Abstract
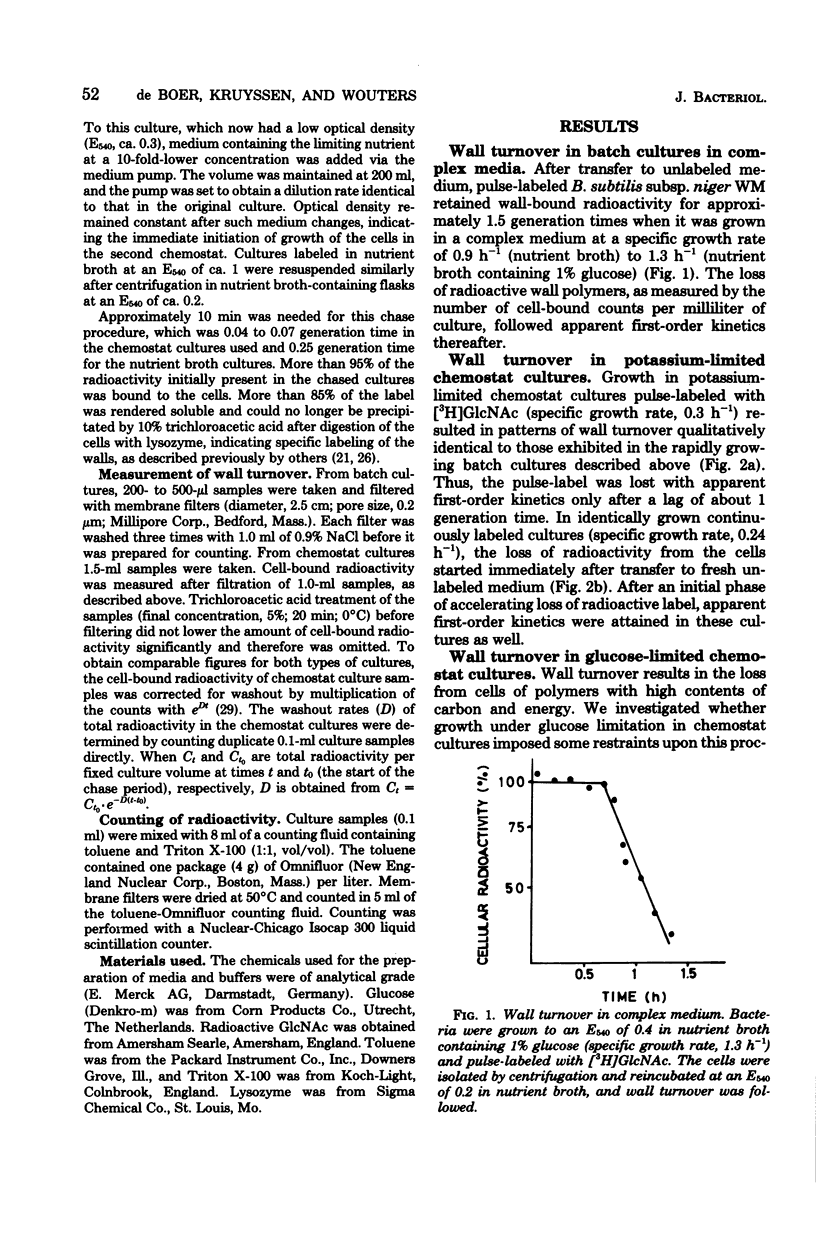
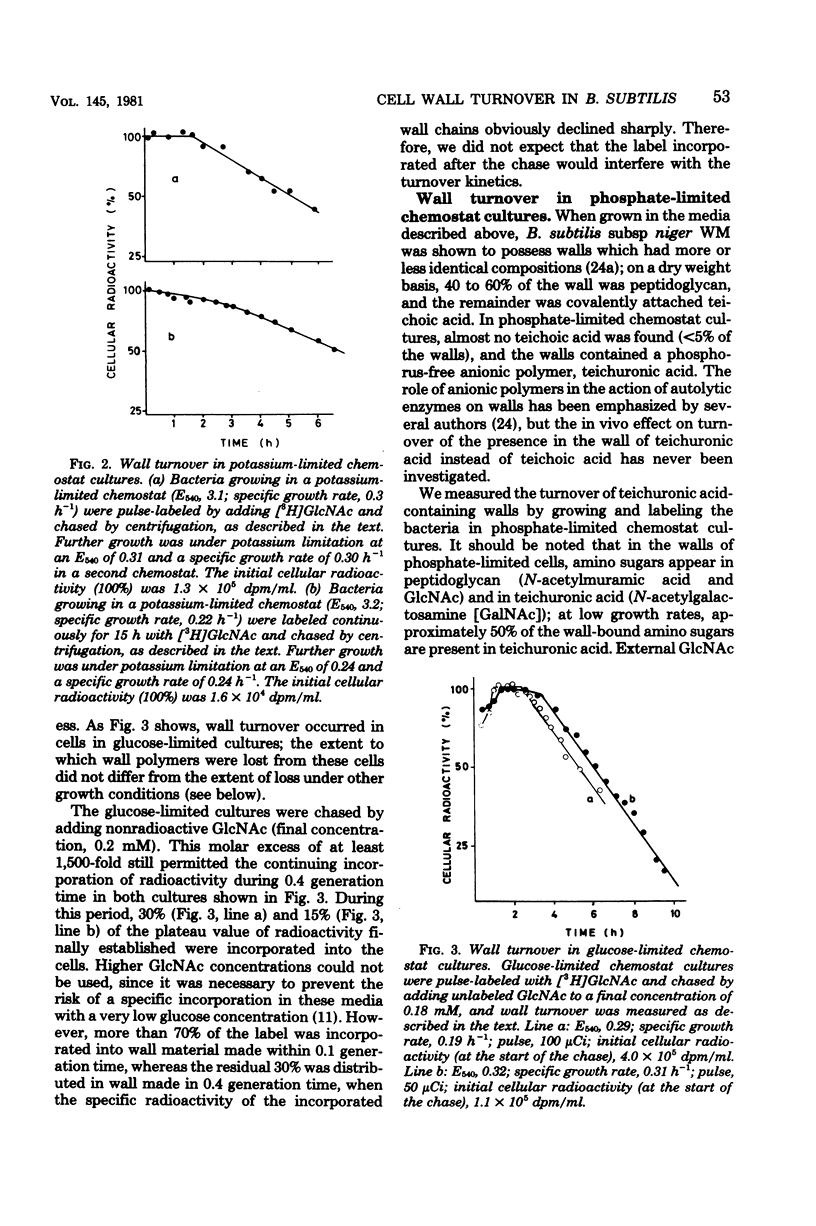
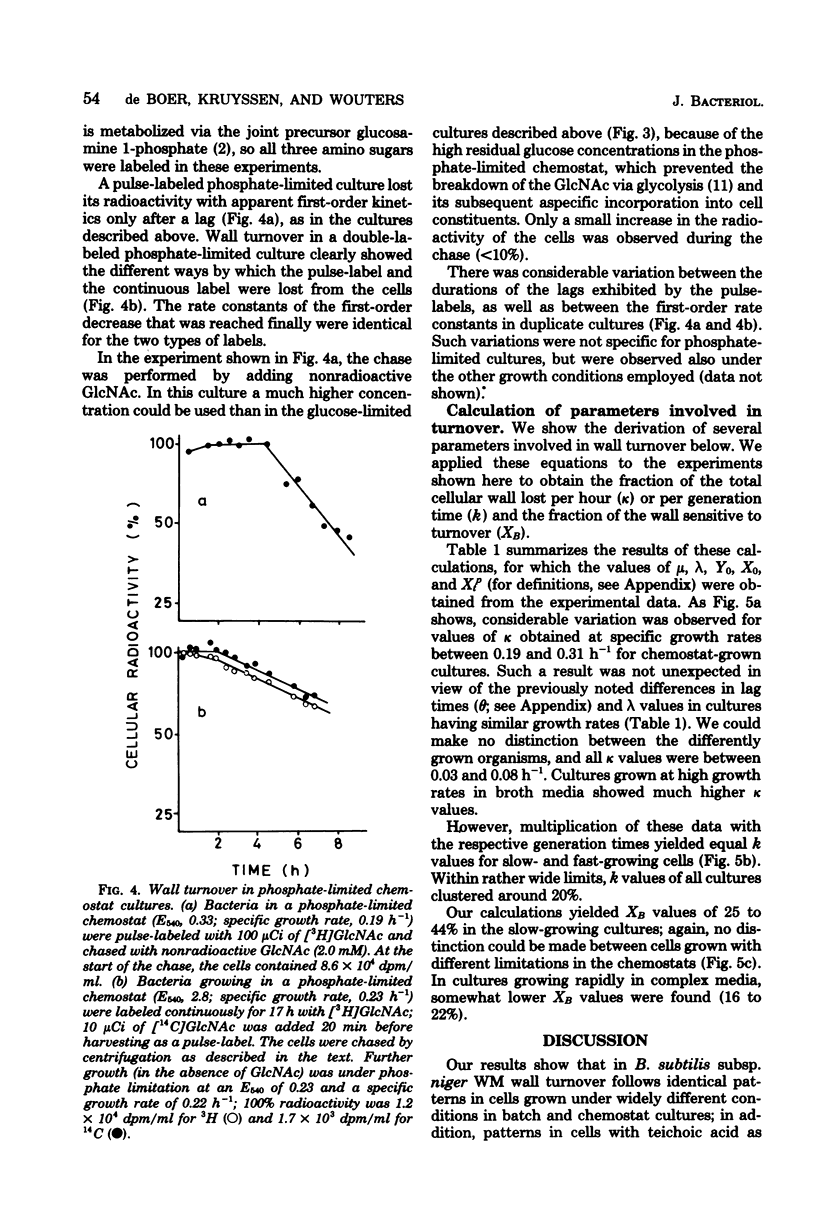
Wall turnover was studied in Bacillus subtilis. The loss of radioactively labeled wall polymers was followed during exponential growth in batch and chemostat cultures. Turnover kinetics were identical under all growth conditions; pulse-labeled wall material was lost with first-order kinetics, but only after exponential growth for 1 generation time after its incorporation. Similarly, continuously labeled cells showed an accelerating decrease in wall-bound radioactivity starting immediately after removal of the labeled precursor and also reached first-order kinetics after 1 generation time. A mathematical description was derived for these turnover kinetics, which embraced the concept of "spreading" of old wall chains (H. M. Pooley, J. Bacteriol. 125:1127-1138, 1976). Using this description, we were able to calculate from our experimental data the rate of loss of wall polymers from cells and the fraction of the wall which was sensitive to turnover. We found that about 20% of the wall was lost per generation time and that this loss was affected by turnover activity located in the outer 20 to 45% of the wall; rather large variations were found with both quantities and also between duplicate cultures. These parameters were quite independent of the growth rate (the specific growth rate varied from 1.3 h-1 in broth cultures to 0.2 to 0.3 h-1 in chemostat cultures) and of the nature of the anionic polymer in the wall (which was teichoic acid in cultures with an excess of phosphate and teichuronic acid in phosphate-limited chemostat cultures). Some implications of the observed wall turnover kinetics for models of wall growth in B. subtilis are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Green R. S., Sturman A. J., Archibald A. R. Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and concanavalin A, and its susceptibility to turnover. J Bacteriol. 1978 Dec;136(3):886–899. doi: 10.1128/jb.136.3.886-899.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. THE INCORPORATION OF LABELLED AMINO SUGARS BY BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:155–158. doi: 10.1042/bj0960155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Burge R. E., Adams R., Balyuzi H. H., Reaveley D. A. Structure of the peptidoglycan of bacterial cell wassl. II. J Mol Biol. 1977 Dec 25;117(4):955–974. doi: 10.1016/s0022-2836(77)80007-7. [DOI] [PubMed] [Google Scholar]
- Burge R. E., Fowler A. G., Reaveley D. A. Structure of the peptidogylcan of bacterial cell walls. I. J Mol Biol. 1977 Dec 25;117(4):927–953. doi: 10.1016/s0022-2836(77)80006-5. [DOI] [PubMed] [Google Scholar]
- CHALOUPKA J., RIHOVA L., KRECKOVA P. DEGRADATION AND TURNOVER OF BACTERIAL CELL WALL MUCOPEPTIDES IN GROWING BACTERIA. Folia Microbiol (Praha) 1964 Jan;24:9–15. doi: 10.1007/BF02875894. [DOI] [PubMed] [Google Scholar]
- CLARKE J. S., PASTERNAK C. A. The regulation of amino sugar metabolism in Bacillus subtilis. Biochem J. 1962 Jul;84:185–191. doi: 10.1042/bj0840185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Characterization of degradation products of the cell wall released during growth and sporulation of Bacillus megaterium. Folia Microbiol (Praha) 1974;19(4):292–300. doi: 10.1007/BF02873221. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Doyle R. J., Streips U. N. A proposed functional role for bacterial N-acetylmuramyl-L-alanine amidases. J Theor Biol. 1977 Oct 7;68(3):385–390. doi: 10.1016/0022-5193(77)90067-4. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Dickens B. F., Ingram L. O. Peptidoglycan synthesis and turnover in cell division mutants of Agmenellum. J Bacteriol. 1976 Jul;127(1):334–340. doi: 10.1128/jb.127.1.334-340.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. S., Ward J. B., Rogers H. J. Formation of cell wall polymers by reverting protoplasts of Bacillus licheniformis. J Bacteriol. 1975 Nov;124(2):623–632. doi: 10.1128/jb.124.2.623-632.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. New centrifugation technique for isolating enzymes from large cell structures: isolation and characterization of two Bacillus subtilis autolysins. J Bacteriol. 1972 Mar;109(3):1258–1265. doi: 10.1128/jb.109.3.1258-1265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Ryter A. Peptidoglycan turnover during growth of a Bacillus megaterium Dap- Lys- mutant. J Bacteriol. 1979 Feb;137(2):947–955. doi: 10.1128/jb.137.2.947-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Lindsay B. Relation between cell wall turnover and cell growth in Bacillus subtilis. J Bacteriol. 1977 May;130(2):610–619. doi: 10.1128/jb.130.2.610-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Kruyssen F. J., de Boer W. R., Wouters J. T. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger. J Bacteriol. 1980 Oct;144(1):238–246. doi: 10.1128/jb.144.1.238-246.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Polley H. M., Schlaeppi J. M., Karamata D. Localised insertion of new cell wall in Bacillus subtilis. Nature. 1978 Jul 20;274(5668):264–266. doi: 10.1038/274264a0. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer W. R., Kruyssen F. J., Wouters J. T. Cell wall metabolism of Bacillus subtilis [proceedings]. Antonie Van Leeuwenhoek. 1979;45(2):315–317. doi: 10.1007/BF00418596. [DOI] [PubMed] [Google Scholar]



