Abstract
Centrosomes consist of a pair of centrioles surrounded by an amorphous pericentriolar material (PCM). Proteins that contain a Pericentrin/AKAP450 centrosomal targeting (PACT) domain have been implicated in recruiting several proteins to the PCM. We show that the only PACT domain protein in Drosophila (the Drosophila pericentrin-like protein [D-PLP]) is associated with both the centrioles and the PCM, and is essential for the efficient centrosomal recruitment of all six PCM components that we tested. Surprisingly, however, all six PCM components are eventually recruited to centrosomes during mitosis in d-plp mutant cells, and mitosis is not dramatically perturbed. Although viable, d-plp mutant flies are severely uncoordinated, a phenotype usually associated with defects in mechanosensory neuron function. We show that the sensory cilia of these neurons are malformed and the neurons are nonfunctional in d-plp mutants. Moreover, the flagella in mutant sperm are nonmotile. Thus, D-PLP is essential for the formation of functional cilia and flagella in flies.
Keywords: PACT domain; centrosome; centriole; Drosophila; mitosis
Introduction
Centrosomes are the main microtubule-organizing centers in animal cells, and they consist of a pair of centrioles surrounded by an amorphous pericentriolar material (PCM; Kellogg et al., 1994; Rieder et al., 2001; Bornens, 2002). Proteins that contain a Pericentrin/AKAP450 centrosomal targeting (PACT) domain (Gillingham and Munro, 2000) have been implicated in recruiting several proteins to the centrosome. Pericentrin (also called Kendrin; Flory et al., 2000; Flory and Davis, 2003) is a component of the PCM, and anti-pericentrin antibodies disrupt meiotic and mitotic divisions when injected into frog embryos (Doxsey et al., 1994). The overexpression of Pericentrin in tissue culture cells also leads to mitotic spindle defects (Purohit et al., 1999; Pihan et al., 2001). Pericentrin forms a complex with the γ-tubulin ring complex, and both proteins form a unique structural lattice within the PCM (Dictenberg et al., 1998). Pericentrin also interacts with cytoplasmic dynein, and this interaction is thought to play a role in the recruitment of Pericentrin and γ-tubulin to centrosomes (Purohit et al., 1999; Young et al., 2000). Thus, it is widely believed that Pericentrin is essential for mitosis. In support of this possibility, SPC110, the only PACT domain protein in Saccharomyces cerevisiae, is an essential protein that tethers the microtubule-nucleating Tub4 (γ-tubulin in S. cerevisiae) complex to the inner side of the spindle pole body (Knop and Schiebel, 1997; Nguyen et al., 1998).
The A-kinase anchoring protein AKAP450 (also called CG-NAP; Takahashi et al., 1999) contains a PACT domain and recruits PKA and several other proteins to the centrosome (Keryer et al., 1993, 2003; Takahashi et al., 1999). Displacement of the endogenous AKAP450 from centrosomes in tissue culture cells (by overexpression of the AKAP450 PACT domain) leads to defects in cytokinesis, cell cycle progression, and centriole replication (Keryer et al., 2003). In these analyses, the displacement of AKAP450 from centrosomes did not disrupt the centrosomal localization of Pericentrin or γ-tubulin. However, there may be some functional redundancy between Pericentrin and AKAP450/CG-NAP, as Pericentrin can interact with PKA (Diviani et al., 2000), and AKAP450/CG-NAP can interact with components of the γ-tubulin ring complex (Takahashi et al., 2002).
In Drosophila, the predicted gene CG6735 encodes the only recognizable PACT domain protein (Gillingham and Munro, 2000), and we call this protein the Drosophila Pericentrin-like protein (D-PLP). We find that there are two distinct fractions of D-PLP associated with centrosomes—one that associates with centrioles and another that associates with the PCM. D-PLP is required for the efficient centrosomal recruitment of not just γ-tubulin, but of all six PCM components that we tested. Surprisingly, however, all of these PCM components can eventually be recruited to mitotic spindle poles in d-plp mutants, and mutants are viable and exhibit few (if any) mitotic defects. However, mutant flies are severely uncoordinated, a phenotype often associated with defects in mechanosensory neuron function. We show that the mechanosensory cilia in these neurons are abnormal in d-plp mutants, and the cells can no longer respond to external stimuli. Moreover, d-plp mutant sperm are nonmotile, suggesting that D-PLP is essential for the proper function of all cilia and flagella in flies.
Results
Characterization of the d-plp gene
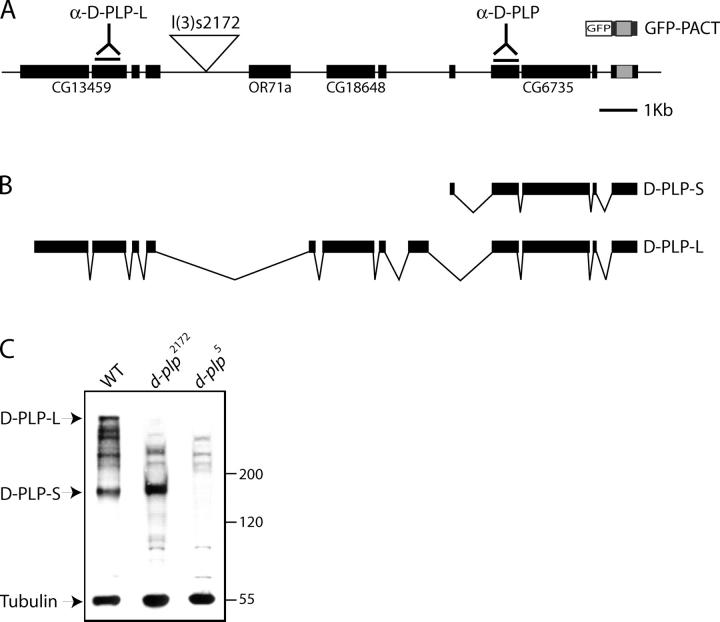
The predicted protein encoded by the CG6735 gene contains a COOH-terminal PACT domain (Fig. 1 A). It was shown previously that the COOH-terminal 226 amino acids of this protein fused to GFP (Fig. 1 A; GFP-PACT) localizes to centrosomes when ectopically expressed in human tissue culture cells (Gillingham and Munro, 2000). A full-length cDNA has been isolated for CG6735 (Fig. 1 B; D-PLP-S), and recently a longer cDNA has been isolated that incorporates two other predicted genes in this region (Fig. 1 B, D-PLP-L; Kawaguchi and Zheng, 2003). Thus, the d-plp gene encodes at least one large (D-PLP-L) and one small (D-PLP-S) form of D-PLP.
Figure 1.
Characterization of the d-plp gene. (A) A schematic representation of the d-plp genetic region, as originally assigned by the Berkeley Drosophila Genome Project. The coding regions used to generate the anti-D-PLP-L and anti-D-PLP antibodies are indicated, as is the position of the l(3)S2172 P-element insertion. The PACT domain is indicated by the gray box. (B) Structure of the two identified cDNAs encoded by d-plp. (C) A Western blot of extracts of WT, d-plp 2172, and d-plp 5 mutant larval brains probed with anti-D-PLP antibodies. The antibodies recognize a band of ∼400 kD (D-PLP-L) that is strongly reduced in both alleles, and a band of ∼180 kD (D-PLP-S) that is reduced in the d-plp 5 allele. These antibodies also recognize a series of bands of ∼200–350 kD. In the WT lane, some of these bands appear to be alternatively spliced versions or degradation products of D-PLP-L. In the d-plp 5 lane, the residual bands appear to be a nonspecific background, as they are present at an approximately equal intensity in all 18 d-plp mutant alleles (even those that lack detectable D-PLP-L and D-PLP-S [not depicted]; see below). Blots were probed with anti-tubulin antibodies as a loading control.
We raised antibodies against two regions of D-PLP (Fig. 1 A). Anti-D-PLP antibodies should recognize both D-PLP-S and D-PLP-L, whereas anti-D-PLP-L antibodies should recognize only D-PLP-L (Fig. 1, A and B). In Western blots of third instar larval brains, both antibodies recognized a cluster of 2–4 high mol wt bands in the ∼300–450-kD range, and the anti-D-PLP antibodies also recognized a band of ∼180 kD. These proteins appear to be products of the d-plp gene, as their abundance was altered in the brains of d-plp mutant larvae (Fig. 1 C; see below).
The D-PLP protein is associated with centrioles
Both anti-D-PLP and anti-D-PLP-L antibodies stained centrosomes as a very small dot that was located in the middle of the PCM (Fig. 2 A). The GFP-PACT fusion protein also localized to centrosomes as a very small dot that colocalized with the endogenous D-PLP (Fig. 2 B). This suggested that D-PLP and GFP-PACT were primarily associated with centrioles, rather than with the PCM.
Figure 2.
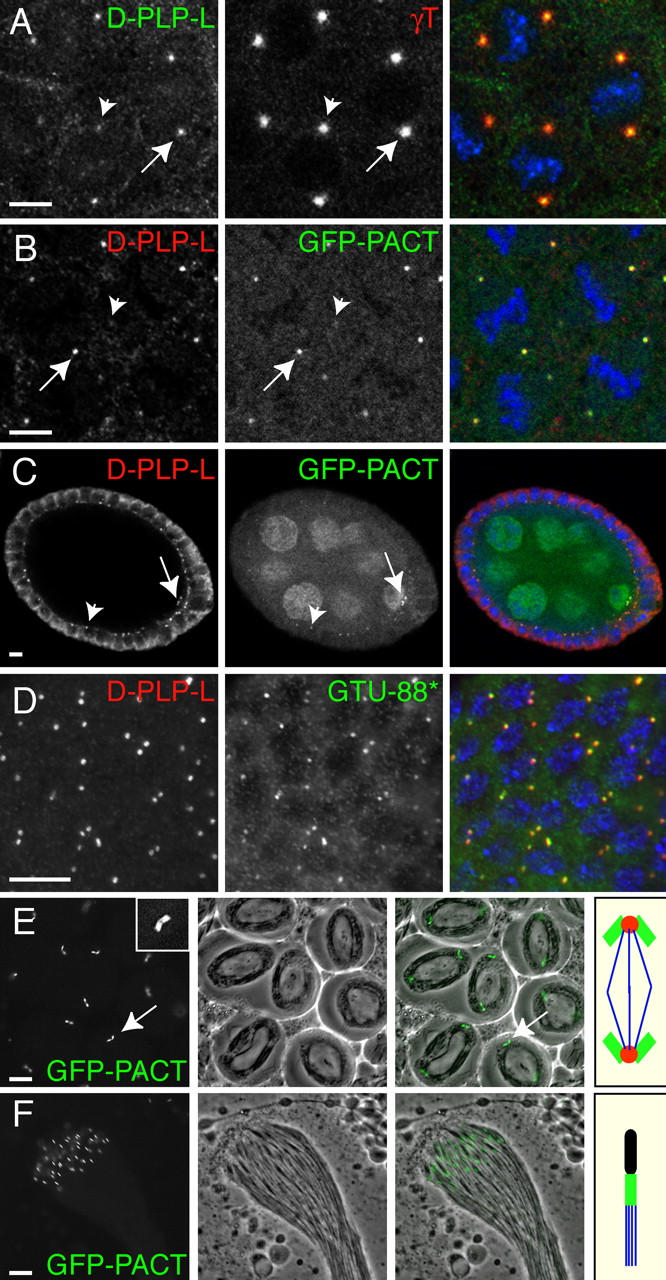
D-PLP and GFP-PACT are associated with centrioles. (A) In fixed WT embryos, anti-D-PLP-L antibodies stain centrosomes as a small dot that is located in the center of the PCM (stained here with anti-γ-tubulin antibodies). (B) GFP-PACT colocalizes with D-PLP in these small dots. Note that the intensity of the D-PLP and GFP-PACT staining varies from centrosome to centrosome (A and B, arrows and arrowheads). This is because the D-PLP and GFP-PACT dots are so small that they often cannot all be visualized in the same optical section. (C) In Drosophila oocytes, D-PLP and GFP-PACT colocalize in small dots that appear to be the centrioles (arrows; see text). Both markers also stain the centrioles in the follicle cells that surround the oocyte and nurse cells (arrowheads). GFP-PACT is also present in the oocyte and nurse cell cytoplasm and nuclei, presumably because this fusion protein is expressed at higher levels than the endogenous protein. (D) GTU88* and anti-D-PLP-L antibodies recognize the centrioles in interphase larval neuroblasts. In A–D, DNA is shown in blue in the merged images. (E) In living WT spermatocytes, GFP-PACT is concentrated in the large pair of orthogonally arranged centrioles at the poles of each meiosis I spindle (arrow; inset shows a 3×-magnified view of a centriole pair). The middle panel shows a phase-contrast image, and the right panel a merged image. The panel on the far right shows a schematic representation of the centrioles (green), PCM (red), and microtubules (blue) in a typical meiosis I spindle. (F) In a WT cyst of maturing sperm, GFP-PACT is concentrated in the centrioles/basal bodies located at the base of each sperm flagellum. The panel on the far right shows a schematic representation of the DNA (black), centriole/basal body (green), and microtubules (blue) in a single sperm. Bars, 10 μm.
We tested this possibility in three ways. First, we examined the localization of D-PLP and GFP-PACT in Drosophila oocytes. In fly oogenesis, a cystoblast undergoes four rounds of incomplete cell division to generate a cyst of 16 cells that remain interconnected by intercellular bridges. EM analyses have shown that the centrioles in the 16-cell cyst lack any associated PCM, and they all migrate into the developing oocyte where they become clustered at the posterior pole (Mahowald and Strassheim, 1970). Although several immunofluorescence analyses have failed to detect any PCM components associated with these centrioles (Theurkauf, 1994; Tavosanis et al., 1997; Wilson et al., 1997), a commercially available ascites fluid containing the anti-γ-tubulin mAb GTU88 has recently been shown to stain oocyte centrioles (Grieder et al., 2000; Bolivar et al., 2001). In our hands, a specific batch of this ascites fluid (that we call GTU88*) stained oocyte centrioles, but several other batches of GTU88 ascites fluid (from the same commercial supplier) did not, and several other anti-γ-tubulin antibodies also did not stain oocyte centrioles (unpublished data). Thus, GTU88* appears to contain a contaminating antibody that recognizes centrioles in oocytes (and in all other Drosophila tissues that we tested; see below). In our hands, oocyte centrioles were stained by GTU88*, by both D-PLP and D-PLP-L antibodies, and by GFP-PACT (Fig. 2 C), but they were not stained by antibodies raised against any other PCM markers we tested (γ-tubulin, CNN, D-TACC, Msps, CP190, or CP60; unpublished data).
Second, we examined the localization of D-PLP and GFP-PACT in Drosophila larval brain cells. All the known PCM markers we tested did not stain the centrosomes during interphase in these cells (unpublished data). However, the GTU88* antibody stained one or two dots (presumably the centrioles) in each cell during interphase, and these dots were also stained with D-PLP or D-PLP-L antibodies (Fig. 2 D) and by GFP-PACT (unpublished data). Interestingly, microtubules were not detectably concentrated around these centrioles during interphase (unpublished data), suggesting that interphase centrosomes do not organize microtubules in these cells.
Third, we examined the localization of D-PLP and GFP-PACT in spermatocytes. These cells have very large centrioles and only a small amount of PCM (Riparbelli et al., 2002). Spermatocyte centrioles were stained with GTU88* (see below), but surprisingly, they were not stained by D-PLP or D-PLP-L antibodies (unpublished data). We suspect that this is due to antibody penetration problems, as in living spermatocytes GFP-PACT strongly localized to the centrioles during meiosis (Fig. 2 E; Rebollo et al., 2004) and to the centrioles at the base of the mature sperm (Fig. 2 F). However, in fixed preparations of testes expressing GFP-PACT, anti-GFP antibodies did not stain the centrioles, even though the centriolar fluorescence of GFP-PACT was still detectable (unpublished data). Thus, GFP-PACT cannot be detected in spermatocyte centrioles with anti-GFP antibodies. This probably explains why anti-D-PLP antibodies cannot detect D-PLP in these unusually large centrioles. Together, these data suggest that D-PLP and GFP-PACT are associated with centrioles in Drosophila.
GFP-PACT is associated with centrioles and with the PCM
To test whether GFP-PACT is stably associated with centrioles, we performed a FRAP analysis in living embryos. We photobleached a small area of an embryo and monitored the recovery of fluorescence over time by time-lapse confocal microscopy (Fig. 3). In all 14 embryos we observed, some GFP-PACT fluorescence recovered rapidly (T1/2 ∼1–2 min) in a relatively broad area around the centrioles (Fig. 3 A; arrowheads). However, the very bright, dot-like fluorescence of GFP-PACT never recovered if the embryos remained in interphase (Fig. 3 A; 8/8 embryos observed in interphase for 8–30 min). However, the very bright, dot-like fluorescence of the centrioles invariably recovered as soon as the centrioles replicated (Fig. 3 B; 6/6 embryos replicated their centrioles within the 10–15-min observation period). Thus, we conclude that there are two populations of GFP-PACT associated with centrosomes: a fraction that is stably associated with centrioles, and a fraction that is associated with the PCM and is in rapid exchange with a cytoplasmic pool.
Figure 3.
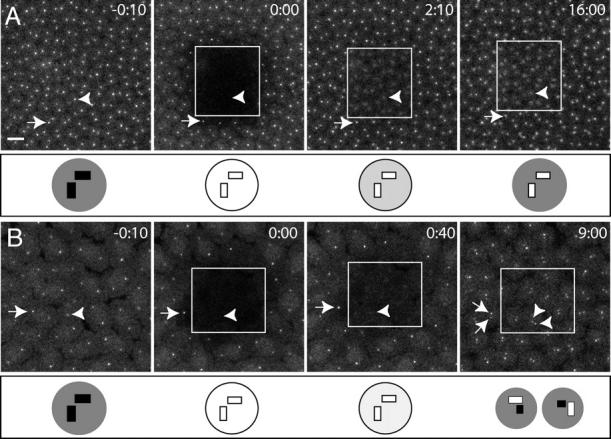
FRAP analysis of GFP-PACT dynamics in living embryos. A small area (white box) of an embryo expressing GFP-PACT was photobleached (at time 0:00), and the recovery of fluorescence was monitored by time-lapse confocal microscopy. Time (in minutes) is shown at the top of each panel. Arrows indicate the position of a centrosome outside of the bleached zone; arrowheads indicate the position of a centrosome within the bleached zone (or its approximate position when it could not be detected). The diagram below each panel shows a schematic interpretation of the fluorescent status of the centrioles and PCM within the bleached zone. (Note that although the centrioles are depicted here as pairs, these pairs cannot be resolved by the light microscope and so appear as a single dot in the embryo). Filled boxes or circles represent fluorescent centrioles or PCM, respectively; white boxes or circles represent bleached centrioles or PCM; circles filled with lighter shades of gray represent bleached PCM that has partially recovered its fluorescence. (A) An embryo that remained in interphase throughout the experiment. The position of the centrosomes can be seen within the bleached zone as an area of diffuse fluorescence (2:10 and 16:00), but no intense spot of centriole staining recovered in the bleached centrosomes. (B) An embryo that replicated its centrosomes during the experiment. The intense centriole staining in the bleached zone recovered as soon as the centrosomes duplicated (arrowheads at 9:00). Bar, 10 μm.
d-plp mutant flies are uncoordinated
To investigate the function of D-PLP, we searched the databases for mutations in the d-plp gene. The lethal line l(3)s2172 contains a P-element inserted within an intron of d-plp (Fig. 1 A). When homozygous, this mutant is lethal, but when transheterozygous with Df(3L)BrdR15, a deficiency that uncovers the d-plp gene, the mutant flies were viable. Thus, there is at least one lethal mutation on the l(3)s2172 chromosome that is not associated with the P-element. We used standard meiotic recombination to separate the P-insertion from the other lethal mutation. We refer to this “cleaned up” recombinant chromosome as d-plp 2172.
Flies that were homozygous for the d-plp 2172 chromosome were viable, but they exhibited a severe “uncoordinated” phenotype and died shortly after eclosion because they immediately got stuck in the food. In Western blots of homozygous third instar larval brain extracts, the levels of D-PLP-L were reduced in mutant brains by >95%, but D-PLP-S was present at normal levels (Fig. 1 C). Therefore, we performed an ethylmethane sulphate mutagenesis screen to generate new d-plp alleles. We screened ∼5,000 mutagenized chromosomes for lethality or for an uncoordinated phenotype when transheterozygous with d-plp 2172. We generated 17 new alleles of d-plp, all of which were viable but uncoordinated when transheterozygous with either d-plp 2172 or Df(3L)BrdR15. Western blotting revealed that all 17 lines had at least reduced levels of D-PLP-L, and five lines had undetectable levels of both D-PLP-L and D-PLP-S (unpublished data; Fig. 1 C). In immunofluorescence analyses of mutant larval brains, no D-PLP protein was detectable at the centrioles of these five mutant lines with either the D-PLP or D-PLP-L antibodies (unpublished data; see below). Thus, these five alleles appear to be protein nulls.
We analyzed the d-plp mutant phenotype in detail in both homozygous d-plp 2172 animals and in animals that were transheterozygous for d-plp 5 (a putative protein null) and the Df(3L)BrdR15 deficiency (for simplicity we refer to these flies as d-plp 5 mutants). We found that d-plp 2172 and d-plp 5 mutants had very similar phenotypes (suggesting that D-PLP-S is largely nonfunctional in the processes we describe here), and in the following figures, we often do not distinguish between them.
D-PLP is required for the efficient recruitment of proteins to the PCM
We analyzed the distribution of γ-tubulin in immunostained preparations of wild-type (WT) and d-plp mutant brains. As discussed above, there is very little (if any) PCM associated with the centrioles of these cells in interphase. However, as WT cells entered mitosis, the levels of γ-tubulin at the centrosome increased dramatically, and the γ-tubulin formed particles in the cytoplasm that were initially recruited to a relatively broad region around the D-PLP–stained centrioles (Fig. 4 A). In d-plp mutant cells, the centrioles were no longer detectable with anti-D-PLP-L antibodies, and the γ-tubulin particles were either weakly accumulated around the centrosomes (centrosome position was confirmed by following the localization of GFP-PACT in d-plp mutants; see below) or were dispersed throughout the cytoplasm (Fig. 4 A).
Figure 4.
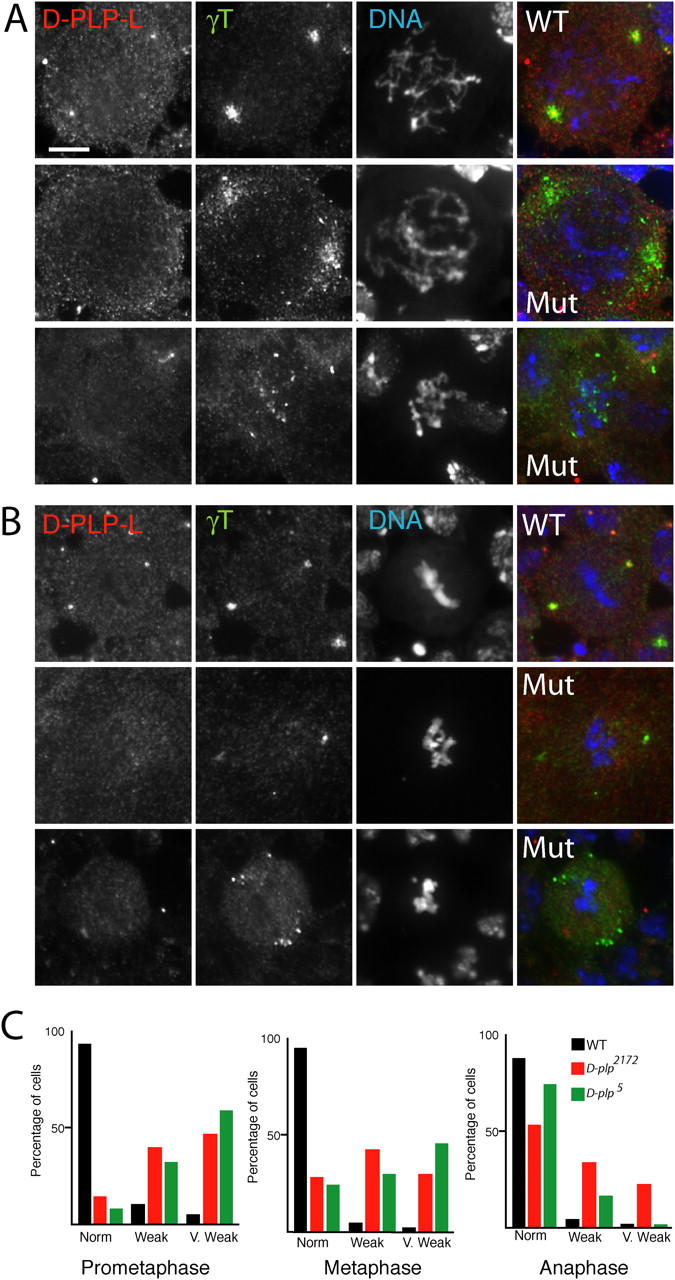
The mitotic recruitment of γ-tubulin to centrosomes is impaired in d-plp mutant cells. The distribution of D-PLP-L (left panels), γ-tubulin (middle panels), and DNA (right panels) is shown in WT and d-plp mutant larval brain cells in prometaphase (A) and metaphase (B). In WT prometaphase cells, γ-tubulin is recruited to a relatively broad area around the centrioles. In mutant prometaphase cells, D-PLP-L is no longer detectable at centrioles, and γ-tubulin is (1) often only weakly recruited to one or two diffuse areas (middle panels); or (2) remains scattered throughout the cell (bottom panels). In WT metaphase cells, γ-tubulin is recruited to a compact area around the centrioles. In mutant metaphase cells, the centrosomal recruitment of γ-tubulin is often weaker than normal (middle panels), and in some cells, particles of γ-tubulin remain scattered in the cytoplasm (bottom panels). Bar, 5 μm. (C) Quantitation of the γ-tubulin localization defects in WT and mutant brain cells. The graphs show the percentages of cells displaying normal, weak, or very weak centrosomal localization of γ-tubulin in WT (black), d-plp 2172 (red), or d-plp 5 (green) cells in prometaphase, metaphase, and anaphase (see Materials and methods for the criteria used for scoring each class). The total number of cells (WT/d-plp 2172/d-plp 5) counted from at least eight different brain squashes was: prometaphase, 51/110/43; metaphase, 139/267/132; anaphase, 40/61/33.
In WT cells, D-PLP remained tightly associated with centrioles from metaphase to telophase, and γ-tubulin also became tightly associated with the centrosomes during this period (Fig. 4 B). In d-plp mutant metaphase cells, γ-tubulin was often only weakly concentrated at centrosomes or was abnormally dispersed in the cytoplasm (Fig. 4 B). Quantification of these phenotypes (Fig. 4 C; see Materials and methods) revealed that the defect in γ-tubulin recruitment was similar in both d-plp 5 and d-plp 2172 mutants, suggesting that D-PLP-L plays the major role in recruiting γ-tubulin to centrosomes. Moreover, the recruitment of γ-tubulin to centrosomes was most aberrant in early mitosis and improved dramatically as cells progressed through cell division. This suggests that there is a D-PLP–independent mechanism that can eventually recruit γ-tubulin to the centrosomes/spindle poles during mitosis.
To test whether D-PLP was required to recruit any other proteins to the centrosome during mitosis, we stained WT and d-plp mutant cells with several other centrosomal markers. The centrosomal recruitment of all the proteins we tested (CNN, D-TACC, CP60; Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200402130/DC1) (Msps and CP190; unpublished data) was disrupted in d-plp 2172 and d-plp 5 mutant cells. Thus, in larval brain cells, D-PLP is required for the efficient recruitment of several proteins to the PCM.
Centriole replication appears to occur normally in d-plp mutants
As D-PLP is associated with centrioles, the failure to efficiently recruit PCM components in d-plp mutants could be due to a failure to properly form centrioles. To determine if centriole replication was disrupted in d-plp mutant cells, we examined the distribution of the centrioles using the GTU88* centriolar marker in d-plp 2172 and d-plp 5 mutant brains. The size and distribution of centrioles in WT and mutant interphase brain cells was indistinguishable, and we obtained a similar result using GFP-PACT as a centriolar marker in living preparations of WT or mutant brains (unpublished data; note that the expression of GFP-PACT did not alter any aspect of the d-plp mutant phenotype, nor did it have any detectable dominant-negative affect). Thus, centriole replication appears to occur normally in d-plp mutant brain cells. The same is also true in d-plp mutant testes (see below).
Mitosis is not dramatically disrupted in d-plp mutant cells
To our surprise, the arrangement of microtubules in d-plp mutant cells was not grossly perturbed, and anaphase cells usually had a normal arrangement of microtubules and chromosomes, even when centrosomal markers were not tightly concentrated at centrosomes (Fig. 5, A, B, and F). Most prophase and metaphase mutant cells also had a normal arrangement of microtubules and chromosomes, although there was a modest increase in the number of cells that had at least one clearly disorganized spindle pole or that were more severely disorganized (Fig. 5, C–F). In mutant cells that also expressed GFP-PACT as a centriolar marker, the centrioles were always at the center of a microtubule aster in prophase cells, and the centrioles were invariably located at the poles of the mitotic spindle from metaphase to anaphase (unpublished data). Thus, the centrioles appear to function as microtubule-organizing centers in d-plp mutant cells even though the recruitment of the PCM is inefficient. This suggests that most d-plp mutant cells assemble their spindles in a centrosome-dependent manner.
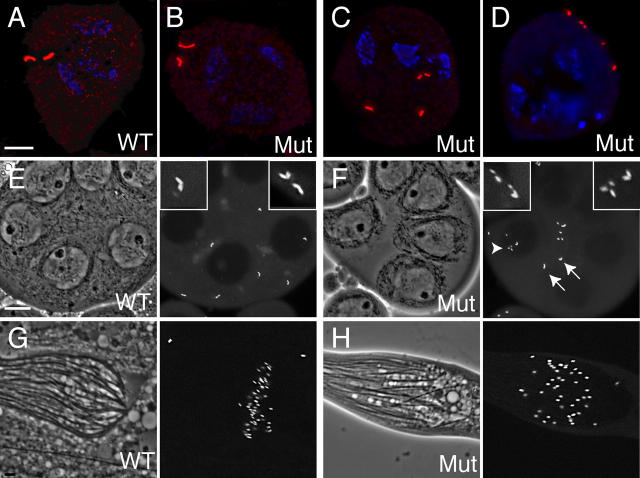
Figure 5.
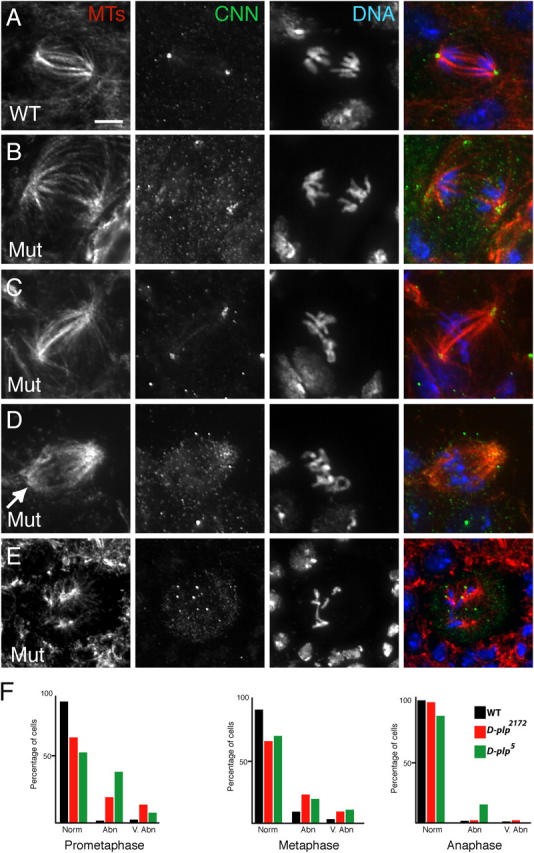
d-plp mutant brain cells have few mitotic spindle defects. (A–E) The distribution of microtubules (red), CNN (green), and DNA (blue) is shown in WT (A) or d-plp mutant (B–E) cells. (A) A WT cell in anaphase. (B) A d-plp mutant cell in anaphase. In this cell, although the recruitment of CNN to the spindle poles is abnormal, the spindle appears morphologically normal and chromosome segregation is unperturbed. (C) A mutant metaphase cell with a normal spindle morphology. (D) A mutant metaphase cell with one pole that is not tightly focused (arrow). (E) A mutant metaphase cell in which the arrangement of the spindle microtubules is highly abnormal. (F) Graphs showing the percentage of WT (black), d-plp 2172 (red), or d-plp 5 (green) mutant cells with normal, abnormal, or very abnormal spindle morphologies at various stages of the cell cycle. The total number of cells (WT/d-plp 2172/d-plp 5) counted from six different brain squashes was: prometaphase, 84/111/98; metaphase, 215/279/257; anaphase, 73/75/68. Bar, 5 μm.
Compared with controls, mutant cells did not have higher levels of either polyploidy/aneuploidy during metaphase or lagging chromosomes during anaphase (unpublished data). In addition, the mitotic index was no higher in mutant cells (1.4 ± 0.4, n > 5,000 for d-plp 5; 1.3 ± 0.6; n > 8,000 for d-plp 2172) than in control cells (1.4 ± 0.6; n > 7,000). Thus, although the recruitment of several proteins to centrosomes was impaired in mutant cells, this did not lead to dramatic defects in mitosis.
d-plp 2172 mutant sensory neurons have ciliary defects
The uncoordinated phenotype seen in d-plp mutant flies is often associated with defects in mechanosensory transduction (Kernan et al., 1994). Type I sensory organs contain neurons with specially modified cilia that transduce proprioceptive and auditory stimuli, and these cilia are required for normal sensory transduction (Dubruille et al., 2002; Han et al., 2003). We found that GFP-PACT was concentrated at the centrioles/basal bodies in all of the ciliated sensory neurons that we observed (chordotonal, olfactory, taste, and mechanosensory; unpublished data), so we wondered whether it might be required for proper cilia formation in these cells.
To assess sensory neuron function, we recorded sound-evoked potentials (SEPs) from Johnston's organ—the large, auditory, chordotonal organ in the second antennal segment. Johnston's organ contains ∼200 sensory units, or scolopidia, which respond to sound-induced vibrations in the distal antennal segments (Caldwell and Eberl, 2002). Cilia extending from each of two or three sensory neurons in a scolopidium are attached at their distal tips to a dendritic cap that transmits the vibration to the cilia. To record sound-evoked responses, individual d-plp 2172 mutant flies or their heterozygous siblings were exposed to a standard sound stimulus while recording extracellular potentials with electrodes placed in the antenna and head (Eberl et al., 2000). Heterozygous flies had normal responses to each sound pulse (Fig. 6 A). In contrast, most (16/26) homozygous mutant flies had no sound-evoked responses, and the amplitude of the response was greatly reduced in those flies that did respond (Fig. 6 B).
Figure 6.
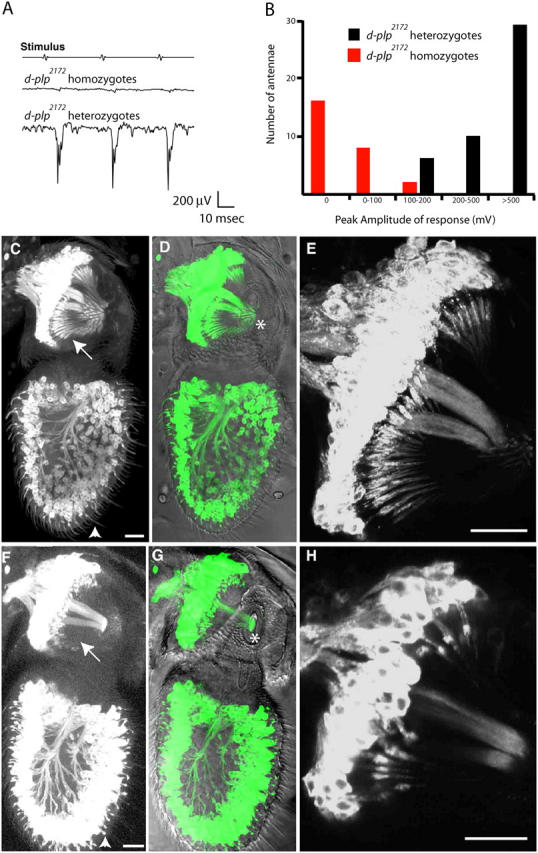
d-plp2172 mutants have defects in mechanosensory neuron function. (A) A trace of the SEPs originating from the auditory chordotonal organ of a d-plp 2172 homozygous and a d-plp 2172 heterozygous adult. (B) The distribution of the peak SEP amplitudes in d-plp 2172 homozygotes (red) and d-plp 2172 heterozygotes (black). (C–H) Micrographs show the chordotonal neurons in the second and third antennal segments of WT flies (C–E) or d-plp 2172 homozygous mutant flies (F–H). The neurons express the mCD8-GFP membrane marker (left panel; green in image merged with a DIC view); a magnified view or the second segment is shown in E and H. In d-plp 2172 heterozygous antennae, the extended outer segment cilia of the mechanosensory chordotonal neurons in the second antennal segment (arrows) and the chemosensory neurons in the third antennal segment (arrowheads) are clearly visible. In d-plp 2172 homozygous mutant antennae, most of these extended cilia are either absent or short and kinked, and the chordotonal cilia of the second segment fail to attach to the cuticle that connects the second and third antennal segments (asterisk). Bars, 20 μm.
To see if this defect in sensory neuron function could be due to defects in the cilia, we visualized the cilia by labeling the plasma membrane of all neurons with the mCD8-GFP marker (Lee and Luo, 1999). In d-plp 2172 heterozygous antennae, the ciliary outer segments of the mechanosensory chordotonal neurons in the second antennal segment and of the chemosensory neurons in the third antennal segment were clearly visible (Fig. 6, C–E). By contrast, in d-plp 2172 homozygous mutant antennae the sensory cilia in both the chordotonal organs and the olfactory bristles were absent or severely shortened and kinked (Fig. 6, F–H). It seems likely that all ciliated sensory neurons, including those innervating tactile and proprioceptive bristles, are similarly affected. Thus, the uncoordinated phenotype of d-plp mutant flies seems to be caused by cilia defects in the type I sensory neurons.
d-plp mutants have centriole defects during spermatogenesis
Apart from the type I sensory neurons, the only other cells in flies that have cilia or flagella are the sperm (Han et al., 2003). Therefore, we analyzed whether flagella function was abnormal in d-plp mutant sperm. During normal spermatogenesis, each spermatogonium goes through four rounds of mitotic division to generate a cyst of 16 primary spermatocytes (Fuller, 1993). These cells contain four large centrioles arranged as two orthogonal pairs (Fig. 7 A). These centrioles are ∼10-fold larger and are much more structurally elaborate than those found in any other cells in Drosophila (Gonzalez et al., 1998). In d-plp 2172 and d-plp 5 mutant testes, morphologically normal primary spermatocytes were formed that each contained two pairs of large, orthogonally arranged centrioles (Fig. 7 B), suggesting that centriole replication occurs normally during these premeiotic rounds of cell division in d-plp mutants.
Figure 7.
d-plp mutants exhibit centriole defects during spermatogenesis. (A–D) The distribution of centrioles (red; stained here with GTU88*) is shown in immature (A and B) or mature (C and D) WT (A) or mutant (C and D) primary spermatocytes. In immature spermatocytes, the centrioles in both WT (A) and mutant (B) cells are arranged as two orthogonal pairs, demonstrating that centriole replication has occurred normally during the mitotic divisions that generated these cells. This orthogonal arrangement is maintained in WT cells as they enter meiosis I (see Fig. 2 E), but in the majority of mutant cells (C and D) the centrioles lose their orthogonal arrangement and start to partially fragment. DNA is stained in blue. (E and F) Centriole fragmentation is also seen in living mutant spermatocytes. Phase-contrast images show part of a cyst of WT (E) or mutant (F) primary spermatocytes, whereas the fluorescent images show the distribution of centrioles (visualized here with GFP-PACT). Arrows highlight normal centriole pairs, arrowheads highlight centriole fragments. Insets show a 3×-magnified view of selected centrioles. (G) In WT cysts of maturing sperm, the centrioles/basal bodies (visualized with GFP-PACT) are located at the base of the sperm flagella. In mutant cysts of maturing sperm, basal bodies and flagella are present, but the cyst is often disorganized. Bars: 5 μm (A–D), 10 μm (E–H).
However, as the spermatocytes matured, the centrioles in mutant spermatocytes often lost their orthogonal arrangement and partially fragmented (Fig. 7, C and D). This was confirmed in living spermatocytes using GFP-PACT as a centriolar marker (Fig. 7, E and F). As a result of this fragmentation, mutant spermatocytes often formed multipolar meiosis I spindles, with each spindle pole organized by at least one centriole (or centriole “fragment”; see Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200402130/DC1). Thus, D-PLP appears to be essential for maintaining the structural integrity of the large centrioles that form in spermatocytes.
Although meiosis was highly abnormal in mutant spermatocytes, at least some cells developed into relatively normal-looking sperm. However, the distribution of centrioles and nuclei in the cysts was often disorganized (Fig. 7, G and H). Moreover, although the mutant sperm contained flagella, these were invariably nonmotile (see Materials and methods). However, an EM analysis revealed that the mutant sperm tails appeared structurally normal (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200402130/DC1).
Discussion
The PACT domain proteins Pericentrin and AKAP450/CG-NAP are amongst the best-studied centrosomal proteins. These proteins are thought to function by recruiting a small number of specific proteins, such as γ-tubulin and PKA, to the PCM, and they are widely believed to play important roles in several aspects of cell division (Keryer et al., 1993, 2003; Doxsey et al., 1994; Dictenberg et al., 1998; Takahashi et al., 1999, 2002; Diviani et al., 2000; Young et al., 2000). Our analyses on D-PLP, the only PACT domain protein in flies, reveal several important insights into the function of this conserved family of proteins in vivo.
D-PLP is associated with both the centrioles and with the PCM
Previous reports have suggested that Pericentrin and AKAP450 are concentrated in the PCM (Keryer et al., 1993; Doxsey et al., 1994; Gillingham and Munro, 2000). However, we find that D-PLP is most strongly associated with the centrioles. Two different anti-D-PLP antibodies stain centrosomes as a very small dot at the center of the PCM, and both antibodies recognize the centrioles in Drosophila oocytes and in interphase larval brain cells—cells where the centrioles appear to lack any associated PCM. However, our FRAP analysis of GFP-PACT in living embryos suggests that there are two distinct fractions of D-PLP at centrosomes: a fraction that stably associates with centrioles (and is only incorporated into centrioles when they replicate) and a fraction in the PCM that is in rapid exchange with a cytoplasmic pool. Although the localization of GFP-PACT may not accurately reflect the localization of the endogenous D-PLP protein, it has previously been shown that the overexpression of the PACT domain can displace endogenous PACT domain proteins from the centrosome, strongly arguing that this domain binds to the same sites in the centrosome as the endogenous protein (Gillingham and Munro, 2000; Keryer et al., 2003). Moreover, we find that D-PLP and GFP-PACT colocalize in oocytes, embryos, and larval brain cells in a manner that is distinct from that seen with any other centrosomal markers. Thus, we believe that GFP-PACT is likely to be a reliable marker of D-PLP localization.
We speculate that other PACT domain proteins will also be concentrated in the centrioles as well as in the PCM. Although several previous reports have concluded that these proteins are components of the PCM, some of this data is consistent with a localization in centrioles (Doxsey et al., 1994; Keryer et al., 2003). Pericentrin, for example, is concentrated at the basal body of Xenopus sperm nuclei, whereas other PCM markers, such as γ-tubulin, are not (Stearns and Kirschner, 1994). In addition, the centriolar fraction of PACT domain proteins may be difficult to detect by indirect immunofluorescence methods in some systems because of antibody penetration problems. In most Drosophila cells, centrioles are unusually small and simple structures, but in spermatocytes they are larger and more elaborate, and more closely resemble the centrioles found in typical vertebrate cells (Gonzalez et al., 1998). In Drosophila spermatocytes, neither D-PLP antibody stains the centrioles. This appears to be due to antibody penetration problems, as GFP-PACT is concentrated in these centrioles, but cannot be detected with anti-GFP antibodies.
D-PLP is required for the efficient centrosomal recruitment of several PCM components, but is not essential for mitosis
We find that D-PLP is essential for the efficient centrosomal recruitment of all six PCM components that we tested, and we have not found a PCM component whose concentration at the centrosome is not perturbed in d-plp mutant cells. This suggests that D-PLP plays an important part in recruiting most (and possibly all) PCM components to the centrosome. However, as mitosis proceeds, the recruitment of PCM components to the centrosome in mutant cells improves, and by the time the cells have entered anaphase, ∼50–80% of cells have a normal centrosomal concentration of PCM markers. A possible explanation is that the d-plp mutations are not nulls, and that a small amount of residual D-PLP function can eventually recruit proteins to centrosomes. However, we think this unlikely, as we have generated 17 alleles of d-plp, several of which appear to be protein nulls by Western blotting and immunofluorescence criteria (using two different anti-D-PLP antibodies). γ-Tubulin can be detected at centrosomes and mitosis does not appear to be appreciably perturbed in any of these 17 alleles (unpublished data). This suggests that there is a mechanism of recruiting PCM proteins to the centrosomes/spindle poles that is independent of D-PLP.
How might D-PLP recruit proteins to the centrosome? Our observation that D-PLP is associated with both the centrioles and the PCM raises a number of possibilities. D-PLP could function to directly link the centrioles to the PCM: in the absence of D-PLP, no PCM is initially recruited to the centrioles, but a D-PLP–independent mechanism eventually recruits the PCM to the spindle poles as mitosis progresses. Alternatively, the centriolar- and PCM-associated fractions of D-PLP may have separate functions, and D-PLP could interact with PCM components in the cytoplasm and so target them to the centrosome. Finally, D-PLP may play no direct role in recruiting proteins to the PCM, but could simply provide structural integrity to the PCM.
Perturbing Pericentrin function in Xenopus eggs or in tissue culture cells leads to defects in spindle assembly (Doxsey et al., 1994; Purohit et al., 1999; Pihan et al., 2001), whereas perturbing AKAP450 function leads to defects in cytokinesis, centriole replication, and cell cycle progression (Keryer et al., 2003). Recently, the large form of D-PLP (called CP309 in this paper) was shown to be essential for microtubule nucleation from purified Drosophila centrosomes in vitro (Kawaguchi and Zheng, 2003). Surprisingly, however, we find that cell division is not dramatically perturbed in d-plp mutant larval brain cells. Centriole replication appears to occur normally, and even in cells that largely fail to recruit centrosomal proteins to the spindle poles, mitosis occurs relatively normally. This finding is consistent with several previous observations. In cnn and asterless (asl) mutant brain cells, for example, there is a dramatic reduction in the amount of γ-tubulin recruited to centrosomes during mitosis, but these cells have few mitotic defects (Giansanti et al., 2001; Megraw et al., 2001). It seems that several cell types can organize bipolar spindles in the absence of centrosomes (Heald et al., 1996; Khodjakov et al., 2000). Therefore, we speculate that PACT domain proteins may be dispensable for cell division in vivo in all higher eukaryotic organisms.
D-PLP is essential for proper cilia/flagella formation
Although D-PLP is not essential for viability, d-plp mutants are invariably uncoordinated, a phenotype often associated with defects in mechanosensory transduction (Kernan et al., 1994). Type I sensory organs contain sensory neurons with specially modified cilia that transduce proprioceptive and auditory stimuli. Recently, mutations in several genes have been identified that cause defects in cilia formation in these sensory neurons, and these mutations have an uncoordinated phenotype that is very similar to that seen in d-plp mutants (Dubruille et al., 2002; Han et al., 2003). We find that the chordotonal sensory neurons in d-plp mutant flies lack normal cilia and are almost completely nonfunctional, suggesting that cilia defects in the mechanosensory neurons cause the uncoordinated phenotype in d-plp mutants. Moreover, the flagella of sperm cells, which are the only other cells in Drosophila that contain cilia/flagella, are nonmotile, suggesting that D-PLP is required for the proper function of all cilia/flagella in flies.
What is the essential role of D-PLP in flagella and cilia? We propose that D-PLP has a role in maintaining the structural integrity of centrioles/basal bodies in cells that form cilia or flagella. In support of this possibility, the large centrioles formed in d-plp mutant spermatocytes often lose their orthogonal arrangement and partially fragment during development. Such a centriolar “fragmentation” has, to our knowledge, not been described before, and it suggests that the structural integrity of the centrioles in these cells is compromised in the absence of D-PLP. In vertebrates, cilia are important in many processes, including sperm motility, sensory neuron function, phototransduction, and the generation of left/right asymmetry during development. We predict that knocking out PACT domain protein function in a vertebrate organism would lead to defects in all of these cilia-dependent processes.
Materials and methods
Fly strains
Flies were maintained on standard maize meal Drosophila medium at 25°C. w 67 was used as a WT stock and as the parental stock to generate transgenic lines. The Df(3L)Brd15 deficiency and the P-element insertion line l(3)s2172 were obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN).
Ethylmethane sulphate mutagenesis
New mutations in the d-plp gene were generated by feeding ru, st, e, ca males ethylmethane sulphate (25 mM in 1% sucrose) using standard procedures (Roberts, 1986).
Generation of flies expressing a GFP-PACT fusion protein
A 1.5-kb DNA fragment encoding GFP fused to the COOH-terminal 226 amino acids of CG6735 (which contains the PACT domain) was amplified by PCR from a plasmid described previously (Gillingham and Munro, 2000). The amplified product was then subcloned into pWRpUbq (Lee et al., 2001); full cloning details are available on request. Transgenic flies were generated using standard methods (Roberts, 1986).
Production of antibodies
PCR was used to amplify the DNA encoding aa 8–351 of the predicted protein encoded by CG13459, and aa 683–974 of the D-PLP-S cDNA (referred to as SD04227 by the Berkeley Drosophila Genome Project). The PCR products were subcloned, in frame, into the pMal vector (New England Biolabs, Inc.), and the resulting maltose-binding protein fusion proteins were purified according to the manufacturer's instructions. The purified fusion proteins were used to generate antibodies in rabbits; Eurogentec performed all injections and bleeds. The antibodies were affinity purified and stored as described previously (Huang and Raff, 1999).
Electrophoresis and immunoblotting
For Western blotting, 20 brains were dissected from WT or d-plp 2172 homozygous mutant third instar larvae in PBS and were homogenized in SDS sample buffer (Laemmli, 1970). The proteins were separated in 3–8% gradient precast acrylamide gel NuPAGE® (Invitrogen) and then were transferred by electroblotting to a nitrocellulose membrane (Millipore). Western blotting was performed as described previously (Huang and Raff, 1999).
Brain and testis immunostaining
For γ-tubulin staining, brains were prepared and treated as described previously (Gonzalez and Glover, 1993). All other immunostaining of brains or testes was performed as described previously (Williams and Goldberg, 1994), except for the staining of microtubules, where we used a different protocol (Bonaccorsi et al., 2000). To quantify γ-tubulin and CNN at centrosomes, we scored at least eight brains from WT, d-plp 2172, or d-plp 5/Df(3L)Brd R15 mutants. The following criteria were used to assign each mitotic cell to one of three classes: “normal” cells contained two well-focused poles of γ-tubulin staining; “weak” cells contained at least one pole that had either weak staining or an unusually diffuse staining of γ-tubulin; “very weak” cells had either a very weak staining or a very diffuse staining of γ-tubulin at both poles. In brains, we determined the mitotic index as the ratio of phosphohistone H3–positive (mitotic) cells per total Hoechst-stained cells. Anti-D-PLP antibodies were used at 1 μg/ml; antibodies to γ-tubulin (GTU88; Sigma–Aldrich), α-tubulin (DM1a; Sigma-Aldrich), and phosphohistone H3 (Upstate Biotechnology) were used at dilutions of 1:500, 1:1,000, and 1:1,000, respectively. All dilutions of primary antibodies were made in PBS + 0.05% Triton X-100 or PBS + BSA. Secondary antibodies coupled to the appropriate fluorophore (Alexa® 488, Cy3, or Cy5; Molecular Probes, Inc.) were diluted to 1:1,000 in PBS + 0.05% Triton X-100. Note that for all γ-tubulin staining, we used a batch of the GTU88 mAb that did not recognize centrioles in any tissues.
Live testis analysis
Testes from third instar larvae, pupae, or adults were dissected in PBS and transferred to a small drop of PBS on a clean coverslip. Testes from third instar larvae were gently squashed between a coverslip and a slide, whereas testes from pupae or adults were first cut with a pair of tungsten needles and then gently squashed. To test for sperm motility, freshly eclosed d-plp mutant males were moved to a clean vial of food. Although unable to move in a coordinated manner, mutant flies could live for several days under these conditions. Testes were dissected from WT and mutant males, the seminal vesicle and associated testes were cut with a pair of dissecting needles, and the sperm were observed under a dissecting microscope (model M6; Leica) for any sign of motility.
Embryo staining
We fixed 0–4-h-old embryos in methanol and processed them for immunostaining as described previously (Huang and Raff, 1999).
Electron microscopy
Testes of WT and d-plp 2172 mutant pupae were dissected and fixed for 4 h in cold PBS + 2% glutaraldehyde. The samples were osmicated in 1% OsO4 and subsequently en bloc stained in uranyl acetate. After dehydration, they were embedded in Araldite. Thin (∼50 nm) sections were stained in uranyl acetate and lead citrate and viewed in an electron microscope (CM100; FEI/Philips) operated at 80 kV. Images were recorded onto Kodak 4489 sheet film and subsequently scanned to obtain digital images.
FRAP analysis
We observed living embryos expressing GFP-PACT with a microscope (model E800; Nikon) using a Radiance confocal system (Bio-Rad Laboratories). Using our own macro (written by Alex Sossick), we manually selected a small area of an embryo and then photobleached it with two passes of the scanning laser on 100% power. The whole embryo was then imaged using normal laser power (usually 2–5% of full power), and the images acquired during the recovery period were imported into NIH Image for analysis.
Recording of SEPs
Near-field sound stimuli were generated to approximate Drosophila courtship song. SEPs were amplified and recorded as described previously (Eberl et al., 2000). Recordings were made from heterozygote and homozygote d-plp 2172 flies in alternating sequence.
Immunofluorescence analysis of chordotonal cilia
We generated a line of flies that carried the d-plp 2172 mutation and expressed mCD8-GFP under the control of the elav C155 Gal4 driver. Heterozygote and homozygote d-plp 2172 pupae were selected on the basis of eye pigmentation at 30–50 h post-pupariation. Antennae were dissected and fixed in 4% formaldehyde in PBS with 0.3% Triton X-100 (PBST) for 2 h at RT. Tissues were washed three times for 10 min in PBST and briefly in PBS before mounting in 80% glycerol.
Image acquisition and presentation
The imaging of all brain and testes preparations was performed using a microscope (Axioskop 2; Carl Zeiss MicroImaging, Inc.) with a CoolSnap HQ™ camera (Photometrics) with MetaMorph® software (Universal Imaging Corp.). Pictures of brain squashes show a single optical section that was determined to have the brightest centrosome staining at both poles of the spindle. Deconvolution and final projection of the fixed testes images (Fig. 7, A–D) were performed with Huygens professional 2.5 (Scientific Volume Imaging) after collection of 40 optical sections at 0.1-μm intervals. The imaging of live and fixed embryos was performed on a Radiance confocal microscope system (Bio-Rad Laboratories). All images were imported into Adobe Photoshop®, and all control and experimental images were processed in exactly the same way. Fluorescence images of antennae were collected using a confocal microscope (TCS SP2; Leica), and Z-projections were made using the maximum projection algorithm.
Online supplemental material
Fig. S1 shows the localization of CNN, D-TACC, and CP60 in WT and d-plp mutant brain cells in metaphase. Fig. S2 shows the meiosis defects observed in d-plp mutant spermatocytes. Fig. S3 shows an EM cross section of WT and d-plp mutant sperm flagella. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200402130/DC1.
Acknowledgments
We thank Sean Munro for bringing the PACT domain to our attention and for supplying the initial GFP-PACT construct. We thank Shinichi Kawaguchi and Yixian Zheng for generously providing the sequence of the D-PLP-L cDNA before publication, Carly Dix for her help in analyzing the 17 new d-plp alleles, and Jeremy Skepper at the EM facility in the Department of Anatomy for cutting and analyzing the EM sections shown in Fig. 7. We also thank Alex Sossick for writing the FRAP macro for the confocal system, and members of the Raff lab for helpful comments on the manuscript.
This work was supported by a Wellcome Trust Senior Research Fellowship (J.W. Raff), a Wellcome Trust Prize Studentship (M. Martinez-Campos), an EMBO post-doctoral Fellowship (R. Basto), and National Institutes of Health grant DC02780 (J. Baker and M. Kernan).
M. Martinez-Campos and R. Basto contributed equally to this paper.
The online version of this article includes supplemental material.
References
- Bolivar, J., J.R. Huynh, H. Lopez-Schier, C. Gonzalez, D. St Johnston, and A. Gonzalez-Reyes. 2001. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development. 128:1889–1897. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi, S., M.G. Giansanti, and M. Gatti. 2000. Spindle assembly in Drosophila neuroblasts and ganglion mother cells. Nat. Cell Biol. 2:54–56. [DOI] [PubMed] [Google Scholar]
- Bornens, M. 2002. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14:25–34. [DOI] [PubMed] [Google Scholar]
- Caldwell, J.C., and D.F. Eberl. 2002. Towards a molecular understanding of Drosophila hearing. J. Neurobiol. 53:172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg, J.B., W. Zimmerman, C.A. Sparks, A. Young, C. Vidair, Y. Zheng, W. Carrington, F.S. Fay, and S.J. Doxsey. 1998. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviani, D., L.K. Langeberg, S.J. Doxsey, and J.D. Scott. 2000. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol. 10:417–420. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J., P. Stein, L. Evans, P.D. Calarco, and M. Kirschner. 1994. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 76:639–650. [DOI] [PubMed] [Google Scholar]
- Dubruille, R., A. Laurencon, C. Vandaele, E. Shishido, M. Coulon-Bublex, P. Swoboda, P. Couble, M. Kernan, and B. Durand. 2002. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development. 129:5487–5498. [DOI] [PubMed] [Google Scholar]
- Eberl, D.F., R.W. Hardy, and M.J. Kernan. 2000. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 20:5981–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory, M.R., and T.N. Davis. 2003. The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics. 82:401–405. [DOI] [PubMed] [Google Scholar]
- Flory, M.R., M.J. Moser, R.J. Monnat, Jr., and T.N. Davis. 2000. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA. 97:5919–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, M. 1993. Spermatogenesis. The Development of Drosophila melanogaster. M. Bate and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 71–147.
- Giansanti, M.G., M. Gatti, and S. Bonaccorsi. 2001. The role of centrosomes and astral microtubules during asymmetric division of Drosophila neuroblasts. Development. 128:1137–1145. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., and S. Munro. 2000. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, C., and D.M. Glover. 1993. Techniques for studying mitosis in Drosophila. The Cell Cycle: A Practical Approach. P. Fantes and R. Brooks, editors. IRL Press, Oxford. 143–174.
- Gonzalez, C., G. Tavosanis, and C. Mollinari. 1998. Centrosomes and microtubule organisation during Drosophila development. J. Cell Sci. 111:2697–2706. [DOI] [PubMed] [Google Scholar]
- Grieder, N.C., M. de Cuevas, and A.C. Spradling. 2000. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 127:4253–4264. [DOI] [PubMed] [Google Scholar]
- Han, Y.G., B.H. Kwok, and M.J. Kernan. 2003. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13:1679–1686. [DOI] [PubMed] [Google Scholar]
- Heald, R., R. Tournebize, T. Blank, R. Sandaltzopoulos, P. Becker, A. Hyman, and E. Karsenti. 1996. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 382:420–425. [DOI] [PubMed] [Google Scholar]
- Huang, J., and J.W. Raff. 1999. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18:2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, S.I., and Y. Zheng. 2003. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol. Biol. Cell.15:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg, D.R., M. Moritz, and B.M. Alberts. 1994. The centrosome and cellular organization. Annu. Rev. Biochem. 63:639–674. [DOI] [PubMed] [Google Scholar]
- Kernan, M., D. Cowan, and C. Zuker. 1994. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 12:1195–1206. [DOI] [PubMed] [Google Scholar]
- Keryer, G., R.M. Rios, B.F. Landmark, B. Skalhegg, S.M. Lohmann, and M. Bornens. 1993. A high-affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II in the centrosome of human cells. Exp. Cell Res. 204:230–240. [DOI] [PubMed] [Google Scholar]
- Keryer, G., O. Witczak, A. Delouvee, W.A. Kemmner, D. Rouillard, K. Tasken, and M. Bornens. 2003. Dissociating the centrosomal matrix protein AKAP450 from centrioles impairs centriole duplication and cell cycle progression. Mol. Biol. Cell. 14:2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., R.W. Cole, B.R. Oakley, and C.L. Rieder. 2000. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 10:59–67. [DOI] [PubMed] [Google Scholar]
- Knop, M., and E. Schiebel. 1997. Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 16:6985–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of the structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., F. Gergely, K. Jeffers, S.Y. Peak-Chew, and J.W. Raff. 2001. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3:643–649. [DOI] [PubMed] [Google Scholar]
- Lee, T., and L. Luo. 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 22:451–461. [DOI] [PubMed] [Google Scholar]
- Mahowald, A.P., and J.M. Strassheim. 1970. Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J. Cell Biol. 45:306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw, T.L., L.R. Kao, and T.C. Kaufman. 2001. Zygotic development without functional mitotic centrosomes. Curr. Biol. 11:116–120. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., D.B. Vinh, D.K. Crawford, and T.N. Davis. 1998. A genetic analysis of interactions with Spc110p reveals distinct functions of Spc97p and Spc98p, components of the yeast γ-tubulin complex. Mol. Biol. Cell. 9:2201–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan, G.A., A. Purohit, J. Wallace, R. Malhotra, L. Liotta, and S.J. Doxsey. 2001. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 61:2212–2219. [PubMed] [Google Scholar]
- Purohit, A., S.H. Tynan, R. Vallee, and S.J. Doxsey. 1999. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo, E., S. Llamazares, J. Reina, and C. Gonzalez. 2004. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biol. 2:E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., S. Faruki, and A. Khodjakov. 2001. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11:413–419. [DOI] [PubMed] [Google Scholar]
- Riparbelli, M.G., G. Callaini, D.M. Glover, and C. Avides Mdo. 2002. A requirement for the abnormal spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J. Cell Sci. 115:913–922. [DOI] [PubMed] [Google Scholar]
- Roberts, D. 1986. Drosophila, A Practical Approach. IRL Press, Oxford. 316 pp.
- Stearns, T., and M. Kirschner. 1994. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell. 76:623–637. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., H. Shibata, M. Shimakawa, M. Miyamoto, H. Mukai, and Y. Ono. 1999. Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J. Biol. Chem. 274:17267–17274. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., A. Yamagiwa, T. Nishimura, H. Mukai, and Y. Ono. 2002. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring γ-tubulin ring complex. Mol. Biol. Cell. 13:3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis, G., S. Llamazares, G. Goulielmos, and C. Gonzalez. 1997. Essential role for γ-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J. 16:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W.E. 1994. Microtubules and cytoplasm organization during Drosophila oogenesis. Dev. Biol. 165:352–360. [DOI] [PubMed] [Google Scholar]
- Williams, B.C., and M.L. Goldberg. 1994. Determinants of Drosophila zw10 protein localization and function. J. Cell Sci. 107:785–798. [DOI] [PubMed] [Google Scholar]
- Wilson, P.G., Y. Zheng, C.E. Oakley, B.R. Oakley, G.G. Borisy, and M.T. Fuller. 1997. Differential expression of two γ-tubulin isoforms during gametogenesis and development in Drosophila. Dev. Biol. 184:207–221. [DOI] [PubMed] [Google Scholar]
- Young, A., J.B. Dictenberg, A. Purohit, R. Tuft, and S.J. Doxsey. 2000. Cytoplasmic dynein-mediated assembly of pericentrin and γ tubulin onto centrosomes. Mol. Biol. Cell. 11:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]