Abstract
The exocyst is an octameric protein complex required to tether secretory vesicles to exocytic sites and to retain ER tubules at the apical tip of budded cells. Unlike the other five exocyst genes, SEC3, SEC5, and EXO70 are not essential for growth or secretion when either the upstream activator rab, Sec4p, or the downstream SNARE-binding component, Sec1p, are overproduced. Analysis of the suppressed sec3Δ, sec5Δ, and exo70Δ strains demonstrates that the corresponding proteins confer differential effects on vesicle targeting and ER inheritance. Sec3p and Sec5p are more critical than Exo70p for ER inheritance. Although nonessential under these conditions, Sec3p, Sec5p, and Exo70p are still important for tethering, as in their absence the exocyst is only partially assembled. Sec1p overproduction results in increased SNARE complex levels, indicating a role in assembly or stabilization of SNARE complexes. Furthermore, a fraction of Sec1p can be coprecipitated with the exoycst. Our results suggest that Sec1p couples exocyst-mediated vesicle tethering with SNARE-mediated docking and fusion.
Introduction
Carrier vesicles mediate the transport of proteins and lipids between the various compartments along the secretory and endocytic pathways of eukaryotic cells. Each donor compartment gives rise to a specific set of transport vesicles. To ensure fidelity in transport, each class of vesicles must recognize and fuse only with its correct target organelle. To achieve such specificity, each stage of transport relies on a set of proteins that form the core molecular machinery essential for membrane tethering, docking, and bilayer fusion. Some of the components involved, such as the Rabs, Sec1p/Munc18-like (SM), and SNARE proteins, are members of large protein families in which different members carry out related functions at different stages of transport within the cell (Toonen and Verhage, 2003; Ungar and Hughson, 2003).
The initial recognition and binding of the two membrane compartments has been termed tethering. Tethering contributes to the specificity of intracellular transport by linking only appropriate membranes to initiate their docking and fusion. In contrast to the Rabs, SM proteins, and SNAREs, the various tethering proteins that act at different stages of transport share little or no sequence homology. Each stage of transport thus appears to rely on a somewhat different molecular mechanism for tethering (Guo et al., 2000; Whyte and Munro, 2002).
The exocyst was first identified as a complex composed of eight different subunits required for exocytosis in yeast (TerBush and Novick, 1995; TerBush et al., 1996). One subunit, Sec3p, stably associates with the plasma membrane at specialized exocytic sites, whereas other subunits become localized only while secretory vesicles are delivered to these sites (Salminen and Novick, 1989; Ayscough et al., 1997; Finger et al., 1998). Therefore, we proposed that the exocyst complex assembles as vesicles arrive at the exocytic sites, and thereby establishes an initial connection between the plasma membrane and the secretory vesicles. Tethering may be regulated by Sec4p, a member of the Rab family of small GTPases. Rab GTPases play important roles in many aspects of membrane trafficking. In their GTP-bound form, Rabs bind to downstream effectors to regulate their function. Sec4p is present on the secretory vesicles and it binds to the Sec15p subunit of the exocyst in its GTP-bound form, and may thereby promote exocyst function (Walch-Solimena et al., 1997; Guo et al., 1999).
Productive membrane tethering is followed by membrane docking; a stronger, less reversible interaction of the two membrane bilayers engaged in fusion. Central to membrane docking is the function of the SNARE proteins (Ungar and Hughson, 2003). For each membrane fusion step at least one SNARE protein is embedded in the membrane of the transport vesicle (v-SNARE) and in the target membrane (t-SNARE). These SNARE proteins engage each other in a highly stable SNARE complex via their coiled-coil domains. As SNARE complexes form, the two membranes are pulled into very close proximity, which is necessary to initiate membrane fusion. In the yeast exocytic reaction, the v-SNARE Snc forms a complex with the two t-SNAREs, Sso and Sec9p (Rossi et al., 1997). In vitro, SNAREs alone are capable of mediating the fusion of liposomes, albeit at a nonphysiologically slow rate (Weber et al., 1998). However, in vivo, SNAREs are likely to work together with other essential factors to promote membrane fusion.
The closest functional link to SNARE-mediated docking and membrane fusion has so far been demonstrated for the SM family of proteins (Toonen and Verhage, 2003). SM family proteins are essential for membrane fusion and, like SNAREs, act downstream of the tethering reaction. Furthermore, they bind to the SNARE protein(s) that act at the corresponding stage of membrane traffic (Carr et al., 1999; Sato et al., 2000; Yamaguchi et al., 2002). Sec1p is essential for yeast exocytosis and binds to the Sso/Sec9p t-SNARE complex (Scott et al., 2004) as well as the fully assembled Snc/Sso/Sec9p SNARE complex (Carr et al., 1999). However, unlike several other SM family proteins, Sec1p does not bind directly to the unassembled syntaxin-like t-SNARE, Sso. The role of Sec1p and other SM family proteins in SNARE function remains incompletely understood; however, stimulation of SNARE-mediated liposome fusion by Sec1p was recently demonstrated (Scott et al., 2004).
In addition to its essential role in vesicle fusion, the yeast exocytic apparatus is also required for the proper targeting of secretory vesicles to specific subdomains of the plasma membrane. As the sites of exocytosis shift with the cell cycle, so too must the localization of the exocytic machinery. Polarized targeting of yeast secretory vesicles is a two-step process. Secretory vesicles bearing activated Sec4p are transported along actin cables toward regions of active surface growth, such as the bud early in the cell cycle or the neck separating the mother and daughter cell late in the cycle (Walch-Solimena et al., 1997; Pruyne et al., 1998). The exocyst proteins then further restrict the tethering of these vesicles to small subdomains of the plasma membrane; for example to the apical bud tip during early bud formation (Finger et al., 1998; Wiederkehr et al., 2003). The localization of Sec4p, most exocyst subunits, and Sec1p to sites of polarized secretion depends on each other in a hierarchical manner that reflects their order of function in the fusion reaction. Thus, inhibition of Sec4p function leads to a loss of polarized localization of secretory vesicles, the exocyst subunit Sec8p and Sec1p, whereas loss of exocyst function blocks proper localization of Sec1p (Ayscough et al., 1997; Walch-Solimena et al., 1997; Finger et al., 1998; Carr et al., 1999; Grote et al., 2000). The exception to this rule is the exocyst subunit Sec3p. Its localization to sites of exocytosis is independent of the function of both the secretory pathway and the actin cytoskeleton, and Sec3p was therefore proposed to act as a spatial landmark, defining exocytic sites (Finger et al., 1998). SEC3 is also unique among the structural genes encoding exocyst subunits in that, under certain conditions, it is not essential for growth and secretion (Wiederkehr et al., 2003). Consistent with its role as a spatial landmark, Sec3p is required for the correct targeting of the exocyst and secretory vesicles during polarized secretion.
Surprisingly, the absence of the Sec3p protein also leads to a defect in the inheritance of the ER into the yeast bud (Wiederkehr et al., 2003). ER tubules form and are delivered into the bud, but fail to be anchored at the tip and ultimately recede back into the mother cell. Although the molecular details of the connection between Sec3p and the ER are still unclear, the results suggest that this role of Sec3p is not directly connected to its role in exocytosis.
Here, we find a close functional connection between Sec1p, Sec4p, and the exocyst in yeast exocytosis. Overproduction of Sec1p or Sec4p not only rescues the partial secretion defect of the sec3Δ mutant, but also bypasses the need for the otherwise essential exocyst genes, SEC5 and EXO70. The sec3Δ, sec5Δ, and exo70Δ mutants differ with respect to their phenotypes, suggesting subunit-specific roles in vesicle targeting and ER inheritance. Sec1p overproduction increases the levels of SNARE complexes in vivo, which could explain mechanistically how Sec1p is able to promote exocytosis downstream of a partially defective exocyst. We also find that Sec1p binds to the exocyst and may thus establish a functional link between membrane tethering and SNARE-mediated vesicle docking.
Results
Sec1p and Sec4p suppress the growth and secretion defect of a sec3Δ mutant
Recent results have demonstrated that several tethering complexes can physically interact with the Rab proteins, SM proteins, and t-SNAREs that act in the corresponding fusion reaction (Guo et al., 1999; Sato et al., 2000; Seals et al., 2000; Siniossoglou and Pelham, 2002). For example, Sec4p was found to interact with the exocyst subunit Sec15p (Guo et al., 1999). Furthermore, Sec4p, the exocyst, and Sec1p are all concentrated at sites of polarized secretion (Walch-Solimena et al., 1997; Finger et al., 1998; Carr et al., 1999). We speculate that in addition to its function in membrane tethering, the exocyst may also bring together the various components of the exocytic machinery to facilitate exocytosis. Sec3p is the only nonessential subunit of the exocyst, and it plays a role mainly in targeting secretory vesicles to subdomains of the plasma membrane (Wiederkehr et al., 2003). The absence of Sec3p also leads to a partial defect in exocytosis that may be the result of the inability of this mutant strain to concentrate essential protein components at sites of polarized secretion. Hence, overproduction of a limiting component might bypass the need for Sec3p or possibly other exocyst subunits in exocytosis. Therefore, we tested whether overproduction of Sec1p, Sec4p, or the t-SNAREs, Sso or Sec9p, could suppress the slow growth and partial secretion defect of a sec3Δ mutant.
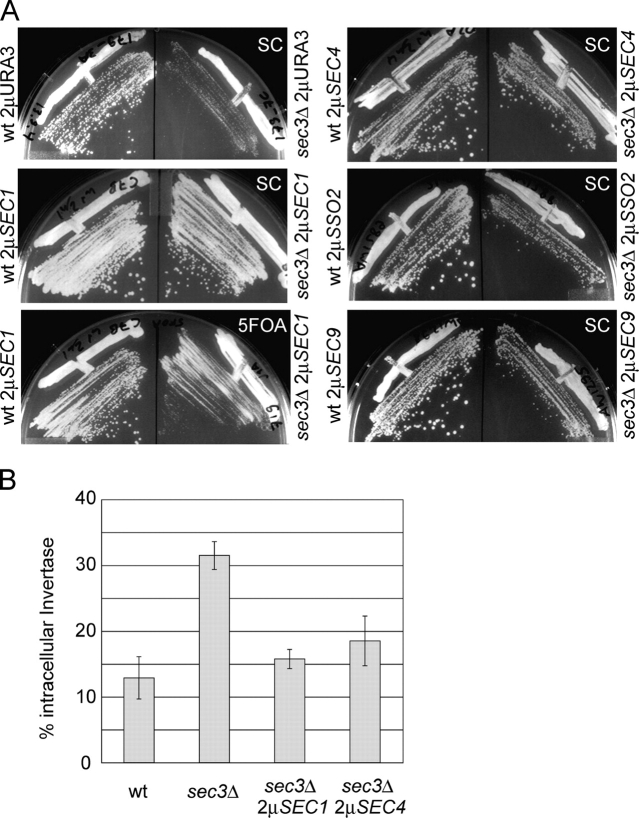
Multi-copy plasmids used to overexpress the genes of interest were introduced into a sec3Δ/SEC3 heterozygous diploid strain. The transformants were then sporulated and dissected. After dissection and marker analysis, wild-type and sec3Δ mutant haploids that retained the URA3 based multi-copy plasmid were struck out for single colonies on synthetic complete (SC)-Ura plates at 25°C. Overproduction of Sec1p or Sec4p clearly suppressed the growth defect of sec3Δ cells (Fig. 1 A and Table I). However, the suppressed sec3Δ strains remain temperature-sensitive at 37°C (unpublished data and Table I). As expected, a control strain overproducing Sec3p restored growth at all temperatures. An empty plasmid control (Fig. 1 A) or multi-copy plasmids carrying SEC5 or SEC6, encoding two other subunits of the exocyst, had no effect on sec3Δ growth. Interestingly, the multi-copy SSO2 or SEC9 plasmids also improved sec3Δ growth but less strikingly than either SEC1 or SEC4 (Fig. 1 A). These genetic results show that Sec1p, Sec4p and, to a lesser extent SNAREs, can compensate for the absence of Sec3p from the exocyst complex suggesting a functional connection between the exocyst, Sec1p, Sec4p, and SNARE proteins.
Figure 1.
Sec1p and Sec4p overexpression stimulate growth and secretion of a sec3Δ mutant strain. (A) Wild-type (left) and sec3Δ mutant strains (right) carrying the indicated multi-copy (2μ) plasmids were grown on SC plates for 3 d at 25°C. After the loss of the 2μSEC1 plasmid on the 5FOA plate (bottom left), the previously suppressed sec3Δ strain is slow growing comparable to the strain with the control empty plasmid (top left). (B) Invertase secretion was measured in wild-type, sec3Δ, and sec3Δ strains suppressed by either 2μSEC1 or 2μSEC4. To derepress invertase, the cells were shifted to low glucose (0.1%) containing SC medium at 25°C. After 90 min incubation in this medium, invertase activity was measured. The graph shows the amount of intracellular invertase as a percentage of the total newly synthesized invertase. For each strain the average and SD from four independent experiments are shown.
Table I. Growth rates.
| Genotype
|
Doubling time at 25°C
|
Increase over wild-type
|
Growth at 37°C |
|---|---|---|---|
| min | % | ||
| wild-type | 180 ± 9 | 0 | yes |
| wild-type 2μURA3 empty | 177 ± 4 | −2 | yes |
| sec3Δ | 272 ± 10 | 34 | no |
| sec3Δ 2μURA3 empty | 278 ± 7 | 35 | no |
| sec3Δ 2μSEC1 | 188 ± 14 | 5 | no |
| sec3Δ 2μSEC4 | 202 ± 14 | 11 | no |
| sec5Δ 2μSEC1 | 240 ± 23 | 25 | no |
| sec5Δ 2μSEC4 | 254 ± 17 | 29 | no |
| exo70Δ 2μSEC1 | 187 ± 12 | 4 | yes |
| exo70Δ 2μSEC4 | 233 ± 7 | 23 | yes (very slow) |
Phenotypic characterization of the suppressed sec3Δ mutant strains
As mentioned earlier in this paper, sec3Δ cells have a partial defect in secretion. Therefore, we tested the extent to which the defect in the secretion of the derepressible, secreted protein invertase was suppressed when either Sec1p or Sec4p were overproduced. Overproduction of either Sec1p or Sec4p clearly improved secretion from a sec3Δ strain (Fig. 1 B). The sec3Δ strain accumulated 32% of the newly synthesized invertase in an intracellular pool, whereas in a wild-type strain only 13% was intracellular, corresponding to the amount of invertase in transit along the secretory pathway. In the sec3Δ strain overproducing Sec1p, the average value of intracellular invertase measured was 16%, which is not significantly different from the wild type. In the sec3Δ strain overproducing Sec4p, slightly more (18.5%) of the invertase was intracellular after derepression. Sec1p and Sec4p efficiently suppress the secretion defect of sec3Δ strains and allow them to grow at almost wild-type rates (Fig. 1 and Table I).
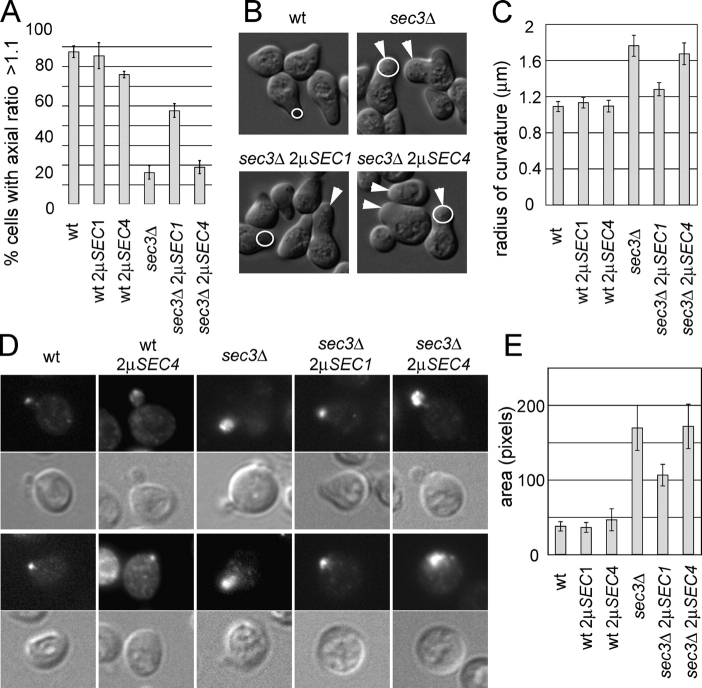
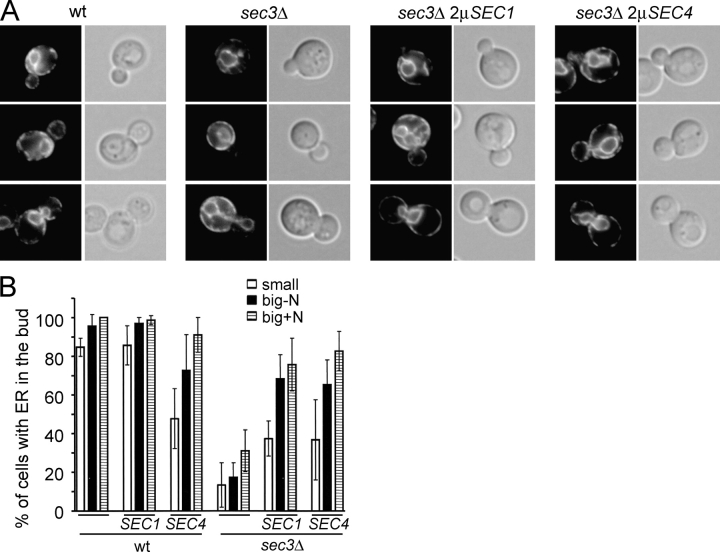
In an earlier study we found several phenotypes of sec3Δ cells suggesting a defect in polarized secretion. Although Sec4p is concentrated in a very small area at the bud tip of wild-type cells, it is broadly distributed in the buds of sec3Δ cells. Unlike the elongated wild-type cells, sec3Δ cells are also round and are unable to extend normal mating projections (Wiederkehr et al., 2003). Therefore, we tested whether overproduction of Sec1p or Sec4p, in addition to stimulating secretion, would also restore the polarity of sec3Δ cells. The sec3Δ cells overproducing Sec4p were round and showed defects in mating projection formation similar to sec3Δ cells (Fig. 2, A–C). As Sec4p was overexpressed the Sec4p staining was stronger, but was still distributed broadly in the bud as in sec3Δ cells (Fig. 2, D and E). In a wild-type background, Sec4p overexpression did not significantly affect the focal localization of Sec4p in the bud, although a fraction of the cells expressing very high levels of Sec4p showed additional cytoplasmic Sec4p staining. Surprisingly, overproduction of Sec1p led to a partial restoration of these sec3Δ defects. A much larger fraction of sec3Δ cells overproducing Sec1p were elongated, similar to the morphology of wild-type cells (Fig. 2 A). The sec3Δ cells carrying the SEC1 multi-copy plasmid were also better at forming mating projections than sec3Δ cells, although quite a few cells in the culture still showed aberrant, rounded projections (Fig. 2, B and C). Sec4p localization remained partially delocalized in sec3Δ cells overexpressing Sec1p, but was more restricted at sites of polarized secretion than in the corresponding sec3Δ strain (Fig. 2, D and E). It was surprising to find that overproduction of Sec1p restored secretion to a similar extent as Sec4p, yet unlike Sec4p also partially restored polarity. The final parameter we examined was the inheritance of cortical ER into the yeast bud. The sec3Δ cells extend ER tubules into the bud, but the cortical ER fails to be established in the daughter cells. Overproduction of either Sec1p or Sec4p in the sec3Δ cells failed to completely restore inheritance of the ER into the bud. Although most small buds still lacked cortical ER, in both cases overproduction did improve ER inheritance, as a significant fraction of the cells were able to establish cortical ER by the time the cells were large budded (Fig. 3, A and B). Tubule number, dynamics, and orientation appeared normal (Table II).
Figure 2.
Polarity defects of suppressed sec3Δ strains. (A) Percentage of yeast mother cells with wild-type-like elongated morphology was determined as described in Materials and methods. For each dataset the yeast strains were grown in parallel and DIC images were taken to measure length to width ratios. The average and SD were derived from four independent datasets. At least 50 cells were measured per strain and dataset (total >200 cells per strain). (B) Comparison of morphology after α-factor treatment. The indicated yeast strains of mating type a were incubated in the presence of α-factor for 6 h in SC medium. DIC pictures of representative examples of shmooing yeast cells are shown. Arrowheads point to cells extending aberrant round shmoos typical for sec3Δ cells. (C) To quantitatively compare the shmoos of the different yeast mutants, the curvature of the shmoo tips was measured as indicated with the white circles in B. For each strain 100 shmoos were measured for the analysis. The mean radius of shmoo curvature with a 99% confidence interval is shown. (D) Sec4p localization in yeast buds (top panels) and the corresponding DIC pictures (bottom panels). (E) Sec4p-positive areas in the bud were measured. For each strain the mean values and the 99% confidence interval from 100 measured cells are shown.
Figure 3.
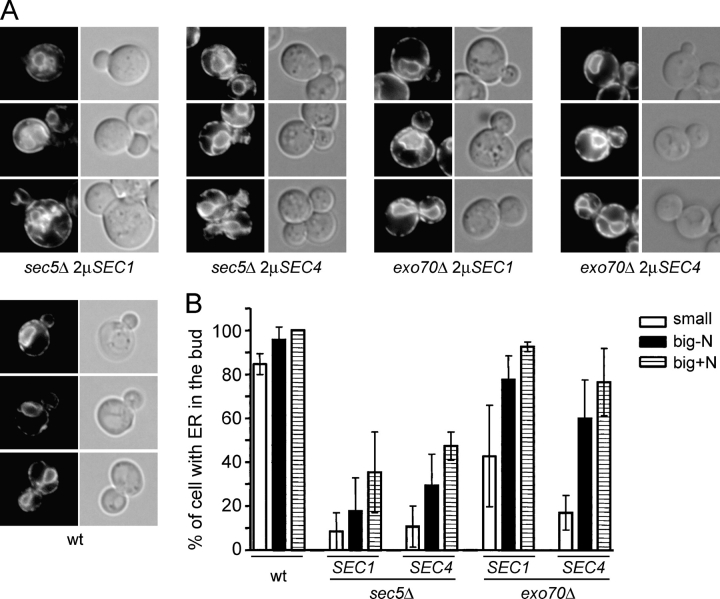
ER inheritance defects are partially restored in suppressed sec3Δ strains. (A) Hmg1-GFP (left) was used as a marker to follow the inheritance of the cortical ER during yeast bud growth of the indicated yeast strains. Corresponding DIC pictures (right) were used to determine the relative bud size. The cells were grouped into three categories: Small buds 0.3–0.5 times the diameter of the mother cell (top panels), buds with diameters larger than 0.5 times the mother cell (middle panels), and large buds where the nucleus had migrated into the bud but was still connected between the mother and daughter cell (bottom panels). (B) Quantification of the ER inheritance defect as described in Materials and methods. The graph shows the fraction of cells with small buds (white bars), large buds (black bars), and large buds containing nuclear elements (striped bars) that have established a cortical ER. For each strain and category >100 cells were examined.
Table II. ER tubule analysis.
| Strain description | Tubule number | Tubule dynamic | Tubule orientation |
|---|---|---|---|
| wt | 2 | + | + |
| wt 2μSEC1 | 2 | + | + |
| wt 2μSEC4 | 2 | + | + |
| sec3Δ | 3 | + | + |
| sec3Δ 2μSEC1 | 3 | + | + |
| sec3Δ 2μSEC4 | 2 | + | + |
| sec5Δ 2μSEC1 | 4 | + | + |
| sec5Δ 2μSEC4 | 2 | + | + |
| exo70Δ 2μSEC1 | 3 | + | + |
| exo70Δ 2μSEC4 | 3 | + | + |
Yeast strains were analyzed to determine the average number of ER tubules per cell, the tubule dynamic of each, and whether or not at least one tubule is oriented towards the bud. At least eight movies were analyzed for each strain (see supplemental materials, available at http://www.jcb.org/cgi/content/full/jcb.200408001/DC1).
Sec1p or Sec4p can bypass the requirement for Sec5p and Exo70p in exocytosis
Given the efficient suppression of the secretion defect of a sec3Δ mutant, we determined if overproduction of Sec1p or Sec4p could bypass the requirement for any other exocyst subunits. Dissection of sec6Δ/SEC6, sec8Δ/SEC8, sec10Δ/SEC10, sec15Δ/SEC15, or exo84Δ/EXO84 heterozygous diploids strains overproducing either Sec1p or Sec4p did not result in any viable haploid strains disrupted for these exocyst genes. However, dissection of sec5Δ/SEC5 and exo70Δ/EXO70 strains gave rise to viable haploid sec5Δ and exo70Δ strains in the presence of either the SEC1 or the SEC4 multi-copy plasmid (Fig. 4 A). The sec5Δ and exo70Δ strains were strictly dependent on Sec1p or Sec4p overproduction for viability. Tetrads in which the multi-copy plasmids were lost during sporulation only gave rise to two wild-type haploid strains. Furthermore, no sec5Δ and exo70Δ colonies were observed after selection against the URA3 plasmid marker on 5-fluoroorotic acid (5FOA) plates (Fig. 4 A). Only wild-type cells, which do not require the URA3-based plasmids, grew on SC plates containing 5FOA.
Figure 4.
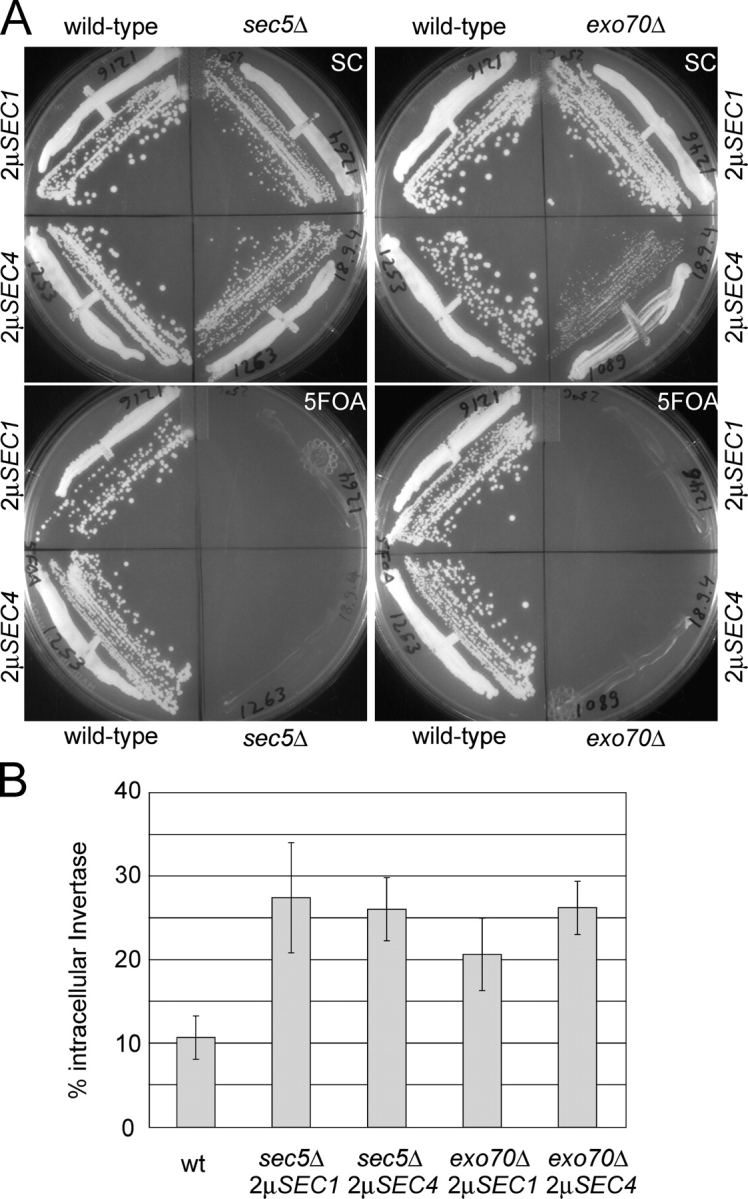
Sec1p or Sec4p overproduction can bypass a deletion of the essential exocyst genes SEC5 and EXO70. (A) Wild-type, sec5Δ, and exo70Δ strains were struck for single colonies on either SC or SC plates containing 5FOA (to select against the plasmid) as indicated. The growth of sec5Δ mutants (left plates) and exo70Δ (right plates) in the presence (SC plates) or absence of the plasmid (5FOA plates) was directly compared with the growth of a corresponding wild-type strain. On each plate the mutant strains are shown on the right. (B) Invertase secretion defects of suppressed sec5Δ and exo70Δ strains were measured as described in Fig. 1 B.
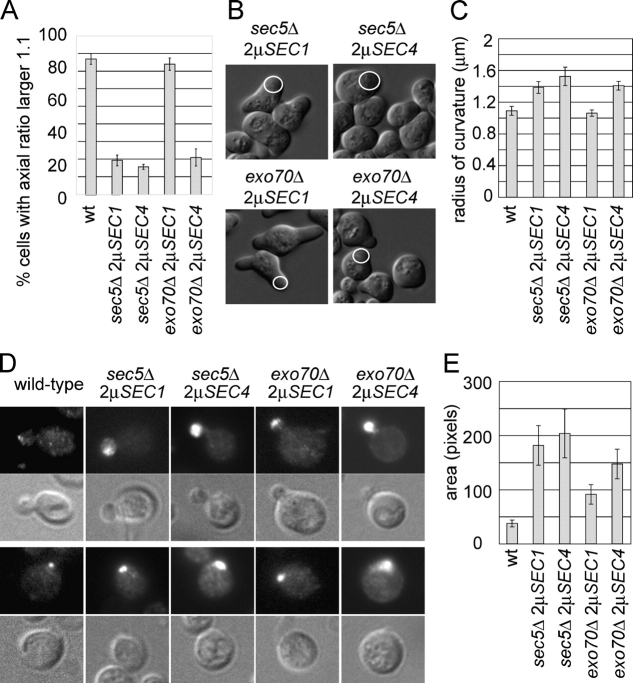
Sec1p and Sec4p are approximately equally efficient suppressors of the sec5Δ mutant (Fig. 4 A and Table I). However, in the case of the exo70Δ mutant, Sec1p was a clearly better suppressor than Sec4p (Fig. 4 A and Table I). The exo70Δ mutants overexpressing Sec1p grew very well, with a growth rate in liquid SC media close to that of the corresponding wild-type strain, and were not temperature sensitive for growth. In contrast, the exo70Δ mutant overexpressing Sec4p grows slowly both at 25 and 37°C. Both sec5Δ strains are tightly temperature sensitive for growth at 37°C (Table I).
Phenotypic analysis of sec5Δ and exo70Δ mutant strains
We used invertase as a marker to measure the secretory defects of these strains. All of the suppressed mutants had only a weak secretory defect, accumulating 20–30% of the derepressed invertase in an intracellular pool (Fig. 4 B). Improvement of secretion by Sec1p or Sec4p is a likely explanation for the restoration of viability of the sec5Δ and exo70Δ mutants.
Although the exocyst works as a complex in secretion, specific subunits might confer different aspects of exocyst function. Therefore, we tested whether Sec5p and Exo70p, like Sec3p, are required for polarized secretion and ER inheritance. Cells lacking SEC5 have the broad Sec4p distribution and morphology defects observed for the sec3Δ cells (Fig. 5). The sec5Δ mutants also have a severe ER inheritance defect, similar to the sec3Δ strain. At each stage during bud growth, a large fraction of the sec5Δ cells have little or no cortical ER (Fig. 6, A and B), although the number of tubules is equal or higher than in the wild-type cells and tubule dynamics and orientation appear normal (Table II). In summary, Sec5p appears to be as important for polarized secretion and ER inheritance as Sec3p. With regard to its Sec4p localization and morphology phenotypes, the exo70Δ strain overproducing Sec4p is similar to the sec3Δ and the suppressed sec5Δ mutant strains (Fig. 5). However, ER inheritance is only delayed in this mutant strain, as the defect is restricted to small budded cells (Fig. 6, A and B). By the time larger buds have formed, most exo70Δ cells have inherited cortical ER. Tubule number, dynamics, and orientation appear normal (Table II). This distinction from the sec3Δ and sec5Δ mutants is even more striking in an exo70Δ mutant overproducing Sec1p, where ER inheritance is close to normal even in small budded cells (Fig. 6, A and B). In contrast, the sec3Δ and sec5Δ mutant overproducing Sec1p have very dramatic defects in ER inheritance, suggesting that the function of the Exo70p is less directly linked to ER inheritance than Sec3p and Sec5p. In addition, exo70Δ cells overproducing Sec1p are mostly elongated, similar to wild-type yeast cells (Fig. 5 A). Furthermore, the mating projections of the exo70Δ strain overproducing Sec1p are even more pronounced than those of the wild-type cells or wild-type cells overproducing Sec1p. Of all the mutants analyzed here, Sec4p localization was most highly polarized in the exo70Δ 2μSEC1 cells, although compared with wild-type cells, Sec4p was still partially delocalized (Fig. 5, D and E). Sec1p overproduction appears to improve polarized secretion, as in both the sec3Δ and exo70Δ mutant backgrounds Sec1p, but not Sec4p, clearly improves the morphology of the cells. The differences observed for the various strains, especially when overproducing Sec1p, show that Exo70p contributes differently to polarized secretion and ER inheritance than do Sec3p or Sec5p (Table III). In summary, Sec5p and Exo70p carry out essential functions in the exocyst, but their function can be bypassed when secretion is stimulated by the overproduction of either Sec1p or Sec4p.
Figure 5.
Analysis of polarity phenotypes of the suppressed sec5Δ and exo70Δ strains. The phenotypic analysis was performed as described in Fig. 2. (A) The percentage of elongated cells. (B) DIC images of mutant cells with shmoos. (C) Curvature of shmoo tips. (D) Polarized localization of Sec4p in yeast buds. (E) Quantification of Sec4p distribution in the bud.
Figure 6.
Cortical ER inheritance in sec5Δ and exo70Δ mutant strains. The appearance of the ER marker Hmg1-GFP in the yeast bud during ER inheritance. (A) Hmg1-GFP fluorescence pictures (left) were taken at different stages of yeast bud growth. The three categories were as described in Fig. 3. DIC pictures of the small budded (top), large budded (middle), and large budded cells with nuclear ER (bottom) are shown on the right. (B) Quantification of the ER inheritance phenotype of suppressed sec5Δ and exo70Δ strains as described in Fig. 3 B.
Table III. Phenotypic analysis.
| Strain description | Secretion | Polarity | ER | SNARE |
|---|---|---|---|---|
| wt | + | + | + | + |
| wt 2μSEC1 | N.D. | + | + | ++ |
| wt 2μSEC4 | N.D. | + | +b | + |
| sec3Δ | +/− | − | − | +/− |
| sec3Δ 2μSEC1 | +a | +/− | +b | ++ |
| sec3Δ 2μSEC4 | +a | − | +b | +(/−) |
| sec5Δ 2μSEC1 | +/− | − | − | ++ |
| sec5Δ 2μSEC4 | +/− | − | − | +/− |
| exo70Δ 2μSEC1 | +/− | + | +b | ++ |
| exo70Δ 2μSEC4 | +/− | − | +c | + |
Yeast mutant strains are compared according to the following phenotypes: secretion of invertase; polarity phenotypes as shown in Fig. 2 and Fig. 5; inheritance of the cortical ER into the mother cells; and SNARE complex levels. N.D., not determined.
Secretion in these suppressed strains is close to normal (Fig. 1 B).
Mild ER inheritance defect.
Inheritance of cortical ER delayed but close to normal in cells with large buds (Fig. 6, A and B).
The sec3Δ, sec5Δ, and exo70Δ mutants show defects in exocyst assembly
Our working model of exocyst function is that the complex assembles to mediate vesicle tethering at the plasma membrane. By this model, the stably assembled exocyst assures that vesicles are tethered to the correct subdomain of the plasma membrane to allow the vesicles to undergo membrane fusion. Several of the temperature-sensitive exocyst mutants form a less stable complex or are missing specific subunits from the complex (TerBush and Novick, 1995). We analyzed the assembly state of the exocyst in the deletion mutants. For this purpose endogenous Sec8p was myc epitope tagged and isolated from different mutant backgrounds. In the absence of Sec3p, Sec5p, or Exo70p there was a clear reduction in the yield of exocyst subunits that were coprecipitated with Sec8myc (Fig. 7). A large fraction of the exocyst complex (50–80%) was isolated by immunoprecipitation of Sec8myc from a wild-type strain (Fig. 7 A, lane 4). Although similar amounts of Sec8myc were isolated from a sec3Δ strain, only 2–6% of Sec6p, Sec10p, or Sec15p was co-isolated (Fig. 7 A, lane 5). No background of exocyst subunits was observed when the isolation was conducted in parallel from an untagged control strain (Fig. 7 A, lane 3). These results demonstrate that in a sec3Δ strain only a small fraction of the exocyst is assembled and sufficiently stable to be isolated by immunoprecipitation. Therefore, Sec3p is important for exocyst assembly or stability. Overproduction of Sec1p or Sec4p improves secretion in a sec3Δ strain, but has no effect on the coprecipitation of the other exocyst subunits with Sec8myc (Fig. 7 A, lanes 6 and 7). Similar effects on exocyst assembly state were observed with the sec5Δ and exo70Δ mutants (Fig. 7, C and D). Only 2–6% of Sec6p, Sec10p, and Sec15p was co-isolated with Sec8myc from these strains, regardless of the suppressing plasmid (Fig. 7 C, lanes 6 and 12; Fig. 7 D, lane 6). Overproduction of Sec1p or Sec4p in a wild-type background had no effect on exocyst isolation (Fig. 7 C, lanes 5 and 11).
Figure 7.
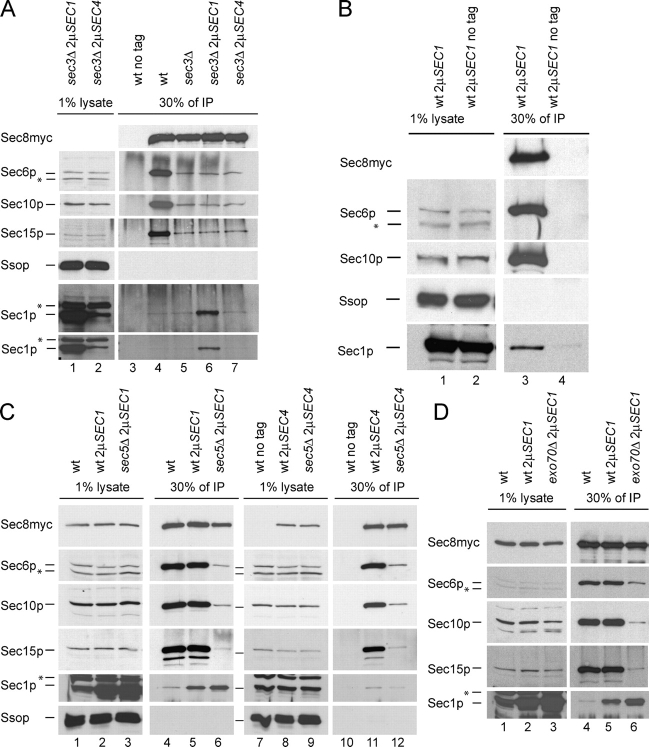
Co-isolation of Sec1p and exocyst subunits with myc epitope tagged Sec8p. The exocyst was isolated from lysates of wild-type (B and controls in A, C, and D), sec3Δ (A), sec5Δ (C), and exo70Δ (D) mutant strains as described in Materials and methods. Except for the control strains (no tag), all strains express Sec8p with a carboxy-terminal 13myc epitope. For the exocyst isolation, myc epitope–tagged Sec8p was immunoprecipitated with a monoclonal myc antibody. Co-immunoprecipitation of Sec1p, Sec6p, Sec10p, Sec15p, and Ssop was detected by Western blotting. Mutant genotypes (wt, sec3Δ, sec5Δ, or exo70Δ) and the presence of multi-copy plasmids (2μSEC1 or 2μSEC4) are indicated on the top of each lane. 30% of the immunoprecipitates (IP, right panels) is compared with 1% of the lysates (left panels). Major nonspecific bands in the lysates are marked with an asterisk. Two exposures of the Sec1p Western blot are shown in A.
The above results concerning exocyst assembly in different mutant backgrounds are consistent with two possible interpretations. Either in these mutants Sec8myc binds more weakly to an otherwise fully assembled exocyst, or the absence of Sec3p, Sec5p, or Exo70p has a more global effect on the binding of exocyst subunits to each other. To distinguish between these possibilities, we also isolated the exocyst using a myc tag on Sec10p, another subunit of the exocyst. In a wild-type background, isolation of the exocyst using Sec10myc was similarly efficient as with Sec8myc. A large fraction of Sec6p, Sec8p, and Sec15p was co-isolated with the Sec10myc subunit from a wild-type lysate. However, ∼10 times less Sec6p or Sec8p was co-isolated with Sec10myc from lysates of the different mutant strains (Fig. 8, lanes 5, 8, and 13). The results show that the exocyst is largely unassembled or less stably assembled in these mutant strains. Nonetheless, in all cases Sec10p still efficiently bound Sec15p. The amount of Sec15p in a Sec10myc immunoprecipitation was only slightly reduced in the sec3Δ, sec5Δ, and exo70Δ mutants relative to wild type (Fig. 8, lanes 5, 8, and 13). Therefore, Sec10p and Sec15p form a subcomplex that is little affected by the absence of Sec3p, Sec5p, or Exo70p from the complex. The abundance of Sec6p, Sec8p, Sec10p, and Sec15p in the lysate was not affected in the different mutants (Fig. 7 and Fig. 8). Therefore, the absence of Sec3p, Sec5p, or Exo70p does not result in proteolysis of these other exocyst subunits. The results show that the assembly or stability of the complex is affected in these mutants, although a Sec10p–Sec15p subcomplex and possibly other subcomplexes remain intact.
Figure 8.
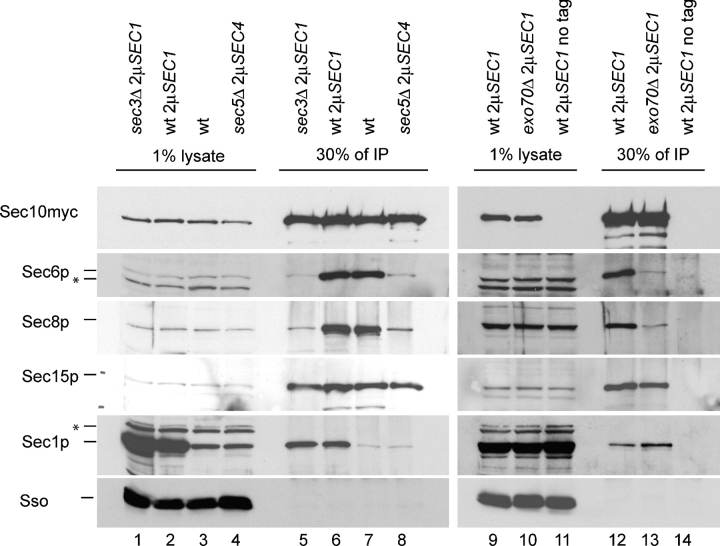
Co-isolation of Sec1p and exocyst subunits with myc epitope–tagged Sec10p. The exocyst was isolated from a wild-type (lanes 6, 7, and 12), sec3Δ (lane 5), sec5Δ (lane 8), and exo70Δ (lane 13) mutant strain via a carboxy-terminal 13myc epitope–tagged Sec10p. Immunoprecipitations were performed as described in Fig. 7. Mutant genotypes (wt, sec3Δ, sec5Δ, or exo70Δ) and the presence of multi-copy plasmids (2μSEC1 or 2μSEC4) are indicated on the top of each lane. 1% of lysates (left lanes) are compared with 30% of the immunoprecipitate (IP, right lanes). The antigens detected by Western blot analysis are marked on the left. Nonspecific bands are marked with an asterisk.
Sec1p binds to the exocyst
The Sec1p homologue Vps33p is a bona fide subunit of the class C/HOPS tethering complex required for vacuole-to-vacuole fusion (Sato et al., 2000; Seals et al., 2000). Given this result and the strong genetic interactions seen between SEC1 and exocyst mutants, we tested whether Sec1p is physically connected to the exocyst. We consistently observed that a minor fraction (0.2–0.4%) of Sec1p coprecipitated with the exocyst (Fig. 7 and Fig. 8). The same amount of Sec1p was co-isolated with the exocyst from sec3Δ cells and sec3Δ, sec5Δ, or exo70Δ mutants suppressed by Sec4p overproduction, where only a small fraction of the exocyst is in its assembled state (Fig. 7 A, compare lane 4 with lanes 5 and 7; Fig. 7 C, lanes 11 and 12; Fig. 8, lanes 7 and 8). Upon overproduction, an increased amount of Sec1p coprecipitated with the exocyst, although the relative fraction bound to the exocyst appeared to be very similar (Fig. 7, C and D, lanes 5 and 6; Fig. 8, lanes 5, 6, 12, and 13). When overproduced, the amounts of Sec1p bound to the exocyst were similar in sec3Δ and wild-type lysates (Fig. 8, lanes 5 and 6). Compared with the wild type, even increased amounts of Sec1p were co-isolated in sec5Δ and exo70Δ mutant strains overproducing Sec1p (Fig. 7, C and D, lanes 5 and 6; Fig. 8, lanes 12 and 13). The coprecipitation was specific, as no signal above background was detected in myc precipitates from untagged strains expressing Sec1p at endogenous levels (Fig. 7 A, lane 3; Fig. 7 C, lane 10) as well as from Sec1p-overproducing strains (Fig. 7 B, lane 4; Fig. 8, lane 14). Although binding of Sec1p to the exocyst is likely more transient than the interaction between Vps33p and the rest of the class C/HOPS complex, our results suggest a similar functional connection between these two tethering complexes and their corresponding SM family member.
Prior results from our laboratory showed that Sec1p binds to SNARE complexes (Carr et al., 1999). Therefore, we tested whether the exocyst, possibly via its interaction with Sec1p, can associate with SNARE proteins. However, we could not detect any of the syntaxin-like SNARE Sso in the exocyst immunoprecipitates (Fig. 7 and Fig. 8). These results imply that Sec1p can bind to the exocyst independent of assembled SNARE complexes.
Sec1p increases the levels of SNARE complexes
Members of the SM family bind to SNARE proteins and may regulate SNARE complex assembly, stability, or function (Kosodo et al., 2002; Peng and Gallwitz, 2002; Toonen and Verhage, 2003; Scott et al., 2004). Ongoing membrane traffic is essential for SNARE complex formation, and temperature-sensitive sec4 and exocyst mutants lead to the rapid loss of exocytic SNARE complexes after a shift to the restrictive temperature (Carr et al., 1999; Grote et al., 2000). Therefore, we tested how SNARE complex levels are affected by the absence of Sec3p, Sec5p, or Exo70p, as well as by Sec1p or Sec4p overproduction. For this analysis, steady-state levels of SNARE complexes were measured in the different mutants (Fig. 9). The SNARE complexes were isolated using an antibody against the v-SNARE Snc, and the relative amount of Sso in the immunoprecipitates was determined. Consistent with our earlier results, ∼1% of Sso was co-isolated with Snc from a wild-type strain (Grote et al., 2000). Upstream inhibition of membrane traffic and the concomitant slowed formation of SNARE complexes leads to a decrease in steady-state levels as Sec18p-mediated disassembly of SNARE complexes continues. As secretory function is only partially affected in the sec3Δ mutant, SNARE complex levels were only reduced in this strain (Fig. 9 B, lane 6). In a sec3Δ strain about half as much Sso was isolated in SNARE complexes together with Snc (40% ± 10; n = 5), compared with a wild-type strain. Consistent with its ability to restore secretion, Sec4p overproduction also restored the amount of SNARE complexes that can be isolated from a sec3Δ mutant background (Fig. 9 B, lane 8).
Figure 9.
Sec1p overexpression increases SNARE complex levels. SNARE complexes were isolated from cleared yeast lysates by immunoprecipitation using the anti-Snc pAb as described in Materials and methods. The amount of Ssop bound to Sncp was compared by Western blot using a biotinylated polyclonal anti-Sso antibody followed by streptavidin coupled to HRP. The lysates were prepared from wt (A), sec3Δ (B), sec5Δ (C), or exo70Δ (D) strains carrying either a multi-copy 2μSEC1 or 2μSEC4 plasmid. In each panel a wild-type control without plasmid is included. The SNARE complex isolations grouped in each panel were performed in parallel. 30% of the immunoprecipitations (IP) and 1% lysate controls were run on 12% SDS-PAGE gels.
Surprisingly, overproduction of Sec1p not only restored the levels of SNARE complexes in a sec3Δ strain, but yielded complex levels that were actually about twofold higher (190% ± 30; n = 4) than in a wild-type strain (Fig. 9 B, lane 7). Such increased levels of SNARE complexes were also observed in sec5Δ and exo70Δ (Fig. 9 C, lane 5; Fig. 9 D, lane 5). Importantly, increased SNARE complex levels were seen upon overproduction of Sec1p in a wild-type strain as well (201% ± 18, n = 5; Fig. 9 A, lane 5), indicating that the effect is intrinsic to Sec1p overproduction rather than a response to the deletion mutations. Overproduction of Sec4p didn't have this effect in wild-type cells (118% ± 13, n = 3; Fig. 9 A, lane 6). Also sec5Δ or exo70Δ strains overproducing Sec4p had SNARE complex levels similar to the wild-type strain (Fig. 9, C and D, lane 6).
In summary, the results with the sec3Δ strain show that overproduction of Sec4p can increase SNARE complex levels in a strain partially defective for tethering, presumably by restoring the flux of membrane through the pathway. However, Sec1p is more likely to play a direct role in SNARE function. The unexpected finding that SNARE complex levels are actually higher than normal in both mutant and wild-type strains overproducing Sec1p indicates that Sec1p can either increase assembly or slow disassembly of SNARE complexes. The bypass of the exocyst mutants sec3Δ, sec5Δ, and exo70Δ by Sec1p could be due to the increased SNARE complex levels under conditions where tethering is partially inhibited.
The Sec3, Sec5, and Exo70 proteins are apparently less essential for membrane traffic than the other five exocyst subunits. These results and the phenotypic analysis of the mutants described here show that different subunits are preferentially important for different aspects of exocyst function. As Sec1p binds to both the exocyst and SNARE complexes and can increase SNARE complex levels in vivo, we propose that Sec1p creates a functional link between exocyst-mediated vesicle tethering and SNARE complex–mediated vesicle docking and fusion.
Discussion
At each stage of membrane traffic, a small set of proteins works in concert to efficiently and specifically fuse the appropriate membrane compartments. In this work, we have explored the interactions between several components of the yeast exocytic machinery. Through this analysis we found that SEC1 and SEC4 are excellent high copy number suppressors of the growth and secretion defects resulting from the loss of Sec3p, a component of the exocyst complex. We have gone on to determine whether these suppressors could bypass the requirement for other exocyst components as well. Five of the exocyst subunits remain essential, even when Sec1p or Sec4p are overproduced. Therefore, overproduction of Sec1p or Sec4p does not bypass the need for exocyst-mediated tethering in yeast exocytosis. However, we do find that SEC5 and EXO70, two other exocyst genes, are no longer essential when Sec1p or Sec4p function is increased. These results are particularly surprising in the case of the SEC5 gene because protein–protein interaction studies had indicated that Sec5p is a central component of the complex that physically interacts with several essential exocyst subunits (Guo et al., 1999). These results led us to analyze the impact of the loss of Sec3p, Sec5p, and Exo70p on exocyst structure and function.
In all the deletion mutants analyzed here, the exocyst is largely disassembled. This assembly defect may reflect a reduced stability of the protein complex in the absence of Sec3p, Sec5p, or Exo70p. Complex assembly and stability may play an important role in exocyst function. Our working model is that the exocyst assembles as secretory vesicles arrive at exocytic sites on the plasma membrane. Formation of the exocyst complex thereby creates a link between secretory vesicles and the plasma membrane. This exocyst-dependent vesicle tether must then keep the vesicle in place until it is docked and committed to fusion. As exocyst assembly is partially defective in these mutants, the connection between the secretory vesicle and the plasma membrane may be unstable. The mutant exocyst complexes may frequently disassemble before the SNARE complex is able to complete vesicle docking. As a result, a significant fraction of the secretory vesicles fail to fuse, leading to the observed accumulation of secretory cargo in the mutant strains.
How does the function of Sec3p, Sec5p, and Exo70p differ from that of the other five subunits, which cannot be deleted? One possibility is that these three subunits may be largely regulatory in nature, whereas the other five subunits fulfill a “core” function of the exocyst that cannot be bypassed. In this regard it is interesting to note that in mammalian cells, Sec5 binds to the Ras family GTPase Ral, and this interaction may regulate exocyst assembly (Moskalenko et al., 2002). This is consistent with our conclusion that Sec5p is important for exocyst stability, although in yeast no upstream regulators of Sec5p function are currently known. Sec3p and Exo70p are known to bind to small GTPases of the Rho family and could therefore also be regulatory subunits of the exocyst (Adamo et al., 1999; Guo et al., 2001). By analogy to the regulation of Sec5 by Ral, Rho GTPases may bind to Sec3p or Exo70p to regulate the assembly or stability of the exocyst complex. Rho proteins may increase exocyst stability preferentially at the tip of small buds and at the mother-bud neck toward the end of the cell cycle to ensure localized cell surface expansion at these specific sites.
In the sec3Δ, sec5Δ, and exo70Δ mutant backgrounds the secretion and growth defects can be fully or partially suppressed by overproduction of Sec1p or Sec4p. Therefore, both proteins act as positive regulators of exocytosis. Although Sec4p was already known to promote exocyst function, we have obtained several results that give new insights into the molecular mechanism by which Sec1p acts to facilitate membrane fusion. Previously we demonstrated that Sec1p binds to exocytic SNARE complexes (Carr et al., 1999). Here, we find that overproduction of Sec1p increases SNARE complex levels several fold over those observed in control wild-type cells. Increased SNARE complex levels could be important in exocyst mutants where tethering may be short lived due to defects in complex stability.
In addition to finding that Sec1p can promote an increase of SNARE complex levels, we also find that a fraction of Sec1p can be coprecipitated with the exocyst. These results lead us to speculate that Sec1p forms a link between exocyst-mediated tethering and SNARE complex formation or stabilization. Some SM family proteins have been shown to bind to the corresponding syntaxin-type SNARE (Sato et al., 2000; Yamaguchi et al., 2002; Toonen and Verhage, 2003). These interactions may be important to localize SM function to the correct target membrane. In contrast, yeast Sec1p binds to the assembled t-SNARE complex or the fully assembled SNARE complex (Carr et al., 1999; Scott et al., 2004). We propose that it is the interaction between Sec1p and the exocyst that serves to localize and possibly activate Sec1p at appropriate exocytic sites on the plasma membrane. This proposal is supported by our earlier results showing that the normally polarized distribution of Sec1p is lost when exocyst function is inhibited (Carr et al., 1999; Grote et al., 2000).
In conjunction with its role in membrane tethering, the exocyst is also involved in polarized vesicle targeting. Furthermore, Sec3p is required for ER inheritance, a biological process not directly connected to exocytosis (Wiederkehr et al., 2003). Therefore, we have used a basic phenotypic analysis of the suppressed sec3Δ, sec5Δ, and exo70Δ mutants to establish how these three exocyst subunits contribute to the different aspects of exocyst function (Table III). We find that Sec5p, in addition to Sec3p, is required for ER inheritance. By extension, other exocyst subunits may play a role in ER inheritance as well. In contrast, Exo70p appears to have a function that is more restricted to exocytosis. The loss of Sec3p, Sec5p, or Exo70p from the complex leads to changes linked to defects in polarized cell surface expansion. In the sec3Δ and exo70Δ mutant, Sec1p (but not Sec4p) overproduction partially restores the polarity defects. The results show that in these two mutant backgrounds, Sec1p may be able to work together with the partially defective exocyst to restore polarized secretion.
In general, our analysis shows that there is no clear correlation between the secretion, vesicle targeting, and ER inheritance defects, but that different subunits are more or less important for different aspects of exocyst function (Table III). Our findings also show that different subunits of the exocyst are more or less essential for exocytosis. Thus, the exocyst should not be regarded as a large molecular structure with one function, but rather as a complex composed of different proteins that contribute differentially to the multiple functions of the complex. Based on our results, we also propose that one important function of the exocyst is to recruit Sec1p to exocytic sites and thereby promote SNARE-mediated vesicle docking and fusion.
Materials and methods
Yeast strains and media
Saccharomyces cerevisiae strains used in this study are listed in Table IV. For all experiments described here the strains were grown in SC growth medium at 25°C. SC medium was made as described in Wiederkehr et al. (2003). Agar plates containing 5FOA (1 mg/ml final concentration) were used to select cells able to lose plasmids carrying the URA3 gene as a marker. Before streaking the cells on 5FOA-containing plates, the strains were grown 2 d on SC plates containing uracil so the plasmid could be lost from strains that do not depend on the overproduction of Sec1p or Sec4p for viability.
Table IV. Yeast strains.
| Strain | Genotype |
|---|---|
| NY2449 | Matα leu2-3,112 ura3-52 his3Δ200 |
| NY2474 | Matα leu2-3,112 ura3-52 |
| NY2448 | Matα sec3Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 |
| NY2475 | Matα sec3Δ::KanMX4 leu2-3,112 ura3-52 |
| NY2476 | Matα sec5Δ::KanMX4 leu2-3,112 ura3-52 2μSEC1:URA3 |
| NY2477 | Matα sec5Δ::KanMX4 leu2-3,112 ura3-52 2μSEC4:URA3 |
| NY2478 | Matα exo70Δ::KanMX4 leu2-3,112 ura3-52 2μSEC1:URA3 |
| NY2479 | Matα exo70Δ::KanMX4 leu2-3,112 ura3-52 2μSEC4:URA3 |
| NY2485 | Mata leu2-3,112::hmg1-GFP:LEU2 ura3-52 |
| NY2486 | Mata sec3Δ::KanMX4 leu2-3,112::hmg1-GFP:LEU2 ura3-52 |
| NY2487 | Matα sec5Δ::KanMX4 leu2-3,112::hmg1-GFP:LEU2 ura3-52 his3Δ200 2μSEC1:URA3 |
| NY2488 | Mata sec5Δ::KanMX4 leu2-3,112::hmg1-GFP:LEU2 ura3-52 his3Δ200 2μSEC4:URA3 |
| NY2489 | Mata exo70Δ::KanMX4 leu2-3,112::hmg1-GFP:LEU2 ura3-52 his3Δ200 2μSEC1:URA3 |
| NY2490 | Mata exo70Δ::KanMX4 leu2-3,112::hmg1-GFP:LEU2 ura3-52 his3Δ200 2μSEC4:URA3 |
| NY2491 | Matα leu2-3,112 ura3-52 his3Δ200 SEC8-myc13x:His5p |
| NY2492 | Matα sec3Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC8-myc13x:His5p |
| NY2493 | Matα sec5Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC8-myc13x:His5p 2μSEC1:URA3 |
| NY2494 | Matα sec5Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC8-myc13x:His5p 2μSEC4:URA3 |
| NY2495 | Matα exo70Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC8-myc13x:His5p 2μSEC1:URA3 |
| NY2496 | Matα leu2-3,112 ura3-52 his3Δ200 SEC10-myc13x:His5p |
| NY2497 | Matα sec3Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC10-myc13x:His5p 2μSEC1:URA3 |
| NY2498 | Matα sec5Δ::KanMX4 leu2-3,112 ura3-52 his3Δ200 SEC10-myc13x:His5p 2μSEC4:URA3 |
Heterozygous diploid strains were constructed in our strain background by replacing the entire coding region of one copy of the exocyst genes with the KanMX4 module as described previously (Wiederkehr et al., 2003). Standard techniques were used for the sporulation and tetrad analysis of these yeast strains. For each of the eight exocyst mutant strains, between 10 and 16 tetrads were analyzed. Included in the analysis were only those tetrads in which the viable strains had retained the plasmid after dissection. Additional markers such as HMG1-GFP or the myc-tagged SEC8 or SEC10 were introduced by crossing a wild-type strain containing the new marker with the different suppressed mutant strains.
Plasmids and DNA manipulations
SEC1 was cloned as a 3.4-kb HindIII–SphI fragment into YEp24 (pNB680). SEC4 was cloned as a 1.4-kb EcoRI–BamHI fragment into a multi-copy vector as described previously (Salminen and Novick, 1987). Overexpression of Sec1p and Sec4p was confirmed by Western blotting. Description of the construct used for the in vivo localization of the ER marker HMG1-GFP can be found elsewhere (Wiederkehr et al., 2003). For the integration of the HMG1-GFP marker at the LEU2 locus, the vector pSFN1015 was linearized with BstEII.
For the myc tagging of SEC8 or SEC10, long oligonucleotide primers were designed to remove the stop codon and fuse these genes in frame with multiple myc tags as described previously (Longtine et al., 1998).
Invertase secretion
The invertase secretion and activity assay was performed as described previously (Wiederkehr et al., 2003).
Morphological analysis
The length and width of yeast cells was measured on differential interference contrast (DIC) pictures using the NIH Image 1.62 program. The ratio of length to width is a measure for the elongated shape of the yeast mother cells, and therefore the buds were not included in the measurements. Cells with an axial ratio <1.1 were considered round. The percentage of cells with an axial ratio >1.1 is shown in the graphs. For the shmooing reaction, the density of the yeast cultures was adjusted to equal density (OD600 = 0.25) and 10 μg/ml α-factor was added to the Mata yeast strains. DIC pictures were taken 6 h after α-factor addition. The radius of curvature of the shmoo tips was analyzed using the OpenLab 3.1.4 program.
Fluorescence microscopy
Cells were examined on an Axioplan2 microscope (Carl Zeiss MicroImaging, Inc.) with a 63× Plan Neofluor apochromatic oil-immersion objective lens (N.A. 1.4). Pictures and videos of cells were taken with a cooled CCD camera (ORCA ER; Hamamatsu Corporation) at RT (21–23°C). The images were analyzed with OpenLab software. To visualize the ER, yeast strains expressing the marker Hmg1-GFP were grown to an OD600 of 0.2–0.3. The cell suspension was mixed with an equal volume of 0.6% NuSieve GTG low melting temperature agarose (FMC BioProducts) and mounted on a glass slide. Hmg1-GFP was visualized using the FITC filter set. For quantitation of the ER inheritance defect, cells at different stages of bud growth were grouped together. The three different categories were defined as described in the legend for Fig. 3 (A and B). For the purpose of quantitation of cortical ER inheritance, we only considered the ER apposed to the plasma membrane in the bud and not cytoplasmic tubules or diffuse fluorescence in the bud. Sec4p immunofluorescence labeling and quantification was performed as described in Wiederkehr et al. (2003).
Exocyst isolation
The exocyst was isolated by immunoprecipitation from yeast strains expressing myc-tagged Sec8p or Sec10p from their endogenous promoter. After growth in SC medium, 50 OD600 units of cells were harvested and washed in 50 ml ice-cold washing buffer (10 mM Tris, pH 7.4, and 10 mM NaN3). The washed cells were then resuspended in 5 ml spheroblasting buffer (50 mM NaPO4, pH 7.4, 1.4 M sorbitol, 35 mM β-mercaptoethanol, 10 mM NaN3, and 100 μg/ml Zymolyase [Seikagaku Corporation]) and incubated at 30°C for 30 min. The cell suspension was then layered on top of a 5-ml 1.5 M sorbitol solution (50 mM NaPO4, pH 7.4, 1.5 M sorbitol, 10 mM NaN3, and protease inhibitors). The cells were spun through the sorbitol cushion for 10 min at 1,000 g. The pellets were resuspended in cold lysis buffer (20 mM Pipes, pH 6.8, 100 mM NaCl, 1 mM EDTA, 1% Nonidet-P40 (IGEPAL CA 630), 1 mM DTT, and protease inhibitors). All further steps were performed on ice or at 4°C. The lysates were cleared by centrifugation for 10 min at 10,000 g and were adjusted for equal protein concentrations (4 mg/ml). 1 ml of lysate was used per immunoprecipitation. After adding 3 μl of an anti-myc mAb (Babco), the lysates were incubated for 3 h at 4°C. Finally, protein A–Sepharose (45 μl 50% slurry; Sigma-Aldrich) was added, followed by another hour of incubation at 4°C. After washing the beads, the bound proteins were separated on 8% SDS-PAGE protein gels.
SNARE complex isolation
SNARE complexes were isolated by immunoprecipitation as described previously (Carr et al., 1999). Where mentioned, protease inhibitors were used at the following concentrations: 6 μg/ml antipain, 2 μg/ml aprotinin, 2 μg/ml chymostatin, 8 μg/ml leupeptin, 12 μg/ml pepstatin A, and 1 mM PMSF (Sigma-Aldrich).
Online supplemental material
Videos show ER tubule dynamics in the indicated mutant strains. For all videos, Hmg1-GFP was used as marker to follow ER dynamics. Videos were captured at one frame per 3 s and are played at 10 frames/s. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200408001/DC1.
Acknowledgments
We thank Cordelia Vahadji and Chave Carr for preliminary observations, Jeff Coleman and Charles Boyd for yeast strains, Eric Grote for advice during this study, and Michèle Wiederkehr-Adam for carefully reading the manuscript.
This work was supported by grants from the National Institutes of Health to P. Novick (GM35370) and S. Ferro-Novick (CA 46128). A. Wiederkehr was supported by a fellowship from the Swiss National Science Foundation.
Abbreviations used in this paper: 5FOA, 5-fluoroorotic acid; DIC, differential interference contrast; SC, synthetic complete; SM, Sec1p/Munc18-like proteins.
References
- Adamo, J.E., G. Rossi, and P. Brennwald. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell. 10:4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, C.M., E. Grote, M. Munson, F.M. Hughson, and P.J. Novick. 1999. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger, F.P., T.E. Hughes, and P. Novick. 1998. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 92:559–571. [DOI] [PubMed] [Google Scholar]
- Grote, E., C.M. Carr, and P.J. Novick. 2000. Ordering the final events in yeast exocytosis. J. Cell Biol. 151:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., D. Roth, C. Walch-Solimena, and P. Novick. 1999. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., M. Sacher, J. Barrowman, S. Ferro-Novick, and P. Novick. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10:251–255. [DOI] [PubMed] [Google Scholar]
- Guo, W., F. Tamanoi, and P. Novick. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353–360. [DOI] [PubMed] [Google Scholar]
- Kosodo, Y., Y. Noda, H. Adachi, and K. Yoda. 2002. Binding of Sly1 to Sed5 enhances formation of the yeast early Golgi SNARE complex. J. Cell Sci. 115:3683–3691. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., A. McKenzie III, D.J. Demarini, N.G. Shah, A. Wach, A. Brachat, P. Philippsen, and J.R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14:953–961. [DOI] [PubMed] [Google Scholar]
- Moskalenko, S., D.O. Henry, C. Rosse, G. Mirey, J.H. Camonis, and M.A. White. 2002. The exocyst is a Ral effector complex. Nat. Cell Biol. 4:66–72. [DOI] [PubMed] [Google Scholar]
- Peng, R., and D. Gallwitz. 2002. Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 157:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D.W., D.H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. [DOI] [PubMed] [Google Scholar]
- Rossi, G., A. Salminen, L.M. Rice, A.T. Brunger, and P. Brennwald. 1997. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem. 272:16610–16617. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and P.J. Novick. 1987. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 49:527–538. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and P.J. Novick. 1989. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 109:1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T.K., P. Rehling, M.R. Peterson, and S.D. Emr. 2000. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 6:661–671. [DOI] [PubMed] [Google Scholar]
- Scott, B.L., J.S. Van Komen, H. Irshad, S. Liu, K.A. Wilson, and J.A. McNew. 2004. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J. Cell Biol. 167:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals, D.F., G. Eitzen, N. Margolis, W.T. Wickner, and A. Price. 2000. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA. 97:9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou, S., and H.R. Pelham. 2002. Vps51p links the VFT complex to the SNARE Tlg1p. J. Biol. Chem. 277:48318–48324. [DOI] [PubMed] [Google Scholar]
- TerBush, D.R., and P. Novick. 1995. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D.R., T. Maurice, D. Roth, and P. Novick. 1996. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Toonen, R.F., and M. Verhage. 2003. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 13:177–186. [DOI] [PubMed] [Google Scholar]
- Ungar, D., and F.M. Hughson. 2003. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19:493–517. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena, C., R.N. Collins, and P.J. Novick. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, T., B.V. Zemelman, J.A. McNew, B. Westermann, M. Gmachl, F. Parlati, T.H. Sollner, and J.E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. [DOI] [PubMed] [Google Scholar]
- Whyte, J.R., and S. Munro. 2002. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115:2627–2637. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., Y. Du, M. Pypaert, S. Ferro-Novick, and P. Novick. 2003. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell. 14:4770–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, T., I. Dulubova, S.W. Min, X. Chen, J. Rizo, and T.C. Sudhof. 2002. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev. Cell. 2:295–305. [DOI] [PubMed] [Google Scholar]