fig. 7, a and b). This finding suggested that the intrinsic pathway was involved in anoikis.
Figure 7.
αvβ5-mediated anoikis is independent of FADD. (a and b) Anoikis of αv-transduced H357 cells suspended in presence of caspase inhibitors z-IETD-fmk (caspase 8), z-LEHD-fmk (caspase 9), and z-VAD-fmk (pan caspase). (b) SEM of three experiments. (c) Flow cytometric profiles of αv-expressing H357 cells before and after transduction with eGFP-dNFADD-AU1. GFP fluorescence is shown. (d) SEM of two experiments comparing the percentage of anoikis after 0 or 72 h in suspension. Parental H357 cells were compared with cells transduced with αv (H357αv) alone or in combination with dnFADD. (e) Effect of z-VAD-fmk (CI) and dnFADD on MDCK anoikis was measured by flow cytometry of annexin V–positive cells. Data are from a single representative experiment.
To determine whether or not anoikis was indeed death receptor independent, we introduced dnFADD into αv-expressing H357 cells. The dnFADD construct, tagged with eGFP, was subcloned into pBabe puro and used to infect H357 cells. A high level of expression of dnFADD in over 90% of cells was achieved (Fig. 7 c). Suspension experiments were performed comparing parental H357 cells, H357 cells expressing αv alone, and H357 cells expressing both αv and dnFADD. Expression of dnFADD had no effect on anoikis of αv-expressing H357 cells (Fig. 7 d). As a positive control, dnFADD was also introduced into MDCK cells; it inhibited anoikis even more effectively than z-VAD-fmk (Fig. 7 e). This finding suggests that FADD does not play a role in αvβ5-mediated anoikis.
Inhibition of caspase 8 increases Akt activity
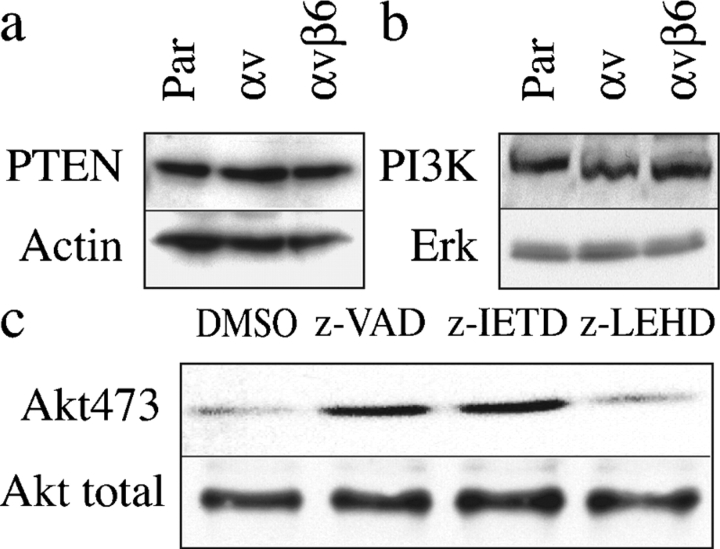
We investigated potential mechanisms by which Akt phosphorylation was down-regulated when αvβ5-expressing H357 cells were held in suspension for 72 h. PTEN is a negative regulator of Akt-dependent cell survival (Stambolic et al., 1998); however, there was no difference in PTEN levels in parental cells or cells expressing αvβ5 or αvβ6 (Fig. 8 a). In addition, there was no difference in expression of PI3 kinase, as judged by the level of the p110α subunit detected on Western blots (Fig. 8 b), nor in the total level of Akt (Fig. 8 c).
Figure 8.
Mechanisms by which Akt phosphorylation could be regulated. (a and b) Western blots of parental H357 cells (par) or cells transduced with αv alone or αv and β6 were suspended for 72 h and probed with antibodies to PTEN (a) or PI3 kinase (p110α subunit; b). Actin (a) and Erk MAPK (b) were loading controls. (c) Western blots of H357 cells transduced with αv probed with anti-phosphoAkt473 and total Akt. Cells were suspended for 72 h with 100 μM z-VAD-fmk, z-IETD-fmk, z-LEHD-fmk, or DMSO. Amount of protein loaded per track was greater than sixfold higher than in blots shown in Figs. 4–6.
To investigate the relationship between caspases and Akt phosphorylation, αvβ5-expressing cells were suspended for 72 h in the presence or absence of caspase inhibitors. The pan caspase inhibitor z-VAD-fmk and the caspase 8 inhibitor z-IETD-fmk increased the level of phosphoAkt, whereas the caspase 9 inhibitor z-LEHD-fmk did not (Fig. 8 c). We conclude that caspase 8 is upstream of Akt in αvβ5-mediated anoikis.
Discussion
When the αv integrin subunit was introduced into αv-negative H357 cells it formed a functional heterodimer on the cell surface with the β5 subunit. Expression of αvβ5 induced anoikis, which could be blocked by integrin ligation or caspase inhibition but not by blocking FADD death receptor signaling. The effect was not specific for H357 cells because a second SCC line was also stimulated to undergo anoikis by overexpression of αv. Anoikis was dependent on the β5 cytoplasmic domain and was not observed in cells expressing αvβ6. Anoikis of parental H357 cells was induced with a PI3 kinase inhibitor and correlated with a failure of αvβ5-expressing cells to activate Akt in suspension. Resistance to anoikis was achieved by constitutive activation of Akt or caspase inhibition. Caspase 8 inhibition resulted in increased phosphoAkt levels, placing caspase 8 upstream of Akt. We conclude that αvβ5 confers susceptibility to anoikis in SCC cells and that this is overridden when β5 is removed from the cell surface through loss of αv or up-regulation of the β6 integrin subunit. Up-regulation of β6 is a common feature of human SCCs, and we propose that this is a means of allowing growth of tumor cells in the absence of a basement membrane. This is a novel way in which up-regulation of αvβ6 contributes to cancer progression.
The ability of integrin ligation to suppress apoptosis is well established, and several different mechanisms have been reported, reflecting a degree of cell type and integrin specificity (for review see Jost et al., 2001; Miranti and Brugge, 2002). Ligation of integrins can induce antiapoptotic proteins, such as Bcl-2 (Matter and Ruoslahti, 2001), or suppress proapoptotic proteins, such as Bax (Gilmore et al., 2000; Valentijn et al., 2003). Ligation of α5β1 or αvβ3 increases Bcl-2 expression through NFκB activation (Scatena et al., 1998). Integrin activation of FAK leads to increased expression of inhibitors of apoptosis proteins, which bind to and inhibit executioner caspases (Sonoda et al., 2000).
Previous work on anoikis has demonstrated the involvement of death receptor signaling and the subsequent activation of the extrinsic death pathway via caspase 8 activation (Frisch, 1999a; Rytömaa et al., 2000). αvβ5-mediated anoikis could not be inhibited with dnFADD, suggesting that unlike anoikis of endothelial cells and simple epithelial cells (Frisch, 1999a; Rytömaa et al., 1999; Hood et al., 2003), SCC anoikis is independent of death receptor signaling. In endothelial cells, αvβ5 ligation protects against FADD-mediated apoptosis (Hood et al., 2003), demonstrating that the same integrin can regulate apoptosis by distinct mechanisms, dependent on cellular context. αvβ5-mediated anoikis could be blocked with inhibitors of caspase 8 and 9. Caspase 8 can be activated independently of the extrinsic death pathway in cells with unligated integrins (Stupack et al., 2001). Caspase 8 cleaves Bid (a Bcl-2 family member), triggering mitochondrial cytochrome c release and activation of caspase 9 (Rytömaa et al., 2000). Caspase 9 may then lead to further cleavage and activation of caspase 8, hence amplifying the signal (Rytömaa et al., 2000).
It has previously been reported that whereas unligated β1 and β3 integrin subunits can directly activate caspase 8, β5 does not (Stupack et al., 2001). In agreement with this report, we were unable to coimmunoprecipitate αvβ5 or αvβ6 with caspase 8 in H357 cells under a variety of conditions (unpublished data). In H357 cells expressing αvβ5 it is more likely that the β5 cytoplasmic tail activates caspase 8 indirectly via effects on the cytoskeleton. In some dying cells, actin, integrins, and caspase 8 have been seen to colocalize in complexes that lack death receptors and FADD (Stupack et al., 2001), and local accumulation of caspase 8 is sufficient to trigger apoptosis. The mechanism of the association of caspase 8 with the cytoskeleton is not known, but newly described proteins Hip and Hippi, homologous to cytoskeleton linker proteins talin and myosin, have been found that include “pseudo-death effector domains” (Gervais et al., 2002).
In contrast to αv-negative H357 cells and cells expressing αvβ6, cells that expressed αvβ5 did not activate Akt in suspension. The pro-survival function of Akt is well established. Activated Akt can phosphorylate and inhibit several different proapoptotic proteins including BAD (Datta et al., 1997) and caspase 9 (Cardone et al., 1998). Active Akt also induces NFκB (Kane et al., 1999), which reduces the release of cytochrome c from mitochondria (Kennedy et al., 1999). Akt is negatively regulated by the phosphatase PTEN, and a lack of PTEN leads to increased resistance to apoptosis (Lu et al., 1999). However, there were no differences in PTEN levels in parental H357 cells or cells expressing αvβ5 or αvβ6.
The mechanism by which unligated αvβ5 triggers anoikis requires activation of caspases 8 and 9 and suppression of Akt activity. Because there was no reduction in total Akt when αvβ5-expressing H357 cells were held in suspension, caspase-dependent cleavage of Akt (Bachelder et al., 2001) does not appear to be involved. Nevertheless, inhibition of caspase 8 did lead to an increase in phosphoAkt, placing caspase 8 upstream of the suppression of Akt activation. Inhibition of caspase 9 did not increase phosphoAkt, leading us to speculate that Akt is upstream of caspase 9 (Cardone et al., 1998).
One striking difference between primary human keratinocytes and SCC cells is that primary keratinocytes undergo terminal differentiation and not anoikis when held in suspension (Gandarillas et al., 1999). Moreover, when unligated integrins are expressed in the suprabasal layers of the epidermis, apoptosis is not stimulated (Carroll et al., 1995). When αv was overexpressed in primary human keratinocytes they retained the ability to terminally differentiate and did not undergo anoikis. It seems possible that the selection pressure placed on clones of αv-transfected H357 cells described previously selected for rare cells that had the ability to terminally differentiate rather than apoptose (Jones et al., 1996).
The similarities between apoptosis and keratinocyte terminal differentiation have previously been discussed and include inhibition of suspension-induced differentiation by integrin ligation (Levy et al., 2000) and by caspase inhibitors (Allombert-Blaise et al., 2003). Akt activation plays a role in driving the later stages of keratinocyte terminal differentiation (Janes et al., 2004). We speculate that by preventing Akt activation, αvβ5 acts as a fail-safe device, triggering anoikis in SCC cells that have lost the ability to differentiate. Resistance to anoikis can potentially enhance epithelial neoplasia by allowing cells to proliferate in the absence of attachment to extracellular matrix and to leave their normal environment and travel to distant body sites, thereby forming metastases. Up-regulation of αvβ6 is a novel mechanism by which tumor cells can avoid anoikis.
Materials and methods
Cell culture
Human epidermal keratinocytes (strain km, passage 2–6) were cultured on a feeder layer of J2 3T3 cells in FAD medium (1 part Ham's F12, 3 parts DME, and 180 μM adenine) supplemented with 10% FCS and HICE cocktail (0.5 μg/ml hydrocortisone, 5 μg/ml insulin, 10−10 M cholera enterotoxin, and 10 ng/ml EGF), essentially as described previously (Levy et al., 1998). The H357 (Sugiyama et al., 1993; a gift from S. Prime, University of Bristol, Bristol, UK) and SCC4 (Evans et al., 2003) cell lines were cultured in FAD, 10% FCS, and HICE without a feeder layer. J2-3T3 cells and puromycin-resistant J2-3T3 cells (J2-puro; Levy et al., 1998) were cultured in DME and 10% donor calf serum. 2.5 μg/ml puromycin was added to the medium of J2-puro cells. MDCK cells and retroviral packaging cells were cultured in DME and 10% FCS.
Retroviral vectors
To generate αvpBabe puro, human αv cDNA (provided by J. Loftus, Scripps Research Institute, La Jolla, CA) was blunted and inserted into SnabI cut, shrimp alkaline phosphatase–treated pBabe puro vector (Levy et al., 1998) using T4 ligase. α4pBabe puro and β6pBabe puro were gifts from J. Marshall and I. Hart (Cancer Research UK, London, UK; Thomas et al., 2001b). M+Akt:ER* and A2M+Akt:ER (Kohn et al., 1998; Mirza et al., 2000) in retroviral vector pWZLneo were gifts of L.M. Martins and J. Downward (Cancer Research UK, London, UK). eGFP-dNFADD-AU1 was excised from pcDNA3-AU1-NF4D (a gift of J. Downward; Rytömaa et al., 1999) with NheI and BamHI, blunted and inserted into SnabI cut, shrimp alkaline phosphatase–treated pBabe puro using T4 ligase.
The chimeric β6/β5 integrin subunit (βX6C5) was constructed as follows. The β6 extracellular and transmembrane domain was made by PCR using β6pBabe puro as a template. The primers used had the BamHI and EcoRI sites added and were as follows: 5′-GGGATCCGCCACCATGGGGATTGAACTGCTTTGCCTGTTCTT-3′; 3′-GGAATTCTTCCAGATGCACAGTA- GGACAACCCCGATGA. The resulting β6 cytoplasmic domain-deleted PCR product (β6ΔC) was cloned into pCR-4-TOPO, cut with EcoRI and BamHI, and ligated into pBabe puro. The cytoplasmic domain of β5 was purchased as part of an I.M.A.G.E. clone (7160-k21; Geneservice) and generated by PCR using primers that had an EcoRI site at the 5′ prime end and SalI at the 3′ end. The primers were as follows: 5′-GGAATTCTGCTTGTCACCATCCACGACCGGAGGGAGTT-3′; 3′-GAGTCGACTCAGTCCACAGTGCCATTGTAGGATTTGTTG. The confirmed sequence was cut using EcoRI and SalI and ligated into β6ΔC pBabe puro giving the βX6C5 chimeric molecule, but with an additional EcoRI site. This site was removed using Mung Bean Nuclease. The cDNA was then religated, bringing the chimeric integrin subunit correctly into frame.
Retroviral transduction
Ecotropic Phoenix packaging cells were transiently transfected with retroviral vectors, and virus-containing supernatants were used to infect AM12 packaging cells, as described previously (Legg et al., 2003). 60–85% of AM12 cells transduced with retrovirally encoded integrin subunits had high surface expression of the integrins following puro selection; these cells were selected by FACS® to achieve high expression in >95% of the population (Levy et al., 1998). Stably transduced AM12 cells were cultured with 2.5 μg/ml puromycin or, in the case of the Akt retroviral vectors, 2 mg/ml G418 (Geneticin; GIBCO BRL).
SCC cells and primary keratinocytes were transduced with retroviral vectors either by coculture with AM12 cells (Levy et al., 1998) or by incubation with AM12 supernatant (Legg et al., 2003). 70–85% of epithelial cells had high surface expression of the retrovirally encoded integrin subunits after puro selection; these cells were further enriched by FACS® as described for AM12 cells.
When cells were transduced with two retroviral vectors, selection was either with puromycin and FACS® or with puromycin and neomycin. SCC and primary keratinocytes transduced with retroviral vectors were cultured with 1 μg/ml puromycin and/or 2 mg/ml G418, as appropriate.
Antibodies
The following mAbs to human integrins were used: HAS4 (α2β1), VM-2 (α3β1), 7.2R (α4; a gift from J. Marshall and I. Hart), 13C2 (αv subunit), PIIW7 (αv subunit), P1D6 (α5β1; Chemicon), GoH3 (α6 integrins), P1F6 (αvβ5), AB1932 (αvβ3; Chemicon), 23C6 (αvβ3), 10D5 (αvβ6; Chemicon), MPF410 (α6 integrins), P5D2 (β1 integrins), and 3E1 (β4 integrin subunit). We used JG22 to detect chick β1 integrins. Actin was detected with AC40 (Sigma-Aldrich), transglutaminase 1 with B.C1 (a gift of R. Rice, University of California, Davis, Davis, CA), and involucrin with SY5. An antibody to AU1 was purchased from Covance and anti-p110α PI3K was purchased from BD Biosciences. Rabbit antibodies were also purchased as follows: ER (HC20; Santa Cruz Biotechnology, Inc.), serine 473–phosphorylated Akt (Biosource International; Cell Signaling Technology), total Akt (Cell Signaling Technology), Erk1/2 (Cell Signaling Technology), phospho-Erk1/2 (Thr202/Tyr204; Cell Signaling Technology), Erk2 (SC-1647; Santa Cruz Biotechnology, Inc.), and PTEN (Cascade Biologics, Inc.). Rabbit antibodies against β5 and β6 used for Western blotting were purchased from Calbiochem. Species-specific secondary antibodies conjugated to Alexa 488 or Alexa 594 were purchased from Molecular Probes, and HRP-conjugated second antibodies were purchased from Amersham Biosciences.
FACS®, flow cytometry, and immunofluorescence staining
Live cells were labeled with antibodies diluted in PBSABC and sorted into FAD, 10% FCS, and HICE medium using a FACSVantage™ machine (Becton Dickinson), as described previously (Levy et al., 1998). Involucrin and transglutaminase 1 expression was examined by flow cytometry of PFA-fixed, saponin-permeabilized cells, as described previously (Gandarillas et al., 1999), using a FACScan™ machine (Becton Dickinson).
DNA content of ethanol-fixed cells stained with 5μg/ml propidium iodide in the presence of RNase A (50 μg/ml) was determined with a FACSCalibur flow cytometer.
Focal adhesions were visualized in cells fixed for 10 min with 4% formaldehyde in PBS and 0.1% Triton X-100, as described previously (Levy et al., 2000). Cells were examined at RT with a confocal laser scanning microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.) and software (Carl Zeiss MicroImaging, Inc.) using a 40/NA 1.2 objective (Carl Zeiss MicroImaging, Inc.).
Adhesion assays
Vitronectin was purified from human plasma (Cheresh and Spiro, 1987) and coated onto 96-well bacterial plates (Evans et al., 2003). Cells were labeled with 5 μM of CellTracker dye (Molecular Probes), harvested with trypsin/EDTA, and resuspended at 4 × 105 cells/ml in TBS containing 1 mM Mn2+. 100 μl of cell suspension was added per well and incubated at 37°C for 30 min. The number of adherent cells was quantitated as described previously (Evans et al., 2003).
Anchorage-independent growth and suspension culture
Anchorage independent growth in soft agar was evaluated as described previously (Jones et al., 1996). 5 × 103 cells per 35-mm dish in 0.3% agar were cultured for 3 wk, and then stained with 1 mg/ml of nitroblue tetrazilium dye (Sigma-Aldrich). All colonies >140 μm in diameter in each of the three randomly chosen 0.8-cm2 fields were scored under a dissecting microscope.
Cells were suspended in medium supplemented with 3.5 g/200 ml of methyl cellulose (Sigma-Aldrich), essentially as described previously (Levy et al., 2000), except that the concentration of HICE cocktail was one tenth that in the normal culture medium. The following inhibitors were used at the concentrations indicated: z-VAD-fmk (100μM; ICN Biomedicals), z-LIEH-fmk (100μM; Sigma-Aldrich), z-LEHD-fmk (100μM; Sigma-Aldrich), LY294002 (50 μM; Sigma-Aldrich), SB203580 (10 μM; Sigma-Aldrich), and U0126 (10 μM; Promega).
Western blotting
Cells were lysed on ice in a modified RIPA buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 5 mM EDTA, and 1% Triton X-100, with protease and phosphatase inhibitor cocktails (Sigma-Aldrich; Hobbs and Watt, 2003). Soluble proteins were resolved by SDS-PAGE on 10% Laemmli gels and transferred onto Immobilon PVDF membranes (Millipore). Antibody labeling was performed as described previously using a chemiluminescence kit (Western Lightning; NEN Life Science Products) for detection (Hobbs and Watt, 2003).
Acknowledgments
We thank K. Nishi, J. Downward, R. Evans, R. Hobbs, D. Owens, S. Broad, and D. Campbell for advice, reagents, and practical help. H357 cells were a kind gift from S. Prime.
S.M. Janes was the recipient of a Medical Research Council Clinical Fellowship. This work was supported by Cancer Research UK.
Abbreviations used in this paper: dn, dominant-negative; ER, estrogen receptor; OHT, 4-hydroxytamoxifen; SCC, squamous cell carcinoma.
References
- Agrez, M., X. Gu, J. Turton, C. Meldrum, J. Niu, T. Antalis, and E.W. Howard. 1999. The αvβ6 integrin induces gelatinase B secretion in colon cancer cells. Int. J. Cancer. 81:90–97. [DOI] [PubMed] [Google Scholar]
- Ahmed, N., J. Niu, D.J. Dorahy, X. Gu, S. Andrews, C.J. Meldrum, R.J. Scott, M.S. Baker, I.G. Macreadie, and M.V. Agrez. 2002. Direct integrin αvβ6-ERK binding: implications for tumour growth. Oncogene. 21:1370–1380. [DOI] [PubMed] [Google Scholar]
- Allombert-Blaise, C., S. Tamiji, L. Mortier, H. Fauvel, M. Tual, E. Delaporte, F. Piette, E.M. DeLassale, P. Formstecher, P. Marchetti, and R. Polakowska. 2003. Terminal differentiation of human epidermal keratinocytes involves mitochondria- and caspase-dependent cell death pathway. Cell Death Differ. 10:850–852. [DOI] [PubMed] [Google Scholar]
- Bachelder, R.E., M.A. Wendt, N. Fujita, T. Tsuruo, and A.M. Mercurio. 2001. The cleavage of Akt/protein kinase B by death receptor signaling is an important event in detachment-induced apoptosis. J. Biol. Chem. 276:34702–34707. [DOI] [PubMed] [Google Scholar]
- Breuss, J.M., J. Gallo, H.M. DeLisser, I.V. Klimanskaya, H.G. Folkesson, J.F. Pittet, S.L. Nishimura, K. Aldape, D.V. Landers, W. Carpenter, et al. 1995. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108:2241–2251. [DOI] [PubMed] [Google Scholar]
- Cardone, M.H., N. Roy, H.R. Stennicke, G.S. Salvesen, T.F. Franke, E. Stanbridge, S. Frisch, and J.C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science. 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- Carroll, J.M., M.R. Romero, and F.M. Watt. 1995. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 83:957–968. [DOI] [PubMed] [Google Scholar]
- Cheresh, D.A., and R.C. Spiro. 1987. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 262:17703–17711. [PubMed] [Google Scholar]
- Datta, S.R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M.E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91:231–241. [DOI] [PubMed] [Google Scholar]
- Davies, J., J. Warwick, N. Totty, R. Philp, M. Helfrich, and M. Horton. 1989. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J. Cell Biol. 109:1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, R.D., V.C. Perkins, A. Henry, P.E. Stephens, M.K. Robinson, and F.M. Watt. 2003. A tumor-associated β1 integrin mutation that abrogates epithelial differentiation control. J. Cell Biol. 160:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch, S.M. 1999. a. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr. Biol. 9:1047–1049. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M. 1999. b. Methods for studying anoikis. Methods Mol. Biol. 129:251–256. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and H. Francis. 1994. Disruption of epithelial cell–matrix interactions induces apoptosis. J. Cell Biol. 124:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch, S.M., K. Vuori, D. Kelaita, and S. Sicks. 1996. A role for Jun-N-terminal kinase in anoikis; suppression by bcl-2 and crmA. J. Cell Biol. 135:1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandarillas, A., L.A. Goldsmith, S. Gschmeissner, I.M. Leigh, and F.M. Watt. 1999. Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp. Dermatol. 8:71–79. [DOI] [PubMed] [Google Scholar]
- Gervais, F.G., R. Singaraja, S. Xanthoudakis, C.A. Gutekunst, B.R. Leavitt, M. Metzler, A.S. Hackam, J. Tam, J.P. Vaillancourt, V. Houtzager, et al. 2002. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat. Cell Biol. 4:95–105. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.P., A.D. Metcalfe, L.H. Romer, and C.H. Streuli. 2000. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 149:431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann, J. 2002. Molecular mechanisms of “detachment-induced apoptosis–Anoikis”. Apoptosis. 7:247–260. [DOI] [PubMed] [Google Scholar]
- Haapasalmi, K., K. Zhang, M. Tonnesen, J. Olerud, D. Sheppard, T. Salo, R. Kramer, R.A. Clark, V.J. Uitto, and H. Larjava. 1996. Keratinocytes in human wounds express αvβ6 integrin. J. Invest. Dermatol. 106:42–48. [DOI] [PubMed] [Google Scholar]
- Hobbs, R.M., and F.M. Watt. 2003. Regulation of interleukin-1α expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. 278:19798–19807. [DOI] [PubMed] [Google Scholar]
- Hood, J.D., R. Frausto, W.B. Kiosses, M.A. Schwartz, and D.A. Cheresh. 2003. Differential αv integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes, S.M., T. Ofstad, D.H. Campbell, F.M. Watt, and D.M. Prowse. 2004. Transient activation of FOXN1 in keratinocytes induces a transcriptional programme that promotes terminal differentiation: contrasting roles of FOXN1 and Akt. J. Cell Sci. In press. [DOI] [PubMed] [Google Scholar]
- Jones, J., M. Sugiyama, P.M. Speight, and F.M. Watt. 1996. Restoration of αvβ5 integrin expression in neoplastic keratinocytes results in increased capacity for terminal differentiation and suppression of anchorage-independent growth. Oncogene. 12:119–126. [PubMed] [Google Scholar]
- Jones, J., F.M. Watt, and P.M. Speight. 1997. Changes in the expression of αv integrins in oral squamous cell carcinomas. J. Oral Pathol. Med. 26:63–68. [DOI] [PubMed] [Google Scholar]
- Jost, M., T.M. Huggett, C. Kari, and U. Rodeck. 2001. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol. Biol. Cell. 12:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, L.P., V.S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601–604. [DOI] [PubMed] [Google Scholar]
- Kennedy, S.G., E.S. Kandel, T.K. Cross, and N. Hay. 1999. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja, A., and J. Downward. 1997. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J. Cell Biol. 139:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, A.D., A. Barthel, K.S. Kovacina, A. Boge, B. Wallach, S.A. Summers, M.J. Birnbaum, P.H. Scott, J.C. Lawrence, Jr., and R.A. Roth. 1998. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273:11937–11943. [DOI] [PubMed] [Google Scholar]
- Koistinen, P., and J. Heino. 2002. The selective regulation of αvβ1 integrin expression is based on the hierarchical formation of αv-containing heterodimers. J. Biol. Chem. 277:24835–24841. [DOI] [PubMed] [Google Scholar]
- Legg, J., U.B. Jensen, S. Broad, I. Leigh, and F.M. Watt. 2003. Role of melanoma chondroitin sulphate proteoglycan in patterning stem cells in human interfollicular epidermis. Development. 130:6049–6063. [DOI] [PubMed] [Google Scholar]
- Levy, L., S. Broad, A.J. Zhu, J.M. Carroll, I. Khazaal, B. Peault, and F.M. Watt. 1998. Optimised retroviral infection of human epidermal keratinocytes: long-term expression of transduced integrin gene following grafting on to SCID mice. Gene Ther. 5:913–922. [DOI] [PubMed] [Google Scholar]
- Levy, L., S. Broad, D. Diekmann, R.D. Evans, and F.M. Watt. 2000. β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell. 11:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y., Y.Z. Lin, R. LaPushin, B. Cuevas, X. Fang, S.X. Yu, M.A. Davies, H. Khan, T. Furui, M. Mao, et al. 1999. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 18:7034–7045. [DOI] [PubMed] [Google Scholar]
- Matter, M.L., and E. Ruoslahti. 2001. A signaling pathway from the α5β1 and αvβ3 integrins that elevates bcl-2 transcription. J. Biol. Chem. 276:27757–27763. [DOI] [PubMed] [Google Scholar]
- Mercurio, A.M., and I. Rabinovitz. 2001. Towards a mechanistic understanding of tumor invasion–lessons from the α6β4integrin. Semin. Cancer Biol. 11:129–141. [DOI] [PubMed] [Google Scholar]
- Miranti, C.K., and J.S. Brugge. 2002. Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4:E83–E90. [DOI] [PubMed] [Google Scholar]
- Mirza, A.M., A.D. Kohn, R.A. Roth, and M. McMahon. 2000. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 11:279–292. [PubMed] [Google Scholar]
- Munger, J.S., X. Huang, H. Kawakatsu, M.J. Griffiths, S.L. Dalton, J. Wu, J.F. Pittet, N. Kaminski, C. Garat, M.A. Matthay, et al. 1999. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 96:319–328. [DOI] [PubMed] [Google Scholar]
- Pasqualini, R., J. Bodorova, S. Ye, and M.E. Hemler. 1993. A study of the structure, function and distribution of β5 integrins using novel anti-β5 monoclonal antibodies. J. Cell Sci. 105:101–111. [DOI] [PubMed] [Google Scholar]
- Ramos, D.M., M. But, J. Regezi, B.L. Schmidt, A. Atakilit, D. Dang, D. Ellis, R. Jordan, and X. Li. 2002. Expression of integrin β6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 21:297–307. [DOI] [PubMed] [Google Scholar]
- Regezi, J.A., D.M. Ramos, R. Pytela, N.P. Dekker, and R.C. Jordan. 2002. Tenascin and β6 integrin are overexpressed in floor of mouth in situ carcinomas and invasive squamous cell carcinomas. Oral Oncol. 38:332–336. [DOI] [PubMed] [Google Scholar]
- Rytömaa, M., L.M. Martins, and J. Downward. 1999. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr. Biol. 9:1043–1046. [DOI] [PubMed] [Google Scholar]
- Rytömaa, M., K. Lehmann, and J. Downward. 2000. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene. 19:4461–4468. [DOI] [PubMed] [Google Scholar]
- Scatena, M., M. Almeida, M.L. Chaisson, N. Fausto, R.F. Nicosia, C.M. Giachelli, P.D. Arora, J. Ma, W. Min, T. Cruz, and C.A. McCulloch. 1998. NF-κB mediates αvβ3 integrin–induced endothelial cell survival. J. Cell Biol. 141:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda, Y., Y. Matsumoto, M. Funakoshi, D. Yamamoto, S.K. Hanks, and T. Kasahara. 2000. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J. Biol. Chem. 275:16309–16315. [DOI] [PubMed] [Google Scholar]
- Stambolic, V., A. Suzuki, J.L. de la Pompa, G.M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J.M. Penninger, D.P. Siderovski, and T.W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 95:29–39. [DOI] [PubMed] [Google Scholar]
- Stupack, D.G., X.S. Puente, S. Boutsaboualoy, C.M. Storgard, and D.A. Cheresh. 2001. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, M., P.M. Speight, S.S. Prime, and F.M. Watt. 1993. Comparison of integrin expression and terminal differentiation capacity in cell lines derived from oral squamous cell carcinomas. Carcinogenesis. 14:2171–2176. [DOI] [PubMed] [Google Scholar]
- Thomas, G.J., M.P. Lewis, I.R. Hart, J.F. Marshall, and P.M. Speight. 2001. a. αvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int. J. Cancer. 92:641–650. [DOI] [PubMed] [Google Scholar]
- Thomas, G.J., M.P. Lewis, S.A. Whawell, A. Russell, D. Sheppard, I.R. Hart, P.M. Speight, and J.F. Marshall. 2001. b. Expression of the αvβ6 integrin promotes migration and invasion in squamous carcinoma cells. J. Invest. Dermatol. 117:67–73. [DOI] [PubMed] [Google Scholar]
- Toker, A., and A.C. Newton. 2000. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271–8274. [DOI] [PubMed] [Google Scholar]
- Valentijn, A.J., A.D. Metcalfe, J. Kott, C.H. Streuli, and A.P. Gilmore. 2003. Spatial and temporal changes in Bax subcellular localization during anoikis. J. Cell Biol. 162:599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier, A., and A. Sonnenberg. 2001. Function and interactions of integrins. Cell Tissue Res. 305:285–298. [DOI] [PubMed] [Google Scholar]
- Watt, F.M. 2002. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21:3919–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]