Abstract
The chromosomal passenger complex of Aurora B kinase, INCENP, and Survivin has essential regulatory roles at centromeres and the central spindle in mitosis. Here, we describe Borealin, a novel member of the complex. Approximately half of Aurora B in mitotic cells is complexed with INCENP, Borealin, and Survivin; and Borealin binds Survivin and INCENP in vitro. A second complex contains Aurora B and INCENP, but no Borealin or Survivin. Depletion of Borealin by RNA interference delays mitotic progression and results in kinetochore–spindle misattachments and an increase in bipolar spindles associated with ectopic asters. The extra poles, which apparently form after chromosomes achieve a bipolar orientation, severely disrupt the partitioning of chromosomes in anaphase. Borealin depletion has little effect on histone H3 serine10 phosphorylation. These results implicate the chromosomal passenger holocomplex in the maintenance of spindle integrity and suggest that histone H3 serine10 phosphorylation is performed by an Aurora B–INCENP subcomplex.
Keywords: mitosis; Aurora B kinase; Survivin; INCENP; TD-60
Introduction
In recent years, it has emerged that chromosomal passengers (Earnshaw and Bernat, 1990), a conserved complex of Aurora B kinase, INCENP, and Survivin (Adams et al., 2000; Kaitna et al., 2000; Kang et al., 2001; Wheatley et al., 2001a; Bolton et al., 2002; Cheeseman et al., 2002; Leverson et al., 2002), are key regulators of mitotic events (for review see Andrews et al., 2003; Carmena and Earnshaw, 2003). In keeping with this role, chromosomal passengers show a dynamic localization pattern during mitosis, appearing on the chromosome arms and inner centromeres from prophase through metaphase and then transferring to the spindle midzone and midbody from anaphase through cytokinesis (Cooke et al., 1987). In yeast, relocalization of the chromosomal passenger complex to the central spindle in early anaphase is mediated by the dephosphorylation of Sli15 (INCENP) by Cdc14 (Pereira and Schiebel, 2003). The requirements for centromere targeting of the chromosomal passengers are still largely unknown, although Aurora A phosphorylation of CENP-A may contribute (Kunitoku et al., 2003).
Aurora B kinase activity peaks in mitosis (Bischoff et al., 1998; Terada et al., 1998), and its regulation involves both specific targeting and modulation of the kinase activity by the other chromosomal passengers. INCENP is a multidomain microtubule-binding protein (Cooke et al., 1987; Wheatley et al., 2001b) that binds to Aurora B through the conserved COOH-terminal IN box (Adams et al., 2000; Honda et al., 2003). Binding stimulates Aurora B to phosphorylate INCENP within the IN box, resulting in further kinase activation in a feedback loop (Bishop and Schumacher, 2002; Cheeseman et al., 2002; Honda et al., 2003). Survivin has been reported to contribute to Aurora B activation in Xenopus laevis and fission yeast (Bolton et al., 2002; Leverson et al., 2002; Petersen and Hagan, 2003), although in vitro no direct stimulation was observed with the human proteins (Honda et al., 2003). Numerous chromosomal and cytoskeletal Aurora B substrates are now being discovered (for review see Andrews et al., 2003; Carmena and Earnshaw, 2003).
Interference with the subunits of the chromosomal passenger complex produces severe mitotic defects. A profound inhibition of chromosome congression to the metaphase plate (Adams et al., 2001; Ditchfield et al., 2003; Hauf et al., 2003) reflects an involvement of Aurora B in the correction of syntelic chromosome attachments (Tanaka et al., 2002; Hauf et al., 2003; Andrews et al., 2004; Lampson et al., 2004). A requirement for chromosomal passengers for spindle assembly checkpoint function was uncovered in yeast through analysis of Ipl1 (Aurora) and Bir1 (Survivin-like) function (Biggins and Murray, 2001; Petersen and Hagan, 2003). Interference with passenger function in vertebrate cells compromises the spindle assembly checkpoint when microtubules are perturbed using taxol or monastrol (Carvalho et al., 2003; Ditchfield et al., 2003; Hauf et al., 2003; Lens et al., 2003). Aurora B, INCENP, and Survivin are also essential for the late stages of cytokinesis (Terada et al., 1998; Kaitna et al., 2000; Severson et al., 2000; Adams et al., 2001; Giet and Glover, 2001; Carvalho et al., 2003; Lens et al., 2003).
A fourth protein, TD-60, was described as a chromosomal passenger based on its localization during mitosis (Andreassen et al., 1991). Aurora B and TD-60 are interdependent for localization (Mollinari et al., 2003), but it is presently unclear if TD-60 is part of the chromosomal passenger complex. TD-60 appears to be a guanosine nucleotide exchange factor that may act on Rac1 (Mollinari et al., 2003).
Here, we report the initial characterization of Borealin, a novel fourth subunit of the chromosomal passenger complex in vertebrates. Functional studies reveal that Borealin is required for targeting of Aurora B, INCENP, Survivin, and TD-60; correction of kinetochore attachment errors; and stability of the bipolar spindle in human cells. Our data suggest that there are at least two distinct chromosomal passenger complexes during mitosis.
Results
Borealin is a novel chromosomal passenger protein
A proteomic screen for novel human proteins associated with histone-depleted mitotic chromosomes (unpublished data) identified the uncharacterized protein FLJ10468. A GFP fusion of FLJ10468 showed a localization pattern during mitosis identical with that of chromosomal passenger proteins. It appeared on the chromosome arms and the inner centromeres in prophase (Fig. 1 A) and concentrated at the inner centromeres during metaphase (Fig. 1 A'). It then relocated to the spindle midzone at anaphase onset and remained at the midbody throughout telophase (Fig. 1 A”) and cytokinesis.
Figure 1.
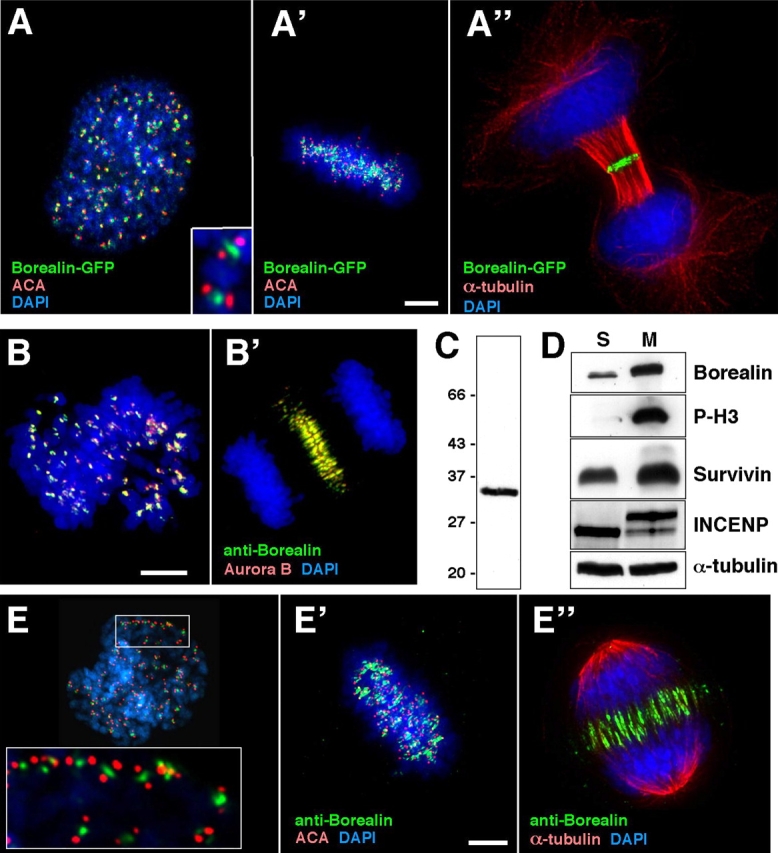
Borealin is a novel chromosomal passenger protein. (A) Localization of Borealin-GFP transiently expressed in HeLa cells at prophase (A), metaphase (A'), and telophase (A”). (B) Aurora B colocalizes with Borealin as detected by affinity-purified anti-Borealin antibody R1647 in HeLa cells. (C) Immunoblot of HeLa cell lysate probed with R1647. (D) Immunoblot showing Borealin levels in S-phase and M-phase cells. (E) R1647 staining confirming that Borealin is a chromosomal passenger protein. Insets in A and E show higher magnification views of Borealin in the inner centromere with ACA staining in red and Borealin in green. Bars, 5 μm.
Affinity-purified rabbit polyclonal antibody R1647 raised against the full-length protein recognized a protein at the expected size (31 kD) on immunoblots of HeLa cell lysates (Fig. 1 C). Indirect immunofluorescence confirmed the dynamic localization of this protein in mitosis (Fig. 1, E–E”). Simultaneous staining for Aurora B confirmed that the two proteins colocalize throughout mitosis (Fig. 1, B and B'; and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200404001/DC1). These results demonstrate that the protein, which we termed Borealin, is a novel chromosomal passenger.
Chromosomal passenger proteins Aurora B, INCENP, and Survivin are expressed in a cell cycle–dependent manner (Li and Altieri, 1999; Honda et al., 2003). Immunoblotting of extracts from HeLa cells arrested in S-phase (Fig. 1 D, S) or mitosis (Fig. 1 D, M) revealed that Borealin levels are also significantly increased in mitosis. Northern blotting detected a corresponding increase in mRNA levels (unpublished data). A slower migrating form of INCENP, presumably the phosphorylated protein, was detected solely in mitotic cells.
Borealin is conserved among vertebrates
The human Borealin gene is located on Chromosome 1 and consists of ten exons encoding a basic protein (pI 9.9) of 280 amino acids. Two putative nuclear localization signals encompass amino acids 4–20 and 146–166. No other conserved amino acid motifs were detected. Database searches revealed relatives to human Borealin in all vertebrates examined (Fig. 2 A). Interestingly, Borealin has an additional paralogue in chicken, X. laevis, and zebrafish (Borealin 2). In contrast, the closest match within the human genome is to a DNA segment on chromosome 7 within an intron of a predicted septin-like protein. However, the absence of EST support and the lack of introns suggest that this ORF is a remnant of a processed pseudogene.
Figure 2.
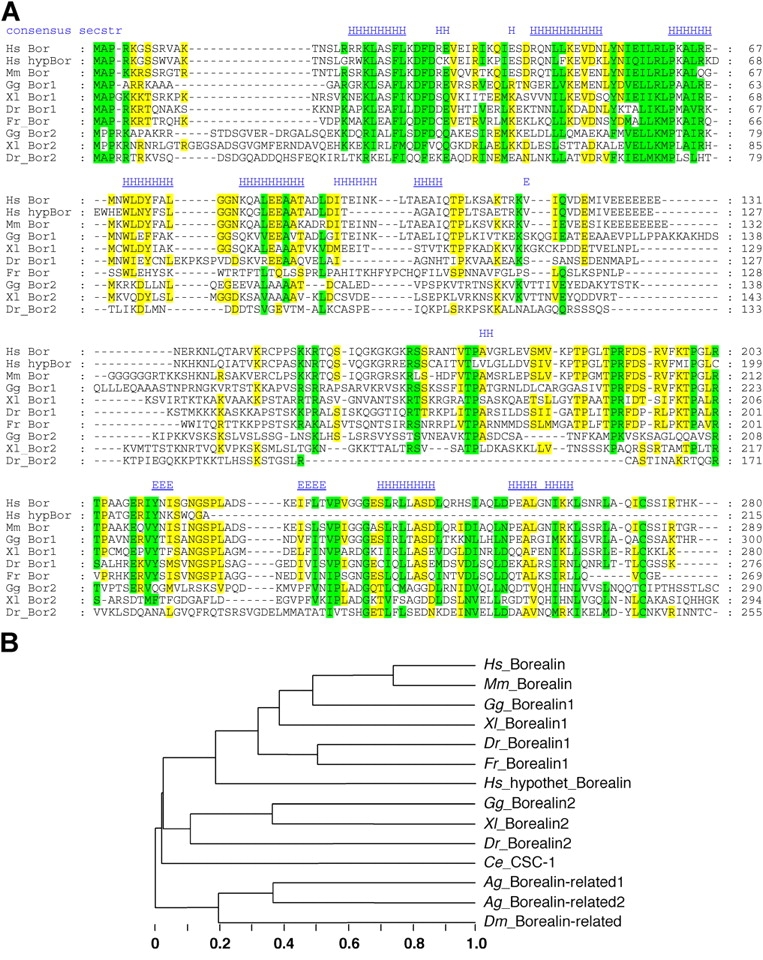
Borealin is conserved among vertebrates with distantly related proteins in insects and nematodes. Multiple sequence alignment and secondary structure (A; Ginalski et al., 2003) plus tentative tree (B; Brodskii et al., 1995) for the vertebrate Borealin family. Colors indicate similarity of >60% (yellow) and >80% (green). Secondary structure predicted consistently for Borealin 1 (blue) is indicated (H, helix; E, strand), with plausible core elements underlined based on their length (H >3; E >2) and presence in all sequences. NCBI/SPTrEMBL accession codes: Human, Hs_Bor, cdc-associated protein 8, NP_060571/Q9NVW5 [cDNA, chromosome 1]; Hs_hypBor, likely pseudogene, Hs7_34123 (5467829–5468473) [genomic DNA chromosome 7]. Mouse, Mm_Bor, BAC37258/Q8BHQ5 [cDNA chromosome 4]. Chicken, Gg_Bor1, (BU135762+BU317198) [EST]; Gg_Bor2, (BU315188+BU328206+ BU324297+BU225870) [EST]. X. laevis, Xl_Bor1 (BJ626030+BJ642424) [EST]; Xl_Bor2, CA973941+CA982691 [EST]. Zebrafish, Dr_Bor1, CK683767 [cDNA]; Dr_Bor2, (BM777478+BM080944). Fugu, Fr_Bor, SINFRUP00000052671 corrected in one exon [pred. protein]. Caenorhabditis, Ce_CSC-1, O45952/CAB07696. Mosquito, Ag_Borealin-related1, XP_309425 [pred. protein]; Ag_Borealin-related2, XP_309424 [pred. protein]. Drosophila, Dm_Borealin- related, CG4454-PB [pred. protein].
Secondary structure predictions suggest that Borealin is built of two distinct domains. Six core helices are predicted for the NH2-terminal domain, whereas the second core contains at least two strands besides three helices at the COOH terminus.
We also detected a remote similarity between Borealin and predicted proteins in mosquito and Drosophila melanogaster. Furthermore, a manual alignment with the Caenorhabditis elegans chromosomal passenger protein CSC-1 revealed weak sequence similarities within two short regions of Borealin. Therefore, we tentatively include these proteins in a Borealin family tree (Fig. 2 B). We have been unable to find Borealin-like proteins in plants and yeasts.
Borealin localization is disturbed in Survivin-depleted cells and in cells expressing a dominant INCENP mutant
To determine whether or not Borealin exhibits functional interactions with the other chromosomal passengers, we exploited the mutual interdependence of these proteins for their localization in vivo. In cells depleted of Survivin by RNAi, Aurora B is localized diffusely in the cytoplasm in mitosis (Carvalho et al., 2003). Therefore, we transfected HeLa cells with Survivin-specific siRNAs and used Aurora B delocalization as a marker to identify Survivin-depleted cells. Borealin was delocalized in all cells in which Aurora B was delocalized (Fig. 3 B), but was localized correctly in cells transfected with control oligo (Fig. 3 A).
Figure 3.
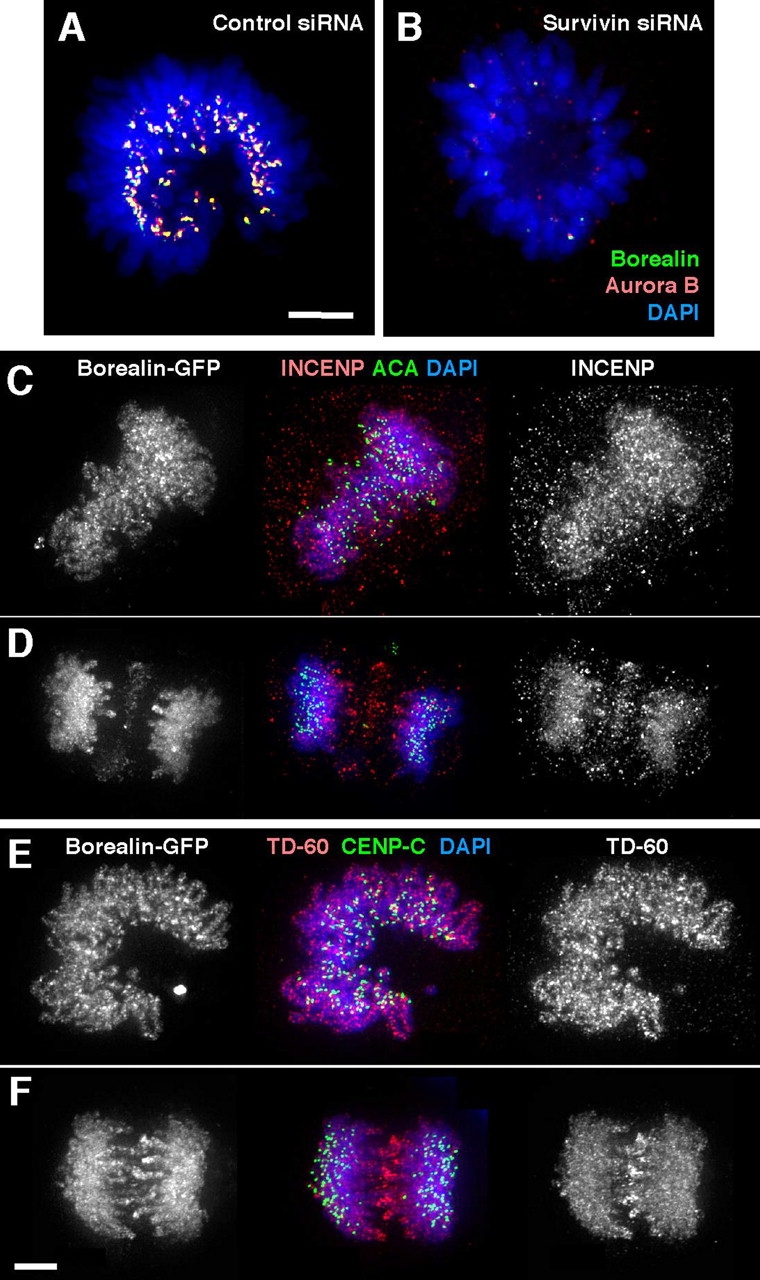
Borealin localization is dependent on the correct localization of other chromosomal passengers. (A and B) Depletion of Survivin by RNA interference results in mislocalization of Aurora B and Borealin from centromeres. (C–F) In cells expressing INCENP1–405, Borealin-GFP and TD-60 are abnormally localized at metaphase and anaphase. Bars, 5 μm.
The chicken INCENP deletion mutant INCENP1–405 disrupts chromosomal passenger localization when overexpressed in vertebrate cells (Mackay et al., 1998). We transfected HeLa cells with the INCENP1–405 construct and identified cells overexpressing the protein by the abnormal localization of endogenous INCENP (Fig. 3 C, right). In these cells, cotransfected Borealin-GFP was diffusely localized on the chromosomes with little or no detectable enrichment at centromeres (Fig. 3 C, left). In anaphase cells expressing INCENP1–405, Borealin-GFP and INCENP remained associated with the chromosomes (Fig. 3 D). Interestingly, TD-60 also showed a diffuse chromosomal localization in the presence of INCENP1–405 (Fig. 3, E and F), as did Survivin and Aurora B (not depicted).
We conclude that Borealin localization is dependent on the correct localization of Survivin and INCENP, suggesting that Borealin is functionally linked with the chromosomal passenger complex.
Borealin is a subunit of the chromosomal passenger complex in vivo and associates with INCENP and Survivin in vitro
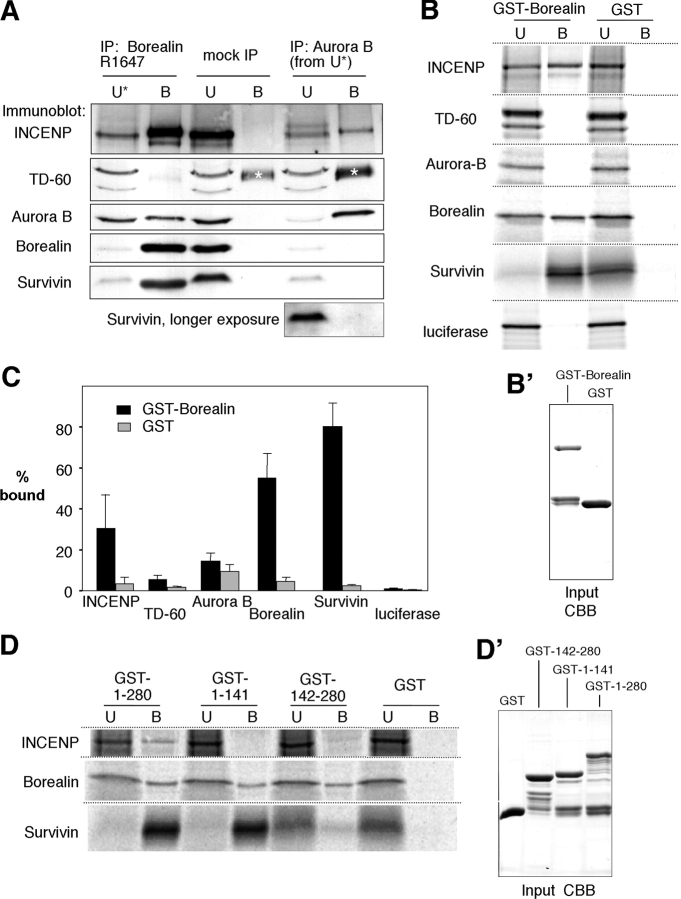
We then investigated whether or not Borealin is a subunit of the chromosomal passenger complex by immunoprecipitating the endogenous protein from mitotic cell lysates. Aurora B, INCENP, and Survivin coimmunoprecipitated with Borealin (Fig. 4 A). About half of Aurora B and most of INCENP and Survivin could thus be depleted from mitotic cell lysates. In contrast, TD-60 did not coimmunoprecipitate with Borealin. To test if the INCENP, Aurora B, and TD-60 remaining soluble after Borealin immunoprecipitation were present in a complex, we subjected the supernatant to a second round of immunoprecipitation with antibody to Aurora B. Under these conditions, INCENP was readily detected on the beads; however, Borealin, Survivin, and TD-60 were not (Fig. 4 A). The TD-60 antibody, made against a COOH-terminal peptide and affinity purified, recognizes bands of 56 and 45 kD in immunoblots of mitotic cells. Preincubation of the antibody with the peptide abolishes both bands (unpublished data), and thus both may be isoforms of TD-60.
Figure 4.
Borealin interacts with subunits of the chromosomal passenger complex in vivo and in vitro. (A) Chromosomal passengers coimmunoprecipitate with endogenous Borealin. Mitotic HeLa cell lysate was incubated with affinity-purified R1647 (IP: Borealin) or preimmune serum R1647 (mock IP) cross-linked to protein A beads. Chromosomal passenger proteins in the unbound (U) and bound (B) fractions were visualized by immunoblotting. The unbound fraction after immunoprecipitation of Borealin was further incubated with anti-Aurora B antibody (IP: Aurora B) cross-linked to protein A beads. Protein loading per lane is equivalent to mitotic lysate from 1.5 × 105 cells. The asterisk denotes residual rabbit IgG heavy chain coeluted from the beads due to incomplete cross-linking. (B) Borealin binds to Survivin, INCENP, and itself in vitro. Proteins were translated in the presence of [35S]methionine and incubated with bacterially expressed GST-Borealin or GST alone bound to glutathione sepharose beads. Bound (B) and unbound (U) fractions were separated by SDS-PAGE, and the proteins were visualized by phosphorimaging. Luciferase was included to check for nonspecific binding of GST-Borealin. (B') GST-Borealin and GST used in the binding reactions (input) stained with Coomassie brilliant blue. (C) Quantification of the binding experiments shown in B. The bars represent the percentage of total translated protein bound to GST-Borealin (black) or GST (gray; n ≥ 3). (D) The NH2- and COOH-terminal halves of Borealin show differential binding properties in vitro. Experiments were performed as described in B, but included GST fusions of Borealin1–141 and Borealin142–280 in addition to full-length Borealin1–280. Equal loading (input) was checked by CBB staining (D').
In vitro binding experiments revealed that GST-Borealin can interact directly with in vitro translated INCENP and Survivin, but not Aurora B or TD-60 (Fig. 4 B). Interestingly, Borealin bound readily to itself. Quantification of the binding experiments revealed that the interaction with Survivin is particularly efficient (Fig. 4 C). When the NH2- and COOH-terminal halves of Borealin (1–141 and 142–280 aa) were used for similar binding experiments (Fig. 4 D), neither half bound INCENP but both halves bound Borealin. Borealin1–141 bound Survivin as efficiently as full-length Borealin, whereas only a weak interaction was detected for Borealin142–280. Thus, the two halves of Borealin interact differentially with subunits of the chromosomal passenger complex.
This analysis suggests that during mitosis, cells may have at least two chromosomal passenger complexes, a holocomplex containing INCENP, Aurora B, Borealin, and Survivin and a subcomplex containing INCENP and Aurora B.
A Borealin fragment selectively perturbs chromosomal passenger targeting to centromeres in vivo
Knowing which regions of Borealin mediate interactions with other chromosomal passengers, we probed the functional significance of these interactions by transiently transfecting HeLa cells with a series of Borealin deletion mutants fused to GFP. Note that full-length Borealin (280 aa) localizes correctly with GFP at either end (Fig. 1, A and B; and Fig. 5 A).
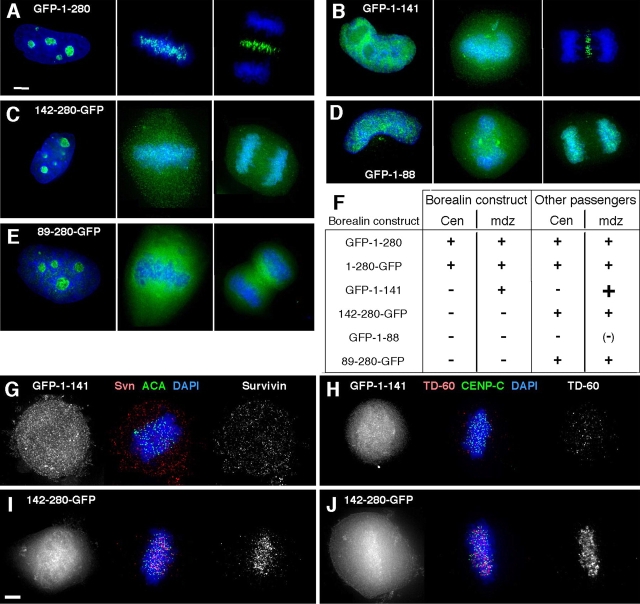
Figure 5.
Transient expression of Borealin deletion mutants identifies functional domains in vivo. (A–E) Localization of deletion mutants expressed as GFP fusions in interphase (left), prometaphase/metaphase (middle), and anaphase (right). HeLa cells were transfected with GFP constructs containing Borealin amino acids 1–280 (A), 1–141 (B), 142–280 (C), 1–88 (D), and 89–280 (E) and processed for fluorescence microscopy after 36 h. (F) Summary of Borealin deletion and endogenous chromosomal passenger localization in mitosis. Cen, centromere; mdz, anaphase spindle midzone. (G–J) Effect of Borealin deletion expression on the localization of endogenous chromosomal passengers at prometaphase/metaphase. Transfected cells were stained for Survivin (G and I) and TD-60 (H and J) 36 h after transfection. Bars, 5 μm.
All deletion mutants (GFP-Borealin1–141, Borealin142–280-GFP, GFP-Borealin1–88, and Borealin89–280-GFP) were nuclear in interphase (Fig. 5, B–E), confirming that the predicted nuclear localization signals, one in each half of the protein, are functional in vivo. The nucleolar localization of these GFP fusion proteins is probably an artifact, as both R1647 and another polyclonal antibody against Borealin (not depicted) fail to stain nucleoli (Fig. S1 A).
None of the deletion mutants localized to centromeres. Therefore, the NH2-terminal 88 amino acids are necessary, but not sufficient, for centromere targeting (Fig. 5, D and E). GFP-Borealin1–141 transferred to the spindle midzone normally, whereas the more extensive deletion GFP-Borealin1–88 did not (Fig. 5, B and D). Both Borealin142–280-GFP and Borealin89–280-GFP failed to localize to the spindle midzone (Fig. 5, C and E).
INCENP, Survivin, Aurora B, and TD-60 were delocalized from centromeres in cells expressing GFP-Borealin1–141 (Fig. 5, G and H) and GFP-Borealin1–88 (not depicted), but not in cells expressing Borealin142–280-GFP (Fig. 5, I and J) or Borealin89–280-GFP (not depicted). Interestingly, GFP-Borealin1–141, although interfering with centromere targeting, did not prevent the other chromosomal passengers from accumulating at the spindle midzone. The chromosomal passengers also transferred normally to the spindle midzone in cells expressing Borealin142–280-GFP and Borealin89–280-GFP. In cells transfected with GFP-Borealin1–88, the few anaphase cells found expressed very low amounts of the deletion construct, suggesting that this protein is toxic. The chromosomal passengers in these cells localized to the midzone, albeit at reduced levels (unpublished data).
These observations suggest that the NH2-terminal half of Borealin, but not the COOH-terminal half, interacts with chromosomal passengers in vivo and can perturb their targeting to centromeres but not to the spindle midzone.
Depletion of Borealin by RNA interference causes spindle abnormalities and failure of cytokinesis
To examine the contribution of Borealin to mitotic regulation, we depleted the protein by RNA interference in HeLa cells. Cells were synchronized in S-phase, released, and subsequently analyzed upon entry into the first round of mitosis after exposure to siRNA. Immunoblotting showed that Borealin was significantly depleted in cells treated with Borealin siRNA (Fig. 6 A). Levels of Survivin and phosphorylated INCENP also fell, but no significant change in Aurora B, Aurora A, TD-60, or histone H3 phosphorylation at serine10 was observed. Indirect immunofluorescence showed that >95% of cells exposed to Borealin siRNA had no detectable Borealin in mitosis. INCENP, Survivin, Aurora B, and TD-60 were all delocalized in Borealin-depleted cells (Fig. 6, D–G). As observed for other chromosomal passengers (Adams et al., 2001; Carvalho et al., 2003), depletion of Borealin caused cells to accumulate in prometaphase with a corresponding decrease in later mitotic stages (Fig. 6 B).
Figure 6.
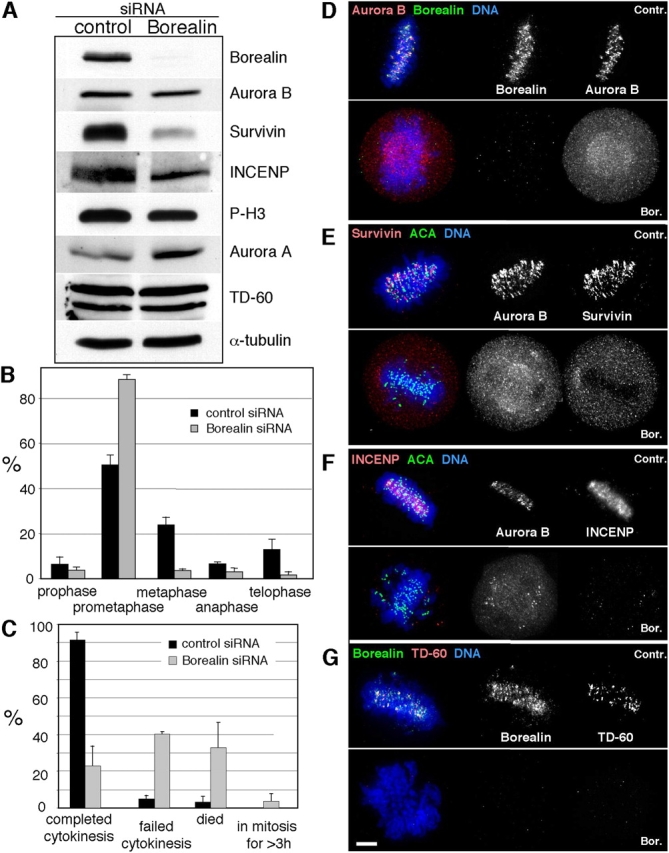
Depletion of Borealin by RNA interference disrupts mitotic progression. (A) Immunoblot showing substantial depletion of Borealin in HeLa cells exposed to Borealin siRNA and effect on the levels of other selected proteins. (B) Distribution of mitotic stages showing an accumulation in prometaphase of cells exposed to Borealin siRNA (n = 3; total number of cells scored: control siRNA, 361; Borealin siRNA, 404). (C) Quantitation of time-lapse experiments examining the effects of Borealin depletion on mitotic progression. Low magnification images of synchronized cells were taken at 1-h intervals 6 h after the release from an S-phase block, and individual cells were categorized according to their fate during the course of the experiment (n = 3; total number of cells scored: control, 158; siRNA, 204). Aurora B (D), Survivin (E), INCENP (F), and TD-60 (G) are mislocalized in Borealin-depleted cells. Bar, 5 μm.
To analyze the effect of Borealin depletion on mitotic progression, cells were grown on grid coverslips. Starting 6 h after their release from the S-phase block, phase-contrast images of several groups of cells were taken at 1-h intervals for a total of 7 h. On coverslips treated with control siRNA, 92% of cells went through mitosis and completed cytokinesis successfully (Fig. 6 C; representative cells shown in Fig. S2 and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200404001/DC1). In contrast, only 23% of cells treated with Borealin siRNA completed cytokinesis, whereas 41% failed cytokinesis, 33% underwent apoptosis, and 3% stayed in mitosis longer than 3 h. The average duration of mitosis was 1.3 ± 0.2 h for control cells and 2.6 ± 0.1 h for cells treated with Borealin siRNA (n = 3).
Closer examination of mitotic phenotypes in Borealin-depleted cells revealed defective interactions between kinetochores and the mitotic spindle. Among the many misaligned chromosomes observed (Fig. 7, B–B”), we noted both syntelic attachments (both kinetochores attached to the same pole; Fig. 7, B” and D) as well as merotelic attachments (one kinetochore attached to both poles; Fig. 7, C–C”). Merotelic attachments cause kinetochores to lag in the spindle midzone during anaphase, and 21 of 21 anaphases observed after Borealin RNAi had such lagging kinetochores (Fig. 7 E).
Figure 7.
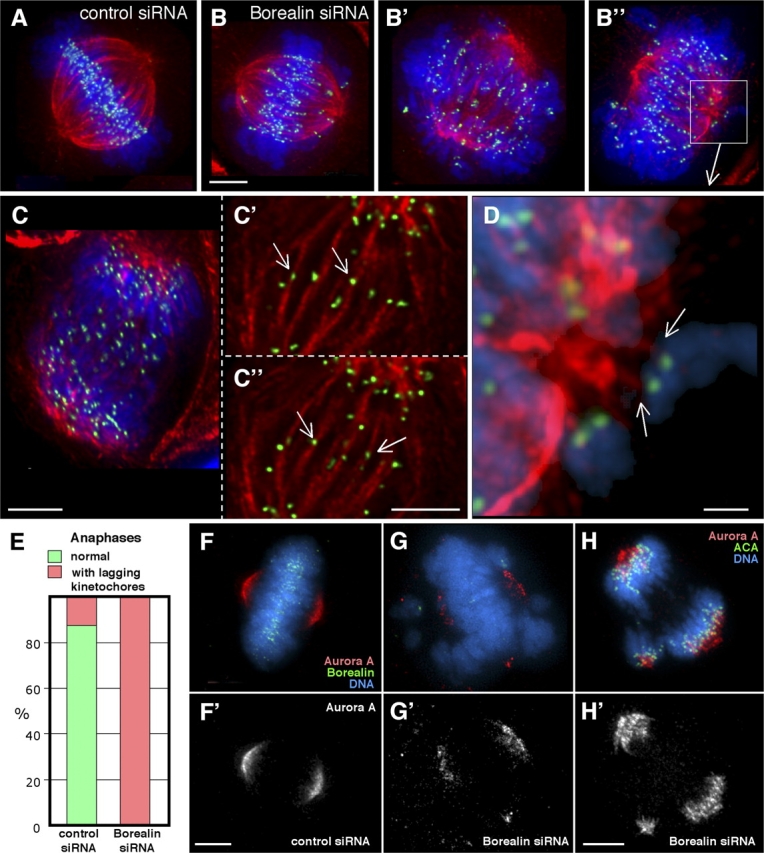
Borealin depletion promotes kinetochore–spindle misattachments and the formation of ectopic spindle poles. (A–D) Mitotic cells exposed to control siRNA with normal bipolar attachment of kinetochores to the spindle (A), and cells treated with Borealin siRNA (B–D) showing syntelic (B and D) and merotelic (C–C”) attachments. (E) Quantitation of anaphases with kinetochores lagging in the spindle midzone (number of cells scored: control siRNA, 41; Borealin siRNA, 21). (F–H) Aurora A staining of cells exposed to Borealin (G and H) or control (F) siRNAs. Bars: (all except D) 5 μm; (D) 1 μm.
In addition to kinetochore misattachments, we also observed spindle abnormalities in Borealin-depleted cells. In addition to a main bipolar spindle, 40% of mitotic cells exhibited ectopic asters containing Aurora A kinase and γ-tubulin (Fig. 7, G and H; and Fig. 8, B–D). Importantly, these extra poles did not represent centrosome amplification resulting from prior failed cytokinesis, as the images shown were collected in the first mitosis following release from the S-phase block. They were also unlikely to be due to the S-phase block itself, as they were only rarely observed in cells transfected with the control oligo (Fig. 8 D).
Figure 8.
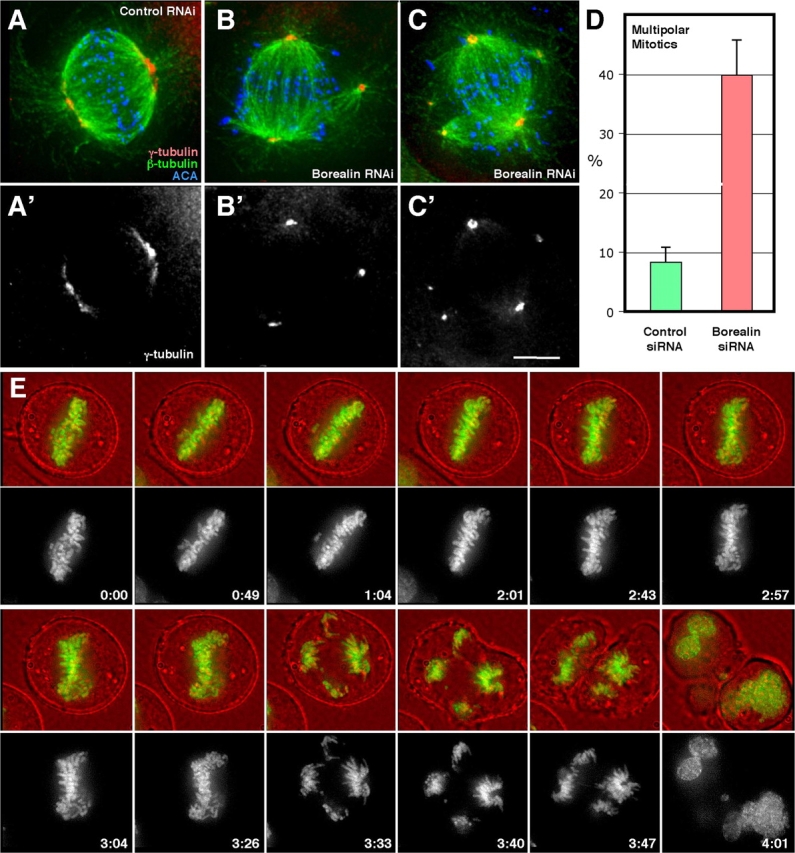
Live imaging of Borealin-depleted cells reveals severe defects in the partitioning of chromosomes in anaphase. (A–C) β- and γ-tubulin costaining of Borealin-transfected cells (B and C) and a control cell (A). (D) Quantification of multipolar mitotic cells (n = 3; total number of cells scored: control siRNA, 138; Borealin siRNA, 144). (E) Selected frames of a representative live cell imaging experiment performed on mitotic HeLa cells expressing histone H2B-GFP treated with Borealin siRNA. Bar, 5 μm.
To investigate the effects of these spindle abnormalities on mitotic chromosome dynamics, we examined cells treated with Borealin siRNA by live cell imaging. A total of 14 HeLa cells expressing histone H2B-GFP were individually followed in mitosis after exposure to siRNA by capturing a stack of DIC and FITC images every 7 min. None of these cells completed mitosis normally.
The most common phenotype, observed in 8 of 14 cells, was extremely unusual. These cells progressed through the early stages of mitosis normally and ultimately formed an apparently normal metaphase plate (Fig. 8 E, frame 2:01). The plate often appeared to become frayed at either end (Fig. 8 E, frame 3:26). Eventually, when the cells entered anaphase, the sister chromatids abruptly segregated from the apparently bipolar metaphase plate in several differing directions toward what appeared to be multiple spindle poles (Fig. 8 E, frame 3:33; and Fig. S4, A, C, and D, available at http://www.jcb.org/cgi/content/full/jcb.200404001/DC1). Other cells had lagging chromosomes in anaphase and clearly failed cytokinesis (Fig. S4 B). None of the seven control cells we followed exhibited any of these defects (unpublished data).
Thus, the chromosomal passenger complex containing Borealin has an essential role in maintaining the bipolar configuration of the mitotic spindle.
Borealin is phosphorylated by Aurora B in vitro
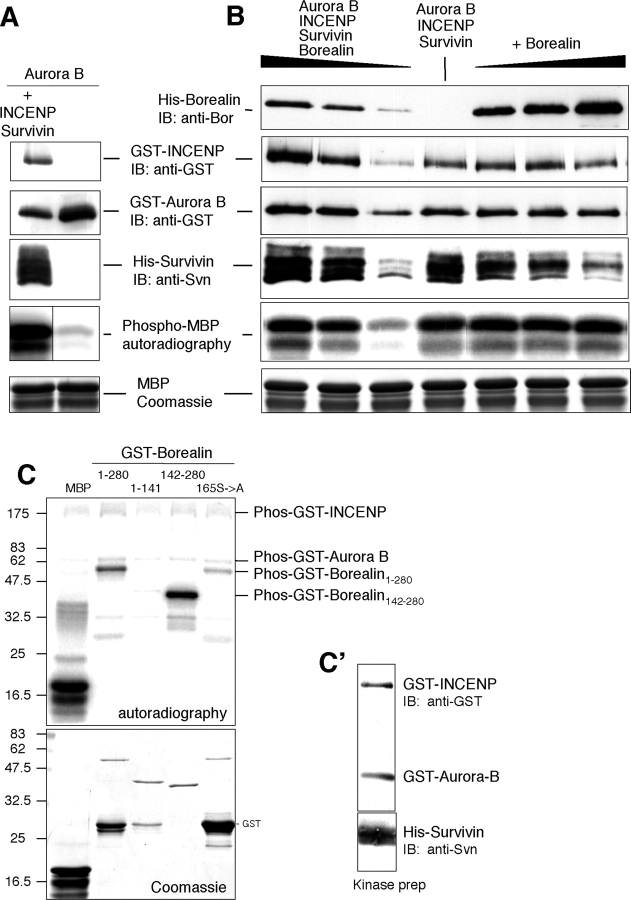
Aurora B kinase activity is stimulated by INCENP binding (Kang et al., 2001; Bishop and Schumacher, 2002; Honda et al., 2003). Therefore, we asked whether or not Borealin had any effect on Aurora B kinase activity in the presence of INCENP and Survivin using myelin basic protein as substrate. We expressed recombinant proteins containing either an NH2-terminal GST (Aurora B and INCENP) or His (Survivin and Borealin) tag in insect cells coinfected with the corresponding baculoviral constructs. As expected, Aurora B kinase activity was greatly increased when it was copurified from insect cells with INCENP and Survivin (using glutathione sepharose beads) compared with Aurora B on its own (Fig. 9 A). We then copurified Aurora B/INCENP/Survivin/Borealin from insect cells and compared the resulting kinase activity with that exhibited by Aurora B in the presence of INCENP and Survivin. Coexpressing Borealin with the other subunits did not alter Aurora B kinase activity appreciably, nor did the addition of separately purified Borealin to Aurora B/INCENP/Survivin (Fig. 9 B).
Figure 9.
Borealin is a substrate for Aurora B kinase in vitro. (A) Kinase activity toward MBP of GST-Aurora B/GST-INCENP/His-Survivin copurified from insect cells using glutathione sepharose beads compared with GST-Aurora B. Black line indicates that intervening lanes have been spliced out. (B) Kinase activity of GST-Aurora B/GST-INCENP/His-Survivin with or without His-Borealin, which was either copurified with the other subunits (left) or added separately in increasing amounts to GST-Aurora B/GST-INCENP/His-Survivin (right). (C) Phosphorylation of GST-Borealin1–280 and GST-Borealin142–280, but not GST-Borealin1–141, by Aurora B. (C') The input of GST-Aurora B/GST-INCENP/His-Survivin was checked by immunoblotting.
When we added bacterially expressed Borealin to Aurora B/INCENP/Survivin, the full-length protein GST-Borealin1–280 was readily phosphorylated (Fig. 9 C). GST-Borealin142–280 was also phosphorylated, but GST-Borealin1–141 was not (Fig. 9 C). A serine to alanine mutation at one predicted phosphorylation site (165 aa) resulted in a reduction, but not the abolition, of phosphorylation, suggesting that Borealin is phosphorylated at more than one site.
Discussion
Borealin is an essential subunit of the chromosomal passenger holocomplex in mitosis
Borealin is a novel 31-kD chromosomal passenger protein with homologues in all vertebrates examined, as well as more distant relatives in mosquito, D. melanogaster, and, possibly, C. elegans.
C. elegans CSC-1 was identified in a genetic screen for mutations affecting chromosome segregation and cytokinesis, and subsequently found to associate with the chromosomal passenger complex (Romano et al., 2003). Similarity between the vertebrate Borealins and CSC-1 is confined to short domains near the NH2 and COOH termini, and the Borealin sequence does not have the prominent imperfect repeat found in CSC-1. Furthermore, the net charge of the two proteins is very different (pI 9.9 for Borealin compared with pI 5.1 for CSC-1), and Borealin binding to Survivin does not require Zn2+ (unpublished data). Thus, it is not clear whether CSC-1 is a true orthologue of Borealin.
Like Aurora B, INCENP, and Survivin (Li et al., 1998; Honda et al., 2003), Borealin is cell-cycle regulated. This confirms the findings of a previous study, which identified eight human transcripts, including that for Borealin, on the basis of their coexpression with known cell cycle genes including CDC2, MCAK, and topoisomerase II (Walker, 2001). Borealin was also one of 50 ESTs identified in a genomic screen for mRNAs expressed in G2/M similar to Aurora A and B (Tien et al., 2004).
Borealin is a novel member of the chromosomal passenger holocomplex, together with Aurora B, INCENP, and Survivin (Adams et al., 2000; Kaitna et al., 2000; Kang et al., 2001; Bolton et al., 2002; Cheeseman et al., 2002; Leverson et al., 2002). Based on our in vitro binding data, Survivin and INCENP are likely to be the major binding partners of Borealin within the complex. Borealin associates with itself in vitro and may be a multimer in the complex. Importantly, almost all of the Survivin is complexed with Borealin in colcemid-arrested cells.
Our data suggest that there are multiple chromosomal passenger complexes during mitosis and that these contain differing stoichiometries of Aurora B, INCENP, Survivin, and Borealin. For example, in addition to a holocomplex containing all four passengers, we obtained evidence for an Aurora B/INCENP subcomplex that does not contain Survivin or Borealin. This subcomplex may be responsible for serine10 phosphorylation of histone H3, as this modification was not significantly decreased following RNAi for Borealin or Survivin (unpublished data). In contrast, the holocomplex is required for the correction of kinetochore attachment errors and for bipolar spindle stability. We believe that complexes containing chromosomal passengers might be highly dynamic, and their composition may vary at differing times and intracellular locations during mitosis.
Borealin may target the passenger holocomplex to centromeres
The dramatic movements of the chromosomal passengers during mitosis are thought to reflect the spatio-temporal regulation of Aurora B kinase activity throughout the mitotic cell (Carmena and Earnshaw, 2003), and the underlying mechanisms are therefore of considerable significance. We observed that when the NH2-terminal half of Borealin is overexpressed in HeLa cells, the endogenous chromosomal passengers are unable to accumulate at centromeres, whereas spindle midzone targeting is unaffected. This effect is unlikely to be due to a disruption of kinase activity of the complex because the ability of INCENP to stimulate Aurora B kinase activity in vitro is unaffected by the presence of excess NH2-terminal Borealin (unpublished data). The selectivity with which the mutant interferes with the localization of the endogenous chromosomal passengers is consistent with a specific role for Borealin in centromere targeting and implies that centromere and spindle midzone targeting may involve distinct mechanisms.
We have used chromosomal passenger complexes copurified from baculovirus-infected Sf-9 cells to ask if the presence of Borealin has a stimulatory or inhibitory effect on the activity of Aurora B kinase. Like INCENP, Borealin is phosphorylated in its COOH-terminal domain by Aurora B in vitro. When comparable amounts of Aurora B, INCENP, and Survivin were used in the assays, the presence of Borealin did not result in a detectable change of kinase activity. Therefore, the major role of Borealin is unlikely to involve direct modulation of Aurora B kinase activity. However, it is still conceivable that Borealin plays a more subtle role, as we did observe a substantial decrease in the levels of a mitosis-specific slower migrating form of INCENP in Borealin-depleted cells.
Borealin is required for kinetochore error correction and stability of the bipolar mitotic spindle
Aurora B has a major role in the correction of kinetochore orientation defects in mitosis, and particularly in the resolution of syntelic attachments (Tanaka et al., 2002; Hauf et al., 2003; Lampson et al., 2004). Therefore, it was not surprising that syntelic chromosomes were observed in cells depleted of Borealin. More surprising was the marked increase in chromosomes exhibiting merotelic attachments, which are not thought to be readily detected by the mitotic checkpoint and have been proposed to be a major source of human aneuploidy (Cimini et al., 2001). These results imply that the chromosomal passenger complex has a significant role in the prevention and/or resolution of merotelic attachments and strengthen the notion of the chromosomal passengers as master overseers of kinetochore orientation during mitosis. The underlying mechanisms remain to be determined, but regulation of factors that influence microtubule behavior such as the KinI kinesin MCAK (Andrews et al., 2004; Kline-Smith et al., 2004; Lan et al., 2004) or its regulators (Ohi et al., 2003) are strong possibilities.
The roles of chromosomal passengers in kinetochore function and cytokinesis are widely documented, but less is known about their role in spindle structure and stability. Here we have shown by live imaging that depletion of Borealin results in a characteristic phenotype in which cells align their chromosomes on what appears to be a nearly normal metaphase plate, but then abruptly undergo multipolar anaphases. In fixed images, small ectopic poles can be seen beside otherwise normal appearing bipolar spindles. These poles, which bind both Aurora A and γ-tubulin, are associated with microtubules, yet the live imaging data suggest that they do not orient significant numbers of chromosomes during prometaphase, as near normal metaphase chromosome alignment is observed. Instead, ectopic asters appear to interact with the chromosomes only after a nearly normal bipolar alignment has been achieved, so that at anaphase onset the chromosomes abruptly leave the metaphase plate in multiple directions.
TD-60: a link between Aurora B and small GTPases?
Our data strongly suggest that there is an intimate functional link between TD-60 and the chromosomal passenger complex. TD-60 colocalizes with the chromosomal passengers (Martineau-Thuillier et al., 1998), and in a recent study, depletion of Aurora B by RNAi was shown to perturb TD-60 localization in cells (Mollinari et al., 2003). We find that whenever passenger localization is perturbed, either by transient expression of dominant-negative INCENP or Borealin mutants or by depletion of Borealin, the localization of TD-60 shows an identical perturbation. The nature of the functional link remains to be determined and may not involve direct binding of TD-60 to the complex, as we have been unable to demonstrate a physical association between TD-60 and the other chromosomal passengers by immunoprecipitation.
TD-60 is structurally homologous to RCC1, a guanosine nucleotide exchange factor, and has been shown to bind Rac1 (Mollinari et al., 2003). Therefore, the available data suggest a role for the small GTPases as either regulators or effectors of Aurora B kinase.
Conclusions
The present study has revealed new levels of complexity and regulation of the chromosomal passengers. The discovery of Borealin has raised the number of components of the vertebrate chromosomal passenger complex to four, and the demonstration of close functional links with TD-60 has strengthened the possibility of regulation of Aurora B kinase by small GTPases. Furthermore, our data suggest that there are at least two chromosomal passenger complexes in mitotic cells. The chromosomal passenger holocomplex containing Borealin is required for a range of kinetochore functions, including the resolution of merotelic attachments. Borealin is also required for the stability of the bipolar spindle during mitosis. Thus, the chromosomal passengers are now firmly established as master regulators of mitotic events.
Materials and methods
Cloning and expression of recombinant proteins
The cDNAs and baculoviral constructs for Aurora B, Survivin, and INCENP have been described previously (Wheatley et al., 2001a; Honda et al., 2003). The cDNA of Borealin and TD-60 were obtained by RT-PCR using total RNA prepared from HeLa S3 cells. The PCR product was introduced into the appropriate vectors and fully sequenced. Recombinant baculovirus-expressing Borealin was generated using the pFastBacHTA vector and the Bac-to-Bac Expression System (Invitrogen). Insect Sf-9 cells were infected with appropriate combinations of the constructs, and complexes containing GST-Aurora B, GST-INCENP, His-Survivin, and His-Borealin were purified on glutathione sepharose beads (Amersham Biosciences) as described previously (Honda et al., 2003). Other recombinant Borealin with an NH2-terminal GST or His tag was expressed overnight at 18°C in Escherichia coli BL21 cells.
In vitro binding assays
Full-length proteins labeled with [35S]methionine were generated from cDNA cloned into pBluescript (Borealin, INCENP, Aurora B, and TD-60) or pcDNA3.1 (Survivin) using a reticulocyte lysate coupled transcription/translation system (Promega). For each binding reaction, 10 μl of transcription/translation mix were added to 90 μl binding buffer (PBS, 5 mM EGTA, 0.1% Triton X-100, 0.5 mM PMSF, and 1 μg/ml CLAP [chymostatin, leupeptin, antipain, and pepstatin A]) containing either GST-Borealin or GST alone prebound to glutathione sepharose beads. Samples were incubated on a rotating wheel for 1 h at 4°C. The supernatant was precipitated with TCA and the beads were washed three times with binding buffer, once with binding buffer without Triton X-100, and then boiled in sample buffer. After SDS-PAGE, proteins labeled with [35S]methionine were detected using a phosphorimager (Storm 860) with ImageQuant software (Amersham Biosciences).
Immunoprecipitations
HeLa cells were harvested after a 20-h colcemid block by mitotic shake-off and lysed in IP buffer (50 mM Tris-HCl, pH 8.0, 0.4 M NaCl, 0.5% NP-40, 0.1% deoxycholate, 30 μg/ml RNase A, 80 U/ml micrococcal nuclease [a gift of J. Allan, University of Edinburgh, Edinburgh, UK], 1 mM PMSF, and 1 μg/ml CLAP) for 20 min on ice. The cleared extract was incubated with affinity-purified anti-Borealin antibody R1647 or preimmune serum R1647 coupled to protein A beads (Dynal) for 5 h at 4°C on a rotating wheel. The beads were washed twice with lP buffer without RNase and micrococcal nuclease and once with 10 mM Tris-HCl pH 7.4, 150 mM NaCl. Supernatant and beads were processed for SDS-PAGE and the proteins were transferred to nitrocellulose membranes for immunoblotting. Immunoprecipitation of Aurora B was carried out under the same conditions using anti–Aurora B antibody (rabbit; Abcam).
Indirect immunofluorescence microscopy and immunoblotting
Indirect immunofluorescence was performed as described previously (Carvalho et al., 2003) using antibodies against Aurora B (mouse; Translab), Survivin (rabbit; Novus), INCENP (rabbit 1186), α-tubulin (mouse; Sigma-Aldrich), β-tubulin (Fant and Merdes, 2002), γ-tubulin (mouse; Sigma-Aldrich), TD-60 (human; a gift of R.L. Margolis, CEA-CNRS, Grenoble, France), Borealin (affinity-purified rabbit 1647), Aurora A (a gift of C. Prigent, Universite de Rennes, Rennes, France), CENP-C (rabbit 558), and appropriate secondary antibodies coupled to FITC, Texas red, or Cy5 (goat; Jackson ImmunoResearch Laboratories). DNA was visualized with 0.5 μg/ml DAPI. Image stacks were captured on a microscope (model IX-70; Olympus) controlled by Delta Vision SoftWorx (Applied Precision) using a 100 or 40× objective. After deconvolution, image stacks were quick-projected and saved as TIFF files.
Immunoblotting was performed as described previously (Carvalho et al., 2003) using antibodies against Aurora B (rabbit; Abcam), TD-60 (affinity-purified rabbit R2316), phospho-H3 (rabbit; Upstate Biotechnology), or others as described for indirect immunofluorescence.
Antibody production
Rabbit polyclonal antibody R1647 was generated against bacterially expressed human His-Borealin by Diagnostics Scotland and affinity-purified against GST-Borealin using standard methods. A polyclonal antibody (R2316) against a COOH-terminal peptide (KEKIKKLPEYNPRT) of human TD-60 was raised in rabbit by Genosphere. The serum was affinity purified against the peptide using the SulfoLink coupling gel according to the manufacturer's instructions (Pierce Chemical Co.).
RNA interference
HeLa cells (ACC57 and DSMZ) at 50% confluency were treated with 2 mM thymidine for 16 h and released into fresh medium without antibiotics for 8 h. Cells were transfected with control or Borealin siRNAs as described previously (Carvalho et al., 2003) 4 h into the release period. A second thymidine block was then applied for 16 h in the presence of the siRNAs. Cells were released into fresh medium and analyzed upon entry into mitosis by immunoblotting or indirect immunofluorescence.
For movies, HeLa cells stably expressing histone H2B-GFP were treated as described in the preceding paragraph and released into fresh medium without phenol red (GIBCO BRL). After 8 h, the medium was supplemented with 10 mM Hepes, pH 7.5, and the coverslips were transferred to a FCS2 chamber (Bioptechs) and kept at 37°C. DIC and FITC three-dimensional image data sets were collected every 7 min as described for indirect immunofluorescence.
Online supplemental material
Fig. S1: Aurora B and Borealin colocalize during mitosis. Fig. S2: examples of cell fates during the time-lapse experiment quantitated in Fig. 6 C. Fig. S3: multinucleate cells observed after depletion of Borealin by RNAi. Fig. S4: selected frames of representative movies of Borealin siRNA-transfected mitotic cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200404001/DC1.
Acknowledgments
We thank Claude Prigent, Bob Margolis, Xavier Fant, and Andreas Merdes for gifts of sera, and Jim Allan for micrococcal nuclease. We also particularly thank Sue Biggins for examining the effects of Borealin expression in Saccharomyces cerevisiae.
R. Gassmann is funded by a studentship from the Darwin Trust of Edinburgh. Work in the W.C. Earnshaw laboratory is funded by The Wellcome Trust, of which W.C. Earnshaw is a Principal Research Fellow. R. Honda acknowledges receipt of a European Molecular Biology Organization long-term fellowship, and both E.A. Nigg and R. Honda were supported by the Max-Planck-Society.
Note added in proof. After this paper was accepted, we learned that the Borealin family proteins had been independently discovered in a study of chromosome-binding proteins in Xenopus extracts. Xenopus Borealin-2 (called Dasra A in that study) was shown to be another component of the chromosomal passenger complex (Sampath, S.C., R. Ohi, O. Leismann, A. Salic, A. Pozniakovsk, and H. Funabiki. 2004. Cell. In press).
A.J. Henzing's present address is Scottish Instrumentation and Resource Centre for Advanced Mass Spectrometry, School of Chemistry, University of Edinburgh, Kings Buildings, West Mains Rd., Edinburgh EH9 3JR, Scotland, UK.
R. Honda's present address is Laboratory of Molecular Biophysics, University of Oxford, South Parks Rd., Oxford OX1 3QU, England, UK.
References
- Adams, R.R., S.P. Wheatley, A.M. Gouldsworthy, S.E. Kandels-Lewis, M. Carmena, C. Smythe, D.L. Gerloff, and W.C. Earnshaw. 2000. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10:1075–1078. [DOI] [PubMed] [Google Scholar]
- Adams, R.R., H. Maiato, W.C. Earnshaw, and M. Carmena. 2001. Essential roles of Drosophila inner centromere protein (INCENP) and Aurora-B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153:865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen, P.R., D.K. Palmer, M.H. Wener, and R.L. Margolis. 1991. Telophase disk: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J. Cell Sci. 99:523–534. [DOI] [PubMed] [Google Scholar]
- Andrews, P.D., E. Knatko, W.J. Moore, and J.R. Swedlow. 2003. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15:672–683. [DOI] [PubMed] [Google Scholar]
- Andrews, P.D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J.R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 6:253–268. [DOI] [PubMed] [Google Scholar]
- Biggins, S., and A.W. Murray. 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15:3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, J.R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, et al. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, J.D., and J.M. Schumacher. 2002. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J. Biol. Chem. 277:27577–27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, M.A., W. Lan, S.E. Powers, M.L. McCleland, J. Kuang, and P.T. Stukenberg. 2002. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell. 13:3064–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodskii, L.I., V.V. Ivanov, I.L. Kalaidzidis, A.M. Leontovich, V.K. Nikolaev, S.I. Feranchuk, and V.A. Drachev. 1995. GeneBee-NET: An Internet based server for biopolymer structure analysis. Biokhimiia. 60:1221–1230. [PubMed] [Google Scholar]
- Carmena, M., and W.C. Earnshaw. 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4:842–854. [DOI] [PubMed] [Google Scholar]
- Carvalho, A., M. Carmena, C. Sambade, W.C. Earnshaw, and S.P. Wheatley. 2003. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J. Cell Sci. 116:2987–2998. [DOI] [PubMed] [Google Scholar]
- Cheeseman, I.M., S. Anderson, M. Jwa, E.M. Green, J.-s. Kang, J.R. Yates, C.S.M. Chan, D.G. Drubin, and G. Barnes. 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 111:163–172. [DOI] [PubMed] [Google Scholar]
- Cimini, D., B. Howell, P. Maddox, A. Khodjakov, F. Degrassi, and E.D. Salmon. 2001. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, C.A., M.M.S. Heck, and W.C. Earnshaw. 1987. The INCENP antigens: movement from the inner centromere to the midbody during mitosis. J. Cell Biol. 105:2053–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting bubR1, Mad2, and CENP-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W.C., and R.L. Bernat. 1990. Chromosomal passengers: Towards an integrated view of mitosis. Chromosoma (Berlin). 100:139–146. [DOI] [PubMed] [Google Scholar]
- Fant, X., and A. Merdes. 2002. The carboxy-terminus of protein 4.1r resembles Beta-tubulin. Cell Biol. Int. 26:371–377. [DOI] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila Aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginalski, K., A. Elofsson, D. Fischer, L. Rychlewski, O.N. Jensen, P. Mortensen, O. Vorm, and M. Mann. 2003. 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics. 19:1015–1018. [DOI] [PubMed] [Google Scholar]
- Hauf, S., R.W. Cole, S. LaTerra, C. Zimmer, G. Schnapp, R. Walter, A. Heckel, J. Van Meel, C.L. Rieder, and J.M. Peters. 2003. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, R., R. Korner, and E.A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 14:3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna, S., M. Mendoza, V. Jantsch-Plunger, and M. Glotzer. 2000. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10:1172–1181. [DOI] [PubMed] [Google Scholar]
- Kang, J., I.M. Cheeseman, G. Kallstrom, S. Velmurugan, G. Barnes, and C.S. Chan. 2001. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 155:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith, S.L., A. Khodjakov, P. Hergert, and C.E. Walczak. 2004. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 15:1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitoku, N., T. Sasayama, T. Marumoto, D. Zhang, S. Honda, O. Kobayashi, K. Hatakeyama, Y. Ushio, H. Saya, and T. Hirota. 2003. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev. Cell. 5:853–864. [DOI] [PubMed] [Google Scholar]
- Lampson, M.A., K. Renduchitala, A. Khodjakov, and T.M. Kapoor. 2004. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 6:232–237. [DOI] [PubMed] [Google Scholar]
- Lan, W., X. Zhang, S.L. Kline-Smith, S.E. Rosasco, G.A. Barrett-Wilt, J. Shabanowitz, D.F. Hunt, C.E. Walczak, and P.T. Stukenberg. 2004. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14:273–286. [DOI] [PubMed] [Google Scholar]
- Lens, S.M.A., R.M.F. Wolthuis, R. Klompmaker, J. Kauw, R. Agami, T. Brummelkamp, G. Kops, and R.H. Medema. 2003. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 22:2934–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson, J.D., H.K. Huang, S.L. Forsburg, and T. Hunter. 2002. The Schizosaccharomyces pombe aurora-related kinase Ark1 interacts with the inner centromere protein Pic1 and mediates chromosome segregation and cytokinesis. Mol. Biol. Cell. 13:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., and D.C. Altieri. 1999. Transcriptional analysis of human survivin gene expression. Biochem. J. 344:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., G. Ambrosini, E.Y. Chu, J. Plescia, S. Tognin, P.C. Marchisio, and D.C. Altieri. 1998. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 396:580–584. [DOI] [PubMed] [Google Scholar]
- Mackay, A.M., A. Ainsztein, D.M. Eckley, and W.C. Earnshaw. 1998. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 140:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau-Thuillier, S., P.R. Andreassen, and R.L. Margolis. 1998. Colocalization of TD-60 and INCENP throughout G2 and mitosis: evidence for their possible interaction in signalling cytokinesis. Chromosoma (Berlin). 107:461–470. [DOI] [PubMed] [Google Scholar]
- Mollinari, C., C. Reynaud, S. Martineau-Thuillier, S. Monier, S. Kieffer, J. Garin, P.R. Andreassen, A. Boulet, B. Goud, J.P. Kleman, and R.L. Margolis. 2003. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev. Cell. 5:295–307. [DOI] [PubMed] [Google Scholar]
- Ohi, R., M.L. Coughlin, W.S. Lane, and T.J. Mitchison. 2003. An inner centromere protein that stimulates the microtubule depolymerizing activity of a KinI kinesin. Dev. Cell. 5:309–321. [DOI] [PubMed] [Google Scholar]
- Pereira, G., and E. Schiebel. 2003. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 302:2120–2124. [DOI] [PubMed] [Google Scholar]
- Petersen, J., and I.M. Hagan. 2003. S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 13:590–597. [DOI] [PubMed] [Google Scholar]
- Romano, A., A. Guse, I. Krascenicova, H. Schnabel, R. Schnabel, and M. Glotzer. 2003. CSC-1: a subunit of the aurora b kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J. Cell Biol. 161:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, A.F., D.R. Hamill, J.C. Carter, J. Schumacher, and B. Bowerman. 2000. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 10:1162–1171. [DOI] [PubMed] [Google Scholar]
- Tanaka, T.U., N. Rachidi, C. Janke, G. Pereira, M. Galova, E. Schiebel, M.J. Stark, and K. Nasmyth. 2002. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 108:317–329. [DOI] [PubMed] [Google Scholar]
- Terada, Y., M. Tatsuka, F. Suzuki, Y. Yasuda, S. Fujita, and M. Otsu. 1998. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 17:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien, A.C., M.H. Lin, L.J. Su, Y.R. Hong, T.S. Cheng, Y.C. Lee, W.J. Lin, I.H. Still, and C.Y. Huang. 2004. Identification of the substrates and interaction proteins of aurora kinases from a protein-protein interaction model. Mol. Cell. Proteomics. 3:93–104. [DOI] [PubMed] [Google Scholar]
- Walker, M.G. 2001. Drug target discovery by gene expression analysis: cell cycle genes. Curr. Cancer Drug Targets. 1:73–83. [DOI] [PubMed] [Google Scholar]
- Wheatley, S.P., A. Carvalho, P. Vagnarelli, and W.C. Earnshaw. 2001. a. INCENP is required for proper targeting of survivin to the centromeres and the anaphase spindle during mitosis. Curr. Biol. 11:886–890. [DOI] [PubMed] [Google Scholar]
- Wheatley, S.P., S.E. Kandels-Lewis, R.R. Adams, A.M. Ainsztein, and W.C. Earnshaw. 2001. b. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp. Cell Res. 262:122–127. [DOI] [PubMed] [Google Scholar]