Abstract
Several determinants of aging, including metabolic capacity and genetic stability, are recognized in both yeast and humans. However, many aspects of the pathways leading to cell death remain to be elucidated. Here we report a role for the actin cytoskeleton both in cell death and in promoting longevity. We have analyzed yeast strains expressing mutants with either increased or decreased actin dynamics. We show that decreased actin dynamics causes depolarization of the mitochondrial membrane and an increase in reactive oxygen species (ROS) production, resulting in cell death. Important, however, is the demonstration that increasing actin dynamics, either by a specific actin allele or by deletion of a gene encoding the actin-bundling protein Scp1p, can increase lifespan by over 65%. Increased longevity appears to be due to these cells producing lower than wild-type levels of ROS. Homology between Scp1p and mammalian SM22/transgelin, which itself has been isolated in senescence screens, suggests a conserved mechanism linking aging to actin stability.
Keywords: actin; senescence; Scp1; apoptosis; ROS
Introduction
Aging is the progressive decline in the ability of an organism to resist stress, damage, and disease. However, variation within populations means that manifestations of age-related decline or functional senescence can be hard to distinguish from maturational events or disease. Yeast has been used as a model organism for studies of cell aging for several years. The ability to rapidly exploit genomic information has led to the identification of several markers that are important in the progression of aging. Furthermore, it seems that a small number of physiological principles underlie the aging process. These include metabolic capacity, resistance to stress, gene dysregulation, and genetic stability. These factors have also been identified as critical in aging in other organisms, including humans.
Coupled to the process of aging is the presence of tightly regulated mechanisms, which ensure that aged cells undergo cell death. Such pathways are critical in multicellular organisms to allow maintenance of tissues and organ structure. However, it is clear that apoptotic pathways that culminate in cell death also occur in single cell organisms. One possible reason for this in yeast is that old cells are less robust and their presence may become deleterious to the well being of a colony. The presence of apoptotic pathways in yeast that resemble those in higher organisms has been well demonstrated (Frohlich and Madeo, 2001; Tissenbaum and Guarente, 2002). As observed in mammalian cells, apoptotic yeast cells show mitochondrial depolarization, high levels of reactive oxygen species (ROS), DNA fragmentation, and phosphoserine exposure at the plasma membrane. There is also good evidence that these apoptotic pathways are part of the normal aging process (Laun et al., 2001; Jazwinski, 2002). The role of the caspase-like protein Yca1p in yeast is less well understood, though there is evidence that caspase-dependent cell death pathways are present and contribute to the process of programmed cell death (Madeo et al., 2002). However, even Δyca1 cells show rapid death in elevated H2O2 levels, demonstrating the presence of other mechanisms to induce cell death.
Several recent studies have reported induction of apoptosis in mammalian cells associated with changes in the actin cytoskeleton (Kim et al., 2003; Morley et al., 2003). To further our understanding of this phenomenon, we have undertaken studies in the budding yeast Saccharomyces cerevisiae. Previous studies in yeast have generated much information on fundamental roles of the actin cytoskeleton because this organism expresses a single form of actin and there is ready availability of mutant actin alleles. Here we demonstrate that a decrease in actin dynamics can induce cell death through the production of ROS from the mitochondria. Conversely, increasing actin dynamics reduces production of ROS and increases cell viability. Finally, we determine that deletion of a single gene, encoding the actin bundling protein Scp1, leads to a reduced production of ROS and a highly significant increase in longevity.
Results and discussion
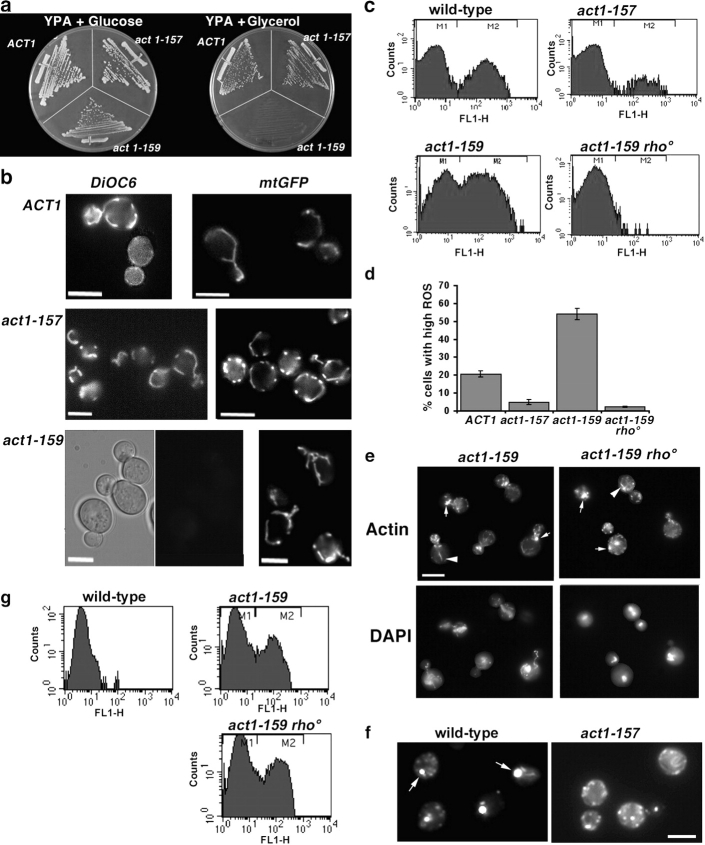
Investigations into the loss in viability of certain actin mutants led to the observation that a reduction in actin dynamics correlates with an inability to grow on the nonfermentable carbon source glycerol. Actin alleles act1-157 (increased actin dynamics) and act1-159 (decreased actin dynamics) and an isogenic parent strain (Belmont and Drubin, 1998; Belmont et al., 1999) were compared for growth on agar plates containing either 2% glucose, the preferred S. cerevisiae carbon source, or 3% glycerol. As shown (Fig. 1 a), strains expressing the act1-159 allele are unable to grow using glycerol as a carbon source. Growth on glycerol requires functional mitochondria (Hampsey, 1997). The presence of mitochondria in these cells was determined using a GFP tag fused to a mitochondrial targeting sequence (Westermann and Neupert, 2000), and mitochondrial function was assessed using the vital stain DiOC6, which requires a membrane potential to be taken up and retained within mitochondria. Interestingly, while in early log phase, all three strains contain mitochondria with a relatively normal morphology, the mitochondria in the act1-159 strain could not be visualized using the DiOC6 dye, indicating that the mitochondria have a significantly reduced membrane potential (Fig. 1 b).
Figure 1.
Actin dynamics correlate with the presence of functional mitochondria and the production of ROS. (a) Comparison of growth on agar plates containing glycerol as a carbon source. (b) Presence of mitochondria was determined using a GFP tag fused to a mitochondrial targeting sequence. Mitochondrial functionality was assessed using the vital stain DiOC6. DIC image is shown to indicate position of cells. Bars, 5 μm. (c) FACS® analysis measured H2O2 levels as an indication of ROS accumulation. The M2 peak represents cells with high levels of ROS. (d) Summary graph of the percentage of cells lying in the M2 peak. Errors are standard deviations from three experiments. (e) Rhodamine-phalloidin staining of actin in act1-159 and act1-159 rho° cells. Corresponding images of DAPI staining show absence of mitochondria in the rho° cells. Arrows indicate large chunks of actin, and arrowheads indicate aberrant actin cables. Bar, 5 μm. (f) Rhodamine-phalloidin staining of actin in wild-type and act1-159 cells. Arrows indicate large chunks of actin. Bar, 5 μm. (g) Caspase levels were assayed following incubation of cells with the caspase binding molecule FITC-VAD-fmk as described. M2 peak represents the cell population with high levels of caspase.
It is well documented in yeast and higher eukaryotes that loss of mitochondrial membrane potential can lead to increased production of ROS, which can then lead to induction of apoptotic-like changes and consequently to cell death (Madeo et al., 1999; Laun et al., 2001). Indeed, there is much evidence in yeast that accumulation of ROS is a major factor in aging (Longo et al., 1996; Nestelbacher et al., 2000). To assess whether these changes also occur in the actin mutant strains, FACS® analysis was undertaken to measure H2O2 levels as an indication of ROS accumulation. As shown (Fig. 1 c), this analysis reveals two populations of cells. The M2 population represents those cells with a high level of ROS accumulation. While the act1-159 mutant has significantly increased ROS levels compared with wild-type cells, the act1-157 mutant has reduced ROS levels. To demonstrate that mitochondria are required for the observed increase in ROS, strains of the act1-159 mutant were generated that lacked mitochondrial DNA (Guthrie and Fink 1991). These yeast strains (designated rho°) did not show the increase in ROS production observed in the parent strain, indicating the importance of the mitochondria for the ROS production observed (Fig. 1 d).
An important consideration is whether the changes to actin are caused by, or are a consequence of, elevated ROS. Actin staining of the act1-159 strain revealed some disorganization of the actin cytoskeleton with fewer, larger chunks of cortical actin and aberrant actin cables (Fig. 1 e). This phenotype was not rescued in the rho° strain that has lower levels of ROS, demonstrating that actin disorganization occurs upstream of the production of ROS from the mitochondria. We also observed that the actin phenotype was exacerbated as act1-159 cells entered stationary phase and that it correlated with an increased incidence of DNA fragmentation (unpublished data). To determine whether this actin “chunk” phenotype correlated with the ROS production, we also investigated actin organization of wild-type and act1-157 cells after 28 d in stationary phase. Here we found a clear difference between the strains, with 45% of wild-type cells containing large actin chunks compared with only 16% of act1-157 cells (Fig. 1 f), demonstrating a role for actin dynamics within stationary phase cells.
To ascertain whether the changes in ROS levels correlated with longevity, we compared viability of the yeast strains over a period of 28 d after entry into stationary phase. This approach allows assessment of chronological lifespan of yeast, which mimics aging of post-mitotic tissues in higher organisms (MacLean et al., 2001). The strain with reduced actin dynamics had all died by 10 d. After 28 d, wild-type cells showed 38.5% (±2.1%) viability, and the strain with increased dynamics showed 51% (±3.3%) viability, an increase of 32%, suggesting a link between actin dynamics, ROS production, and longevity. To demonstrate that the ROS responsible for cell death are derived from mitochondrial activity, viability of the act1-159 strain and act1-159 rho° strain were compared. This measurement was performed on log phase cells because the lack of mitochondria precludes the rho° strains from entering stationary phase. Under these conditions, viability of act1-159 cells was 50.1% (±5.8), and viability of the act1-159 rho° cells was 93.7% (±2.2), demonstrating the importance of mitochondria in production of ROS, which results in cell death.
To determine whether the cell death induced by ROS in response to the actin mutation is due to caspase activation, we incubated yeast with FITC-labeled VAD-fmk. This molecule binds specifically to the catalytic site of metazoan caspases and has been used as a method to detect yeast cells with active caspases using flow cytometry (Madeo et al., 2002). As shown in Fig. 1 f, caspase levels are elevated in strains expressing the act1-159 mutation, correlating with increased cell death of this strain. Interestingly however, the rho° strain lacking mitochondria, which has restored viability under these conditions, still has elevated caspase levels, indicating that high caspase levels alone may not trigger cell death.
One caveat of such studies is that the presence of a mutant allele in cells may for a variety of reasons initiate changes that induce ROS production. To determine whether a reduction in actin dynamics in an otherwise wild-type cell also causes an increase in ROS, we used a yeast strain that is sensitive to the actin stabilizing drug jasplakinolide (KAY339). Previously we have shown that jasplakinolide addition to this strain causes a block in actin dynamics and the actin to accumulate as a single clump (Ayscough, 2000). To analyze the effect of jasplakinolide in this study, the drug was added at low levels overnight to allow concomitant uptake of the dye used for analysis of ROS production. After this time, with 0 and 1 μM jasplakinolide addition, actin was still punctate and polarized, and mitochondrial and nuclear DNA was normal in appearance. However, 2 μM jasplakinolide addition resulted in clumped actin and fragmentation of nuclear DNA, indicative of apoptosis (Fig. 2 a). In addition, a dramatic increase in ROS was measured, indicating that the high ROS levels are a consequence of the changes in the actin cytoskeleton (Fig. 2 b).
Figure 2.
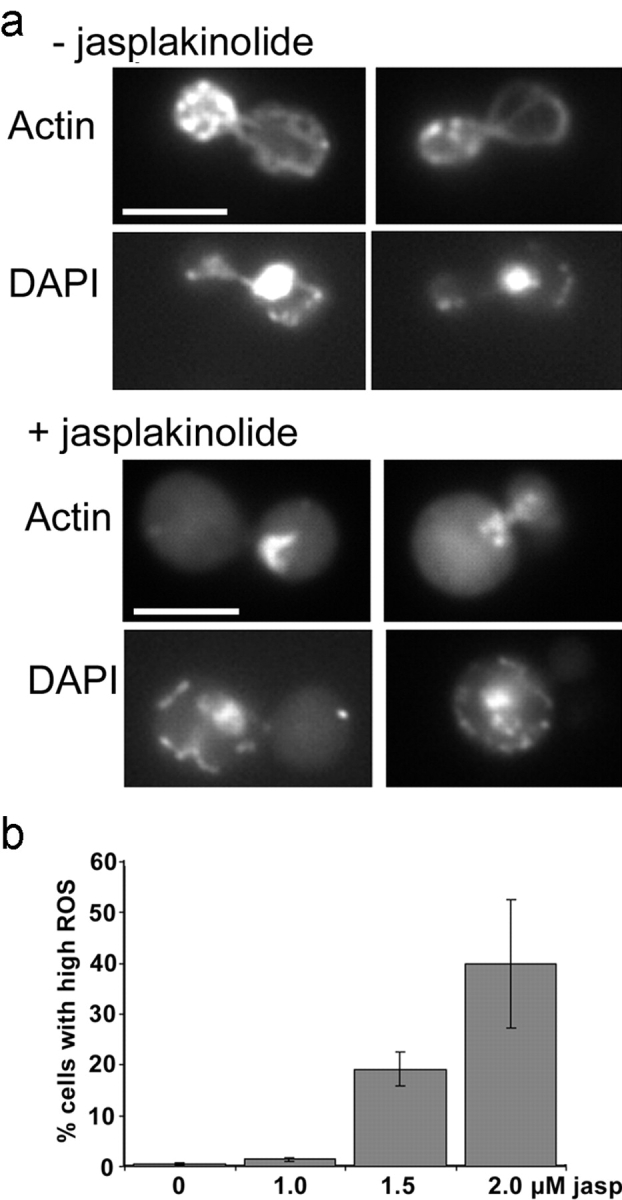
Jasplakinolide addition causes actin clumping, nuclear fragmentation, and increases the level of ROS in yeast cells. (a) KAY339 cells were grown to log phase and half the population was then treated with 2 μM jasplakinolide overnight. Cells were fixed and processed for rhodamine-phalloidin staining to visualize actin, and DAPI was added to allow the organization of DNA to be observed. Bars, 5 μm. (b) Jasplakinolide was added at a range of levels as indicated, and cells were incubated overnight in the presence of H2-DCFDA for analysis by flow cytometry.
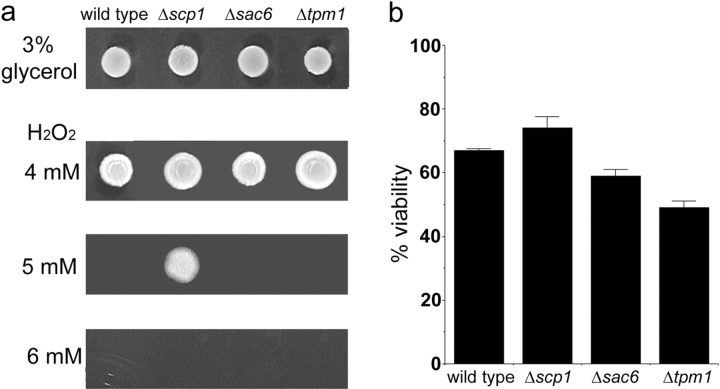
While mutations in actin that increase F-actin stability and addition of drugs that abrogate actin dynamics both result in increased ROS and loss of viability, an important question is whether changes to actin stability may contribute to aging and cell death in the normal lifespan of yeast. To address this possibility, we considered proteins that have been shown to regulate actin dynamics in yeast, in particular proteins that themselves have been shown to confer stability on the actin cytoskeleton. These proteins are the tropomyosins 1 and 2 (Tpm1 and Tpm2), the fimbrin homologue Sac6p (Pruyne and Bretscher, 2000), and most recently described, the small actin binding protein Scp1p, which is related to mammalian SM22/transgelin (Goodman et al., 2003; Winder et al., 2003). Interestingly, the mammalian homologue of Scp1p has been identified in replicative senescence screens that demonstrated that aged cells contain higher levels of the protein than younger cells (Gonos et al., 1998). Comparison of isogenic strains lacking tpm1, sac6, and scp1 revealed that all were able to grow on plates containing glycerol as a carbon source, indicating that all contained functional mitochondria (Fig. 3 a). Growth on hydrogen peroxide plates showed that wild-type, Δtpm1, and Δsac6 cells could grow at 4 mM H2O2; only the Δscp1 strain was able to grow at 5 mM H2O2 (Fig. 3 a). Finally, comparison of viability demonstrated that after 2 d growth in stationary phase, Δtpm1 and Δsac6 cells showed reduced viability compared with wild-type cells, while the strain lacking scp1 showed increased viability (Fig. 3 b). This result indicates that Sac6p and Tpm1p may have other essential roles in the cell that cause viability to be affected following their deletion. Because loss of Scp1p, but not Tpm1p or Sac6p, had an effect on promoting survival on H2O2 and resulted in increased viability, we chose to investigate the role of this protein further.
Figure 3.
Comparison of strains in which genes encoding actin stabilizing proteins tpm1, sac6, and scp1 have been deleted. (a) Growth of strains on plates containing 3% glycerol as the carbon source, or with increasing levels of H2O2. (b) Viability of strains after 2 d in stationary phase. Viability was assessed as described in the Materials and methods.
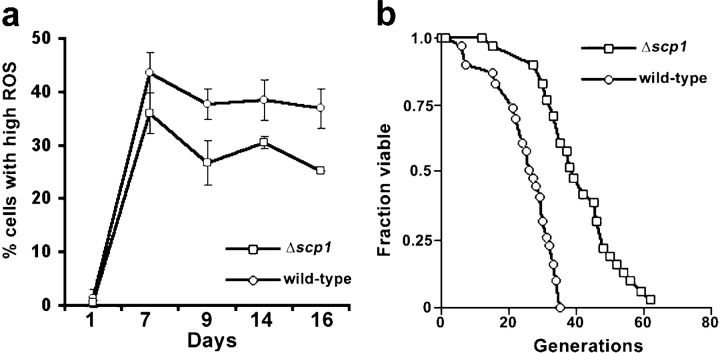
First, levels of ROS were measured in stationary phase cells over a 16-d time course and were found to be lower in strains in which scp1 is deleted, compared with an isogenic parent strain (Fig. 4 a). To investigate whether this difference in ROS is linked to aging in yeast, two assays were performed to assess both chronological and replicative aging. First, cells in which scp1 is deleted were allowed to grow into stationary phase, and their viability was assessed over a 15-d period. Using this approach, we observed a significant increase in viability of Δscp1 cells compared with isogenic wild-type cells, with 79% (±3%) of Δscp1 cells remaining viable after 15 d compared with 42% (±9%) of wild-type cells (an 88% increase). Second, a replicative aging assay was performed in which new daughter cells were separated from their mothers, and the number of new daughter cells that each of these selected cells was able to generate was recorded (Lin et al., 2000). This approach showed a very dramatic decrease in the aging of cells that carried the scp1 deletion (Fig. 4 b). While the replicative capacity of the wild-type cells averages in the order of 24 generations, those lacking scp1 underwent an average of 40 generations, an increase of 67% in replicative capacity. This strongly suggests that Scp1p plays a role in regulating the dynamics of the actin cytoskeleton in yeast to trigger cell death at an appropriate time, and that loss of Scp1p results in increased actin dynamics and an increase in longevity.
Figure 4.
The effect of SCP1 deletion on ROS production and aging. (a) Flow cytometry was undertaken to measure H2O2 levels as an indication of ROS accumulation in wild-type (KAY446) and Δscp1 (KAY448) cells. Depicted here is the percentage of cells at each time point with high levels of ROS. (b) A replicative aging assay was performed in wild-type and Δscp1 cells. In each assay, 32 cells were studied, and each daughter cell produced by a mother cell was counted.
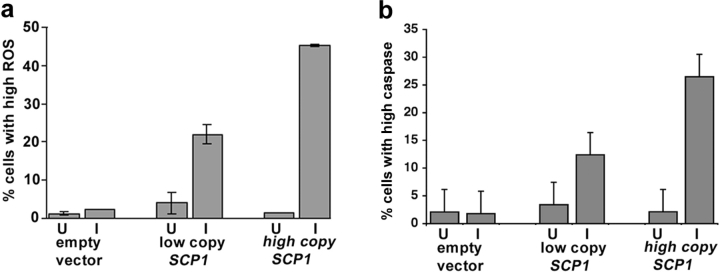
Having determined that a loss of scp1 can increase viability of yeast, we investigated whether the converse were true. Previously we have shown that overexpression of SCP1 does indeed cause inviability in yeast (Winder et al., 2003). However, the mechanism of this was not investigated. As shown (Fig. 5 a), after 26 h induction, the levels of ROS in cells were significantly increased in the presence of increased levels of Scp1p. The strains tested contained either an empty vector (KAY604), a centromeric low copy vector with SCP1 expressed from an inducible promoter (KAY646), or a 2 micron high copy vector with SCP1 expressed from an inducible promoter (KAY647). The level of ROS correlates with the level of SCP1 overexpression, indicating that increased F-actin stability causes ROS accumulation and leads to cell death. Levels of caspase activation were also determined using FACS® analysis in cells overexpressing SCP1, and, as shown (Fig. 5 b), caspase is elevated in cells with the increased levels of SCP1.
Figure 5.
Overexpression of SCP1 induces production of ROS and yeast caspase. Cells expressing wild-type levels of Scp1p, or carrying SCP1 on a centromeric plasmid or on a high copy plasmid were shifted to medium to induce expression of SCP1 from the methionine promoter. U, uninduced; I, induced SCP1 expression after 26 h induction. (a) ROS levels were measured as described in the Materials and methods. Shown here is the percentage of cells with high ROS levels. (b) Caspase levels were assayed by flow cytometry following incubation of cells with the caspase inhibitor FITC-VAD-fmk. Error bars shown are the SEM.
The data we present demonstrate that the actin cytoskeleton can play a central role in regulating apoptosis in yeast. Furthermore, it appears likely that this pathway may be part of a cell aging process in yeast and potentially in mammalian cells. As such, it identifies a further role for the actin cytoskeleton in eukaryotes and reiterates the critical role that actin plays in fundamental cell processes. In yeast, a tight coupling of the mitochondria to the cytoplasmic actin cables has been recognized for many years (Read et al., 1992; Simon et al., 1997). In addition, several proteins, including Mmm1p and Mdm10p, have been identified that directly couple mitochondria to the actin cytoskeleton (Boldogh et al., 1998). However, until now, this link has been proposed to be involved in maintaining mitochondrial morphology and ensuring movement of mitochondria in to the newly growing daughter cell. The work we present suggests that the actin mitochondrial association may also serve to regulate mitochondrial depolarization and subsequent production of ROS. One possible mechanism for coupling is that actin may associate with channels in the mitochondrial membrane to regulate opening or closing. Slowing of actin dynamics might lead to the pores or channels being opened for a prolonged time, thus reducing membrane potential and increasing release of ROS into the cytoplasm. Conversely enhanced dynamics may reduce opening times, reducing release of damaging ROS and promoting lifespan of the organism. Evidence for this model is reported from other studies. The mammalian actin binding protein gelsolin has been shown to regulate closure of the mitochondrial voltage dependent anion channel (VDAC), and this function also correlates with induction of apoptotic changes (Kusano et al., 2000). Furthermore, actin has been shown to modulate gating of VDAC in a Neurospora crassa in vitro system (Xu et al., 2001). In agreement with our results, data from this study demonstrated that stabilization of F-actin blocked the ability to modulate opening of the channel.
In summary, we have demonstrated the importance of actin cytoskeleton dynamics in regulating the production of ROS in yeast and have determined that coupling of actin and the mitochondria is central to this mechanism. Importantly, we also show that deletion of the SCP1 gene encoding an actin bundling protein significantly increases the lifespan of yeast, indicating that this pathway is also part of a pathway regulating aging processes.
Materials and methods
Strains, plasmids, and growth of yeast
Yeast strains used were KAY159 (MATa ura3-52, leu2-3,112, cry1, tub2-201, ACT1::HIS3, his3Δ200); KAY375 (MATa ura3-52, leu2-3,112, ade4, ade2, tub2-201, act1-159::HIS3, his3Δ200); KAY603 (MATa ura3-52, leu2-3,112, ade2-101, tub2-201, act1-159::HIS3, his3Δ200, can1Δ); KAY339 (MATa Δste6::hisG, Δpdr5::TRP1, snq2::hisG, Δerg6::LEU2, ura3-52, his3-Δ200 leu2-Δ1, trp1-Δ63, lys2-801 ade2-101); KAY446 (MATa, his3Δ, leu2Δ, met15Δ, ura3Δ); KAY448 (KAY446+ Δscp1::KanMx); KAY593 (KAY446+ Δsac6::KanMx); and KAY679 (KAY446+ Δtpm1::KanMx). KAY446 was transformed with pKA256 (empty vector), pKA280 (CEN, pMET-SCP1), or pKA281 (2μ pMET-SCP1) to generate strains KAY604, KAY646, and KAY647, respectively. Plasmids are described elsewhere (Winder et al., 2003).
Yeast were grown with shaking at 30°C in liquid YPD (1% yeast extract, 2% peptone, 2% glucose) or synthetic medium (lacking uracil; Sigma-Aldrich). Solid media was made by addition of 2% agar. For glycerol plates, the glucose was replaced with 3% glycerol. Overexpression of SCP1 was induced by switching cells onto media lacking methionine (Winder et al., 2003). The rho° strain of KAY375 was made as previously described (Guthrie and Fink, 1991). Jasplakinolide was added to amounts indicated from a 5 mM stock in DMSO.
Fluorescence procedures
Rhodamine-phalloidin and DAPI staining was performed as previously described (Hagan and Ayscough, 2000). Staining of mitochondria with DiOC6 was performed as published (Pringle et al., 1989). Visualization of mitochondria using GFP on a mitochondrial localization peptide was achieved by transformation of cells with PVTU100U-mtGFP (Westermann and Neupert, 2000). Cell images were recorded from a BX60 Olympus microscope with a Roper Scientific Micromax camera and captured on a Macintosh 7300 computer. Images were processed using Adobe Photoshop 7.0® software.
ROS production.
Cells were incubated overnight in the presence of 5 μg/ml 2',7'-dichloro dihydrofluorescein diacetate (H2-DCFDA; Molecular Probes). Cells were sonicated and analyzed using a Becton Dickinson flow cytometer. Parameters were set at excitation and emission settings of 304 and 551 nm (filter FL-1), respectively. For each experiment, two peaks of fluorescence were observed. The second peak represents cells with high levels of ROS. Experiments were repeated at least three times independently. Representative datasets are depicted. Errors shown are standard deviations. In vivo caspase levels were measured as previously described (Madeo et al., 2002) using a staining solution containing FITC-VAD-fmk (CaspACE™; Promega).
Aging assays
Chronological aging assay.
Cells were grown in YPD medium on a shaker with high aeration (220 rpm) at 30°C. Samples were sonicated before cell number determination, in triplicate, with a Schärfe Systems TT cell counter. Serial dilutions were plated on YPD plates, and numbers of growing colonies were counted in triplicate platings.
Reproductive capacity was assessed using the methods previously described (Lin et al., 2000). In brief, cells from log phase cultures were plated at low density. Daughter cells were isolated as buds that emerged from mother cells and removed using a Singer Instruments MSM manual micromanipulator. Life span was determined by removing all subsequent daughter cells generated. The average reproductive lifespan was calculated from the dissection of budding cells from 32 starting cells for each strain tested.
Acknowledgments
Thanks to Peter Piper for critical reading of manuscript; David Drubin and Lisa Belmont (University of California, Berkeley, CA) for yeast strains; and B. Westermann (Ludwig Maximilians University, Munich, Germany) for mitochondrial GFP plasmid.
This work was supported by a Medical Research Council (MRC) senior research fellowship to K.R. Ayscough (G117/394), MRC career establishment grant to S.J. Winder (G104/51660), a Wellcome Trust studentship to L.N. Carpp, and a Biotechnology and Biological Sciences Research Council grant to K.R. Ayscough and S.J. Winder (17/C12769).
P. Timpson's present address is The Garvan Institute, Darlinghurst, Sydney, Australia.
Abbreviations used in this paper: ROS, reactive oxygen species; Tpm, tropomyosin.
References
- Ayscough, K.R. 2000. Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol. 10:1587–1590. [DOI] [PubMed] [Google Scholar]
- Belmont, L.D., and D.G. Drubin. 1998. The yeast V159N actin mutant reveals roles for actin dynamics in vivo. J. Cell Biol. 142:1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont, L.D., G.M. Patterson, and D.G. Drubin. 1999. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell Sci. 112:1325–1336. [DOI] [PubMed] [Google Scholar]
- Boldogh, I.R., N. Vojtov, S. Karmon, and L.A. Pon. 1998. Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p. J. Cell Biol. 141:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich, K.U., and F. Madeo. 2001. Apoptosis in yeast: a new model for aging research. Exp. Gerontol. 37:27–31. [DOI] [PubMed] [Google Scholar]
- Gonos, E.S., A. Derventzi, M. Kveiborg, G. Agiostratidou, M. Kassem, B.F.C. Clark, P.S. Jat, and S.I.S. Rattan. 1998. Cloning and identification of genes that associate with mammalian replicative senescence. Exp. Cell Res. 240:66–74. [DOI] [PubMed] [Google Scholar]
- Goodman, A., B.L. Goode, P. Matsudaira, and G.R. Fink. 2003. The Saccharomyces cerevisiae calponin/transgelin homolog Scp1 functions with fimbrin to regulate stability and organization of the actin cytoskeleton. Mol. Biol. Cell. 14:2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, G., and G.R. Fink. 1991. Generation of rho° yeast strains. Methods Enzymol. 194:150–151. [Google Scholar]
- Hagan, I.M., and K.R. Ayscough. 2000. Fluorescence microscopy in yeast. Protein Localization by Fluorescence Microscopy: A Practical Approach. V.J. Allan, editor. Oxford University Press, Oxford, UK. 179–205.
- Hampsey, M. 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast. 13:1099–1133. [DOI] [PubMed] [Google Scholar]
- Jazwinski, S.M. 2002. Growing old: metabolic control and yeast aging. Annu. Rev. Microbiol. 56:769–792. [DOI] [PubMed] [Google Scholar]
- Kim, S., S.G. Hwang, I.C. Kim, and J.S. Chun. 2003. Actin cytoskeletal architecture regulates nitric oxide-induced apoptosis, dedifferentiation and cyclooxygenase-2 expression in articular chondrocytes via mitogen-activated protein kinase and protein kinase C pathways. J. Biol. Chem. 278:42448–42456. [DOI] [PubMed] [Google Scholar]
- Kusano, H., S. Shimizu, R.C. Koya, H. Fujita, S. Kamada, N. Kuzumaki, and Y. Tsujimoto. 2000. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 19:4807–4814. [DOI] [PubMed] [Google Scholar]
- Laun, P., A. Pichova, F. Madeo, J. Fuchs, A. Ellinger, S. Kohlwein, I. Dawes, and K. Froehlich. 2001. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 39:1166–1173. [PubMed] [Google Scholar]
- Lin, S.J., P.A. Defossez, and L. Guarente. 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 289:2126–2128. [DOI] [PubMed] [Google Scholar]
- Longo, V., E. Gralla, and J. Valentine. 1996. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. J. Biol. Chem. 271:12275–12280. [DOI] [PubMed] [Google Scholar]
- MacLean, M., N. Harris, and P.W. Piper. 2001. Chronological lifespan of stationary phase yeast cells; a model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms. Yeast. 18:499–509. [DOI] [PubMed] [Google Scholar]
- Madeo, F., E. Froehlich, M. Ligr, M. Grey, S. Sigrist, D. Wolf, and K. Froehlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo, F., E. Herker, C. Maldener, S. Wissing, S. Lachelt, M. Herlan, M. Fehr, K. Lauber, S.J. Sigrist, S. Wesselborg, and K.U. Frohlich. 2002. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 9:911–917. [DOI] [PubMed] [Google Scholar]
- Morley, S.C., G.P. Sun, and B.E. Bierer. 2003. Inhibition of actin polymerization enhances commitment to and execution of apoptosis induced by withdrawal of trophic support. J. Cell. Biochem. 88:1066–1076. [DOI] [PubMed] [Google Scholar]
- Nestelbacher, R., P. Laun, D. Vondrakova, A. Pichova, C. Schuller, and M. Breitenbach. 2000. The influence of oxygen toxicity on yeast mother cell-specific aging. Exp. Gerontol. 35:63–70. [DOI] [PubMed] [Google Scholar]
- Pringle, J.R., R.A. Preston, A.E.M. Adams, T. Stearns, D.G. Drubin, B.K. Haarer, and E.W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357–435. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:571–585. [DOI] [PubMed] [Google Scholar]
- Read, E.B., H.H. Okamura, and D.G. Drubin. 1992. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection. Mol. Biol. Cell. 3:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, V.R., S.L. Karmon, and L.A. Pon. 1997. Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell Motil. Cytoskeleton. 37:199–210. [DOI] [PubMed] [Google Scholar]
- Tissenbaum, H., and L. Guarente. 2002. Model organisms as a guide to mammalian ageing. Dev. Cell. 2:9–19. [DOI] [PubMed] [Google Scholar]
- Westermann, B., and W. Neupert. 2000. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in S. cerevisiae. Yeast. 16:1421–1427. [DOI] [PubMed] [Google Scholar]
- Winder, S.J., T. Jess, and K.R. Ayscough. 2003. SCP1 encodes an actin-bundling protein in yeast. Biochem. J. 375:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., J.G. Forbes, and M. Colombini. 2001. Actin, modulates the gating of Neurospora crassa VDAC. J. Membr. Biol. 180:73–81. [DOI] [PubMed] [Google Scholar]