Abstract
We show here that β1 integrins selectively modulate insulin-like growth factor type I receptor (IGF-IR) signaling in response to IGF stimulation. The β1A integrin forms a complex with the IGF-IR and insulin receptor substrate-1 (IRS-1); this complex does not promote IGF-I mediated cell adhesion to laminin (LN), although it does support IGF-mediated cell proliferation. In contrast, β1C, an integrin cytoplasmic variant, increases cell adhesion to LN in response to IGF-I and its down-regulation by a ribozyme prevents IGF-mediated adhesion to LN. Moreover, β1C completely prevents IGF-mediated cell proliferation and tumor growth by inhibiting IGF-IR auto-phosphorylation in response to IGF-I stimulation. Evidence is provided that the β1 cytodomain plays an important role in mediating β1 integrin association with either IRS-1 or Grb2-associated binder1 (Gab1)/SH2-containing protein-tyrosine phosphate 2 (Shp2), downstream effectors of IGF-IR: specifically, β1A associates with IRS-1 and β1C with Gab1/Shp2. This study unravels a novel mechanism mediated by the integrin cytoplasmic domain that differentially regulates cell adhesion to LN and cell proliferation in response to IGF.
Keywords: laminin; prostate; Gab1; IRS-1; PI 3-kinase
Introduction
Neoplastic prostate growth and metastasis are regulated by interactions between cells and the surrounding ECM proteins (Parise et al., 2000). Members of the laminin (LN) family are the first ECM proteins synthesized during embryonic development (Ekblom et al., 2003). LN-1, composed of α1β1γ1 subunits, is an important component of the epithelial basement membranes, and contributes to epithelial cell adhesion and polarization (Malinda and Kleinman, 1996). LN-1 plays an important role in regulating the functions of normal and malignant human prostate epithelial cells (Bello-DeOcampo et al., 2001). LN interactions with several cells are mediated by integrins (Mercurio, 1995). Integrins are heterodimeric receptors consisting of an α and β subunit mediating cell–cell and cell–ECM interactions (Hynes, 2002). Members of the β1 subfamily and the α6β4 integrin have been reported to function as LN receptors and to play a role in tumor growth (Juliano, 1993; Mercurio, 1995; Colognato and Yurchenco, 2000).
The integrin cytoplasmic domains modulate several signal transduction pathways (Fornaro and Languino, 1997). The cytoplasmic domain of β1 exists in five different spliced forms and is known in its most widely expressed form, i.e., β1A, to modulate cell proliferation and migration as well as receptor localization (Fornaro and Languino, 1997). The β1C integrin, an alternatively spliced variant of the β1 subfamily that contains a unique 48–amino acid sequence in its cytoplasmic domain inhibits normal and cancer cell proliferation (Fornaro and Languino, 1997). This cytoplasmic variant is expressed in nonproliferative, differentiated epithelium and is selectively down-regulated in prostate and breast carcinoma (Fornaro and Languino, 1997; Manzotti et al., 2000).
Insulin-like growth factors (IGFs) and the IGF type I receptor (IGF-IR) are important modulators of growth and differentiation, play a crucial role in the establishment and maintenance of the transformed phenotype (Baserga, 2000), and are potential targets for anticancer treatment (Surmacz, 2003). The IGF axis appears to contribute to prostate cancer progression, but the mechanisms responsible for this process have not been studied (Reiss et al., 1998). IGF-I and IGF-II bind to IGF-IR and stimulate several signaling pathways including phosphatidylinositol 3-kinase (PI 3-kinase) and MAPK (Valentinis and Baserga, 2001; LeRoith and Roberts, 2003); both pathways are also known to be activated by integrin engagement (Damsky and Ilic, 2002). IGF-I induces association of β1 integrins and IGF-IR and increases adhesion of myeloma cells to fibronectin (FN; Tai et al., 2003). It has also been shown to increase adhesion of breast cancer cells to LN and this increase is inhibited by α-IR3, an antibody to the IGF-IR (Dunn et al., 1998).
The present work unravels a novel mechanism that regulates cell adhesion to LN-1 in response to IGF without affecting cell proliferation or tumor growth and is mediated by the association of β1 integrins with IGF-IR and with IGF-IR downstream effectors.
Results
IGFs stimulate adhesion to LN-1 of β1C-, but not of β1A-expressing cells
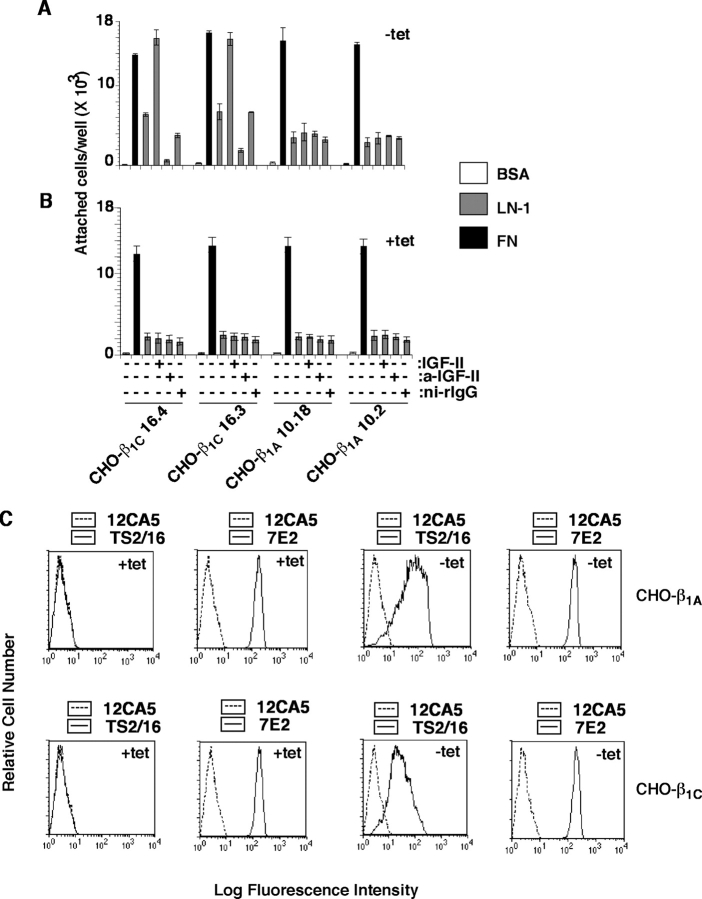
To investigate the effect of β1 integrins on IGF-IR–mediated functions, we used CHO and PC3 cells expressing either β1A or β1C integrin cytoplasmic variants under the control of a tetracycline (tet)-regulated promoter (Fornaro et al., 2000, 2003). As shown in Fig. 1 (A and B), addition of IGF-II caused a eightfold increase in adhesion to LN-1 of CHO cell transfectants expressing β1C (−tet) compared with cells that did not express β1C (+tet). IGF-II had no effect on cell adhesion to FN. Preincubation of cells with Ab to IGF-II prevented IGF-II mediated increased adhesion to LN-1 in β1C expressing cells (Fig. 1 A). In contrast, IGF-II had no effect on cell adhesion in the presence of exogenously expressed β1A integrin (Fig. 1, A and B). Comparable levels of surface expression of exogenous β1A and β1C evaluated using an mAb to human β1, TS2/16, were consistently observed (Fig. 1 C). Flow cytometry (FACS®) analysis performed using mAb 7E2 (Brown and Juliano, 1985) revealed comparable levels of expression of endogenous β1 integrin in the β1A and β1C transfectants (Fig. 1 C), thus suggesting that the observed effect on cell adhesion to LN-1 was not due to changes in endogenous β1 integrin levels.
Figure 1.
IGF stimulates adhesion to LN-1 of β1C-expressing cells. β1C-CHO (clones 16.3 and 16.4) and β1A-CHO (clones 10.18 and 10.2) clones were cultured in the absence (A) or in the presence (B) of tet for 48 h. Cells were labeled using 51Cr-sodium chromate for 1 h at 37°C. 51Cr-labeled cells were incubated in the presence or in the absence of purified rabbit Ab to IGF-II or ni-rIgG for 1 h on ice. Cells were plated on BSA, LN-1, or FN at 37°C for 2 h in the presence or in the absence of IGF-II. Attached cells were washed, lysed, and the amount of 51Cr associated with the attached cells was measured by liquid scintillation counting. Data are expressed as mean ± SEM. (C) Representative β1C-CHO and β1A-CHO clones were cultured in the absence or in the presence of tet for 48 h and analyzed by FACS® using a β1 integrin Ab TS2/16 specific to human β1 or 7E2 specific to hamster β1 or, as negative control, 12CA5.
Because our previous studies have shown that the β1C variant is down-regulated in prostate carcinoma (Fornaro et al., 1999; Moro et al., 2004), we analyzed the ability of normal or neoplastic prostate cells to adhere to LN-1 in response to IGF-I. IGF-I stimulated adhesion to LN-1 of normal human prostate epithelial cells (PrEC), but did not increase adhesion to LN-1 of human prostate cancer, PC3, cells (Fig. 2 A). To further confirm that IGF-stimulated cell adhesion to LN-1 is mediated by β1C integrin in prostate epithelial cells, we used 267B1, a nontumorigenic prostate epithelial cell line, that expresses β1C (Fig. 2 B) and adheres to LN-1 (not depicted). We down-regulated the expression of β1C in 267B1 cells using a ribozyme (RZ) specific for β1C (RZ-β1C; Fig. 2 C). The RZ specifically inhibited β1C expression but did not have any effect on the levels of other proteins, such as Akt or IGF-IR (Fig. 2 C). As shown in Fig. 2 D, down-regulation of β1C integrin completely prevented IGF-I stimulated cell adhesion to LN-1, but did not have any effect on cell adhesion to FN.
Figure 2.
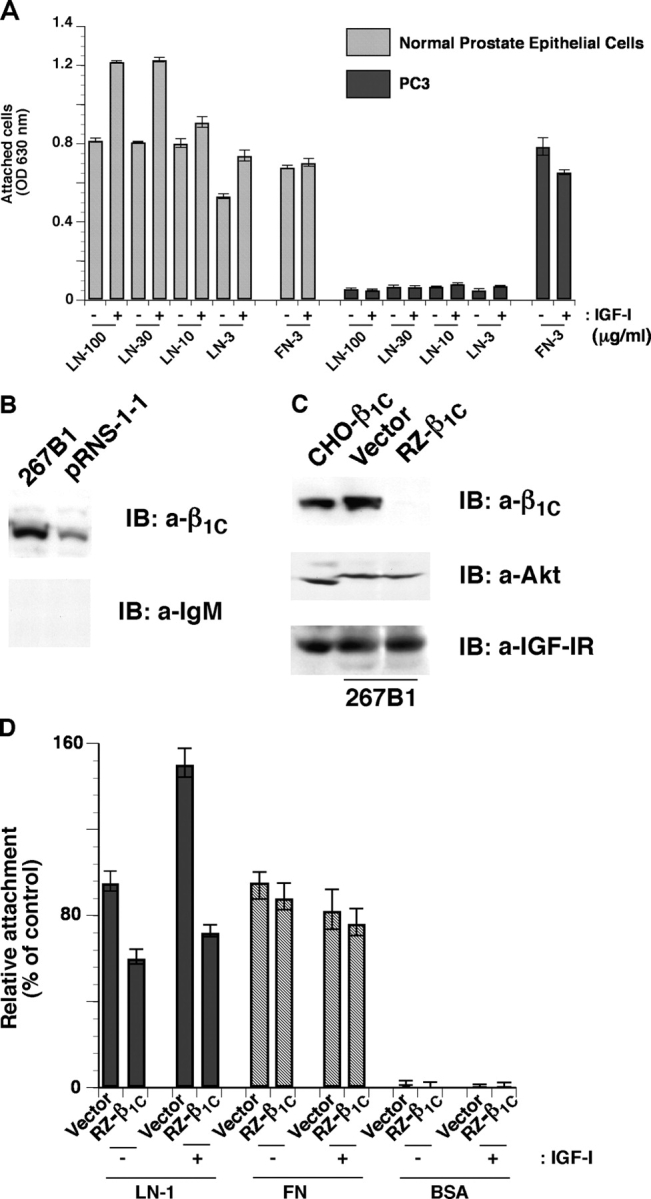
Down-regulation of β1C expression in 267B1 cells inhibits IGF-I–stimulated cell adhesion to LN-1. (A) PrEC and PC3 cells were serum starved for 12 h, detached, and plated on LN-1 or FN at 37°C for 2 h in the presence or in the absence of IGF-I. Cell adhesion was analyzed by crystal violet staining. (B) pRNS-1-1 and 267B1 cells were lysed and immunoblotted with mAb to β1C or normal IgM. (C) 267B1 cells were transiently transfected with pBJ1-RZ-β1C or vector alone, lysed, and immunoblotted with mAb to β1C, Ab to Akt, or Ab to IGF-IR. CHO-β1C cell lysate was used as a positive control. (D) 267B1 cells were transiently transfected with pBJ1-RZ-β1C or vector alone were detached and seeded on BSA or LN-1 or FN-coated plates at 37°C for 2 h in the presence or in the absence of IGF-I and stained with β-gal. Cell attachment was expressed as percentage of cells transfected with vector and attached to LN-1 in the absence of IGF-I, set at 100. Data are expressed as mean ± SEM. The experiments were repeated at least twice with similar results.
Re-expression of β1C integrin in PC3 cells caused a significant increase in cell adhesion to LN-1 in the presence of IGF-II (Fig. 3 A) and IGF-I (Fig. 3 C). In contrast, IGF-II had no effect on cell attachment to LN-1 in β1A expressing cells (Fig. 3 B). Similarly, IGF-I caused an increase in cell adhesion to LN-1 in the presence of β1C, but not of β1A integrin (Fig. 3 C). Because both IGF-I and IGF-II bind IGF-IR (Baserga, 2000) and gave comparable results (not depicted), they were used interchangeably. Cell adhesion to LN-1 stimulated by IGF-I was inhibited by an Ab to LN-1 (Fig. 3 D) and no differences between β1C and β1A expressing cells were observed when the cells attached to FN (Fig. 3, A–D). In conclusion, cells expressing elevated levels of β1C show a small, enhanced adhesion to LN-1, which is significantly stimulated in the presence of IGF-I or IGF-II. In contrast, cells expressing β1A retain the low baseline level of adhesion to LN-1, and their adhesion is not responsive to IGFs.
Figure 3.
β1C enhances IGF-I– and II–mediated PC3 cell adhesion to LN-1. (A–D) β1A- and β1C-PC3 clones were cultured in the presence or in the absence of tet for 48 h. Cells were detached and plated on BSA or LN-1 or FN at 37°C for 2 h in the presence of IGF-II (A and B), of IGF-I (C and D), or of Ab to LN-1 (D) or ni-rIgG (D). In A, the differences in cell adhesion to LN-1 in the presence or in the absence of tet are statistically significant (*P ≤ 0.03). In C, the differences in cell adhesion to LN-1 between β1A and β1C expressing cells are statistically significant (*P ≤ 0.024). The experiments were repeated at least three times with similar results using two clones each of β1A- and β1C-PC3 cells. (E and F) β1A- and β1C-PC3 clones were cultured in the presence or in the absence of tet for 48 h. Cells were serum starved for 12 h, detached, and plated on LN-1 (E), FN or BSA (F) in serum-free medium at 37°C for 2 h in the presence or in the absence of IGF-I or GoH3 or rtIgG. Cell adhesion was analyzed by crystal violet staining. Data are expressed as mean ± SEM. (G) Surface expression of endogenous or exogenous β1A or β1C integrin or endogenous α6 integrin was analyzed in PC3 cells by FACS® using Ab to human β1, TS2/16, chicken β1, W1B10, α6, GoH3; or, as negative controls, mIgG, rtIgG, or 12CA5.
The α6β1 integrin is the major LN-1 receptor and inhibition of α6β1 functions prevents acinar morphogenesis in prostate epithelial cells (Cooper et al., 1991; Shaw and Mercurio, 1993; Mercurio, 1995; Bello-DeOcampo et al., 2001). As shown in Fig. 3 E, addition of an inhibitory Ab to α6 (GoH3; Sonnenberg et al., 1987) prevented IGF-I mediated increased cell adhesion to LN-1 in PC3-β1C transfectants. Neither GoH3 nor normal rat IgG (rtIgG) inhibited cell adhesion to FN (Fig. 3 F). Comparable levels of surface expression of exogenous β1A or β1C in PC3 cells were consistently observed in all experiments after tet removal (Fig. 3 G). As shown in Fig. 3 G, induction of exogenous β1A or β1C expression did not change the levels of endogenous α6 integrin as analyzed by FACS® using GoH3. In conclusion, the α6 integrin is the predominant receptor that mediates IGF-stimulated cell adhesion to LN-1 of β1C expressing cells.
Expression of β1C inhibits IGF-stimulated cell proliferation and tumor growth
We investigated whether β1 integrins could interfere with PC3 cell proliferative response to IGFs. We found that both IGF-I and IGF-II increased PC3 cell proliferation in the presence of β1A, whereas expression of β1C prevented IGF-I or IGF-II stimulation of cell proliferation (Fig. 4 A). Based on the finding that β1 integrin is essential for tumor growth (Bloch et al., 1997) and IGF-IR promotes transformation (Baserga, 2000), we hypothesized that β1C would prevent tumor growth given its ability to inhibit IGF-stimulated cell proliferation. Because prostate cancer cells form tumors in an IGF-IR–dependent manner when injected subcutaneously in nude mice (Burfeind et al., 1996; Reiss et al., 1998), we injected PC3 cell transfectants expressing either β1A or β1C and followed tumor growth. Tumor formation by PC3-β1A cells was not affected by the presence or absence of tet in the drinking water and there were no differences in tumor formation and growth at all the examined time points (Fig. 4 B). The tumors that formed in the tet-deprived animals injected with PC3-β1C cells were significantly smaller in size compared with those in animals given tet (Fig. 4 C). PC3-β1C cells showed tumor formation at significantly later time points indicating that the cells were alive (not depicted). These data show that β1C significantly reduced prostate cancer cell proliferation and tumor growth, in vivo.
Figure 4.

β1A and β1B differentially affect IGF-mediated cell proliferation and tumor growth. (A) β1A- and β1C-PC3 clones were cultured in the absence of tet for 48 h. Cells were plated on a 96-well plate in serum-free medium with or without IGF-I or IGF-II at 37°C. After 72 h of incubation, cell proliferation was analyzed using SRB. As control, sulforhodamine B (SRB) incorporation of cells attached to FN-coated substrates was measured in parallel (black bars). (B and C) PC3-β1A (B) and PC3-β1C (C) clones were injected subcutaneously in athymic male Balb/c mice. Mice were given water supplemented with either 5% sucrose to induce β1A or β1C expression, or 5% sucrose plus 100 μg/ml tet. The graphs show kinetics of tumor growth. Tumor growth is expressed as tumor volume in cubic millimeters. Data are the mean ± SEM of 10 animals per group. *P ≤ 0.001 at 10, 12, 14, and either 16 or 17 d from injection. These experiments were repeated three times with similar results.
IGF-IR and β1C act synergistically to support cell adhesion to LN-1
To analyze the role of IGF-IR in mediating cell adhesion to LN-1, we used R− cells derived from mouse embryos with a targeted disruption of the IGF-IR gene, and R+ cells are obtained by transfection of IGF-IR in R− cells (Sell et al., 1994). R− and R+ cells were transiently transfected using human β1A or β1C integrin cDNA. The expression levels of the transfected integrins were analyzed by FACS® and found to be comparable (Fig. 5 A). Expression of β1C, but not of β1A integrin in R+ cells significantly increased cell adhesion to LN-1 and this increase was further stimulated by IGF-II (Fig. 5 B). Transfection of β1C integrin in R− cells showed no increase in cell adhesion to LN-1, suggesting that β1C integrin alone is unable to bind to LN-1 (Fig. 5 C). Comparing cell adhesion of R− and R+ cells to LN-1 and FN, revealed that these cells adhere equally well to LN-1 and FN (not depicted). This excludes the possibility that IGF-IR alone is sufficient to stimulate cell adhesion to LN-1. These results show that neither β1A alone, nor β1C alone, nor IGF-IR alone, nor IGF-IR and β1A support cell adhesion to LN-1, and that the synergistic activity of both IGF-IR and β1C is required to support cell adhesion to LN-1.
Figure 5.

IGF-IR and β1C act synergistically to support cell adhesion to LN-1. (A) R− and R+ cells were transiently transfected with human β1A or β1C. Surface expression of β1A or β1C was analyzed by FACS® using TS2/16 or 12CA5 as a negative control. Thick line, TS2/16; thin line, 12CA5. (B) R+ cells transiently transfected with β1A or β1C were detached and seeded on BSA or LN-1–coated plates at 37°C for 2 h in the presence or in the absence of IGF-II and stained with β-gal. (C) R− and R+ cells (106) transiently transfected with β1A or β1C were incubated with or without P4C10 (a-β1) or 1C10 (neg-cont) on ice for 1 h. Cells were plated on BSA or LN-1 or FN at 37°C for 2 h and stained with β-gal. Attachment of cells transfected with β1-integrin cDNA was expressed as percentage of cells transfected with pBJ (B) or pBJ-β1A (C) that were attached to LN-1, set at 100. The experiments were repeated at least twice with similar results. Data are expressed as mean ± SEM.
β1C decreases IGF-stimulated tyrosine phosphorylation of IGF-IR and β1A–IGF-IR association
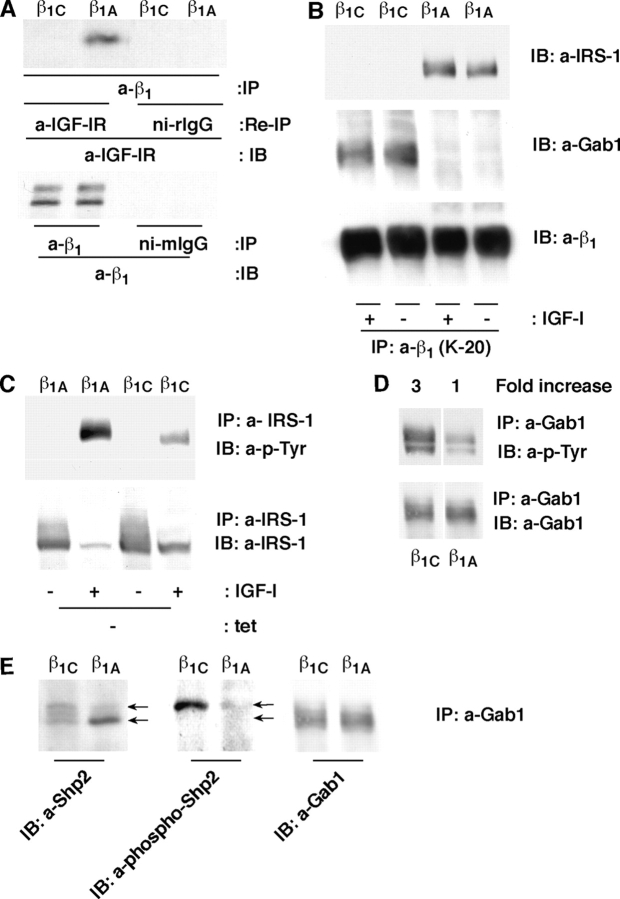
Because β1 integrins have been shown to associate with IGF-IR (Tai et al., 2003), we investigated whether β1A or β1C would differentially associate with IGF-IR using PC3 transfectants. The results showed that β1A, but not β1C associates with IGF-IR (Fig. 6 A). IGF-IR belongs to the family of receptor tyrosine kinases that undergo tyrosine phosphorylation upon ligand binding (LeRoith and Roberts, 2003). IGF-I increased IGF-IR tyrosine phosphorylation in β1A expressing PC3 cells, whereas expression of β1C significantly prevented IGF-stimulated tyrosine phosphorylation of IGF-IR resulting in inhibition of association between β1A and IGF-IR (Fig. 6, B and C).
Figure 6.
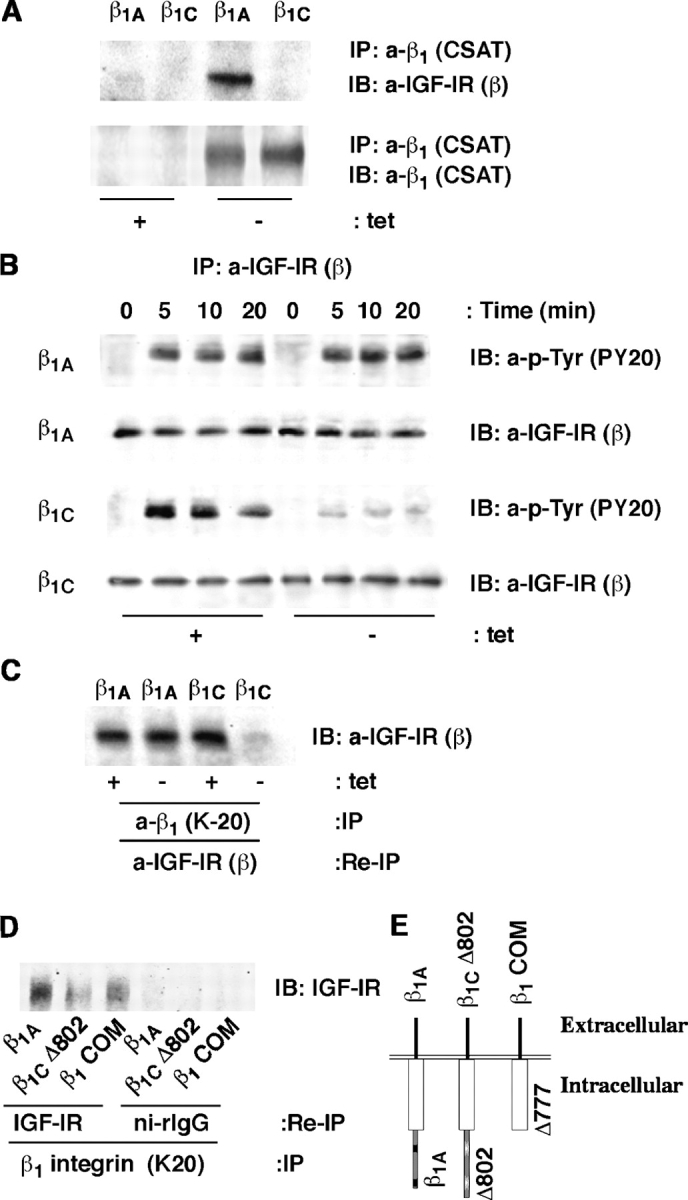
β1C inhibits IGF-IR tyrosine phosphorylation and β1A association with IGF-IR. β1A- and β1C-PC3 clones were cultured in the presence or in the absence of tet for 48 h, stimulated with IGF-I for 10 min at 37°C, lysed. Exogenous β1 was immunoprecipitated using CSAT (A) and endogenous β1 using K-20 (C). In A, immunoblotting (IB) was performed using CSAT or Ab to IGF-IR-β. (B) Cells were incubated with or without IGF-I for 0, 5, 10, and 20 min in suspension at 37°C. Immunoprecipitation was performed using Ab to IGF-IR-β. Immunoprecipitates were separated on SDS-PAGE and immunoblotted with PY20 or Ab to IGF-IR-β. In C, the endogenous β1 immunoprecipitates were reprecipitated and immunoblotted with Ab to IGF-IR-β. (D) CHO clones expressing the full-length β1A integrin or β1COM or β1CΔ802 truncated mutant were cultured for 48 h. Cells were lysed and proteins were immunoprecipitated with K-20. The immunocomplexes were dissociated and proteins were reimmunoprecipitated with Ab to IGF-IR or ni-rIgG. The immunoprecipitates were separated using SDS-PAGE and immunoblotted with Ab to IGF-IR. (E) Schematic representation of β1C cytoplasmic domain deletion mutants. The experiments were repeated at least twice with similar results.
To analyze whether the β1C integrin unique 48–amino acid cytoplasmic domain has an inhibitory effect on IGF-IR–β1A association, we used CHO transfectants expressing full-length β1A integrin or the β1COM mutant, that preserves the sequence shared by β1A and β1C integrins or the β1CΔ802 truncated mutant shown in Fig. 6 E. As expected, expression of full-length β1A integrin showed a strong association with IGF-IR (Fig. 6 D). The β1COM mutant retained the property to associate with IGF-IR showing that the common sequence between β1A and β1C integrin is only partially responsible for this association. The Δ802 significantly inhibited the association with IGF-IR, showing that the 778–802 domain mediates the β1C inhibitory effect on the association between IGF-IR and β1A. The results suggest an important role of the β1 cytoplasmic domain in the regulation of IGF-IR tyrosine phosphorylation and of β1A–GF-IR association.
β1A associates with and supports tyrosine phosphorylation of IRS-1, whereas β1C associates with and supports tyrosine phosphorylation of Gab1 and Shp2
Upon tyrosine phosphorylation, IGF-IR becomes the docking site for SH2 domain-containing proteins (LeRoith and Roberts, 2003), such as insulin receptor substrate-1 (IRS-1). IRS-1 is known to mediate several functions, predominantly proliferation and transformation, stimulated by the IGF-IR (Surmacz, 2003). Grb2-associated binder1 (Gab1) is another downstream effector of IGF-IR, as well as of the insulin receptor, and shares functional and structural homology with IRS-1 (Winnay et al., 2000; Gu and Neel, 2003). To investigate whether the β1 cytoplasmic domain would affect the downstream signaling proteins via direct association with β1A or β1C, we used CHO transfectants, where β1A was found to be associated with the IGF-IR (Fig. 7 A, top). Our results showed that IRS-1 associates with β1A and that β1C associates with Gab1 (Fig. 7 B). Cell transfectants expressed similar levels of exogenous β1 integrins (Fig. 7 A, bottom), IRS-1, or IGF-IR (not depicted).
Figure 7.
β1A associates with and supports tyrosine phosphorylation of IRS-1, whereas β1C associates with and supports tyrosine phosphorylation of Gab1 and Shp2. (A) CHO clones expressing either β1C or β1A integrin were lysed and β1 integrin was immunoprecipitated using K-20. (A) The β1 immunoprecipitates were reprecipitated with Ab to IGF-IR or ni-rIgG and immunoblotted with polyclonal Ab to IGF-IR (top). As control, proteins from CHO cell lysates were immunoprecipitated with Ab to β1 integrin or ni-mIgG and immunoblotted with Ab to β1 integrin (Ab13, bottom). (B) CHO clones expressing either β1C or β1A integrin were incubated with or without IGF-I for 10 min, lysed, and proteins were immunoprecipitated with Ab to β1 integrins (K-20) and immunoblotted with Ab to IRS-1 (top), Gab1 (middle), or β1 integrin (bottom). (C–E) CHO clones expressing either β1C or β1A integrin were incubated with or without IGF-I for 10 min, lysed, and proteins were immunoprecipitated with Ab to IRS-1 (C) or Gab1 (D and E). Immunoprecipitates were separated using SDS-PAGE and immunoblotted with Ab to p-Tyr (PY20) (C and D), IRS-1 (C), Gab1 (D and E), Shp2 (E), or phospho-Shp2 (E). (F) β1A- and β1C-CHO clones were transiently transfected with wt-Shp2 or Shp2 C/S or vector alone. Cells were then cultured in the absence of tet for 48 h, plated on LN-1 and incubated for 2 h at 37°C in the presence or in the absence of IGF-I. After incubation, cells were fixed and stained with β-gal. Attachment of cells transfected with wt-Shp2 or Shp2 C/S cDNA was expressed as percentage of the number of attached cells transfected with vector alone, set at 100 in the absence of IGF-I. Data are expressed as mean ± SEM. (G) β1A- and β1C-CHO clones were transiently transfected with either HA-tagged wt-Shp2 or FLAG-tagged Shp2 C/S or vector alone. ERK1 was used as loading control. Cells were lysed after 48 h and immunoblotted with Ab to HA for wt-Shp2 or Ab to FLAG for Shp2 C/S. The experiments were repeated at least three times with similar results. White lines indicate that intervening lanes have been spliced out.
To study the effect on IRS-1 or Gab1 tyrosine phosphorylation of the differential association of β1A and β1C with IRS-1 or Gab1 respectively, we used CHO transfectants. As shown in Fig. 7 C, IGF-I increased the tyrosine phosphorylation of IRS-1 in β1A expressing CHO cells, whereas expression of β1C significantly prevented IGF-stimulated tyrosine phosphorylation of IRS-1. In contrast, we found that expression of β1C significantly increased tyrosine phosphorylation of Gab1 (Fig. 7 D). Gab1, upon tyrosine phosphorylation, recruits and activates SH2-containing protein-tyrosine phosphate 2 (Shp2) phosphatase that causes dephosphorylation of Shp2 substrates including IGF-IR (Neel et al., 2003). In β1C, but not in β1A expressing cells, Gab1-associated Shp2 showed increased tyrosine phosphorylation levels (Tyr542) as evaluated by mobility shift assay and using a phospho-Ab specific to Shp2 (Fig. 7 E).
To explore the role of Shp2 in IGF-I mediated cell adhesion to LN-1, CHO cells expressing β1A or β1C were transiently transfected with either HA-tagged wt-Shp2 or FLAG-tagged Shp2 C/S, a dominant negative form of Shp2 (Zhang et al., 2002). As shown in Fig. 7 F, enhanced cell adhesion to LN-1 in the presence of IGF-I in β1C expressing CHO cells was completely prevented by transfection of Shp2 C/S, as compared with vector alone or wt-Shp2. The expression levels of the transfected wt-Shp2 or Shp2 C/S cDNAs were analyzed in cell lysates and found comparable in β1A and β1C expressing cells (Fig. 7 G).
Overall, these data show that β1A and β1C differentially associate with IRS-1 and Gab1 and that Shp2 activation is required for cell adhesion to LN in response to IGFs.
PI 3-kinase mediates IGF-stimulated adhesion to LN-1
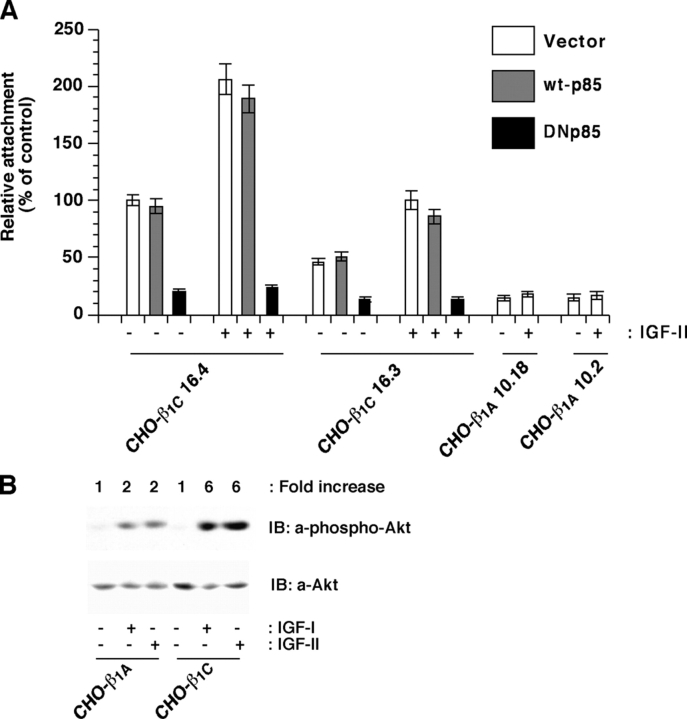
PI 3-kinase is a downstream effector that mediates IGF-IR as well as integrin-stimulated signaling; PI 3-kinase activation requires Shp2 in some pathways activated by receptor-tyrosine kinases such as IGF-IR and PDGF receptor, but not by others such as EGF receptor (Reiss et al., 2001; Neel et al., 2003; Tai et al., 2003). We investigated the role of PI 3-kinase in cell adhesion stimulated by IGF-II in cell transfectants expressing β1C integrin. We transiently transfected CHO clones expressing β1C integrin with dominant negative form of p85 (DNp85), wild-type p85 (wt-p85), or vector alone. As shown in Fig. 8 A, IGF-II stimulated cell adhesion to LN-1 in β1C expressing cells in either vector or wt-p85 transfected cells, but cell adhesion to LN-1 was significantly inhibited upon transfection of DNp85. In our previous work, we have shown that antibody-mediated engagement of β1 integrins activates the PI 3-kinase/Akt pathway (Fornaro et al., 2000). We found threefold higher activation of Akt, a PI 3-kinase downstream signaling molecule, in response to IGF-I or IGF-II stimulation in β1C expressing cells as compared with β1A expressing cells (Fig. 8 B). Our results indicate that PI 3-kinase mediates β1C integrin effect on cell adhesion to LN-1 in response to IGF.
Figure 8.
PI 3-kinase mediates IGF-stimulated adhesion to LN-1. (A) β1C-CHO clones were transiently transfected with vector alone (pcDNA3), wt-p85, or DNp85. Cells were cultured in the absence of tet for 48 h. 1.5 × 105 cells were plated per well coated with LN-1 and incubated for 2 h at 37°C in the presence or in the absence of 100 ng/ml IGF-II. Cells were washed, fixed, and stained with β-gal. Attachment of cells transfected with PI 3-kinase cDNA was expressed as percentage (average and SD) of the number of attached cells that were transfected with vector alone, set at 100. Data are expressed as mean ± SEM. (B) β1A- or β1C-CHO clones were stimulated with IGF-I or IGF-II for 10 min, lysed and immunoblotted with Ab to Akt or Ab to phospho-Akt. Experiments were repeated at least twice with similar results. Results using representative clones are shown.
Inhibition of IGF-IR prevents IGF-stimulated cell adhesion to LN-1
To confirm a direct involvement of IGF-IR in IGF-stimulated cell adhesion to LN-1 in the presence of β1C integrin, CHO cells expressing β1A or β1C were transiently transfected with 486/STOP, a dominant negative form of IGF-IR, known to inhibit IGF-IR signaling (Dunn et al., 1998). As shown in Fig. 9 A, enhanced cell adhesion to LN-1 in the presence of IGF-II in β1C integrin expressing CHO cells was completely prevented by transfection of 486/STOP, as compared with vector alone. The expression level of the mutant receptor was analyzed in culture medium and found comparable in β1A and β1C expressing cells transfected with 486/STOP (unpublished data). Additional evidence confirming the involvement of IGF-IR in adhesion to LN-1 of PC3 cells expressing β1C was that an antibody known to inhibit IGF-IR, α-IR3, prevented cell adhesion to LN-1 in the presence of IGF-I, whereas ni-mIgG had no effect (Fig. 9 B). This effect was specific to LN-1 because no differences were observed when the cells were attached to FN (Fig. 9 C). In conclusion, IGF-IR binding to IGF is necessary to achieve maximal levels of cell adhesion to LN-1 in response to IGFs.
Figure 9.
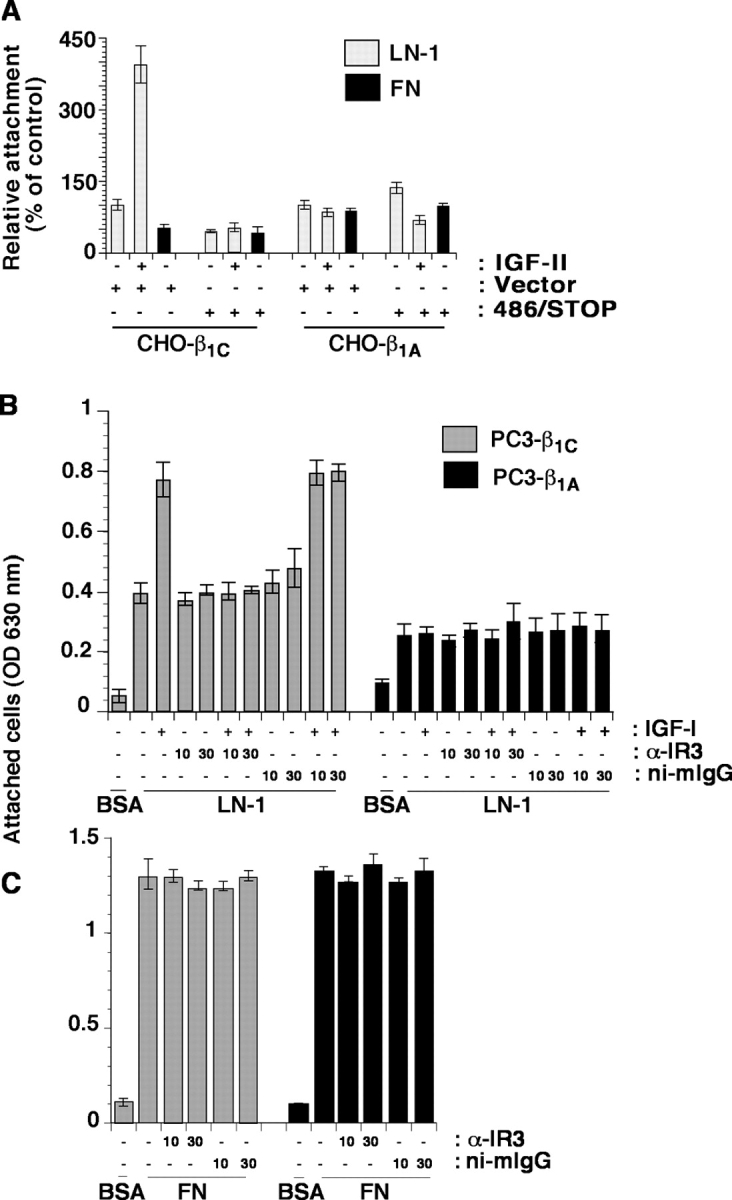
IGF binding to IGF-IR mediates adhesion of β1C expressing cells to LN-1. (A) β1A- and β1C-CHO clones were transiently transfected with 486/STOP or vector alone. Cells were cultured in the absence of tet for 48 h. Cells were plated on either LN-1 or FN and incubated for 2 h at 37°C in the presence or in the absence of IGF-II. After incubation, cells were fixed and stained with β-gal. The number of attached cells transfected with 486/STOP cDNA was expressed as percentage of the number of attached cells transfected with vector alone, set at 100. (B and C) β1C- and β1A-PC3 clones were cultured in the absence of tet for 48 h. Cells were plated on BSA or LN-1 (B) or FN (C) at 37°C for 2 h in the presence or in the absence of IGF-I or α-IR3 or ni-mIgG. The experiments were repeated at least three times with similar results. Data are expressed as mean ± SEM.
Discussion
The novelty of this work is that β1 integrin modulation of IGF-IR functions is controlled by the β1 cytodomains that recruit specific IGF-IR downstream effectors, either IRS-1 or the Gab1–Shp2 phosphatase complex, to the cell surface.
The finding that the association between β1A integrins and IGF-IR regulate cell adhesion to a basement membrane protein in response to IGF stimulation without affecting cell proliferation or tumor growth, is novel. Although it was known that integrins and growth factor receptors act synergistically and are associated, it was not known that their cross-talk determines the specificity of their activities (Yamada and Even-Ram, 2002). The relevance of this work is that the β1A–IGF-IR complex by limiting cancer cell adhesion to the basement membrane, presumably allows the tumor mass to expand and invade. A direct involvement of IGF-IR in cell adhesion to LN has been shown earlier (Dunn et al., 1998) supporting the evidence of a synergistic and overlapping activity of integrins and IGF-IR in cell adhesion. Our discovery was made possible by the serendipitous finding that β1C, a β1A cytoplasmic variant that does not associate with IGF-IR, increases cell adhesion to LN-1. This variant form causes disruption of the β1A–IGF-IR association via a 25–amino acid domain which is uniquely found in the β1C integrin. Several studies have demonstrated that structural differences in the intracellular domains are expected to be important determinants of the specificity of a variety of integrin-mediated events (Fornaro and Languino, 1997). Although the cytodomain was not believed to affect ligand specificity, our data prove that the integrin cytodomain affects ligand specificity in a substrate-dependent manner. We show an inhibitory effect on IGF-IR activation without a wide change in integrin ligand binding, because cell adhesion to FN is unaffected in response to β1C expression.
Here, as shown in Fig. 10, we demonstrate that the β1A–IGF-IR complex preserves IGF-IR phosphorylation and association with IRS-1, a molecule necessary to promote stimulation of IGF-IR signaling pathways important for cell proliferation and transformation (Reiss et al., 2000). In contrast, β1C does not associate with either IGF-IR or with IRS-1 and this results in failure to respond to a canonical IGF stimulation. The β1C integrin inhibits IGF-stimulated tyrosine phosphorylation of IGF-IR and cell proliferation, as well as tumor growth by associating with Gab1, and increasing phosphorylation of Gab1. Gab1, a member of the IRS-1 family of adaptor proteins, is known to be phosphorylated in response to several growth factor receptors' activation, enhances cell growth and cell transformation, but has also a negative role on cell survival through interaction with the Shp2 tyrosine phosphatase (Gu and Neel, 2003; Holgado-Madruga and Wong, 2003). β1C is likely to achieve the goal of preventing IGF-IR and IRS-1 tyrosine phosphorylation via activation of Gab1 and consequent recruitment of a Gab1 binding protein, Shp2 (Gu and Neel, 2003), to IGF-IR and IRS-1 (Myers et al., 1998). Gab1 activates PI 3-kinase, a molecule known to mediate cell adhesion by inhibiting the tyrosine phosphorylation of IRS-1 (Reiss et al., 2001). PI 3-kinase is a downstream effector that mediates IGF-IR as well as integrin-stimulated signaling; PI 3-kinase activation requires Shp2 in some pathways activated by receptor-tyrosine-kinases such as IGF-IR and PDGF receptor, but not by others such as EGF receptor (Reiss et al., 2001; Neel et al., 2003; Tai et al., 2003). Our data show that both Shp2 and PI 3-kinase mediate β1C-Gab1 effect on cell adhesion. On the same line, a previous set of studies by Clemmons's group had shown that inhibition of αVβ3 integrin binding to its ligands prevents IGF-stimulated tyrosine phosphorylation of IGF-IR and IRS-1 as well as cell proliferation to occur via Shp2 activation (Zheng and Clemmons, 1998). In contrast, this is the first work showing Gab1 recruitment to the cell surface by integrins, because Gab1 has only been shown to bind different growth factor receptors (Gu and Neel, 2003).
Figure 10.
A model for the biological effects of the cross-talk between IGF-IR and β1 integrins. This model shows that the β1C integrin forms a complex with and activates Gab1/Shp2; this results in recruitment of Shp2 to IGF-IR and consequently, IGF-IR dephosphorylation. The signaling events stimulated by β1C expression results in increased cell adhesion to laminin, reduced cell proliferation and inhibition of tumor growth. When down-regulation of β1C occurs, IGF-IR remains tyrosine phosphorylated and associated with β1A integrin, and this results in increased tumor growth and cell proliferation, but in reduced cell adhesion to LN.
Neoplastic cells have a surrounding matrix markedly different from the ECM surrounding normal cells (Parise et al., 2000). In prostate cancer, like in breast carcinoma, a complete loss of basement membranes has been described even in well-differentiated tumors affecting its various components including LN-1 (Fuchs et al., 1989; Nerlich et al., 1998). LN-1 is found in normal human prostate glands (Cress et al., 1995; Davis et al., 2001; Brar et al., 2003) and in adult mouse prostate (Falk et al., 1996), but its expression is lost in basement membranes surrounding primary carcinoma and metastatic lymph node lesions (Brar et al., 2003). Although normal tissues display expression of both β1C and LN-1, down-regulation of both molecules occurs during prostate cancer progression (Fornaro et al., 2001b), indicating that disruption of basement membranes and dysregulation of integrin variant expression are coordinated events in neoplastic transformation and that their expression is essential to sustain IGF stimulation of cell adhesion. Indeed, assembly of LN polymers requires expression of the LN receptor β1 integrin (Bouvard et al., 2001).
Further elucidation of the role of integrin–IGF-IR association as well as of their downstream signaling pathways will provide a better understanding of the mechanisms that contribute to prostate cancer progression.
Materials and methods
Reagents and antibodies
Mouse LN-1 and lipofectamine 2000 were purchased from Invitrogen; BSA was purchased from Sigma-Aldrich; recombinant human IGF-I was purchased from R&D Systems; and IGF-II was purchased from Austral Biologics. Human FN was purified as described previously (Fornaro et al., 2003).
The following murine mAbs were used: to human β1 integrin TS2/16 (American Type Culture Collection); K-20 (Immunotech); P4C10 (CHEMICON International, Inc.); 13 and clone 18 to β1 integrin (BD Biosciences); 7D5/BF10 to β1C integrin (Fornaro et al., 2001a); to chicken β1 integrin W1B10 (Sigma-Aldrich); CSAT (Developmental Studies Hybridoma Bank [DSHB]); to hamster β1 integrin 7E2 (DSHB). In addition, mAb to FLAG (Sigma-Aldrich); mAb 12CA5 to HA (American Type Culture Collection); 1C10 to vascular endothelial surface protein (Life Technologies); α-IR3 to IGF-IR (Oncogene); PY20 to phosphotyrosine (Santa Cruz Biotechnology, Inc.); rat mAb to α6 GoH3 (CHEMICON International, Inc.). The following rabbit polyclonal Abs were used: to IGF-IR-β (Santa Cruz Biotechnology, Inc.); to Gab1 and to IRS-1 (Upstate Biotechnology); to hIGF-II (PeproTech); to Shp2 (Santa Cruz Biotechnology, Inc.); to phospho-Akt (Ser 473), to Akt, to phospho-Shp2 (Tyr542; Cell Signaling); to ERK1 (Santa Cruz Biotechnology, Inc.); to LN-1 described previously (Tsiper and Yurchenco, 2002; provided by P. Yurchenco, Robert Wood Johnson Medical School, Piscataway, NJ). Non-immune Abs were ni-mIgG (Pierce Chemical Co.), ni-rIgG and mIgM (Sigma-Aldrich), and rtIgG (Cappel).
Cell lines and transfectants
Normal human PrEC cells were obtained from Clonetics and maintained as the manufacturer recommended. SV40 immortalized nontumorigenic human prostate epithelial 267B1 and pRNS-1-1 cells were grown in keratinocyte serum-free medium with 5 ng/ml human recombinant EGF and 0.05 mg/ml bovine pituitary extract (Parda et al., 1993; Peehl et al., 1997).
CHO stable cell transfectants expressing either human β1A or β1C integrin were cultured as described previously (Fornaro et al., 2000). PC3 stable cell transfectants expressing chimeric β1A (clones 8 and 11) or β1C (clones 17 and 19) integrin (chicken extracellular and human intracellular) were generated using the tet-regulated expression system and cultured as described previously (Fornaro et al., 2003). CHO-β1A, CHO-β1C, PC3-β1A, and PC3-β1C clones were cultured for 48 h in growth medium in the absence of 1 μg/ml tet to induce the expression of β1A or β1C integrin. R− cells, mouse embryo fibroblasts obtained from IGF-IR knockout mice; and R+ cells, previously established by stably transfecting R− cells with the human wild-type IGF-IR cDNA (Sell et al., 1994), provided by R. Baserga (Thomas Jefferson University, Philadelphia, PA).
Integrin surface expression was analyzed by FACS® as described previously (Fornaro et al., 2000, 2003). The pECE-β1COM plasmid containing the β1 integrin area of the cytoplasmic domain shared by β1A and β1C truncated at threonine residue 777, has been described previously (Retta et al., 1998; provided by G. Tarone, University of Torino, Torino, Italy). CHO cells were electroporated using 10 μg pECE-β1COM along with 1 μg pFneo. G418-resistant clones were pooled and analyzed for cell surface expression of human β1COM integrin by FACS® using TS2/16 or 12CA5, as a negative control, as described previously (Fornaro et al., 2000).
The β1C nucleotide sequence (Languino and Ruoslahti, 1992) was used to design the RZ specific for the β1C cytoplasmic domain. A double-stranded DNA encoding the RZ-β1C was obtained by annealing two synthetic single-stranded oligodeoxyribonucleotides containing flanking ClaI restriction sites and spanning a 55-bp sequence. The resulting double-stranded DNA encoding the RZ-β1C was subcloned into the ClaI site of the mammalian expression vector pBJ-1 and its sequence is as follows: 5′-cgCGGTTTACCCTGTGCAAAGCAGGAGTGCCTGAGTAGTCAGAGAGA-CAGCGGGT-3′. Lowercase letters correspond to the restriction sites, whereas the underlined sequence corresponds to β1C regions (2420-2434 and 2437-2451) flanking the sequence encoding the catalytic domain of the RZ. The RZ activity was initially tested in in vitro cell-free assays using 32P-labeled β1C mRNA.
Prostate tumor growth
PC3-β1A and PC3-β1C transfectants were induced to express β1A or β1C integrin. Cells were detached using 0.05% trypsin/0.53 mM EDTA, washed, and resuspended in RPMI. Cells (106) were inoculated subcutaneously into the flank of 6–8-wk-old male athymic Balb/c mice (Charles River Laboratories). Mice were given water supplemented with 5% sucrose or 5% sucrose plus 100 μg/ml tet to regulate the exogenous β1 integrin expression. Tumor size was determined using a caliper every other day. Tumor volume was calculated using the formula (vol = 0.5236 × [width]2 × [length]) and expressed as tumor volume in cubic millimeters. 10 mice/group were used in each experiment.
Transient transfection
All transient transfections were performed using Lipofectamine 2000. R− and R+ cells were transiently transfected with 2 μg pCMV-β-galactosidase (β-gal) and either 10–30 μg pBJ-β1A or 20–30 μg pBJ-β1C. CHO clones expressing β1A or β1C were transiently transfected with 2 μg β-gal and either 20 μg pCMV-HA-wt-Shp2 or 20 μg pCMV-FLAG-Shp2 C/S (Zhang et al., 2002) cDNA, provided by B. Neel (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA). β1C-CHO clones were transiently transfected with 2 μg β-gal and 20 μg of vector alone (pcDNA3), 20 μg DNp85 (pSRα-Δp85α) or 20 μg wt-p85 (Hara et al., 1994) cDNA, provided by R. Kalb (Yale University School of Medicine, New Haven, CT). Cells were cultured in the absence of tet in growth medium for 48 h. In a separate set of experiments, β1A- and β1C-CHO clones were transiently transfected with 2 μg β-gal and either 20 μg of vector alone (pcDNA3) or 20 μg 486/STOP (pCVNIGFIRsol cDNA; provided by R. Baserga). Cells were cultured in the absence of tet in growth medium for 48 h. 267B1 cells were transiently transfected with 2 μg β-gal and either 20 μg pBJ1 or 20 μg pBJ1-RZ-β1C cDNA. Cells were harvested 48 h after transfection and used in adhesion assays as described below. In parallel, transfected cells were seeded on 48-well plates and stained for β-gal expression to determine transfection efficiency as described previously (Manes et al., 2003).
Cell adhesion assay
CHO cell adhesion to LN-1 or BSA (100 μg/ml), or 10 μg/ml FN was performed as described previously (Languino et al., 1993) in the presence or absence of 100 ng/ml IGF-II. Where specified, cells were incubated with either Ab to IGF-II or, as a negative control, 0.1 μg/ml ni-rIgG for 1 h on ice.
Alternatively, cell adhesion assays of 267B1, CHO clones, R− and R+ cells to BSA or LN-1 (100 μg/ml), or 10 μg/ml FN after being transiently transfected with cDNA constructs were performed by incubating cells with the coated substrates for 2 h at 37°C in the presence or in the absence of IGF-II or IGF-I (100 ng/ml). After adhesion, β-gal staining was performed as described previously (Manes et al., 2003).
For PC3 clones and PrEC, 80,000 cells were allowed to adhere to plates coated with different substrates as indicated in each figure legend for 2 h at 37°C in the presence or in the absence of IGF-I or IGF-II (100 ng/ml), α-IR3 or ni-mIgG (10 or 30 μg/ml), Ab to LN-1 or ni-rIgG (30 or 100 μg/ml), or GoH3 or rtIgG (20 μg/ml). Cells were fixed and stained with crystal violet (0.5%) and OD was measured at 630 nm (Manes et al., 2003).
Analysis of β1 integrin association with IGF-IR, IRS-1, or Gab1
PC3 and CHO clones were induced to express β1A or β1C integrin. Cells were detached and stimulated with 100 ng/ml IGF-I for 10 min, washed and lysed. Proteins were immunoprecipitated by incubating with K-20 and protein A-Sepharose. Immunocomplexes were dissociated and reprecipitated with Ab to IGF-IR or ni-rIgG as described previously (Fornaro et al., 2000). Proteins were separated by SDS-PAGE and immunoblotted using Abs to β1 integrin (clone 18), to IGF-IR-β, to IRS-1 or to Gab1. As control, expression levels of IRS-1 or β1 integrin or IGF-IR were analyzed using respective Abs. Immunoprecipitation of chicken β1 integrin was performed using CSAT Ab as described previously (Marcantonio and Hynes, 1988). CHO clones expressing wild-type full-length β1A or different truncated forms of β1 integrin were allowed to grow to 70% confluency. The cells were lysed and association between β1 integrin and IGF-IR was studied as described above.
Analysis of tyrosine phosphorylation of IGF-IR, IRS-1, or Gab1
PC3 or CHO clones were detached and trypsin was neutralized with soybean trypsin inhibitor. Cells were stimulated with 100 ng/ml IGF-I, washed, lysed, and proteins were immunoprecipitated with IGF-IR, IRS-1 or Gab1 Abs and protein A-Sepharose. Immunoprecipitates were then separated by SDS-PAGE under reducing conditions and immunoblotted with PY20, IGF-IR-β, IRS-1 or Gab1, and visualized by ECL.
Immunoblotting
pRNS-1-1 and 267B1 cells were grown to 70% confluency, lysed, and immunoblotted with mAb to β1C or mIgM (5 μg/ml) as described previously (Fornaro et al., 2001a). 267B1 cells transiently transfected with RZ-β1C or vector were lysed and immunoblotted with mAb to 5 μg/ml β1C or Ab to Akt or Ab to 0.2 μg/ml IGF-IR (Fornaro et al., 2000). CHO clones expressing β1A or β1C were starved overnight, stimulated with IGF-I or IGF-II for 10 min, lysed, and immunoblotted with Ab to Akt or Ab to phospho-Akt (Fornaro et al., 2000). CHO clones expressing β1A or β1C were transiently transfected with HA-tagged wt-Shp2 or FLAG-tagged Shp2 C/S. Cells were lysed and immunoblotted with Ab to 0.5 μg/ml HA or Ab to 0.5 μg/ml FLAG or Ab to 0.2 μg/ml ERK1.
Analysis of Gab1 association with either Shp2 or phospho-Shp2
CHO clones were induced to express β1A or β1C integrin. Cells were detached and stimulated with 100 ng/ml IGF-I for 10 min, washed and lysed; proteins were immunoprecipitated by incubating with Ab to 1 μg Gab1 and protein A-Sepharose (Fornaro et al., 2000). Proteins were separated by SDS-PAGE and immunoblotted with Ab to 0.2 μg/ml Gab1 or 0.2 μg/ml Shp2 or phospho-Shp2 (1:1,000 dilution).
SRB assay
Proliferation was measured using SRB assay. PC3 clones were induced to express β1A or β1C integrin, serum starved for 12 h, detached, and seeded on 96-well plates (7.5 × 103 cells per well) in the presence or in the absence of IGF-I or IGF-II (100 ng/ml). Cells were incubated for 72 h without a medium change. After incubation, cells were fixed with 10% TCA at 4°C for 1 h, washed five times with tap water and stained with 0.2% SRB for 15 min. The plates were then washed five times with 1% acetic acid, dried, and stained cells in each well were solubilized with 10 mM Tris base. The absorbance in each well was measured at 540 nm. SRB results were confirmed by cell counting.
Statistical analysis
Differences in cell adhesion to LN were measured using t test. Tumor volume was measured for each mouse on multiple days; to analyze the data, a mixed-effect general linear model for repeated measurements was applied. Analysis was operated using SAS (version 8.2; SAS Institute, Inc.).
Acknowledgments
The CSAT hybridoma developed by Dr. A. Horwitz and the 7E2 hybridoma developed by Dr. R. Juliano were obtained from the DSHB under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Dept. of Biological Sciences, Iowa City. We acknowledge the generous help of Dr. R. Baserga for R− and R+ cells and 486/STOP plasmid. We are grateful to Dr. Guido Tarone for providing us the β1COM integrin cDNA; Dr. P. Yurchenco for Ab to LN (E8); Dr. R. Kalb for DNp85 and wt-p85; and Dr. B. Neel for wt-Shp2 and Shp2 C/S. We would like to thank Drs. Chung-Cheng Hsieh and Qin Liu for help with statistical analysis.
This work was supported by grants from National Institutes of Health, RO1 CA-89720 (to L.R. Languino) and from Army, PCRP DAMD17-98-1-8506 (to L.R. Languino) and by a Consiglio Nazionale delle Ricerche fellowship IBBE (to L. Moro; bando n.203.04.17).
Abbreviations used in this paper: β-gal, β-galactosidase; FN, fibronectin; Gab1, Grb2-associated binder1; IGF, insulin-like growth factor; IGF-IR, IGF type 1 receptor; IRS-1, insulin receptor substrate-1; LN, laminin; PI 3-kinase, phosphatidylinositol 3-kinase; RZ, ribozyme; Shp2, SH2-containing protein-tyrosine phosphatase 2; SRB, Sulforhodamine B; tet, tetracycline.
References
- Baserga, R. 2000. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 19:5574–5581. [DOI] [PubMed] [Google Scholar]
- Bello-DeOcampo, D., H.K. Kleinman, N.D. Deocampo, and M.M. Webber. 2001. Laminin-1 and α6β1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate. 46:142–153. [DOI] [PubMed] [Google Scholar]
- Bloch, W., E. Forsberg, S. Lentini, C. Brakebusch, K. Martin, H.W. Krell, U.H. Weidle, K. Addicks, and R. Fassler. 1997. β1 integrin is essential for teratoma growth and angiogenesis. J. Cell Biol. 139:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard, D., C. Brakebusch, E. Gustafsson, A. Aszodi, T. Bengtsson, A. Berna, and R. Fassler. 2001. Functional consequences of integrin gene mutations in mice. Circ. Res. 89:211–223. [DOI] [PubMed] [Google Scholar]
- Brar, P.K., B.L. Dalkin, C. Weyer, K. Sallam, I. Virtanen, and R.B. Nagle. 2003. Laminin α-1, α-3, and α-5 chain expression in human prepubertal benign prostate glands and adult benign and malignant prostate glands. Prostate. 55:65–70. [DOI] [PubMed] [Google Scholar]
- Brown, P.J., and R.L. Juliano. 1985. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 228:1448–1450. [DOI] [PubMed] [Google Scholar]
- Burfeind, P., C.L. Chernicky, F. Rininsland, and J. Ilan. 1996. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc. Natl. Acad. Sci. USA. 93:7263–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., and P.D. Yurchenco. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218:213–234. [DOI] [PubMed] [Google Scholar]
- Cooper, H.M., R.N. Tamura, and V. Quaranta. 1991. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the α6β1 integrin. J. Cell Biol. 115:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress, A.E., I. Rabinovitz, W. Zhu, and R.B. Nagle. 1995. The α6β1 and α6β4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 14:219–228. [DOI] [PubMed] [Google Scholar]
- Damsky, C.H., and D. Ilic. 2002. Integrin signaling: it's where the action is. Curr. Opin. Cell Biol. 14:594–602. [DOI] [PubMed] [Google Scholar]
- Davis, T.L., A.E. Cress, B.L. Dalkin, and R.B. Nagle. 2001. Unique expression pattern of the α6β4 integrin and laminin-5 in human prostate carcinoma. Prostate. 46:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, S.E., M. Ehrlich, N.J. Sharp, K. Reiss, G. Solomon, R. Hawkins, R. Baserga, and J.C. Barrett. 1998. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 58:3353–3361. [PubMed] [Google Scholar]
- Ekblom, P., P. Lonai, and J.F. Talts. 2003. Expression and biological role of laminin-1. Matrix Biol. 22:35–47. [DOI] [PubMed] [Google Scholar]
- Falk, M., K. Salmivirta, M. Durbeej, E. Larsson, M. Ekblom, D. Vestweber, and P. Ekblom. 1996. Integrin α6B β1 is involved in kidney tubulogenesis in vitro. J. Cell Sci. 109:2801–2810. [DOI] [PubMed] [Google Scholar]
- Fornaro, M., and L.R. Languino. 1997. Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix Biol. 16:185–193. [DOI] [PubMed] [Google Scholar]
- Fornaro, M., G. Tallini, D.Q. Zheng, W.M. Flanagan, M. Manzotti, and L.R. Languino. 1999. p27kip1 acts as a downstream effector of and is coexpressed with the β1C integrin in prostatic adenocarcinoma. J. Clin. Invest. 103:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro, M., C.A. Steger, A.M. Bennett, J.J. Wu, and L.R. Languino. 2000. Differential role of β1C and β1A integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/mitogen-activated protein kinase pathways. Mol. Biol. Cell. 11:2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro, M., M. Lovecchio, P. Jose, D.-Q. Zheng, L. Moro, and L.R. Languino. 2001. a. Epitope-specific antibodies to the β1C integrin cytoplasmic domain variant. Exp. Mol. Pathol. 70:275–280. [DOI] [PubMed] [Google Scholar]
- Fornaro, M., T. Manes, and L.R. Languino. 2001. b. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 20:321–331. [DOI] [PubMed] [Google Scholar]
- Fornaro, M., J. Plescia, S. Chheang, G. Tallini, Y.-M. Zhu, M. King, D.C. Altieri, and L.R. Languino. 2003. Fibronectin protects prostate cancer cells from tumor necrosis factor alpha-induced apoptosis via the AKT/Survivin pathway. J. Biol. Chem. 278:50402–50411. [DOI] [PubMed] [Google Scholar]
- Fuchs, M.E., M.K. Brawer, M.A. Rennels, and R.B. Nagle. 1989. The relationship of basement membrane to histologic grade of human prostatic carcinoma. Mod. Pathol. 2:105–111. [PubMed] [Google Scholar]
- Gu, H., and B.G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13:122–130. [DOI] [PubMed] [Google Scholar]
- Hara, K., K. Yonezawa, H. Sakaue, A. Ando, K. Kotani, T. Kitamura, Y. Kitamura, H. Ueda, L. Stephens, T.R. Jackson, et al. 1994. 1-phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl. Acad. Sci. USA. 91:7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga, M., and A.J. Wong. 2003. Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol. Cell. Biol. 23:4471–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R.O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. [DOI] [PubMed] [Google Scholar]
- Juliano, R.L. 1993. The role of β1 integrins in tumors. Semin. Cancer Biol. 4:277–283. [PubMed] [Google Scholar]
- Languino, L.R., and E. Ruoslahti. 1992. An alternative form of the integrin β1 subunit with a variant cytoplasmic domain. J. Biol. Chem. 267:7116–7120. [PubMed] [Google Scholar]
- Languino, L.R., J. Plescia, A. Duperray, A.A. Brian, E.F. Plow, J.E. Geltosky, and D.C. Altieri. 1993. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1 dependent pathway. Cell. 73:1423–1434. [DOI] [PubMed] [Google Scholar]
- LeRoith, D., and C.T. Roberts, Jr. 2003. The insulin-like growth factor system and cancer. Cancer Lett. 195:127–137. [DOI] [PubMed] [Google Scholar]
- Malinda, K.M., and H.K. Kleinman. 1996. The laminins. Int. J. Biochem. Cell Biol. 28:957–959. [DOI] [PubMed] [Google Scholar]
- Manes, T., D.Q. Zheng, S. Tognin, A.S. Woodard, P.C. Marchisio, and L.R. Languino. 2003. αvβ3 integrin expression up-regulates cdc2, which modulates cell migration. J. Cell Biol. 161:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzotti, M., P. Dell'Orto, P. Maisonneuve, M. Fornaro, L.R. Languino, and G. Viale. 2000. Down-regulation of β1C integrin in breast carcinomas correlates with high proliferative fraction, high histological grade, and larger size. Am. J. Pathol. 156:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio, E.E., and R.O. Hynes. 1988. Antibodies to the conserved cytoplasmic domain of the integrin β1 subunit react with proteins in vertebrates, invertebrates, and fungi. J. Cell Biol. 106:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio, A.M. 1995. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 5:419–423. [DOI] [PubMed] [Google Scholar]
- Moro, L., E. Perlino, E. Marra, L.R. Languino, and M. Greco. 2004. Regulation of β1C and β1A integrin expression in prostate carcinoma cells. J. Biol. Chem. 279:1692–1702. [DOI] [PubMed] [Google Scholar]
- Myers, M.G., Jr., R. Mendez, P. Shi, J.H. Pierce, R. Rhoads, and M.F. White. 1998. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 273:26908–26914. [DOI] [PubMed] [Google Scholar]
- Neel, B.G., H. Gu, and L. Pao. 2003. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284–293. [DOI] [PubMed] [Google Scholar]
- Nerlich, A.G., A. Lebeau, H.G. Hagedorn, U. Sauer, and E.D. Schleicher. 1998. Morphological aspects of altered basement membrane metabolism in invasive carcinomas of the breast and the larynx. Anticancer Res. 18:3515–3520. [PubMed] [Google Scholar]
- Parda, D.S., P.J. Thraves, M.R. Kuettel, M.S. Lee, P. Arnstein, M.E. Kaighn, J.S. Rhim, and A. Dritschilo. 1993. Neoplastic transformation of a human prostate epithelial cell line by the v-Ki-ras oncogene. Prostate. 23:91–98. [DOI] [PubMed] [Google Scholar]
- Parise, L.V., J. Lee, and R.L. Juliano. 2000. New aspects of integrin signaling in cancer. Semin. Cancer Biol. 10:407–414. [DOI] [PubMed] [Google Scholar]
- Peehl, D.M., S.T. Wong, R.G. Seller, S. Jin, and J.S. Rhim. 1997. Loss of response to epidermal growth factor and retinoic acid accompanies the transformation of human prostatic epithelial cells to tumorigenicity with v-Ki-ras. Carcinogenesis. 18:1643–1650. [DOI] [PubMed] [Google Scholar]
- Reiss, K., C. D'Ambrosio, X. Tu, C. Tu, and R. Baserga. 1998. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin. Cancer Res. 4:2647–2655. [PubMed] [Google Scholar]
- Reiss, K., J.Y. Wang, G. Romano, F.B. Furnari, W.K. Cavenee, A. Morrione, X. Tu, and R. Baserga. 2000. IGF-1 receptor signaling in a prostatic cancer cell line with a PTEN mutation. Oncogene. 19:2687–2694. [DOI] [PubMed] [Google Scholar]
- Reiss, K., J.Y. Wang, G. Romano, X. Tu, F. Peruzzi, and R. Baserga. 2001. Mechanisms of regulation of cell adhesion and motility by insulin receptor substrate-1 in prostate cancer cells. Oncogene. 20:490–500. [DOI] [PubMed] [Google Scholar]
- Retta, S.F., F. Balzac, P. Ferraris, A.M. Belkin, R. Fassler, M.J. Humphries, G. De Leo, L. Silengo, and G. Tarone. 1998. β1-integrin cytoplasmic subdomains involved in dominant negative function. Mol. Biol. Cell. 9:715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell, C., G. Dumenil, C. Deveaud, M. Miura, D. Coppola, T. DeAngelis, R. Rubin, A. Efstratiadis, and R. Baserga. 1994. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 14:3604–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, L.M., and A.M. Mercurio. 1993. Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J. Cell Biol. 123:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg, A., H. Janssen, F. Hogervorst, J. Calafat, and J. Hilgers. 1987. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J. Biol. Chem. 262:10376–10383. [PubMed] [Google Scholar]
- Surmacz, E. 2003. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 22:6589–6597. [DOI] [PubMed] [Google Scholar]
- Tai, Y.T., K. Podar, L. Catley, Y.H. Tseng, M. Akiyama, R. Shringarpure, R. Burger, T. Hideshima, D. Chauhan, N. Mitsiades, et al. 2003. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of β1-integrin and phosphatidylinositol 3′-kinase/AKT signaling. Cancer Res. 63:5850–5858. [PubMed] [Google Scholar]
- Tsiper, M.V., and P.D. Yurchenco. 2002. Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. J. Cell Sci. 115:1005–1015. [DOI] [PubMed] [Google Scholar]
- Valentinis, B., and R. Baserga. 2001. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 54:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnay, J.N., J.C. Bruning, D.J. Burks, and C.R. Kahn. 2000. Gab-1-mediated IGF-1 signaling in IRS-1-deficient 3T3 fibroblasts. J. Biol. Chem. 275:10545–10550. [DOI] [PubMed] [Google Scholar]
- Yamada, K.M., and S. Even-Ram. 2002. Integrin regulation of growth factor receptors. Nat. Cell Biol. 4:E75–E76. [DOI] [PubMed] [Google Scholar]
- Zhang, S.Q., W.G. Tsiaras, T. Araki, G. Wen, L. Minichiello, R. Klein, and B.G. Neel. 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22:4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., and D.R. Clemmons. 1998. Blocking ligand occupancy of the αVβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA. 95:11217–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]