Abstract
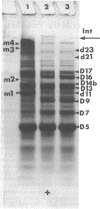
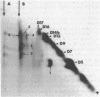
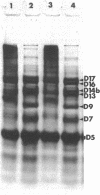
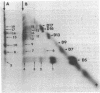
The arrangement of the amphiphilic protein spiralin and of the other major polypeptides in the Spiroplasma citri cell membrane was investigated by one- and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The analyses were performed on untreated membranes for the detection of disulfide bonds and on membranes treated with dimethylsuberimidate and dithiobis(succinimidyl propionate). All membranes were depleted of the bulk of extrinsic proteins. Spiralin monomers and oligomers (mainly dimers) were detected. Almost all the oligomers appeared to be stabilized by intermolecular disulfide bonds. Components D7 (39,000 daltons), D9 (51,000 daltons), D13 (69,000 daltons), D14b (76,000 daltons), D16 (89,000 daltons), and D17 (95,000 daltons), which are the other (presumably intrinsic) main polypeptides of the S. citri membrane, were also involved in homooligomers stabilized by disulfide bonds. However, in contrast to spiralin, larger amounts of D7, D9, and D14b were involved in high-molecular-weight multimers (molecular weight, greater than 400 X 10(3) after cross-linking with dithiobis(succinimidyl propionate). Extensive cross-linking with dimethylsuberimidate showed that spiralin was the polypeptide least readily integrated to large covalent complexes. These results suggest that spiralin probably does not form a two-dimensional network in the S. citri membrane depleted of the bulk of extrinsic proteins.
Full text
PDF
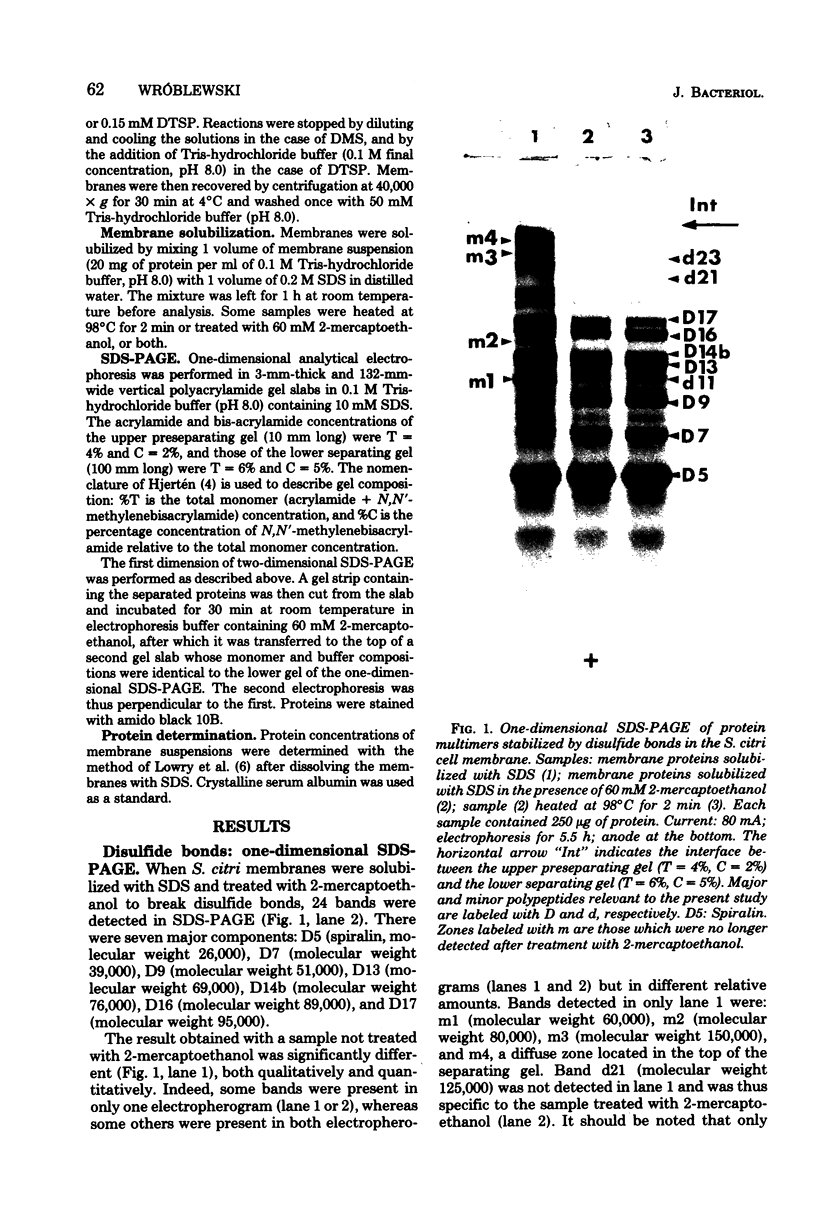



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole R. M., Tully J. G., Popkin T. J., Bové J. M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from "stubborn" disease of citrus. J Bacteriol. 1973 Jul;115(1):367–384. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Garoff H., Simons K. Solubilization of the Semliki Forest virus membrane with sodium deoxycholate. Biochim Biophys Acta. 1976 Jun 17;436(2):319–334. doi: 10.1016/0005-2736(76)90197-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Maddy A. H. A critical evaluation of the analysis of membrane proteins by polyacrylamide gel electrophoresis in the presence of dodecyl sulphate. J Theor Biol. 1976 Oct 21;62(2):315–326. doi: 10.1016/0022-5193(76)90123-5. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Razin S., Hasin M., Ne'eman Z., Rottem S. Isolation, chemical composition, and ultrastructural features of the cell membrane of the mycoplasma-like organism Spiroplasma citri. J Bacteriol. 1973 Dec;116(3):1421–1435. doi: 10.1128/jb.116.3.1421-1435.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Cross-linking of the proteins in the outer membrane of Escherichia coli. Biochim Biophys Acta. 1977 Apr 18;466(2):245–256. doi: 10.1016/0005-2736(77)90222-x. [DOI] [PubMed] [Google Scholar]
- Smith A. P., Loh H. H. Effect of bisimidate cross-linking reagents on synaptosomal plasma membrane. Biochemistry. 1978 May 2;17(9):1761–1765. doi: 10.1021/bi00602a029. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]
- Wróblewski H. Amphilphilic nature of spiralin, the major protein of the Spiroplasma citri cell membrane. J Bacteriol. 1979 Nov;140(2):738–741. doi: 10.1128/jb.140.2.738-741.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróblewski H., Johansson K. E., Hjérten S. Purification and characterization of spiralin, the main protein of the Spiroplasma citri membrane. Biochim Biophys Acta. 1977 Mar 1;465(2):275–289. doi: 10.1016/0005-2736(77)90079-7. [DOI] [PubMed] [Google Scholar]