Abstract
Apoptosis in response to developmental cues and stress stimuli is mediated by caspases that are regulated by the Bcl-2 protein family. Although caspases 2 and 9 have each been proposed as the apical caspase in that pathway, neither is indispensable for the apoptosis of leukocytes or fibroblasts. To investigate whether these caspases share a redundant role in apoptosis initiation, we generated caspase-2−/−9−/− mice. Their overt phenotype, embryonic brain malformation and perinatal lethality mirrored that of caspase-9−/− mice but were not exacerbated. Analysis of adult mice reconstituted with caspase-2−/−9−/− hematopoietic cells revealed that the absence of both caspases did not influence hematopoietic development. Furthermore, lymphocytes and fibroblasts lacking both remained sensitive to diverse apoptotic stimuli. Dying caspase-2−/−9−/− lymphocytes displayed multiple hallmarks of caspase-dependent apoptosis, including the release of cytochrome c from mitochondria, and their demise was antagonized by several caspase inhibitors. These findings suggest that caspases other than caspases 2 and 9 can promote cytochrome c release and initiate Bcl-2–regulated apoptosis.
Keywords: apoptosis; caspase-2; caspase-9; Bcl-2; cytochrome c
Introduction
Apoptosis, which is critical for development and tissue homeostasis, is executed by caspases (Adams, 2003). The 10 or so mammalian caspases include both “effectors” (3, 6, and 7), which efficiently digest vital proteins, and “initiators” (e.g., 2, 8, and 9), which proteolytically activate the effectors. Many cell “stress” stimuli, e.g., cytokine deprivation and genome damage, and developmental cues, trigger a common pathway of caspase activation regulated by the Bcl-2 protein family (Adams, 2003). Until recently, the sole apical initiator in that pathway was assumed to be caspase-9, which is activated in a complex termed the “apoptosome” by the scaffold protein Apaf-1 and its cofactor cytochrome c. Evidence that the Bcl-2 family regulates permeabilization of mitochondria argued that cytochrome c release and the ensuing caspase-9 activation were central to the “stress” response. For some neuronal cells, this model is supported, as mice lacking Apaf-1 or caspase-9 die perinatally with brain overgrowth caused by a defect in neuronal apoptosis (Adams, 2003). The apoptosome is not, however, universally essential for Bcl-2–regulated apoptosis, because certain neuronal (Honarpour et al., 2001), hematopoietic, and fibroblastoid cells (Marsden et al., 2002) lacking Apaf-1 or caspase-9 readily undergo apoptosis in response to diverse insults and, at least in lymphocytes, that apoptosis requires caspase activity (Marsden et al., 2002). Hence, there must be apoptotic pathways regulated by the Bcl-2 family that require the activation of caspases other than caspase-9 (Adams, 2003).
Evidence is also accumulating that certain caspases can contribute to mitochondrial damage and hence may be activated before apoptosome formation (Guo et al., 2002; Lassus et al., 2002; Marsden et al., 2002; Robertson et al., 2002). In particular, caspase-2 has been implicated in cytochrome c release (Guo et al., 2002; Lassus et al., 2002; Robertson et al., 2002) and seems to be necessary for cellular demise in some transformed cell lines (Lassus et al., 2002). However, because apoptosis is not markedly impaired in caspase-2–deficient mice (Bergeron et al., 1998; O'Reilly et al., 2002), caspase-2 cannot have a major nonredundant role in apoptosis.
These discordant findings might be reconciled if caspase-2 acts redundantly with caspase-9, each activating distinct but converging pathways. If so, loss of both caspases should markedly attenuate apoptosis. We address that hypothesis here by studies on mice lacking both caspases 2 and 9.
Results and discussion
To generate mice lacking both caspases 2 and 9, we first intercrossed animals deficient in caspase-2 (O'Reilly et al., 2002) with caspase-9+/− mice (Kuida et al., 1998). As expected from the severe caspase-9−/− phenotype (Hakem et al., 1998; Kuida et al., 1998), intercrosses of the resulting caspase-2+/− 9+/− mice yielded no weaned progeny lacking caspase-9, irrespective of caspase-2 status (67 progeny genotyped). Mice of all other genotypes appeared at the expected Mendelian ratios and were healthy and fertile (unpublished data).
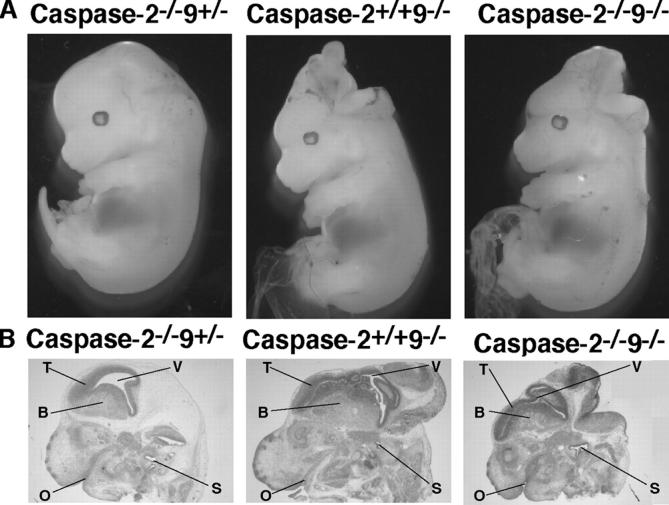
To investigate whether caspase-2 deficiency exacerbated the neuronal overgrowth characteristic of the caspase-9 deficiency (Hakem et al., 1998; Kuida et al., 1998), embryos from the intercrosses were examined at E14.5, when all genotypes appeared in the expected ratios. Brain over-growth resembling that previously described and observed in caspase-2+/+9−/− littermates (Fig. 1) appeared in 6/11 caspase-2−/−9−/− and 2/5 caspase-2+/+9−/− embryos but never in those expressing caspase-9 (n = 47). All other organs appeared normal. Although the brain abnormalities cannot be quantified, we conclude that caspase-2 loss does not substantially exacerbate the brain phenotype due to caspase-9 deficiency and that other organs develop normally to at least E14.5 without either caspases 2 or 9.
Figure 1.
Loss of caspase-2 does not aggravate the overt defects caused by caspase-9 deficiency. (A) Similar overt phenotype of E14.5 caspase-2−/−9−/− and caspase-9−/− embryos. (B) Similar histologic presentation of caspase-2−/−9−/− and caspase-9−/− embryos. Sagittal brain sections were stained with hematoxylin and eosin. For comparison, littermate embryos lacking only caspase-2, which are indistinguishable from wild-type embryos, are shown. O, oral cavity; B, basal ganglia; T, dorsal telencephalon; V, lateral ventricle; S, semicircular canals.
Bcl-2–regulated apoptosis, which is critical for the physiological death of hematopoietic cells (Marsden and Strasser, 2003), can occur independently of caspase-9 (Marsden et al., 2002). To study how caspase-2−/−9−/− hematopoietic cells respond to the physiological death cues in healthy mice, C57BL/6-Ly5.1 mice were reconstituted with fetal liver–derived hematopoietic stem cells from E14.5 offspring of the intercrosses (Ly5.2+). 10 wk later, the thymocytes were all derived from donor (Ly5.2) cells, irrespective of donor genotype (Fig. 2 A). The absence of caspases 2 and 9 did not augment cell numbers or perturb cell subset composition in the thymus, spleen, lymph nodes, or bone marrow (Fig. 2, B–D; and not depicted). Western blot analysis on reconstituted organs (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200312030/DC1) confirmed the absence of caspase-2 and/or -9 and revealed no compensatory up-regulation of caspases 1, 3, 6, 7, 8, or 9. Thus, whereas overexpression of Bcl-2 in the hematopoietic compartment, or absence of its antagonist Bim, promotes cell accumulation (Bouillet et al., 1999; Marsden et al., 2002), programmed death in that compartment appears unaffected by loss of both caspases 2 and 9.
Figure 2.
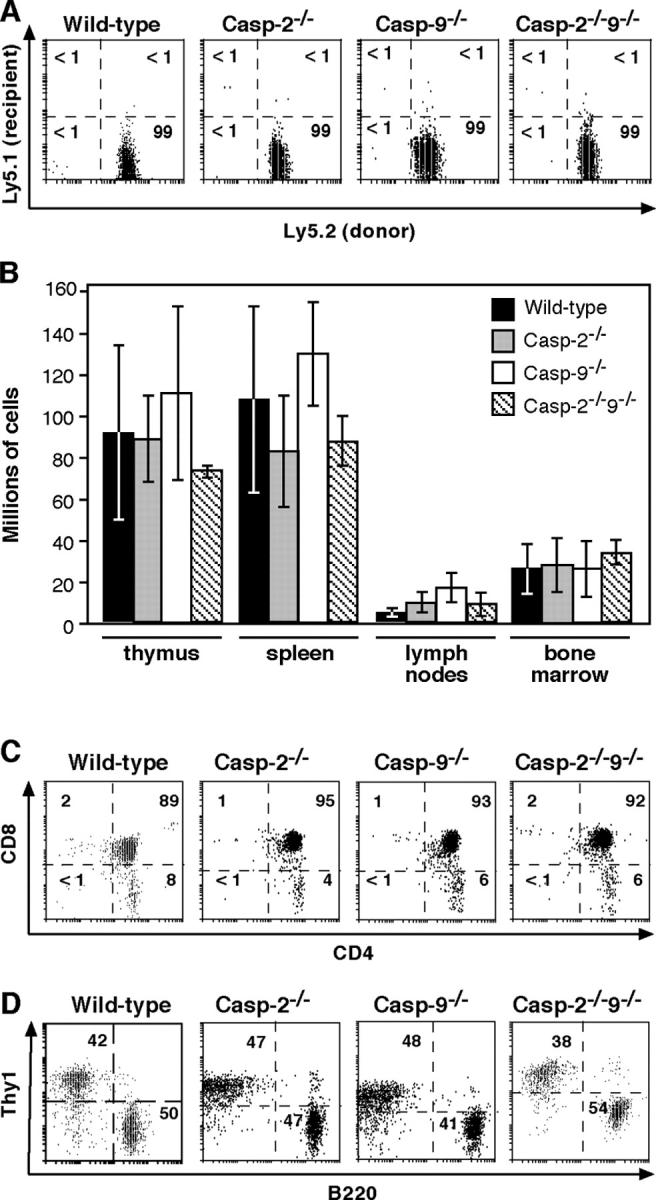
Normal hematopoietic development from caspase-2 −/− 9 −/− hematopoietic stem cells. Ly5.1 mice that had been lethally irradiated (2 × 5.5Gy) were reconstituted with fetal liver cells and analyzed 10–15 wk later. (A) Staining of thymocytes with antibodies to Ly5.1 (recipient) versus Ly5.2 (donor) cells. (B) Normal cellularities of thymus, lymph nodes (pooled inguinal, axillary, brachial and mesenteric), spleen and bone marrow in animals reconstituted with caspase-2−/−9−/−cells. Results represent means ± SD for >3 mice per genotype. (C) Normal composition of the thymus in the reconstituted mice. (D) Normal proportions of B lymphocytes (B220+ Thy-1−) and T cells (Thy-1+ B220−) in lymph nodes of the reconstituted mice. Results are representative of >3 mice per genotype.
To explore further whether caspases 2 and 9 are essential for Bcl-2–regulated cell death, we analyzed the responses to diverse cytotoxic insults of donor-derived B and T lymphocytes and CD4+8+ thymocytes purified from reconstituted animals. The death on cytokine withdrawal of mature B or T cells, activated T lymphoblasts or thymocytes was unimpaired by the absence of caspase-2, caspase-9, or both (Fig. 3, A–C and Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200312030/DC1). Similarly, mature T cells remained sensitive to dexamethasone (Fig. 3 A). In response to γ irradiation (Fig. 3 D; Fig. S2 B) or treatment with dexamethasone, PMA, or etoposide (Fig. S2, C–H), thymocytes lacking caspase-9 were slightly more resistant than wild-type cells, but concomitant deficiency for caspase-2 provided no additional protection, and the lack of caspase-2 alone had no significant effect. This held over a range of doses of each stimulus (Fig. S2, B, D, F, H; and not depicted). Thus, in lymphocytes caspase-9 deficiency at most merely slows rather than prevents cell death, and concomitant caspase-2 deficiency does not further reduce the rate.
Figure 3.
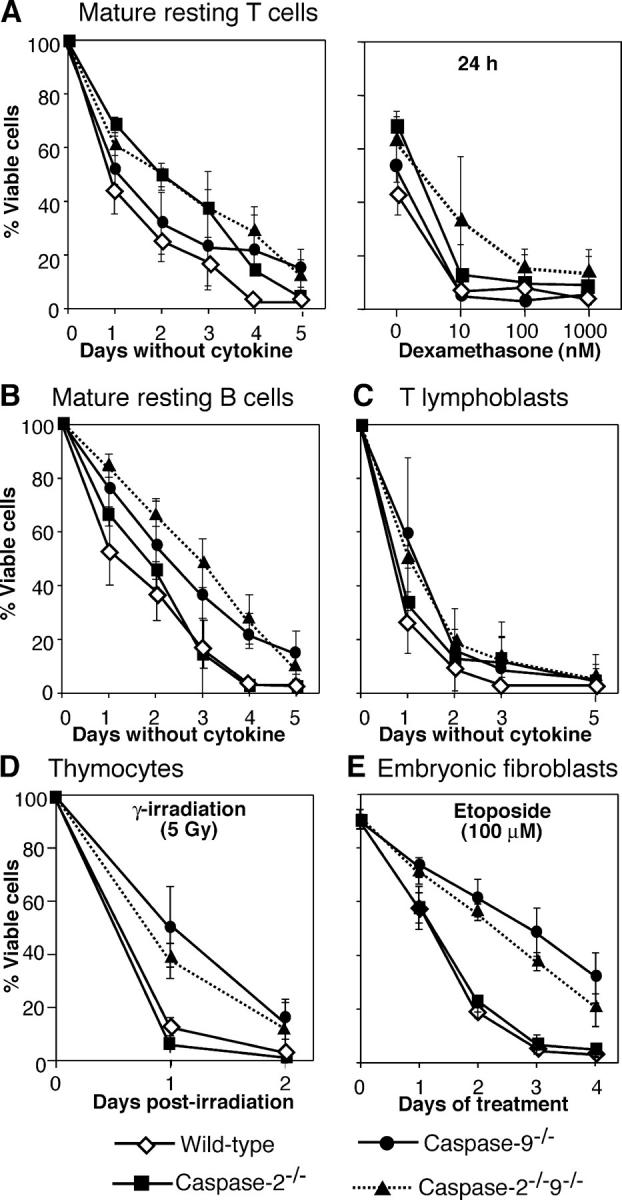
Deficiency of caspases 2 and 9 does not prevent apoptosis in lymphocytes or fibroblasts. To test the sensitivity of lymphocytes to apoptotic stimuli, donor-derived (Ly5.2+) (A) lymph node T cells (Thy-1+), (B) lymph node B cells (CD45R-B220+) and (D) CD4+8+ thymocytes were purified from the reconstituted mice, or (C) T lymphoblasts were generated by mitogenic activation of spleen cells. (E) Early passage embryonic fibroblasts from E14.5 embryos. In all cases, viability was measured by staining cells with PI and FACS analysis. Results represent means ± SD of >3 mice per genotype.
To explore how loss of caspases 2 and 9 affected cells that were not of hematopoietic origin, we studied embryonic fibroblasts lacking caspase-2, caspase-9, or both. When exposed to etoposide, caspase-9−/− fibroblasts died somewhat more slowly than wild-type cells, but the protection was transient and loss of caspase-2 provided no additional survival advantage (Fig. 3 E). Thus, caspase-2 deficiency does not delay the death of caspase-9–deficient fibroblasts.
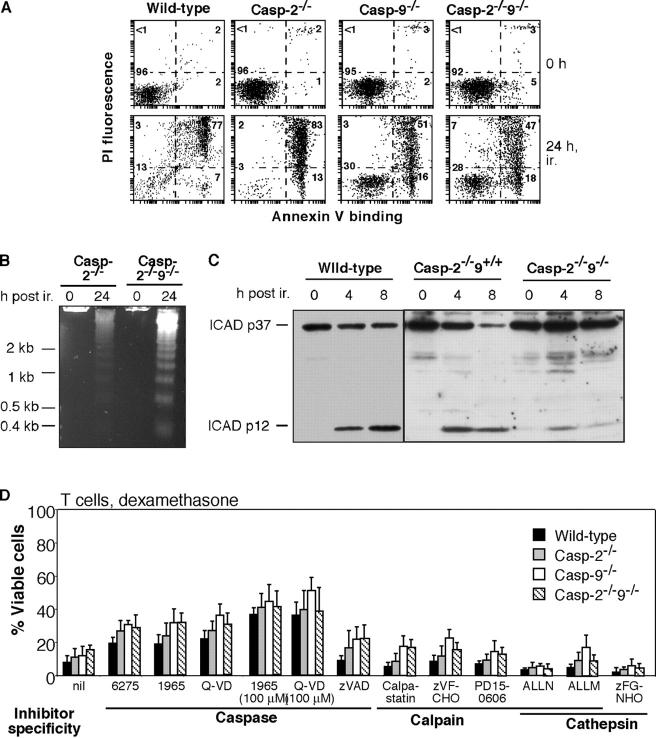
Dying caspase-9–deficient cells display the hallmarks of apoptosis (Marsden et al., 2002). Concomitant absence of caspase-2 did not prevent apoptosis. Two of its classic features, exposure of phosphatidylserine and DNA fragmentation, appeared in caspase-2−/−9−/− thymocytes subjected to γ irradiation (Fig. 4, A and B). It is likely that effector caspases contributed to their death, because the well-characterized caspase substrates ICAD, spectrin, and gelsolin (Fig. 4 C, Fig. S3, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200312030/DC1) were all processed appropriately, albeit to a lower extent than in dying wild-type cells. ICAD is thought to be processed only by caspases 3 or 7 (McIlroy et al., 1999); caspase-3 is required to generate the 41-kD product of gelsolin (Slee et al., 2001), and although either caspases or calpains can generate the 150-kD fragment of spectrin, only caspases give the 120-kD fragment (Methot et al., 2004). In dying caspase-2−/−9−/− cells, all three substrates yielded the expected caspase-dependent products (Fig. 4 C; Fig. S3, A and B). Moreover, the gelsolin processing was ablated in irradiated caspase-2−/−9−/− thymocytes treated with either of two structurally unrelated caspase inhibitors, IDN-1965 (Fig. S3 C) or Q-VD-OPh (not depicted). Thus, caspases are strongly implicated in the death of these cells.
Figure 4.
Dying caspase-2 −/− 9 −/− cells display hallmarks of apoptosis and are protected by caspase inhibitors but not calpain/cathepsin inhibitors. (A–C) Ly5.1−CD4+8+ thymocytes sorted from reconstituted mice were cultured following 5 Gy γ irradiation (ir.) (A) Staining with FITC-conjugated Annexin V plus PI reveals phosphatidylserine exposure before loss of membrane integrity irrespective of genotype. (B) DNA “laddering” indicative of inter-nucleosomal fragmentation demonstrated by gel electrophoresis of genomic DNA. (C) Western blots showing processing of ICAD in dying caspase-2−/−9−/− cells. (D) The death of caspase-2−/−9−/− cells is antagonized by inhibitors of caspases but not of calpains or cathepsins. Ly5.1−Thy1+ mature T lymphocytes from the lymph nodes of reconstituted mice were cultured for 24 h in the presence of 100 nM dexamethasone plus the caspase inhibitors zVAD-fmk, IDN-6275 (6275), IDN-1965 (1965), or Q-VD-OPh (Q-VD), the calpain inhibitors z-VF-CHO or PD-150606, a cell permeable peptide of the calpain inhibitor calpastatin, the dual calpain/cathepsin inhibitors ALLM and ALLN or the cathepsin inhibitor z-FG-NHO-Bz-pOMe (zFG-NHO) (each at 50 μM). Cell death was quantified by PI staining and FACS analysis. Results represent mean ± SD of at least three independent experiments for each genotype. Vehicle: 0.4% DMSO.
Processing of synthetic as well as physiological caspase substrates is lower in cell lysates lacking caspase-9 (Marsden et al., 2002). Similarly, with the fluorogenic caspase substrate DEVD-aminomethylcoumarin, dying caspase-2−/−9−/− or caspase-9−/− thymocytes had only ∼10–20% of the DEVDase activity of dying wild-type or caspase-2−/− lysates, but the activity was completely blocked by the caspase inhibitor zVAD-fmk (Fig. S3 D). It was unaffected by ALLN, a potent inhibitor of calpains and cathepsins, arguing against any contribution of these proteases in processing this caspase substrate.
To further explore whether the death of caspase-2−/−9−/− cells requires caspases, we examined the ability of four chemically distinct caspase inhibitors to impair their death (Fig. 4 F). Three of them, Q-VD-OPh (Caserta et al., 2003), IDN-1965, and IDN-6275 (Wu and Fritz, 1999), delayed apoptosis substantially up to 24 h after dexamethasone treatment of T cells (Fig. 4 D). zVAD-fmk had a smaller inhibitory effect, probably due to its reportedly inferior stability, membrane permeability, and performance in culture (Nicholson, 1999).
Non-caspase proteases, in particular calpains and cathepsins, have been proposed to contribute to apoptosis in certain circumstances (Jaattela and Tschopp, 2003). To determine whether either participated in the apoptosis of caspase-2−/−9−/− cells, we tested six inhibitors reported to impair apoptosis under certain conditions: the calpain inhibitors z-VF-CHO and PD150606 (Squier and Cohen, 1997), a cell-permeable peptide of the natural calpain inhibitor calpastatin (Altznauer et al., 2004), the dual calpain and cathepsin inhibitors ALLM and ALLN (Ding et al., 2002), and the selective cathepsin inhibitor z-FG-NHO-Bz-pOMe. In contrast to the caspase inhibitors, none of these inhibitors had any anti-apoptotic activity at doses in the range where others have reported efficacy (Fig. 4 D), and none cooperated with IDN-1965 to enhance its antagonism of apoptosis (not depicted). Hence, it appears unlikely that either calpains or cathepsins act in tandem with the caspase cascade to cause apoptosis in these cells.
Cytochrome c release in thymocytes seems to depend on caspase activity (Marsden et al., 2002), and caspase-2 has been implicated in mitochondrial disruption in certain cells (Guo et al., 2002; Lassus et al., 2002; Robertson et al., 2002). Hence, we examined whether caspase-2 was the sole caspase responsible for mitochondrial damage in thymocytes. Western blotting of fractionated cell lysates revealed that cytochrome c release from mitochondria did not require caspase-2 (Fig. 5 A). Furthermore, mitochondrial transmembrane potential in dying thymocytes was lost normally in the absence of caspase-2, or both caspases 2 and 9, although its loss in wild-type thymocytes was attenuated by a caspase inhibitor (Fig. 5 B), as shown previously (Bossy-Wetzel et al., 1998). Hence, both the release of pro-apoptotic molecules from mitochondria and the loss of mitochondrial transmembrane potential can occur independently of caspases 2 and 9.
Figure 5.
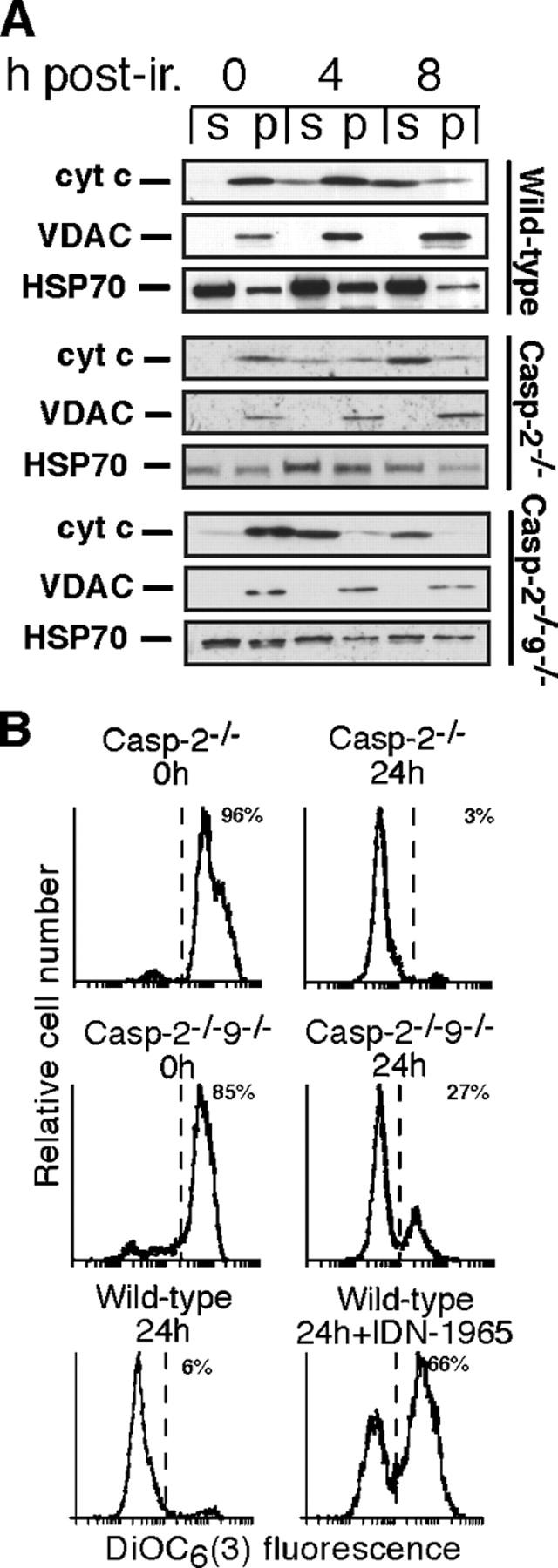
Cytochrome c release and mitochondrial depolarization do not require caspases 2 and 9. Ly5.1−CD4+8+ thymocytes from the reconstituted mice were cultured following 5 Gy γ irradiation (ir.) (A) Subcellular localization of cytochrome c was determined by Western blotting soluble cytosolic (s) and pelleted organelle (p) cell fractions (B). Mitochondrial trans-membrane potential was determined by FACS analysis of cells stained with 40 nM DiOC6(3). The percentages of cells retaining high DiOC6(3) fluorescence are shown. Where indicated, cells were cultured in the presence of IDN-1965 (100 μM).
Our results strongly implicate caspases in the apoptosis of caspase-2−/−9−/− cells and thus imply that there is a Bcl-2–regulated and caspase-mediated pathway that does not require either caspase-2 or -9. Which other caspases might be regulated by Bcl-2? As discussed elsewhere (Adams, 2003), caspases 1, 11, and 12 in mice (or caspases 1, 4, and 5 in humans) are attractive candidates, because, like caspases 2 and 9, their NH2-terminal CARD domain could interact with a cognate scaffold to form an apoptosome-like complex. For example, caspase-12, which is implicated in apoptosis induced by ER stress (Nakagawa et al., 2000) and in cytochrome c–independent apoptosis (Morishima et al., 2002; Rao et al., 2002), forms a large complex on serum starvation (Kilic et al., 2002). In other systems, caspase-11 (Hisahara et al., 2001; Kang et al., 2002) or caspase-1 (Hilbi et al., 1998; Marsden et al., 2002; Rowe et al., 2002) have been implicated in apoptosis. Hence, in different circumstances, various combinations of caspases 1, 11, and 12, and perhaps also caspase-8, might act redundantly with caspases 2 and 9 to initiate apoptosis.
Whereas our results with primary lymphocytes and fibroblasts implicate caspases in addition to caspases 2 and 9 in the initiation of apoptosis, the accompanying paper, Ekert et al. (2004) shows that the hallmarks of apoptosis failed to appear when myeloid progenitor cell lines lacking both caspases 2 and 9 were deprived of growth factor, but that programmed cell death still prevented their clonogenic survival. These findings and those with transformed human cell lines (Lassus et al., 2002) can be reconciled with our conclusion that caspases 2 and 9 are not essential for loss of viability per se, if some cell types but not others use these caspases to accelerate apoptotic cell demolition.
Materials and methods
Mice
Caspase-2+/−9+/− mice were generated by intercrosses of mice deficient in caspase-2 (129/sv) (O'Reilly et al., 2002) with caspase-9+/− (C57BL/6) mice (Kuida et al., 1998). Embryos and 3-wk-old mice were genotyped by PCR. Hematopoietic reconstitution was performed as described previously (Marsden et al., 2002) from fetal liver cells of embryos with a caspase-2−/−, caspase-9−/−, or caspase-2−/−9−/− genotype (all mixed C57/BL6-129Sv, Ly5.2+) or wild-type (C57/BL6 Ly5.2+) embryos.
Microscopic imaging
All microscopy used either a Stemi SV11 or an Axioplan 2 microscope (Carl Zeiss MicroImaging, Inc.). The latter used objective lenses (magnification/numerical aperture: 5×/0.15 and 10×/0.30; Carl Zeiss MicroImaging, Inc.). Images were recorded with a Axiocam and Axiovision software (Carl Zeiss MicroImaging, Inc.).
Flow cytometry
Cells stained with fluorochrome- or biotin-conjugated surface marker-specific antibodies (Marsden et al., 2002) were analyzed using a FACScan (Becton Dickinson). Live and dead cells were discriminated by staining with 2 μg/ml propidium iodide (PI; Sigma-Aldrich). In cell sorting, host-derived (Ly5.1) cells were excluded.
Cell culture
Cells were cultured and T lymphoblasts generated as described previously (Marsden et al., 2002). Before death assays, the viable cells in stimulated spleen cultures were enriched by centrifugation over Ficoll-Paque Plus (Amersham Biosciences). Embryonic fibroblasts, cultured from E14.5 embryos, were used at early passage (n < 5).
Cell death assays
For cell death assays, cells were cultured at 0.2–1 × 106 cells/ml and treated with dexamethasone (Sigma-Aldrich) at 10–8–10–6 M, γ radiation (5 Gy from a 60Co source), PMA (Sigma-Aldrich) at 0.1–10 ng/ml, or etoposide (VP-16, David Bull Laboratories) at 0.1–100 μM. Cell viability was determined by staining with 2 μg/ml PI and analysis on a FACScan (Becton Dickinson). Alternatively, flow cytometry was performed on cells stained cells with FITC-conjugated Annexin-V. To measure mitochondrial transmembrane potential, cultured cells stained for 15 min with 40 nM 3,3′dihexyloxacarbocyanine iodide (DiOC6(3); Molecular Probes) were subjected to flow cytometry. The caspase inhibitors IDN-1965, IDN-6275 (gifts of K. Tomaselli and T. Oltersdorf, IDUN Pharmaceuticals, San Diego, CA), Q-VD-OPh (ICN Biomedicals), and zVAD-fmk (Bachem), as well as the inhibitors zVF-CHO, PD150606, ALLN, ALLM, and z-FG-NHO-Bz-pOMe (all from Calbiochem), were all solubilized in DMSO. Calpastatin peptide (Calbiochem) was solubilized in water.
Subcellular fractionation and Western blotting
Subcellular fractionation and Western blotting were performed as described previously (Marsden et al., 2002).
Online supplemental material
Western blot analysis of thymocytes (Fig. S1 A) and splenocytes (Fig. S1 B) from reconstituted animals demonstrates that no compensatory increase in expression of other caspases was evident in cells lacking caspase-2 and/or caspase-9.
Combined deficiency of caspases 2 and 9 in thymocytes does not prevent apoptosis in response to a variety of death stimuli. Thymocytes were subjected to cytokine withdrawal (Fig. S2 A), cultured after graded doses of γ irradation (Fig. S2 B), or treated with dexamethasone, PMA, or etoposide (Fig. S2, C–H). Caspase-2 deficiency did not influence the sensitivity to these stimuli of cells lacking or expressing caspase-9.
Western blotting of thymocytes sorted from reconstituted mice demonstrates that in dying cells the caspase substrates spectrin (Fig. S3 A) and gelsolin (Fig. S3 B) are processed to the expected caspase-generated products. Cell extracts were also made from cells of the indicated genotypes cultured with or without the caspase inhibitor IDN-1965. Western blotting of these extracts shows the caspase dependence of gelsolin processing in dying cells (Fig. S3 C). Caspase-like DEVDase activity was measured by fluorogenic assay (Fig. S3 D). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200312030/DC1.
Acknowledgments
We thank Dr. K. Kuida for caspase-9+/− mice; Drs. K. Tomaselli and T. Oltersdorf for caspase inhibitors; Drs. L. O'Reilly (antibodies to caspase-2; The Walter and Eliza Hall Institute), R. Anderson (antibodies to HSP70; Peter MacCallum Cancer Centre, Melbourne, Australia), Y. Lazebnik (antibodies to caspases-3, -7, and -9; Cold Spring Harbor Laboratory, Cold Spring Harbor, NY), P. Vandenabeele and M. Kalai (antibodies to caspase-1; University of Ghent, Ghent, Belgium), and D. Kwiatkowski (antibody to gelsolin; Harvard Medical School, Boston, NY); M. Pakusch for technical assistance; J. Morrow, C. Tilbrook, and A. Naughton for animal care; and Dr. F. Battye, D. Kaminaris, V. Lapatis, and C. Tarlinton for cell sorting. We are grateful to Drs. L. Coultas, S. Cory, R. Kluck, T. Thomas, and A. Voss for discussions.
This work was supported by the National Health and Medical Research Council, the Leukemia and Lymphoma Society (SCOR Center), the Cancer Council Victoria, the National Institutes of Health, the Cancer Research Institute, the Commonwealth Dept. of Education, Science and Training and the University of Melbourne.
Abbreviation used in this paper: PI, propidium iodide.
References
- Adams, J.M. 2003. Ways of dying: multiple pathways to apoptosis. Genes Dev. 17:2481–2495. [DOI] [PubMed] [Google Scholar]
- Altznauer, F., S. Conus, A. Cavalli, G. Folkers, and H.U. Simon. 2004. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J. Biol. Chem. 279:5947–5957. [DOI] [PubMed] [Google Scholar]
- Bergeron, L., G.I. Perez, G. Macdonald, L. Shi, Y. Sun, A. Jurisicova, S. Varmuza, K.E. Latham, J.A. Flaws, J.C. Salter, et al. 1998. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12:1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel, E., D.D. Newmeyer, and D.R. Green. 1998. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet, P., D. Metcalf, D.C.S. Huang, D.M. Tarlinton, T.W.H. Kay, F. Köntgen, J.M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- Caserta, T.M., A.N. Smith, A.D. Gultice, M.A. Reedy, and T.L. Brown. 2003. Q-VDOPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 8:345–352. [DOI] [PubMed] [Google Scholar]
- Ding, W.X., H.M. Shen, and C.N. Ong. 2002. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem. Biophys. Res. Commun. 291:321–331. [DOI] [PubMed] [Google Scholar]
- Ekert, P.G., S.H. Read, J. Silke, V.S. Marsden, H. Kaufmann, C.J. Hawkins, R. Gerl, S. Kumar, and D.L. Vaux. 2004. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J. Cell Biol. 165:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y., S.M. Srinivasula, A. Druilhe, T. Fernandes-Alnemri, and E.S. Alnemri. 2002. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 277:13430–13437. [DOI] [PubMed] [Google Scholar]
- Hakem, R., A. Hakem, G.S. Duncan, J.T. Henderson, M. Woo, M.S. Soengas, A. Elia, J.L. de la Pompa, D. Kagi, W. Khoo, et al. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 94:339–352. [DOI] [PubMed] [Google Scholar]
- Hilbi, H., J.E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R.A. Flavell, J. Yuan, P.J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895–32900. [DOI] [PubMed] [Google Scholar]
- Hisahara, S., J. Yuan, T. Momoi, H. Okano, and M. Miura. 2001. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J. Exp. Med. 193:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarpour, N., K. Tabuchi, J.M. Stark, R.E. Hammer, T.C. Sudhof, L.F. Parada, X. Wang, J.A. Richardson, and J. Herz. 2001. Embryonic neuronal death due to neurotrophin and neurotransmitter deprivation occurs independent of Apaf-1. Neuroscience. 106:263–274. [DOI] [PubMed] [Google Scholar]
- Jaattela, M., and J. Tschopp. 2003. Caspase-independent cell death in T lymphocytes. Nat. Immunol. 4:416–423. [DOI] [PubMed] [Google Scholar]
- Kang, S.J., S. Wang, K. Kuida, and J. Yuan. 2002. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 9:1115–1125. [DOI] [PubMed] [Google Scholar]
- Kilic, M., R. Schafer, J. Hoppe, and U. Kagerhuber. 2002. Formation of noncanonical high molecular weight caspase-3 and -6 complexes and activation of caspase-12 during serum starvation induced apoptosis in AKR-2B mouse fibroblasts. Cell Death Differ. 9:125–137. [DOI] [PubMed] [Google Scholar]
- Kuida, K., T.F. Haydar, C.-Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M.S.-S. Su, P. Rakic, and R.A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 94:325–337. [DOI] [PubMed] [Google Scholar]
- Lassus, P., X. Opitz-Araya, and Y. Lazebnik. 2002. Requirement for caspase-2 in stress induced apoptosis before mitochondrial permeabilization. Science. 297:1352–1354. [DOI] [PubMed] [Google Scholar]
- Marsden, V., and A. Strasser. 2003. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21:71–105. [DOI] [PubMed] [Google Scholar]
- Marsden, V., L. O'Connor, L.A. O'Reilly, J. Silke, D. Metcalf, P. Ekert, D.C.S. Huang, F. Cecconi, K. Kuida, K.J. Tomaselli, et al. 2002. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 419:634–637. [DOI] [PubMed] [Google Scholar]
- McIlroy, D., H. Sakahira, R.V. Talanian, and S. Nagata. 1999. Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene. 18:4401–4408. [DOI] [PubMed] [Google Scholar]
- Methot, N., J. Huang, N. Coulombe, J.P. Vaillancourt, D. Rasper, J. Tam, Y. Han, J. Colucci, R. Zamboni, S. Xanthoudakis, et al. 2004. Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics. J. Exp. Med. 199:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima, N., K. Nakanishi, H. Takenouchi, T. Shibata, and Y. Yasuhiko. 2002. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277:34287–34294. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T., H. Zhu, N. Morishima, E. Li, J. Xu, B.A. Yankner, and J. Yuan. 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 403:98–103. [DOI] [PubMed] [Google Scholar]
- Nicholson, D.W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028–1042. [DOI] [PubMed] [Google Scholar]
- O'Reilly, L.A., P. Ekert, N. Harvey, V. Marsden, L. Cullen, D.L. Vaux, G. Hacker, C. Magnusson, M. Pakusch, F. Cecconi, et al. 2002. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ. 9:832–841. [DOI] [PubMed] [Google Scholar]
- Rao, R.V., S. Castro-Obregon, H. Frankowski, M. Schuler, V. Stoka, G. del Rio, D.E. Bredesen, and H.M. Ellerby. 2002. Coupling endoplasmic reticulum stress to the cell death program: an Apaf-1-independent intrinsic pathway. J. Biol. Chem. 277:21836–21842. [DOI] [PubMed] [Google Scholar]
- Robertson, J.D., M. Enoksson, M. Suomela, B. Zhivotovsky, and S. Orrenius. 2002. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 277:29803–29809. [DOI] [PubMed] [Google Scholar]
- Rowe, S.J., L. Allen, V.C. Ridger, P.G. Hellewell, and M.K.B. Whyte. 2002. Caspase-1 deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J. Immunol. 169:6401–6407. [DOI] [PubMed] [Google Scholar]
- Slee, E.A., C. Adrain, and S.J. Martin. 2001. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320–7326. [DOI] [PubMed] [Google Scholar]
- Squier, M.K.T., and J.J. Cohen. 1997. Calpain, an upstream regulator of thymocyte apoptosis. J. Immunol. 158:3690–3697. [PubMed] [Google Scholar]
- Wu, J.C., and L.C. Fritz. 1999. Irreversible caspase inhibitors: tools for studying apoptosis. Methods. 17:320–328. [DOI] [PubMed] [Google Scholar]