Abstract
Arrest of circulating tumor cells in distant organs is required for hematogenous metastasis, but the tumor cell surface molecules responsible have not been identified. Here, we show that the tumor cell α3β1 integrin makes an important contribution to arrest in the lung and to early colony formation. These analyses indicated that pulmonary arrest does not occur merely due to size restriction, and raised the question of how the tumor cell α3β1 integrin contacts its best-defined ligand, laminin (LN)-5, a basement membrane (BM) component. Further analyses revealed that LN-5 is available to the tumor cell in preexisting patches of exposed BM in the pulmonary vasculature. The early arrest of tumor cells in the pulmonary vasculature through interaction of α3β1 integrin with LN-5 in exposed BM provides both a molecular and a structural basis for cell arrest during pulmonary metastasis.
Keywords: metastasis; tumor cell; integrin; laminin; vessel
Introduction
The lung is a frequent site for metastasis of many different tumor types. Tumor cells enter the circulation from the primary tumor and then colonize distant organs. The interactions between the tumor cells and the vessels that allow the initial arrest of the tumor cell in the lung are poorly characterized, although in individual cases contributing molecules have been identified (Abdel-Ghany et al., 2001; Abdel-Ghany et al., 2003). Recently, we developed methods that allow the observation of the early events in metastasis after entry of the cells into the pulmonary circulation (Al-Mehdi et al., 2000). Fluorescent tumor cells were introduced i.v. into mice or rats, and their lungs were isolated and maintained and observed under physiological conditions. Using these methods, we showed that intravascular proliferation rather than early extravasation characterized the initial events in metastatic colony formation. Here, we have adapted these methods to study the initial arrest of tumor cells in the pulmonary vasculature and investigate the role of integrins in this process.
It has long been supposed that integrins play an important role in metastasis. This supposition is based in part on the role of integrins in motility, and in part on data showing that agents that interrupt integrin–ligand interaction also inhibit metastasis. For example, peptides containing the motif RGD (Arg-Gly-Asp) that compete for binding of integrins to fibronectin, or peptides that block binding of integrins to laminin (LN) can inhibit metastasis when coinjected with tumor cells (Humphries et al., 1986; Saiki et al., 1989; Yamamura et al., 1993). Antibodies directed against surface integrins that affect LN binding also reduced lung metastasis (Vollmers et al., 1984). Each integrin is a heterodimer composed of both an α and a β subunit. The ligand-binding domain of the integrin heterodimer is a globular region that requires both subunits to engage the ligand. Thus, antibodies specific for either the α or the β chain can be blocking. Integrins bind to components of ECM such as collagens, fibronectin, and LNs, and can mediate adhesion, spreading, or migration on these substrates (Schwartz, 2001; van der Flier and Sonnenberg, 2001).
Using an attachment assay based on the observation of fluorescent tumor cells in isolated lungs, we have evaluated the involvement of integrins in the arrest of tumor cells in the pulmonary circulation. This report shows for the first time that the α3β1 integrin is an important (but not exclusive) component in that process. The importance of α3β1 integrin in pulmonary vascular attachment by tumor cells raised the question of access to its ligands. LN-5, -8, -10, and -11 are ligands for the α3β1 integrin (Nissinen et al., 1997; Fukushima et al., 1998; Kikkawa et al., 2000; Fujiwara et al., 2001). Each is mainly found in ECM with LN-8/9 and LN-10/11 also present in the stroma of the bone marrow (Siler et al., 2000). How ECM or basement membrane (BM) components could be exposed to tumor cells in pulmonary vessels was not immediately apparent. Unexpectedly, examination of vessels at the sites of tumor cell attachment revealed exposed BM, enabling the binding of α3β1 integrin.
Results
Assay for tumor cell arrest in the pulmonary vasculature
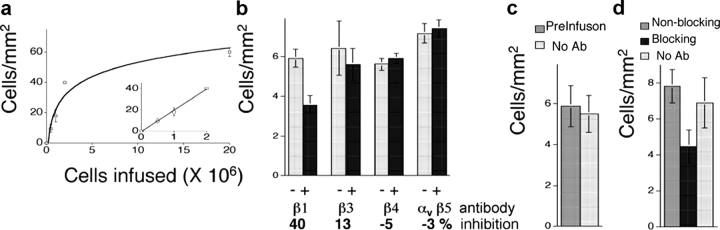
To assess the arrest of tumor cells in the lung, we injected different numbers of fluorescent metastatic HT1080 cells into the renal vein of rats. Immediately after the injection, the isolated, ventilated, and perfused lungs were examined. Fluorescent tumor cells were counted in 60 consecutive nonoverlapping images under low power (10×). The curve of arrested versus injected cells was effectively linear over a range up to 2 × 106 cells. Therefore, injection of cell numbers within the linear range was used in the subsequent experiments to measure pulmonary arrest (Fig. 1 a)
Figure 1.
Inhibition of pulmonary arrest by anti-β integrin antibodies. HT1080-GFP cells were infused into the renal vein of rats, lungs were isolated, and the number of fluorescent cells in a determined area of the surface was counted as described in the Materials and methods section. (a) Number of HT1080-GFP cells arrested on the surface 30 min after infusion of the indicated cell number. Each point represents the results from three rats. Based on this curve, 3 × 105 HT1080-GFP cells were used in subsequent experiments. (b) Inhibition of pulmonary arrest after pretreatment of HT1080 cells with the indicated blocking antibodies. −, treated without antibody; +, treated with antibody. (c) Effect of infusing the anti-β1 blocking antibody before injection of untreated HT1080 cells. (d) Comparison of the effect on pulmonary arrest of anti-β1 blocking antibody to a nonblocking anti-β1 antibody.
Effects of integrins on pulmonary attachment in vivo
HT1080 cells are known to express β1, β3, β4, β5, and β8, and α1, α2, α3, α4, α5, α6, and αv integrin subunits (Petermann et al., 1993). Before i.v. infusion, HT1080-GFP cells were incubated with blocking antibodies to the β1, β3, or β4 integrin subunits or to the αvβ5 integrin. Because blocking antibody to β8 integrin is not available, the effect of this integrin was evaluated with the blocking antibody to αv, the exclusive heterodimeric partner for the β8 integrin subunit (Nishimura et al., 1998). Of the anti-β subunit antibodies tested, only earlier treatment with anti-β1 blocking antibody resulted in a significant reduction in cell attachment (Fig. 1 b). Treatment of HT1080-GFP cells with a nonblocking antibody to β1 integrin did not alter pulmonary arrest, nor did infusion of the blocking antibody into the lung before infusion of the tumor cells (Fig. 1, c and d). These results demonstrated that integrins including the β1 subunit were involved in the arrest of HT1080 in the pulmonary vasculature.
HT1080-GFP cells were incubated with blocking antibodies to the α1, α2, α3, α4, α5, α6, or αv integrin subunits. As shown in Table I, blocking antibodies against the α1, α5, α6, or αv integrin subunits did not affect pulmonary attachment. Anti-α3 integrin subunit antibody had the greatest effect with 34% inhibition, whereas slight reductions of 15 and 16% were seen after the treatments with anti-α2 and α4 integrin subunits, respectively. The effect of blocking the α2 integrin subunit was statistically significant; the effect on the α4 integrin subunit was not. Combining these three antibodies increased the inhibition, but was not entirely additive (unpublished data). Blocking antibodies to α3 (but not α6) integrin subunits similarly reduced pulmonary arrest by two metastatic breast carcinoma cell lines, MDA-MB-231 and MDA-MB-435s (Table II). Because the α3 subunit only forms a complete integrin molecule with the β1 subunit, these data suggest that the α3β1 integrin plays a critical role in the early lodgment of tumor cells in the lung.
Table I.
Effect of anti-α integrin subunit blocking antibodies on pulmonary arrest
| Antibody specificity
|
Control
|
Antibody
|
Inhibition
|
|---|---|---|---|
| cells/mm2 ± SD | cells/mm2 ± SD | % | |
| α1 | 6.00 ± 0.53 | 5.78 ± 0.65 | 4 |
| α2 | 7.23 ± 0.29 | 6.10 ± 0.52 | 15 |
| α3 | 7.42 ± 1.07 | 4.93 ± 1.14 | 34 |
| α4 | 5.95 ± 0.64 | 5.02 ± 0.66 | 16 |
| α5 | 4.65 ± 1.73 | 5.03 ± 2.57 | −8 |
| α6 | 5.94 ± 1.87 | 6.20 ± 1.83 | −5 |
| αv | 6.20 ± 1.50 | 6.00 ± 2.07 | 3 |
HT1080-GFP cells were treated with the indicated blocking antibodies as described in the Materials and methods section. Each value is expressed as cells/mm2 ± SD. The anti-α3 subunit antibody used was P1B5. Similar results were obtained with ASC-1 (unpublished data). The comparison of means using t test was P = 0.03 for α2 integrin subunit blocking antibody and P = 0.05 for α3. The value for α4 was P = 0.15.
Table II.
Effect of α integrin subunit blocking antibodies on pulmonary attachment by breast cancer cell lines
| MDA-MB-231
|
MDA-MB-435s
|
|||||
|---|---|---|---|---|---|---|
| Antibody | Control | Blocking antibody | Inhibition | Control | Blocking antibody | Inhibition |
| α3 | 6.38 ± 0.67 | 4.74 ± 0.42 | 26% | 7.79 ± 1.36 | 5.19 ± 0.92 | 33% |
| α6 | 6.45 ± 0.77 | 6.40 ± 0.61 | 1% | 7.25 ± 1.28 | 7.22 ± 0.88 | 0% |
The effect of anti-α3 or anti-α6 subunit blocking antibodies on the breast carcinoma cell lines MDA-MB-231 and MDA-MB-435s was determined as described in the Materials and methods section. Each value is expressed as cells/mm2 ± SD.
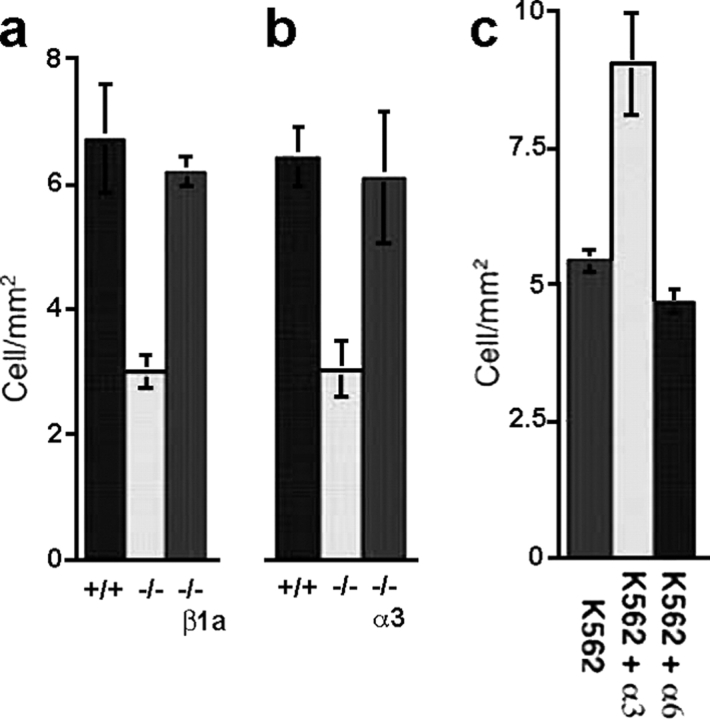
To further evaluate this hypothesis, the effect of genetic elimination of either the α3 or the β1 subunit was tested in pulmonary attachment. The β1 integrin subunit in ESb cells has been eliminated through homologous recombination at both alleles (Stroeken et al., 1998). The deficient ESb cells were ∼40% less effective at pulmonary arrest than the parent ESb cells (Fig. 2 a), similar to the result obtained using HT1080-GFP cells treated with blocking antibodies. Restoration of the β1 integrin by transfection of a β1a cDNA expression vector resulted in the recovery of pulmonary attachment (Fig. 2 a). Likewise, genetic elimination of the α3 integrin subunit using immortalized keratinocytes derived from an α3 integrin-deficient null mouse (MK 5.4.6−/−) resulted in a reduction of 53% in attachment compared with immortalized keratinocytes from a wild-type mouse (MK 1.16+/+; DiPersio et al., 1997, 2000). Restoration of α3 integrin subunit expression by stable transfection (MK 5.4.6−/− + α3) also restored attachment (Fig. 2 b). The role of the α3β1 integrin in cell attachment was also examined in K562 erythroleukemia cells that have only β1 and α5 integrin (Burger et al., 1992). Expression of α3A integrin in K562 cells enhanced cell attachment by 40%, but α6A integrin subunit expression had no effect (Fig. 2 c). These data further support the contention that the α3β1 integrin participates in tumor cell pulmonary attachment.
Figure 2.
The α3β1 integrin contributes to pulmonary arrest in rat lung. (a) Pulmonary attachment by ESb cells (+/+), ESb cells without either β1 integrin allele (−/−), or deficient cells reengineered to express β1a integrin (−/− + β1A). (b) Pulmonary attachment by MK1.16 (+/+), MK5.4.6 (−/−) cell without either α3 integrin, or deficient cells reengineered to express α3 (−/− + α3). (c) Pulmonary arrest of K562 parental cells compared with K562 expressing α3 or α6 integrin subunits.
Anti-α3 or anti-β1 integrin subunit antibody treatment of HT1080-GFP cells also had an inhibitory effect on early colony formation 1 wk after injection. The cells were exposed to the indicated antibody before injection without any subsequent exposure to the antibody, as in the experiments shown in Fig. 1 b. This initial exposure was sufficient to reduce colony formation. The decrease in colony number exceeded the decrease in pulmonary attachment (Table III). Initial pulmonary arrest using the α3β1 integrin appears to play a critical role in subsequent colony formation.
Table III. Inhibition of early colony formation by pre-treatment with anti-integrin subunit antibodies.
| Treatment | Colonies/lung ± SD | Range (# of colonies) |
# of mice | t test against no antibody |
|---|---|---|---|---|
| No antibody | 2.25 ± 1.71 | 0–5 | 12 | - |
| Anti-β1 blocking | 0 | 0 | 7 | 0.001 |
| Anti-β1 nonblocking | 2.43 ± 1.72 | 1–6 | 7 | - |
| Anti-α3 (P1B5) | 0.71 ± 0.76 | 0–2 | 7 | 0.016 |
| Anti-α3 (ASC-1) | 0.71 ± 0.95 | 0–2 | 7 | 0.022 |
| Anti-α6 | 1.86 ± 2.27 | 0–5 | 7 | - |
HT1080-GFP cells were treated with the indicated antibodies for 0.5 h before injection as described in the Materials and methods section. After washing, 5 × 105 cells were injected into the tail vein of nu/nu mice. The numbers of colonies were counted after scanning the entire surface of the left lobe of the isolated mouse lung. A two-tailed t test is shown only for those with a significant difference as compared to the no antibody control.
α3 β 1 integrin ligands in vivo
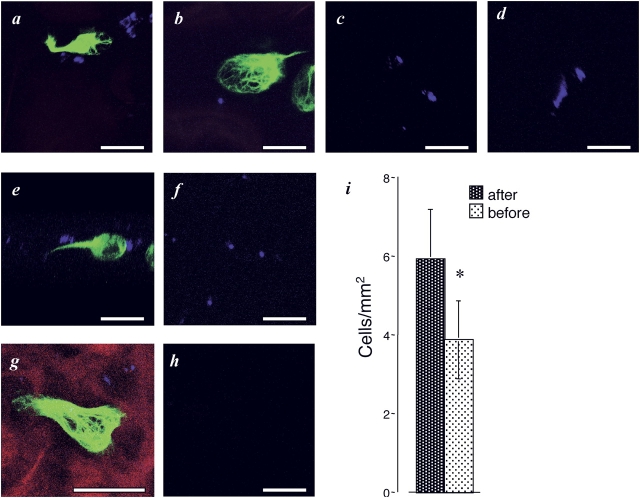
LN-5 is the best-characterized ligand for the α3β1 integrin. Because it is mainly a BM component in the lung, we expected that it would be covered by endothelium and not available to circulating tumor cells in the pulmonary vessels (Mizushima et al., 1998; Coraux et al., 2002). To search for LN-5 in vascular channels, we infused two different fluorescently labeled antibodies to the α3 chain of LN (MIG-1 and CM6) sequentially after the injection of HT1080-GFP-vimentin cells (these mAbs react with rat, but not mouse LN-5). The α3 chain of LN is found in LN-5, -6, and -7 (Colognato and Yurchenco, 2000; Nguyen et al., 2000b). However, LN-5 is considered to be markedly more abundant than the others (Adair-Kirk et al., 2003). Although MIG-1 generally gave a stronger signal than CM6, both anti-LN-5 antibodies stained small areas surrounding the arrested tumor cells (Fig. 3, a and b). A similar signal was obtained after the infusion of antibody against the LN γ2 chain (Fig. 3, e and g). The LN γ2 chain is unique to LN-5 confirming its presence, but not excluding the presence of LN-6 or -7. GFP-vimentin was used to label the cells because the signal from cytoplasmic GFP alone overwhelmed the signal. To test whether these patches existed before the infusion of tumor cells, fluorescently labeled antibodies to either the α3 chain (MIG-1) or the γ2 chain of LN were injected into naive rats. Foci of antibody staining were found in lungs (Fig. 3, c, d, and f). They are infrequent, approximately only on 10–20 high power fields surveyed. Fluorescent antibodies to irrelevant molecules, the cartilage-specific collagen II, or to the platelet integrin αIIb failed to stain any vascular patches (Fig. 3 h). These results have led us to suggest that exposed BM preexists in the pulmonary vasculature.
Figure 3.
LN-5 can act as a ligand in the pulmonary vasculature. Immediately after infusion of HT1080-GFP-vimentin cells into rats, the labeled antibodies to LN-5 were infused. 10 min later, the lungs were isolated and perfused. Panels show representative images obtained with labeled (a) anti-LN α3 chain antibody (MIG-1), or (b) CM6. (c and d) Typical images seen after infusion of the Alexa Fluor® 647–labeled MIG-1 antibody in naive rats. (e) An image after Alexa Fluor®–labeled anti-LN γ2 chain antibody staining. The image is a rotation of a three-dimensional reconstruction showing the direct proximity of the γ2 staining to the cell. (f) LN γ2 chain antibody staining in naive rats. (g) An HT1080-GFP-vimentin cell in a vessel filled with tetramethylrhodamine dextran. LN γ2 staining is seen in blue on the vessel wall. (h) Alexa Fluor® control image. (i) Anti-LN α3 chain antibody (CM6) was infused either before or after infusion of HT1080-GFP cells. When antibody infused before, cell attachment was less, P = 0.019. Bars, 20 μm.
To further test the hypothesis that LN-5 acts as a ligand for the α3β1 integrin on tumor cells, we infused antibodies to the α3 chain (CM6) before the injection of HT1080-GFP tumor cells. These antibodies reduced the extent of pulmonary attachment compared with the pulmonary arrest seen when the antibodies were infused after injection of the tumor cells (Fig. 3 i).
Interaction between tumor cells and vascular BM
We used EM as an alternative means to visualize tumor cell–pulmonary vessel interactions. HT1080-GFP-vimentin cells were labeled with ferritin before injection to allow their identification. 38 tumor cells were identified, all within the pulmonary vessels. Of these, nine cells showed distinct attachment to the vessel wall in EM images. In each of these cases, at the point of contact between the tumor cell and the vessel, the vessel was missing the expected endothelial covering of the basal lamina (Fig. 4, a–c). When the tumor cells were pretreated with a blocking anti-β1integrin subunit antibody, no points of contact were identified (0 out of 33 tumor cells; P = 0.001). Rare patches of exposed BM could be found in the absence of tumor cells (Fig. 4 d). These observations lead to the hypothesis that the foci of exposed BM between endothelial cells may be a prerequisite for tumor cell vascular attachment in the lung.
Figure 4.
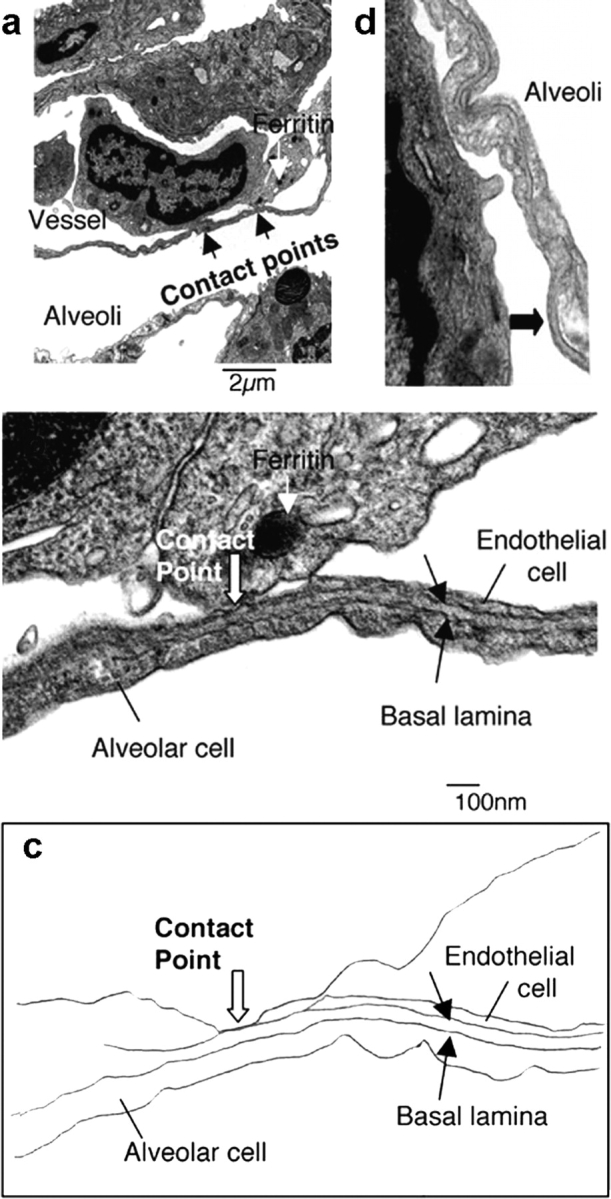
EM shows tumor cells attached to exposed BM. HT1080-GFP-vimentin cells were exposed to ferritin before injection into rats. Lungs were isolated and fixed with glutaraldehyde by perfusion. (a) A representative image from EM with a tumor cell with internalized ferritin within a small vessel and two contact points seen between the tumor cell and the vessel wall. (b) An enlargement of a contact point. At the contact point, the expected overlying endothelium is absent, showing the tumor cell attached to the BM. (c) A tracing of the structures in b. (d) An incidental portion of exposed BM found in lung not involved by tumor.
Discussion
Our analyses have suggested that tumor cells arrest in the pulmonary vasculature through interaction of their α3β1 integrin with exposed BM. We have shown that the α3β1 integrin contributes to metastasis through mediation of early adhesion to the vasculature using a variety of cells, HT1080, MDA-MB-231 and -435s, ESb cells, K562 cells, and immortalized keratinocytes. Interestingly, the α3β1 integrin is commonly expressed by most tumor cells. In some reports, higher expression of the α3β1 integrin correlated with increased metastasis (Morini et al., 2000). Involvement of the β1 integrin subunit in attachment is consistent with data showing reduced metastasis by a lung carcinoma cell line after treatment with blocking antibodies to the β1 integrin subunit (Takenaka et al., 2000). The α3β1 integrin associates with the urokinase receptor and with the tetraspanin CD151, both implicated in metastasis (Scherberich et al., 1998; Testa et al., 1999; Wei et al., 2001; Zhang et al., 2001). In keratinocytes, secretion of matrix metalloproteinase-9, a molecule known to influence metastasis, is dependent on the α3β1 integrin (DiPersio et al., 2000). Our data further indicate that additional receptors are likely to be involved in pulmonary attachment because partial (rather than complete) blockade of attachment was achieved through inhibition of the α3β1 integrin.
The observation that antibodies to components of LN-5 stained foci in pulmonary vessels and that anti-LN α3 chain antibodies block pulmonary arrest provided evidence for LN-5 as a vascular ligand for the tumor cell α3β1 integrins. A potential role for LN-6 is not addressed by these analyses. The structural basis for this staining was shown in EM indicating sporadic absence of overlying endothelial cells in small patches in the pulmonary vessels. Menter et al. (1987) also found EM evidence for direct contact between tumor cells and pulmonary BM. One might expect that these absences could lead to loss of the fluid barrier. However, in the lung fluid integrity is in fact maintained by the tight junctions of the alveolar cells, not the endothelium (Schneeberger-Keeley and Karnovsky, 1968). One might also have expected platelet aggregation. However, the exposure of the surface of the basal lamina or the BM might not expose collagens, or more specifically, their helical domains. In some BMs, the collagens appear to be beneath the basal lamina (Nguyen et al., 2000a). Consistent with this possibility, anti-collagen IV antibody only stained poorly in our hands (unpublished data). This would also explain why the exposed BM regions do not have associated platelet aggregation. Metastasis may favor the lung because of this unique feature of its vessels. Whether arrest in the bone marrow is influenced by the α3β1 integrin binding with LN-8/9 or LN-10/11 remains to be studied. Other organs may also use different adhesion factors. For example, expression of the α5 integrin subunit enhances the arrest of tumor cells in the kidney glomeruli, but does not affect cell attachment in the lung or the liver (Tani et al., 2003).
The α6β4 integrin also binds to LN-5, yet anti-α6 integrin subunit antibody failed to alter attachment in vivo in our experiments. There are other situations in which the α6β4 integrin and the α3β1 integrin function differently. Antibodies to the α3 integrin subunit blocked migration of pancreatic carcinoma cell lines and inhibited adhesion of keratinocytes to LN-5, whereas antibodies to the α6 subunit failed to have these effects (Tani et al., 1997; Hintermann et al., 2001). The signaling pathways through these two integrins are distinct in keratinocytes (Hintermann et al., 2001; Mercurio et al., 2001b). The α6β4 integrin is critical for the formation of hemidesmosomes, whereas the α3β1 integrin regulates adhesion, spreading, and migration in association with the ECM (Borradori and Sonnenberg, 1999). Nonetheless, the α6β4 integrin clearly plays a role in metastasis, if not a demonstrable role in the early arrest of tumor cells in the lung (Mercurio et al., 2001a; Jauliac et al., 2002). Metastasis to the mouse lung is inhibited both by pretreatment of the lung with anti-α6 integrin subunit antibody and treatment of the tumor cells with the antibody (Ruiz et al., 1993). Furthermore, α6 integrin was shown to contribute to survival of breast carcinoma cells as they formed metastatic colonies (Wewer et al., 1997). The α6β1 can also bind LN-5. Some of the actions attributed to the α6 subunit may involve this integrin. Thus, the α6 integrin subunit may augment metastasis, not through enhancing pulmonary arrest, but by facilitating survival or proliferation. Pauli's group has shown that the β4 integrin subunit on tumor cells can ligate to endothelial-bound CLCA1, mediating a signaling cascade that includes FAK activation (Abdel-Ghany et al., 2002). Although they have postulated that this interaction initiates vascular arrest, their data are equally comparable with a model synthesizing our data in which the tumor cell α3β1 integrin interacts with exposed LN-5, allowing subsequent interactions that then signal for intravascular survival and proliferation. CLCA1 may be one of the factors that contribute to pulmonary arrest in addition to the α3β1 integrin.
This model is consistent with the observation that pulmonary metastasis is enhanced by endothelial damage. Both hyperoxia and bleomycin or other chemotherapeutic drugs induce endothelial injury and lead to exposure of the BM (Nicolson and Custead, 1985; Orr et al., 1986; Lichtner and Nicolson, 1987). Orr et al. (1986) demonstrated that endothelial damage with bleomycin promoted pulmonary metastasis, and most of the arrested cells were found attached to the endothelial BM. It has been proposed that tumor cells induce the retraction of endothelial cells, enhancing metastasis (Honn et al., 1989, 1994). Our results suggest that LN-5 is available before interaction with tumor cells, but they do not preclude further retraction after arrest.
These analyses put forward a new model for pulmonary metastasis in which tumor cells use the α3β1 integrin to bind to LN-5 in exposed BM. Colony formation was decreased more extensively than attachment by blockage of the α3β1 integrin subunits, suggesting that in addition to mediating adhesion, the α3β1 integrin may also provide important signaling for other steps required for metastasis.
Materials and methods
Cell lines and culture conditions
HT1080 cells were transfected with pEGFP-C2 (CLONTECH Laboratories, Inc.; Al-Mehdi et al., 2000) and pEGFP-vimentin (a gift from Joe Sanger, University of Pennsylvania, Philadelphia, PA), and were screened for fluorescence after selection with 800 μg/ml geneticin. ESb cells (a spontaneous high metastatic variant of Eb that is a chemically induced T cell lymphoma of DBA/2 mice) and derivatives were gifts from E. Roos (Netherlands Cancer Institute, Amsterdam, Netherlands) and were cultured as described previously (Stroeken et al., 1998). MDA-MB-231 and MDA-MB-435s cell lines were obtained from the American Type Culture Collection and were grown as recommended. Immortalized mouse keratinocyte cell lines (MK cell lines) from wild-type or α3-deficient mice were isolated using a temperature-sensitive mutant of SV-40 large T antigen and cultured as described previously (DiPersio et al., 2000). K562 and K562 expressing the α3 or α6 integrin subunits generated by Weitzman et al. (1997) were gifts from M. Zutter (Vanderbilt University, Nashville, TN) and A. Sonnenberg (Netherlands Cancer Institute).
Monoclonal antibodies
All antibodies used for the tumor cell attachment in vivo assay were purchased from CHEMICON International. The blocking anti-integrin antibodies were as follows: anti-β1 from clone 6S6 (Shang et al., 2001); anti-β3, clone B3A; anti-β4[CD104], clone ASC-3; anti-αvβ5, clone P1F6; anti-αv, clone AV1; anti-α1 I domain, clone FB12; anti-α2, clone P1E6; anti-α3, clone P1B5 and anti-α3, clone ASC (Wayner et al., 1988; Wagner et al., 1991); anti-α4, clone P1H4; anti-α5, clone P1D6; and anti-α6, clone NK1-GoH3. The anti-β1 integrin nonblocking stimulating antibody is clone 21C8.
MIG-1 and CM6 are mouse mAbs raised against different domains of the α3 chain of LN-5. Both antibodies react with rat, but not murine LN-5 (Plopper et al., 1998; Shang et al., 2001). Anti-LN γ2 chain antibody was purchased from CHEMICON International. Antibodies against LNs, or collagen II, were freshly labeled with Zenon mouse IgG labeling kits; Alexa Fluor® 647 (Molecular Probes, Inc.) in a ratio of 10 μg antibody:50 μl labeling reagent:50 μl blocking reagent.
Treatment of cells with antibodies
Cells were harvested, washed with serum-free medium, and resuspended in the same medium containing 0.1% BSA. The suspensions were incubated with antibodies (10 μg/ml) at 4°C for 30 min on a rotator, followed by dilution with equal volume of the same medium. Although some reports have indicated that treatment with anti-α3 integrin subunit antibody can lead to aggregation of keratinocytes (Nguyen et al., 2001), to ensure that aggregation was not occurring in our experiments, we verified that the cells were in single-cell suspension before injection. As additional confirmation, we point out that we did not see cells in aggregates in the lung after injection.
Intravital attachment test
Tumor cells were labeled by either stable GFP expression (HT1080-GFP and HT1080-GFP-vimentin) or vital fluorescence dyes. ESb cells and derivatives were incubated in 100 μM CellTracker™ Green CMFDA (Molecular Probes, Inc.) in a medium containing 10% FBS at 37°C for 30 min. K562 cells, MK cells, and MDA-MB-231 or -435s cells were labeled with the vital dye MitoTracker® Red CMXRos (200 nM; Molecular Probes, Inc.) by 5 min exposure in culture medium. Experiments using CMFDA or MitoTracker® Red on HT1080-GFP cells gave the same results as monitoring GFP. Male rats (∼250 g; Sprague-Dawley) were injected into the renal vein with 3 × 105 cells unless otherwise indicated. For colony assays, female mice (CD-1 nude; Charles River Laboratories) received 5 × 105 cells in the lateral tail vein. 30 min after injection for the attachment assays or 1 wk after injection for the colony assays, the lungs were ventilated, perfused, and isolated under physiological pressures as described previously (Al-Mehdi et al., 1998, 2000). Fluorescent tumor cells were observed in the isolated lungs using an inverted fluorescent research microscope (model DMIRB; Leica), and images were recorded with a camera (Orca; Hamamatsu Photonics) with OpenLab software (Improvision). The quantitative assays were based on counting cells in 60 consecutive and nonoverlapping fields with a 10×/1× lens (1.1 mm2 field/picture). Rats and mice were matched by weight, and each antibody was tested in matched pairs of animals. The results were obtained from at least three independent experiments in all cases.
Confocal scanning laser microscopy
5 min after injection of HT1080-GFP-vimentin cells (106) into the left renal vein, antibody–Alexa Fluor® complexes were introduced through the inferior vena cava. Lungs were isolated as described above. The pulmonary vasculature was labeled by perfusion with tetramethylrhodamine dextran (Molecular Probes, Inc.). The images were captured using a laser scanning system (Radiance 2000; Bio-Rad Laboratories) and a microscope (Eclipse TE300; Nikon). The 2-line Argon/kryotin laser (wavelength 488 nm/568 nm) and the red laser diode (wavelength 638 nm) were used for observation.
Transmission EM observation
HT1080-GFP-vimentin cells were labeled with the electron-dense marker ferritin at 4°C for 10 min. 5 or 30 min after i.v. injection (2 × 106 cells) into rats, the lung was dissected as described above. Afterwards, the lung was washed with PBS, followed by perfusion with 5% glutaraldehyde. Samples were collected from the upper region of the left lung and fixed in 2.5% glutaraldehyde at 4°C. The images were photographed with a transmission electron microscope, 80 kV (JEM-1010; JEOL USA, Inc.)
Acknowledgments
We thank W. Gillies McKenna and Clayton Buck for valuable advice and for reading the manuscript. We also thank Chun Song, Margaret Meuller, and Neelima Shah for technical assistance.
The authors are grateful for support from the National Cancer Institute (National Institutes of Health; grants CA46830 and CA89188).
Abbreviations used in this paper: BM, basement membrane; LN, laminin.
References
- Abdel-Ghany, M., H.C. Cheng, R.C. Elble, and B.U. Pauli. 2001. The breast cancer b4 integrin and endothelial human CLCA2 mediate lung metastasis. J. Biol. Chem. 276:25438–25446. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany, M., H.-C. Cheng, R.C. Elble, and B.U. Pauli. 2002. Focal adhesion kinase activated by β4 integrin ligation to mCLCA1 mediates early metastasis growth. J. Biol. Chem. 277:34391–34400. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany, M., H.C. Cheng, R.C. Elble, H. Lin, J. DiBiasio, and B.U. Pauli. 2003. The interacting binding domains of the b4 integrin and calcium-activated chloride channels (CLCAs) in metastasis. J. Biol. Chem. 278:49406–49416. [DOI] [PubMed] [Google Scholar]
- Adair-Kirk, T.L., J.J. Atkinson, T.J. Broekelmann, M. Doi, K. Tryggvason, J.H. Miner, R.P. Mecham, and R.M. Senior. 2003. A site on laminin a5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J. Immunol. 171:398–406. [DOI] [PubMed] [Google Scholar]
- Al-Mehdi, A.B., G. Zhao, C. Dodia, K. Tozawa, K. Costa, V. Muzykantov, C. Ross, F. Blecha, M. Dinauer, and A.B. Fisher. 1998. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ. Res. 83:730–737. [DOI] [PubMed] [Google Scholar]
- Al-Mehdi, A.B., K. Tozawa, A.B. Fisher, L. Shientag, A. Lee, and R.J. Muschel. 2000. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 6:100–102. [DOI] [PubMed] [Google Scholar]
- Borradori, L., and A. Sonnenberg. 1999. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 112:411–418. [DOI] [PubMed] [Google Scholar]
- Burger, S.R., M.M. Zutter, S. Sturgill-Koszycki, and S.A. Santoro. 1992. Induced cell surface expression of functional α2β1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp. Cell Res. 202:28–35. [DOI] [PubMed] [Google Scholar]
- Colognato, H., and P.D. Yurchenco. 2000. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218:213–234. [DOI] [PubMed] [Google Scholar]
- Coraux, C., G. Meneguzzi, P. Rousselle, E. Puchelle, and D. Gaillaer. 2002. Distribution of laminin 5, integrin receptors, and branching morphogenesis during human fetal lung development. Dev. Dyn. 225:176–185. [DOI] [PubMed] [Google Scholar]
- DiPersio, C.M., K.M. Hodivala-Dilke, R. Jaenisch, J.A. Kreidberg, and R.O. Hynes. 1997. α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 137:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio, C.M., M. Shao, L.D. Costanzo, J.A. Kreidberg, and R.O. Hynes. 2000. Mouse keratinocytes immortalized with large T antigen acquire α3β1 integrin-dependent secretion of MMP-9/gelatinase B. J. Cell Sci. 113:2909–2921. [DOI] [PubMed] [Google Scholar]
- Fujiwara, H., Y. Kikkawa, N. Sanzen, and K. Sekiguchi. 2001. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through α3β1 and α6β1 integrins. J. Biol. Chem. 276:17550–17558. [DOI] [PubMed] [Google Scholar]
- Fukushima, Y., T. Ohnishi, N. Arita, T. Hayakawa, and K. Sekiguchi. 1998. Integrin α3β1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int. J. Cancer. 76:63–72. [DOI] [PubMed] [Google Scholar]
- Hintermann, E., M. Bilban, A. Sharabi, and V. Quaranta. 2001. Inhibitory role of α6β4-associated erB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on α3β1 integrin. J. Cell Biol. 153:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn, K.V., I.M. Grossi, C.A. Diglio, M. Wojtukiewicz, and J.D. Taylor. 1989. Enhanced tumor cell adhesion to the subendothelial matrix resulting from 12(S)-HETE-induced endothelial cell retraction. FASEB. J. 3:2285–2293. [DOI] [PubMed] [Google Scholar]
- Honn, K.V., D.G. Tang, I. Grossi, Z.M. Duniec, J. Timar, C. Renaud, M. Leithauser, I. Blair, C.R. Johnson, C.A. Diglio, et al. 1994. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res. 54:565–574. [PubMed] [Google Scholar]
- Humphries, M.J., K. Olden, and K.M. Yamada. 1986. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 233:467–470. [DOI] [PubMed] [Google Scholar]
- Jauliac, S., C. Lopez-Rodriguez, L.M. Shaw, L.F. Brown, A. Rao, and A. Toker. 2002. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4:540–544. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., N. Sanzen, H. Fujiwara, A. Sonnenberg, and K. Sekiguchi. 2000. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1 and α6β4 integrins. J. Cell Sci. 113:869–872. [DOI] [PubMed] [Google Scholar]
- Lichtner, R.B., and G.L. Nicolson. 1987. Effects of the pyrimido-pyrimidine derivative RX-RA 85 on metastatic tumor cell-vascular endothelial cell interactions. Clin. Exp. Metastasis. 5:219–231. [DOI] [PubMed] [Google Scholar]
- Menter, D.G., J.S. Hatfield, C. Harkins, B.F. Sloane, J.D. Taylor, J.D. Crissman, and K.V. Honn. 1987. Tumor cell-platelet interactions in vitro and their relationship to in vivo arrest of hematogenously circulating tumor cells. Clin. Exp. Metastasis. 5:65–78. [DOI] [PubMed] [Google Scholar]
- Mercurio, A.M., R.E. Bachelder, I. Rabinovitz, K.L. O'Connor, T. Tani, and L.M. Shaw. 2001. a. The metastatic odyssey: the integrin connectin. Surg. Oncol. Clin. N. Am. 10:313–328. [PubMed] [Google Scholar]
- Mercurio, A.M., I. Rabinovitz, and L.M. Shaw. 2001. b. The α6β4 integrin and epithelial migration. Curr. Opin. Cell Biol. 13:541–545. [DOI] [PubMed] [Google Scholar]
- Mizushima, H., N. Koshikawa, K. Moriyama, H. Takamura, Y. Nagashima, F. Hirahara, and K. Miyazaki. 1998. Wide distribution of laminin-5 γ2 chain in basement membranes of various human issues. Horm. Res. 50:7–14. [DOI] [PubMed] [Google Scholar]
- Morini, M., M. Mottolese, N. Ferrari, F. Ghiorzo, S. Buglioni, R. Mortarini, D.M. Noonan, P.G. Natali, and A. Albini. 2000. The α3β1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int. J. Cancer. 87:336–342. [PubMed] [Google Scholar]
- Nguyen, B.P., S.G. Gil, and W.G. Carter. 2000. a. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J. Biol. Chem. 275:31896–31907. [DOI] [PubMed] [Google Scholar]
- Nguyen, B.P., M.C. Ryan, S.G. Gil, and W.G. Carter. 2000. b. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol. 12:554–562. [DOI] [PubMed] [Google Scholar]
- Nguyen, B.P., X.D. Ren, M.A. Schwartz, and W.G. Carter. 2001. Ligation of integrin α3β1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J. Biol. Chem. 276:43860–43870. [DOI] [PubMed] [Google Scholar]
- Nicolson, G.L., and S.E. Custead. 1985. Effects of chemotherapeutic drugs on platelet and metastatic tumor cell-endothelial cell interactions as a model for assessing vascular endothelial integrity. Cancer Res. 45:331–336. [PubMed] [Google Scholar]
- Nishimura, S.L., K.P. Boylen, S. Einheber, T.A. Milner, D.M. Ramos, and R. Pytela. 1998. Synaptic and glial localization of the integrin α8 in mouse and rat brain. Brain Res. 791:271–282. [DOI] [PubMed] [Google Scholar]
- Nissinen, L., L. Pirila, and J. Heino. 1997. Bone morphogenetic protein-2 is a regulator of cell adhesion. Exp. Cell Res. 230:377–385. [DOI] [PubMed] [Google Scholar]
- Orr, F.W., I.Y.R. Adamson, and L. Young. 1986. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res. 46:891–897. [PubMed] [Google Scholar]
- Petermann, A., H. Fees, H. Grenz, S.L. Goodman, and R.B. Sterzel. 1993. Polymerase chain reaction and focal contact formation indicate integrin expression in mesangial cells. Kidney Int. 44:997–1005. [DOI] [PubMed] [Google Scholar]
- Plopper, G.E., S.Z. Domanico, V. Cirulli, W.B. Kiosses, and V. Quaranta. 1998. Migration of breast epithelial cells on laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res. Treat. 51:57–69. [DOI] [PubMed] [Google Scholar]
- Ruiz, P., D. Dunon, A. Sonnenberg, and B.A. Imhof. 1993. Suppression of mouse melanoma metastasis by EA-1, a monoclonal antibody specific for α6 integrins. Cell Adhes. Commun. 1:67–81. [PubMed] [Google Scholar]
- Saiki, I., J. Murata, J. Iida, T. Sakurai, N. Nishi, K. Matsuno, and I. Azuma. 1989. Antimetastatic effects of synthetic polypeptides containing repeated structures of the cell adhesive Arg-Gly-Asp (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR) sequences. Br. J. Cancer. 60:722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberich, A., S. Moog, G. Haan-Archipoff, D.O. Azorsa, F. Lanza, and A. Beretz. 1998. Tetraspanin CD9 is associated with very late-acting integrins in human vascular smooth muscle cells and modulates collagen matrix reorganization. Arterioscler. Thromb. Vasc. Biol. 18:1691–1697. [DOI] [PubMed] [Google Scholar]
- Schneeberger-Keeley, E.E., and M.J. Karnovsky. 1968. The ultrastructural basis of alveolar-capillary membrane permeability to peroxidase used as a tracer. J. Cell Biol. 37:781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A. 2001. Integrin signaling revisited. Trends Cell Biol. 11:466–470. [DOI] [PubMed] [Google Scholar]
- Shang, M., N. Koshikawa, S. Schenk, and V. Quaranta. 2001. The LG3 module of laminin-5 harbors a binding site for integrin α3β1 that promotes cell adhesion, spreading, and migration. J. Biol. Chem. 276:33045–33053. [DOI] [PubMed] [Google Scholar]
- Siler, U., M. Seiffert, S. Puch, A. Richards, B. Torok-Storb, C.A. Muller, L. Sorokin, and G. Klein. 2000. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood. 96:4194–4203. [PubMed] [Google Scholar]
- Stroeken, P.J., E.A. van Rijthoven, M.A. van der Valk, and E. Roos. 1998. Targeted disruption of the β1 integrin gene in a lymphoma cell line greatly reduces metastatic capacity. Cancer Res. 58:1569–1577. [PubMed] [Google Scholar]
- Takenaka, K., M. Shibuya, Y. Takeda, S. Hibino, A. Gemma, Y. Ono, and S. Kudoh. 2000. Altered expression and function of β1 integrins in a highly metastatic human lung adenocarcinoma cell line. Int. J. Oncol. 17:1187–1194. [DOI] [PubMed] [Google Scholar]
- Tani, N., S. Higashiyama, N. Kawaguchi, J. Madarame, I. Ota, Y. Ito, Y. Ohoka, S. Shiosaka, Y. Takada, and N. Matsuura. 2003. Expression level of integrin α5 on tumour cells affects the rate of metastasis to the kidney. Br. J. Cancer. 88:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, T., A. Lumme, A. Linnala, E. Kivilaakso, T. Kiviluoto, R.E. Burgeson, L. Kangas, I. Leivo, and I. Virtanen. 1997. Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am. J. Pathol. 151:1289–1302. [PMC free article] [PubMed] [Google Scholar]
- Testa, J.E., P.C. Brooks, J.M. Lin, and J.P. Quigley. 1999. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 59:3812–3820. [PubMed] [Google Scholar]
- van der Flier, A., and A. Sonnenberg. 2001. Function and interaction of integrins. Cell Tissue Res. 305:285–298. [DOI] [PubMed] [Google Scholar]
- Vollmers, H.P., B.A. Imhof, S. Braun, C.A. Waller, V. Schirrmacher, and W. Birchmeier. 1984. Monoclonal antibodies which prevent experimental lung metastases. Interference with the adhesion of tumour cells to laminin. FEBS Lett. 172:17–20. [DOI] [PubMed] [Google Scholar]
- Wagner, E.A., R.A. Orlando, and D.A. Cheresh. 1991. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 113:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner, E.A., W.G. Carter, R.S. Piotrowicz, and T.J. Kunicki. 1988. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J. Cell Biol. 107:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y., J.A. Eble, Z. Wang, J.A. Kreidberg, and H.A. Chapman. 2001. Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol. Biol. Cell. 12:2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman, J.B., C. Pujades, and M.E. Hemler. 1997. Integrin α chain cytoplasmic tails regulate “antibody-redirected” cell adhesion, independently of ligand binding. Eur. J. Immunol. 27:78–84. [DOI] [PubMed] [Google Scholar]
- Wewer, U.M., L.M. Shaw, R. Albrechtsen, and A.M. Mercurio. 1997. The integrin α6β1 promotes the survival of metastatic human breast cells in mice. Am. J. Pathol. 151:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- Yamamura, K., M.C. Kibbey, S.H. Jun, and H.K. Kleinman. 1993. Effect of matrigel and laminin peptide YIGSR on tumor growth and metastasis. Semin. Cancer Biol. 4:259–265. [PubMed] [Google Scholar]
- Zhang, X.A., A.L. Bontrager, and M.E. Hemler. 2001. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific β1 integrins. J. Biol. Chem. 276:25005–25013. [DOI] [PubMed] [Google Scholar]