Abstract
In many soils plants have to grow in a shortage of phosphate, leading to development of phosphate-saving mechanisms. At the cellular level, these mechanisms include conversion of phospholipids into glycolipids, mainly digalactosyldiacylglycerol (DGDG). The lipid changes are not restricted to plastid membranes where DGDG is synthesized and resides under normal conditions. In plant cells deprived of phosphate, mitochondria contain a high concentration of DGDG, whereas mitochondria have no glycolipids in control cells. Mitochondria do not synthesize this pool of DGDG, which structure is shown to be characteristic of a DGD type enzyme present in plastid envelope. The transfer of DGDG between plastid and mitochondria is investigated and detected between mitochondria-closely associated envelope vesicles and mitochondria. This transfer does not apparently involve the endomembrane system and would rather be dependent upon contacts between plastids and mitochondria. Contacts sites are favored at early stages of phosphate deprivation when DGDG cell content is just starting to respond to phosphate deprivation.
Introduction
Phosphorus is an essential macro element for plant growth and development, but in most soils it is moderately available due to adsorption properties (Raghothama, 1999, 2000). Plant cells have developed safety mechanisms circumventing this shortage, including decrease of their Pi consumption and mobilization of their Pi reserve. Phospholipids are a main form of cellular Pi reserve and their content markedly declines in plants during Pi starvation (Essigmann et al., 1998; Härtel et al., 1998).
In leaves, the most abundant membrane glycerolipids are not phospholipids, but glycolipids such as galactolipids, i.e., monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG). They represent up to 80% of leaf lipids (Joyard et al., 1996). Galactolipids were reported to be localized specifically in plastids and trace amounts of these lipids, which have been detected in the past in other isolated fractions of the cell, such as tonoplast (Haschke et al., 1990), were cautiously considered as possible contamination by plastid membranes. MGDG is synthesized from DAG and UDP-galactose by MGDG synthases, and this enzyme activity is located in the plastid envelope (Douce, 1974). In Arabidopsis thaliana, there are two types of MGDG synthases differing in their NH2-terminal portion: type A with MGD1 and type B with MGD2 and MGD3 (Awai et al., 2001). In MGDG produced by these enzymes, galactose is linked to DAG via a β-glycosidic bond (Carter et al., 1956). On the other hand, two different mechanisms have been reported for the formation of DGDG: either by addition of galactose from UDP-galactose on MGDG with DGD1 or DGD2 enzymes (Kelly and Dormann, 2002; Kelly et al., 2003) or by reaction of two MGDG to form one DGDG and one DAG by the galactolipid–galactolipid galactosyltransferase enzyme (van Besouw and Wintermans, 1978). With DGD1 and DGD2, the inserted galactose is linked by an α-glycosidic bond (Kelly and Dormann, 2002; Kelly et al., 2003), leading to the α-β DGDG structure reported by Carter et al. (1956). The galactolipid–galactolipid galactosyltransferase enzyme generates a β-β DGDG structure because this enzyme activity correlates with the presence of oligogalactolipids carrying several galactose residues with β-glycosidic bonds (Kojima et al., 1990; Xu et al., 2003).
During Pi deprivation, the cellular DGDG content increases (Essigmann et al., 1998; Härtel et al., 1998) and the expression of genes encoding type B MGDG synthases (MGD2 and MGD3; Awai et al., 2001) and DGDG synthases (DGD1 and DGD2; Kelly and Dormann, 2002; Kelly et al., 2003) is stimulated. The induced synthesis of DGDG involves several compartments of the cell. DAG backbone of DGDG was traced back from extraplastidial phosphatidylcholine (PC) (Roughan, 1970; Williams et al., 2000; Kelly et al., 2003), and lipid analyses during the first steps of Pi deprivation indicated that indeed PC was transformed into DGDG via DAG (Jouhet et al., 2003). Galactose insertion for synthesis of newly formed DGDG is expected to occur in plastids because enzymes encoded by MGD2, MGD3, DGD1, and DGD2 can all be addressed to the plastid envelope, very likely to the outer envelope (Awai et al., 2001; Froehlich et al., 2001; Kelly et al., 2003). Consistently, newly synthesized DGDG was proposed to replace missing PC (Härtel and Benning, 2000; Härtel et al., 2000) because (1) PC content is highly reduced; and (2) PC and DGDG adopt similar bilayer conformation in the membranes. These lipid changes cannot be limited to plastid membranes because the bulk of PC is located outside plastids (Dorne et al., 1985). Indeed, a recent report has shown that DGDG accumulates in oat plasma membrane during Pi deprivation (Andersson et al., 2003). However, the plasma membrane represents a low proportion of the cellular membrane surface, and therefore the plasma membrane lipid change cannot solely explain the high amounts of cellular PC and DGDG being affected by Pi deprivation.
Mitochondria are organelles limited by a double membrane like plastids. In plant cells, they represent ∼10% of cell membranes and contribute to 10–20% of total cellular PC (Douce, 1985). During Pi deprivation, mitochondria seem relatively protected from the induced stress. The cellular proportion of diphosphatidylglycerol (DPG), a specific mitochondrial phospholipid, remains relatively constant (Jouhet et al., 2003). Moreover, respiration is not affected except for the cyanide-resistant pathway that is enhanced (Rébeillé et al., 1984). A possible response of the plant cell to Pi deprivation is that DGDG could be transferred to mitochondria as well, and therefore contributes to adaptation of this organelle to the stress conditions. To probe this hypothesis, we cultivated A. thaliana cells with defined levels of Pi and set up a procedure to isolate the mitochondria. In this article, we report that under Pi deprivation, mitochondria contain a high concentration of DGDG in contrast with mitochondria from cells grown with sufficient supply of Pi. The α-β anomeric structure of DGDG present in mitochondria is characteristic of DGDG synthesized through a DGD type enzyme. We further point out that a transfer of DGDG occurs between plastid envelope and mitochondria. This transfer is apparently dependent on contact between plastids and mitochondria. The contacts seem to be favored at early stages of Pi deprivation when DGDG cell content is just starting to respond to Pi deprivation.
Results
DGDG is detected in plastids and in mitochondria of Pi-deprived cells
3 d after the beginning of Pi deprivation, A. thaliana cells grown as a suspension in liquid medium don't divide anymore. Their lipid composition has been modified compared with control cells. Particularly, the level of DGDG has increased from less than 10% up to 30–35% of total glycerolipids during the first day of deprivation, and this level is now stable (Jouhet et al., 2003). To understand where DGDG is localized at this stage, routinely grown Arabidopsis cell cultures were transferred for 3 d in a medium devoid of Pi (−Pi medium) or containing 1 mM Pi (control). Subcellular localization of DGDG was then assayed using antibodies raised against this lipid. Specificity of anti-DGDG antibodies for DGDG was assessed based on absence of reaction with other lipids or proteins of the cell (Maréchal et al., 2002). Fig. 1 A and Fig. S1 (available at http://www.jcb.org/cgi/content/full/jcb.200407022/DC1) show that in control cells, DGDG was only detected in plastids because DGDG-coupled epifluorescence was associated with epifluorescence coupled with the biotin carboxyl carrier protein (BCCP) subunit of acetyl-CoA synthetase, a protein present in the plastid stroma (Alban et al., 1994). In Pi-deprived cells, the situation appears to be more complex because DGDG-associated fluorescence was no more restricted to plastids and was also visible in many small spots distinct from plastids (Fig. 1 B). Some of these spots were present at the periphery of the cell, likely associated with plasma membrane as reported by Andersson et al. (2003). In addition, by comparison with the epifluorescence coupled with dihydropterin pyrophosphokinase (HPPK), a protein from mitochondria (Mouillon et al., 2002), we observed that DGDG-coupled fluorescence was also associated with that of mitochondrial markers (Fig. 1 C). Co-labeling with DGDG antibodies and MitoTracker orange CMTMRos (Molecular Probes, Inc.) confirmed this observation (Fig. 1 D). Therefore, these data indicate that in cells deprived of Pi, DGDG is present outside of plastids, and notably in mitochondria.
Figure 1.
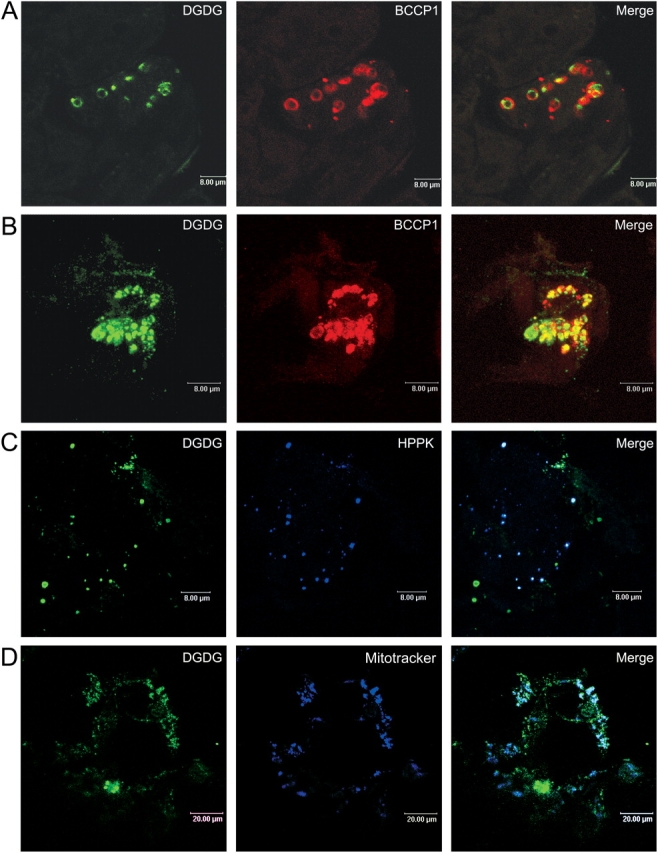
Localization of DGDG in mitochondria of A. thalia na cells deprived of Pi for 3 d. Cells (A, control; B–D, Pi-deprived) were processed for indirect immunofluorescence labeling using anti-DGDG with secondary antibodies coupled to BODIPY and either anti-BCCP1, for chloroplast detection (A and B), or anti-HPPK (C), for mitochondria detection, with secondary antibodies coupled to Alexa 594. In D, mitochondria were visualized by staining with Mitotracker orange CMTMRos. Cells were observed by confocal microscopy. Bars: A–C, 8 μm; D, 20 μm.
Isolation of mitochondria from A. thaliana cells grown without Pi
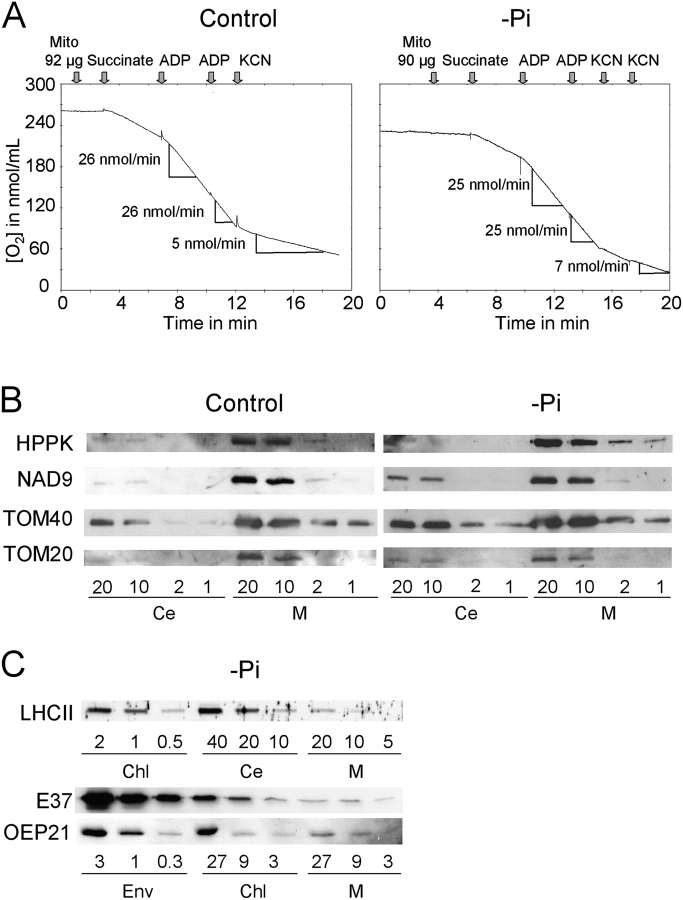
To further investigate their lipid composition, mitochondria were isolated and purified from Arabidopsis cells either deprived of Pi for 3 d or sufficiently provided with Pi (control cells). O2 consumption of isolated mitochondria was analyzed on each purified fraction (Fig. 2 A) and according to Neuburger et al. (1982), data indicated that the preparations were highly enriched in functionally intact mitochondria. To obtain sufficient amounts of lipids for analysis, three mitochondrial preparations obtained in each condition were pooled. Mitochondria purity was further controlled by Western blot on this mix. We detected approximately a fivefold enrichment of the mitochondrial inner membrane protein NAD9 (Lamattina et al., 1993), the outer membrane proteins TOM20 and TOM40 (Werhahn et al., 2001), and of the HPPK matrix protein (Mouillon et al., 2002) in the mitochondrial fractions, as compared with the whole-cell fractions (Fig. 2 B). Taking into consideration that mitochondria may represent 15–20% of total cell protein, the enrichment in mitochondria markers indicated that the isolated mitochondria were rather pure. Nevertheless, in order to ascertain galactolipid content of mitochondria, we measured the cross-contamination of the isolated organelles by plastid membranes classically reported to be enriched in galactolipids. Contamination by chloroplast membranes was measured by following the thylakoid marker LHCII (Vallon et al., 1986), the inner envelope marker E37 (Teyssier et al., 1996), and the outer envelope marker OEP21 (Bolter et al., 1999). Contamination by thylakoids was negligible because mitochondrial fractions contained 40 times less LHCII than chloroplasts (Fig. 2 C). The envelope markers OEP21 and E37 were both five times less abundant in mitochondria than in chloroplasts, and taking into account that envelope proteins likely represent ∼4% of chloroplast total proteins, this indicated that mitochondria (1 mg protein) contained <0.6% envelope proteins (6 μg protein) (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200407022/DC1).
Figure 2.
Characterization of mitochondria fractions isolated from either control or 3 d Pi-deprived A. thaliana cells. (A) To check purity and intactness of isolated mitochondria, succinate oxidation was followed by measuring O2 consumption. On average, each purified fraction consumed ∼280 nmol O2.min−1.mg−1 protein in the presence of succinate and ADP, and O2 consumption was stimulated 2.4 times by addition of ADP. Therefore, fractions were considered to be highly enriched in functionally intact mitochondria. Cyanide resistant pathway was slightly enhanced in Pi-deprived conditions as expected according to Rébeillé et al. (1984). (B) Comparative Western blot analysis of mitochondrial (M) and total cell extract (Ce) using antibodies specific for mitochondrial proteins, HPPK, a matrix protein, NAD9, an inner membrane protein, and TOM20 and TOM40 outer membrane proteins. (C) Western blot analysis of mitochondrial (M), chloroplast (Chl), and total cell extract (Ce) of Pi-deprived cells and of chloroplast envelope (Env) prepared from Arabidopsis plants as in Awai et al. (2001) using antibodies specific for chloroplast membrane proteins, LHCII for thylakoid, E37 for inner envelope membrane, and OEP21 for outer envelope membrane.
Because plasma membrane contains DGDG under Pi-deprived conditions (Andersson et al., 2003), we questioned the possible contamination of mitochondria by plasma membrane. The plasma membrane is characterized by content of sterols, e.g., acylated steryl-glycosides (Norberg and Liljenberg, 1991). We could not detect any acylated steryl-glycosides in mitochondria lipid extracts from Pi-deprived or control cells. Altogether, our results indicate that the mitochondrial fractions we prepared from A. thaliana cells were highly enriched in intact mitochondria and contained little cross-contamination by extramitochondrial membranes.
Mitochondria isolated from Pi-deprived cells contain DGDG
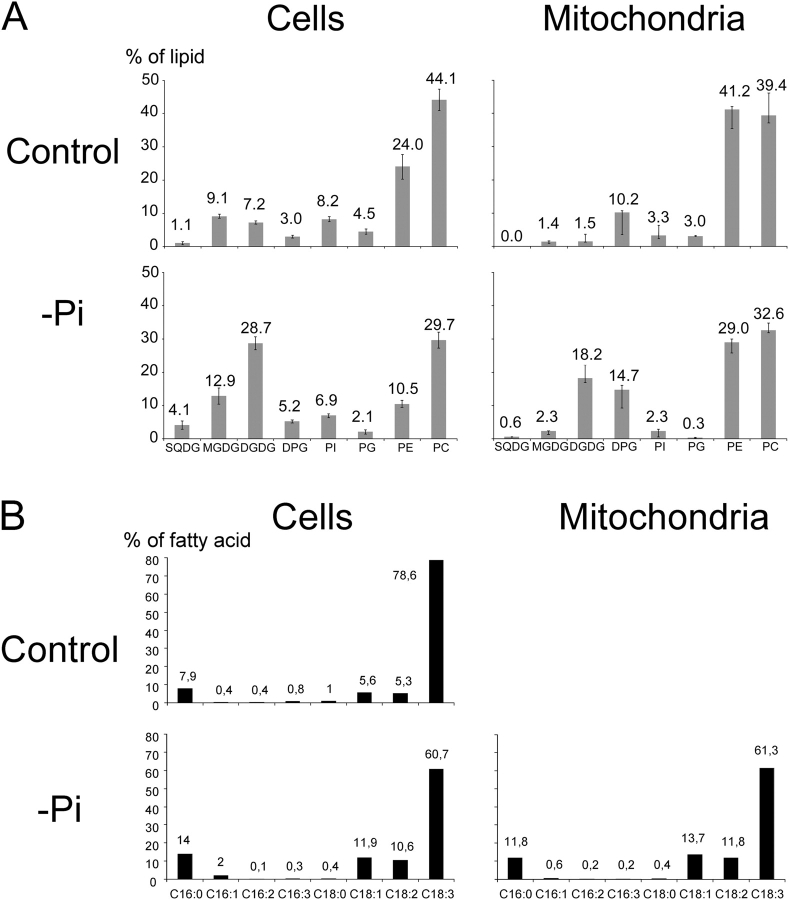
Lipids were extracted from both types of mitochondria and their composition was compared with that of cells. Fig. 3 A shows results of glycerolipid analyses normalized to the total amount of glycerolipid in each fraction. The composition of cells grown with or without Pi was consistent with data published earlier (Essigmann et al., 1998; Härtel et al., 1998; Jouhet et al., 2003). DPG was found at a relatively high level in both types of cells, indicating that mitochondria lipids represent a fair proportion of total cell lipids. In Pi-deprived cells, we mainly observed a decrease in phospholipids and a high increase in DGDG and sulfoquinovosyldiacylglycerol (SQDG). Lipid composition of mitochondria isolated from control cells was similar to that reported earlier, containing mostly phosphatidylethanolamine (PE) and PC (Douce, 1985; Harwood, 1987). Only traces of MGDG and DGDG were detected. The mol percentage of DPG was three times higher in isolated mitochondria than in whole cells. In mitochondria isolated from Pi-deprived cells, the levels of phospholipids (i.e., PC, PE, and PG) were all lower except for DPG, which was present in higher proportion. Contents in MGDG and SQDG were slightly higher than in mitochondria from control cells, but both remained at a low level. By contrast, the level of DGDG was remarkably higher, representing >18% of the −Pi mitochondria lipids. By analyzing the lipid contamination attributable to chloroplast envelope with mitochondrial lipid data, we calculated that the amount of DGDG issued from envelope contamination is much lower than the amount of DGDG measured in the mitochondria fraction (Table S1), indicating that most of the DGDG detected in mitochondria upon 3 d of Pi deprivation was indeed located in mitochondria.
Figure 3.
Glycerolipid analysis of total cell and mitochondria fractions from control and 3 d P i -deprived A. thaliana cells. (A) Glycerolipid composition. SD was calculated on four independent measurements in each case. (B) Fatty acid composition of DGDG isolated either from total cell extracts from 3 d Pi-deprived cells and control cells or from mitochondria fraction from 3 d Pi-deprived cells.
DGDG is present on the surface of mitochondria in Pi-deprived cells
Antibodies raised against DGDG were used to test a possible agglutination of purified mitochondria (Fig. 4). In absence of antibodies, control and −Pi purified mitochondria were visible under light microscope as nonaggregated, whereas they agglutinated in presence of antibodies against mitochondria outer membrane proteins TOM20 and TOM40. When antibodies raised against plastid proteins, like E37 and OEP21, were added to mitochondria, no agglutination was visible whenever OEP21 antibodies did induce chloroplast agglutination. With antibodies raised against DGDG, no agglutination of control mitochondria could be detected, but a strong agglutination was observed with mitochondria prepared from Pi-deprived cells. In conclusion, immunoagglutination assays indicated that in Pi-starved cells, DGDG is accessible on the mitochondrial outer surface to specific antibodies.
Figure 4.
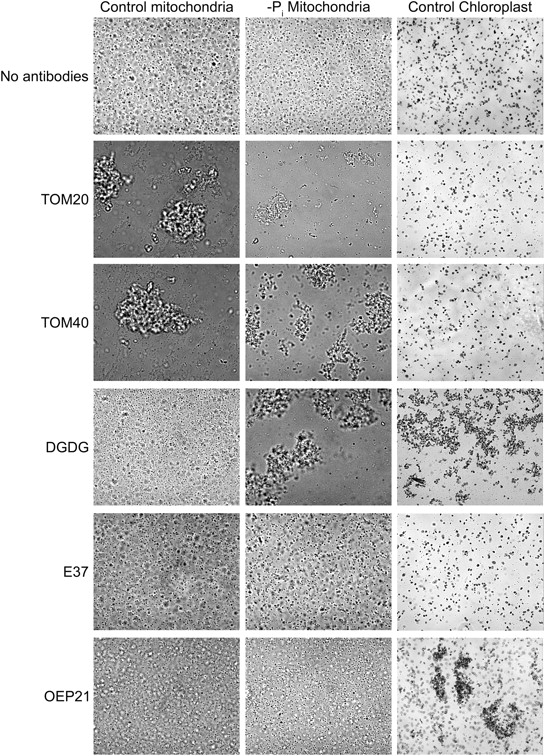
Immunoagglutination assays of isolated mitochondria prepared from either control or 3 d Pi-deprived A. thaliana cells. Mitochondria from Pi-deprived cells, control cells, and chloroplasts were incubated with antibodies as specified. Addition of anti-DGDG lead to agglutination of chloroplasts and of mitochondria of Pi-deprived cells only. Control antibodies were directed against E37 and OEP21 chloroplast inner and outer membrane protein, respectively, and TOM20 and TOM40 outer membrane mitochondrial proteins.
Structure of DGDG present in mitochondria
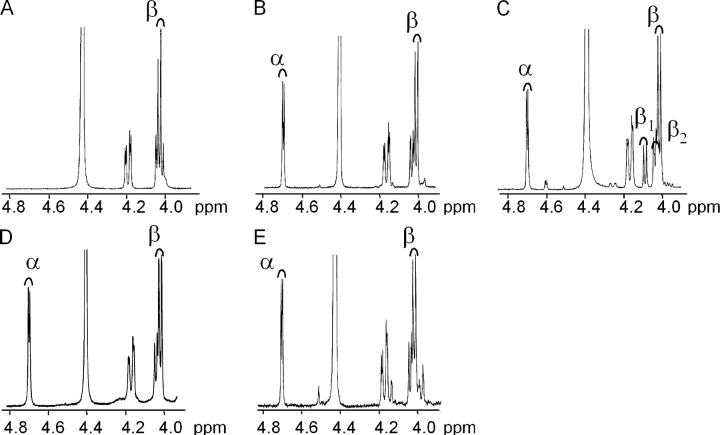
To characterize the overall structure of the mitochondria-associated DGDG, we analyzed its fatty acid composition and its polar head structure. Fig. 3 B shows that fatty acid composition of mitochondrial DGDG was fairly similar to that of Pi-deprived cell DGDG. Compared with DGDG present in control cells, the main characteristic of fatty acid composition of mitochondrial DGDG was an increase in 16C/18C ratio (0.09–0.13) with more 16:0 and an increase in more saturated species of C18, but globally DGDG remained highly enriched in 18:3. The anomeric structure of the polar head of mitochondrial DGDG was resolved by nuclear magnetic resonance (NMR). In 1H-NMR, the anomeric proton in α- or β-glycosidic configuration gives characteristic doublet signals respectively at high chemical shift (∼5.0 ppm) or at low chemical shift (∼4.0 ppm). The exact position of these signals depends on solvent, temperature, and molecular environment. To identify the precise position of these α- and β-doublet signals, several galactolipid molecules were analyzed by NMR and compared. Higher plant MGDG was reported to contain only a β-glycosidic bond (Carter et al., 1956), and indeed Arabidopsis cell MGDG gave a doublet signal at 4.0 ppm (Fig. 5 A). Two forms of DGDG have previously been reported in plants (Kojima et al., 1990; Xu et al., 2003); the main form containing a β-glycosidic bond on the first galactose and an α-glycosidic bond on the second galactose (Carter et al., 1956). DGDG extracted from either control or Pi-deprived Arabidopsis cells corresponded to this main form, with a doublet signal at 4.0 ppm for the β-bond and at 4.7 ppm for the α-bond (Fig. 5, B and D). In addition to the 4.7 ppm α-doublet, DGDG extracted from an isolated fraction of spinach chloroplast envelope contained three doublet signals in the range of the β signal (exact position at 4.0, 4.05, and 4.1 ppm; Fig. 5 C) that were indicative of presence of both α-β and β-β DGDG structures, the later structure very likely resulting from the activation of the galactolipid–galactolipid galactosyltransferase during the course of envelope isolation (Xu et al., 2003). In NMR spectra of mitochondrial DGDG, we observed only doublet signals characteristic for an α-glycosidic bond at 4.7 ppm and for a β-glycosidic bond at 4.0 ppm, with no signal at 4.1 ppm (Fig. 5 E). We concluded from these results that mitochondrial DGDG structure is 1,2-diacyl-3-O-(α-d-galactopyranosyl-(1→6)-O-β-d-galactopyranosyl)-sn-glycerol.
Figure 5.
1H-NMR galactolipid analysis. The α-peak is characterized by a doublet at 4.7 ppm and the β-peak by a doublet at 4.0 ppm. (A) β-MGDG from A. thaliana control cells. (B) α-β DGDG from A. thaliana control cells. (C) DGDG from Spinacia oleracea purified chloroplast envelope. In envelope fraction, two DGDG types are visible: α-β and β1-β2. (D) α-β DGDG from A. thaliana Pi-deprived cells. (E) Mitochondrial α-β DGDG from A. thaliana Pi-deprived cells.
Galactolipid synthesis is not localized in mitochondria
Because DGD1 and DGD2 (that have been located in the chloroplast envelope; Froehlich et al., 2001; Kelly et al., 2003) have been reported to synthesize DGDG with an α-β structure and because up to date, galactolipid synthesis has not been found in another organelle but plastids, one might expect that DGDG is formed in plastids before transfer to mitochondria. We analyzed the galactolipid synthesis capability of mitochondria isolated from Pi-deprived cells. Table I shows that [C14]galactose incorporation into galactolipids was in the range of 3–17 nmol.mg prot−1.h−1 in mitochondria, lower than in chloroplasts (∼25 nmol.mg prot−1.h−1) and far much lower than those previously reported for isolated chloroplast envelope (∼1 μmol.mg prot−1.h−1 as in Block et al. [1983]). Furthermore, the activity in mitochondria decreased when mitochondria were further purified. When reported to the amount of either E37 or OEP21, galactosyltransferase activity correlated with the level of both OEP21 or E37 (Table I), pointing to an association of inner and outer envelope membranes in the mitochondria preparation and indicating that the galactolipid synthesis activity found in the mitochondria fraction was attributable to cross-contamination by plastid envelope membranes.
Table I. Comparison of galactolipid synthesis activity and chloroplast envelope contamination of various fractions isolated from Arabidopsis cells deprived for Pi for 12 h.
|
|
E37
|
OEP21
|
Incorporated galactose
|
|
|---|---|---|---|---|
| Relative units | Relative units | nmol.mg−1.h−1 | Relative units | |
| Chloroplasts | 100 | 100 | 25.9 | 100 |
| Cells | 9.6 | 9.5 | 1.8 | 6.9 |
| Crude mitochondria | 67.2 | 64.5 | 16.6 | 64.1 |
| Purified mitochondria | 13.4 | 14.4 | 2.8 | 10.8 |
Mitochondria fractions were prepared at different stages of purification as described in Materials and methods. Proteins were analyzed by SDS-PAGE and Western blot, and chloroplast envelope contamination was defined by comparing anti-E37 or OEP21 ECL signal intensity of 10, 5, and 2.5 μg protein of a unique chloroplast fraction and 20, 10, and 5 μg of each mitochondria fraction.
Physical contact between mitochondria and chloroplasts
Lipid transfer between organelles and/or membrane vesicles can be activated by contact between membranes (Achleitner et al., 1999; Voelker, 2003). To investigate a possible increase of contact sites between plastids and mitochondria during Pi deprivation, we performed an EM survey of Arabidopsis cell suspensions. In cells that were subcultured into a standard medium for 3 d, we observed numerous round or elongated mitochondria and plastids containing a big starch grain and scarce thylakoid membranes with limited grana stacks. Cells are indeed photosynthetic (Axelos et al., 1992) but not fully autotrophic, and their growth is dependent on presence of sugar. In cells subcultured in −Pi medium for 3 d, mitochondria come across globally similar but plastids look distorted with a fragmented starch grain, further reduced thylakoid membranes, and loose internal membranes. Plastid envelope seems slack as if the inner volume of plastids had been suddenly reduced. When cells are observed only 6 h after subculture, they look roughly similar whenever Pi is present or not. Starch grains in plastids are not quite as developed as in 3-d control cells. Thylakoids are present although scarce. Numerous round or elongated mitochondria can also be observed (Fig. 6 A). In all conditions mitochondria and chloroplasts are rather close to each other, but in Pi-deprived cells tight contacts are more frequent (Fig. 6 B). Indeed, although a close apposition of mitochondria and chloroplasts occurs even under normal growth conditions, the number of contacts between mitochondria and chloroplasts increases approximately three times in Pi-deprived cells compared with control cells (Table II). Analysis of images obtained by a serial cut of a Pi-deprived cell indicates that such contacts cover a rather broad area and are not just localized at focal points between plastids and mitochondria (Fig. 6, B and C). In addition, in cells grown for 6 h in −Pi medium numerous figures of partition are visible on chloroplasts with clear constriction regions. We observed that mitochondria are often present in the vicinity of the chloroplast constrictions (Fig. 6 B). Altogether, the global organization of the cell appears to be modified in the early phase after transfer to Pi-deprived medium. This early response consists particularly in a movement of mitochondria toward chloroplasts or vice-versa and enhanced apposition of mitochondria and plastid membranes.
Figure 6.
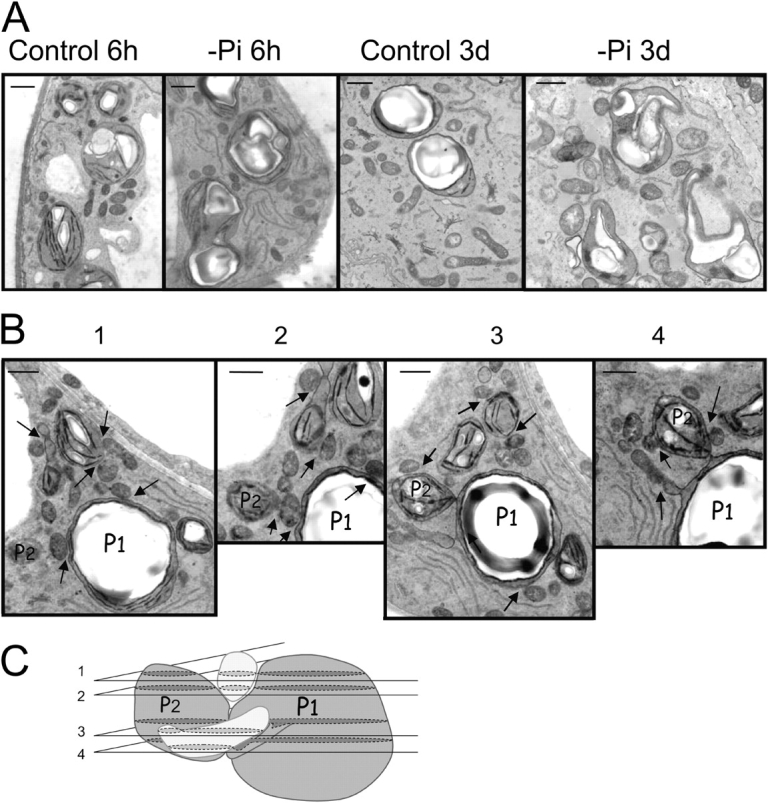
Electron microscopy observation of chloroplasts and mitochondria in A. thaliana cells grown in suspension. (A) Comparison of cells grown for different times (6 h or 3 d) in standard or −Pi medium. (B) Serial cross sections of a cell grown for 6 h in −Pi medium. Arrows indicate position of contact between mitochondria and chloroplasts. (C) Three-dimensional interpretation of relative position of chloroplasts P1 and P2 (referring to P1 and P2 in B) and two mitochondria (in light gray). Numbers of cross sections refer to numbers displayed in B. Bars, 1 μm.
Table II.
Statistical analysis of contacts between chloroplasts and mitochondria and of dividing chloroplasts in A. thaliana cells grown for 6 h in control or −Pi medium
| +Pi 6 h | −Pi 6 h | Comparison | |
|---|---|---|---|
| Total number of chloroplasts | 87 | 84 | - |
| Total mitochondria | 319 | 346 | - |
| Number of contacts between mitochondria and chloroplasts | 40 | 114 | × 3 |
| Number of contacts per chloroplast |
0.46 | 1.36 | × 3 |
| Number of contact per mitochondrium |
0.125 | 0.33 | × 2.5 |
Counting was done on EM observations, considering 30 photos randomly acquired at the same magnification for each sample.
In vitro transfer of DGDG from chloroplast envelope to mitochondria
Considering that (1) the membrane contact was stimulated as early as 6 h after subculture of cells in −Pi medium and that (2) between 6 and 18 h of Pi starvation, the DGDG content of the cells increases (Jouhet et al., 2003), we decided to investigate a possible transfer of galactolipids between chloroplast and mitochondria 12 h after Pi deprivation. We could not detect any transfer of galactolipids from isolated chloroplasts or envelope toward isolated mitochondria. For instance, for 1 h we incubated mitochondria with chloroplasts containing already labeled galactolipids. After separation, mitochondria did not contain any significant amount of labeled galactolipids (0.3 nmol.mg−1 protein representing 1.6% of the initial radioactivity). Moreover, there was no selection amid galactolipids. However, we observed an in vitro transfer of DGDG from mitochondria-associated envelope membranes to mitochondria (Table III). Owing to the presence of contaminating envelope, galactolipids were labeled from UDP-[C14]galactose in mitochondria preparations. Data on Pi-starved fractions show that the level of galactolipid synthesis was dependent of the contamination level of mitochondria by envelope, and that the initial proportion between the various galactolipids was rather stable (Table III). After galactolipid synthesis, mitochondria were further purified on a continuous Percoll gradient to remove extramitochondrial material including the plastid envelope and the radioactive substrate. The labeling of galactolipids in mitochondrial fractions was then compared before and after the purification step. The specific labeling of each galactolipid in mitochondria decreased when mitochondria were purified consistently with a partial and equal removal of both envelope membranes, as monitored by E37 and OEP21 detection. In control mitochondria the reduction of the level of each type of galactolipids was proportional to decrease of E37 and OEP21, indicating that there was no selection among galactolipids (Table S2, available at http://www.jcb.org/cgi/content/full/jcb.200407022/DC1). In contrast, in Pi-starved fractions the reduction of the level of each type of galactolipids was dissimilar; namely the decrease of the DGDG level was less important than that of MGDG and trigalactosyldiacylglycerol (TriGDG). The ratio of DGDG to either MGDG or TriGDG increased after the last purification step, whereas the ratio of MGDG to TriGDG remained about the same (Table III). Altogether, the relative DGDG increase in purified mitochondria of Pi-starved fractions and the level of cross-contamination by envelope decreased indicated that there is a pool of DGDG being transferred from plastid envelope membranes to mitochondria.
Table III.
Relative enrichment of DGDG content in various mitochondria fractions prepared from 12 h P i -deprived A. thaliana cells subsequent to an extra purification step of mitochondria
|
|
MGDG
|
DGDG
|
TriGDG
|
DGDG/MGDG
|
DGDG/TriGDG
|
MGDG/TriGDG
|
OEP21
|
E37
|
|---|---|---|---|---|---|---|---|---|
| nmol.mg−1 protein | nmol.mg−1 protein | nmol.mg−1 protein | Relative units | Relative units | ||||
| Experiment 1 | ||||||||
| Crude mitochondria | 4.08 | 11.26 | 1.09 | 2.8 | 10.3 | 3.7 | 64 | 67 |
| Purified mitochondria | 1.23 | 6.17 | 0.39 | 5.0 | 15.8 | 3.2 | 29 | 31 |
| Experiment 2 | ||||||||
| Purified mitochondria | 0.83 | 1.85 | 0.17 | 2.2 | 10.8 | 4.9 | 14.4 | 13.4 |
| Twice-purified mitochondria | 0.37 | 1.32 | 0.10 | 3.6 | 13.2 | 3.7 | 7.1 | 6.7 |
Galactolipids were initially synthesized either in crude or in purified mitochondria fractions for 1 h. A fraction sample was taken for lipid extraction immediately after the synthesis. The rest of the fraction was layered on a 28% Percoll gradient and purified as described for the second step of mitochondria purification in Materials and methods. Each fraction was tested for chloroplast envelope contamination as in Table I.
Discussion
MGDG and DGDG are unique plant lipids that are almost restricted to plastid membranes in plants grown under normal conditions. In the past few years, it has been shown that the level of DGDG highly and specifically increases in plant cells grown in Pi-deprived conditions (Härtel et al., 1998). In such plants, DGDG was found in the plasma membrane (Andersson et al., 2003), a membranous compartment disconnected from plastid membranes but dynamically connected to the overall endomembrane system, where major phospholipid syntheses (PC, PE) occur. A series of evidence presented in this paper shows that in Pi-deprived conditions, large amounts of DGDG are present in mitochondria. Immunofluorescence labeling, lipid analyses, and immunoagglutination indicated the presence of DGDG in Arabidopsis mitochondria in vivo and in vitro. We put forth much effort to differentiate cross-contamination of mitochondrial preparations by plastid membranes, especially envelope membranes, and the presence of DGDG in purified mitochondria. Considering that mitochondrial DGDG raises from only traces to ∼18% when cells are starved for Pi for 3 d, we concluded that purified mitochondria become highly enriched in DGDG under Pi shortage. Indeed, the ratios of DGDG/MGDG and DGDG/SQDG are much higher in mitochondria than in whole cells, and we calculated that the low level of MGDG and SQDG in isolated mitochondria can be considered as the result of cross-contamination by plastid membranes. On the other hand, considering the abundance of mitochondria in the cells, as estimated by the amount of DPG, the DGDG content of mitochondria is fully significant at the cell level, potentially explaining a part of the important increase of DGDG previously detected in plants grown in Pi-deprived conditions (Härtel et al., 1998). Because the convoluted inner membrane has a much larger surface area as compared with the outer membrane, it is generally considered that the lipid composition of the whole mitochondrion more closely resembles that of the inner membrane than that of the outer membrane (Douce, 1985). Immunoagglutination of mitochondria indicates that DGDG is present at the outer surface of mitochondria. However, if the high mol percentage of DGDG is reported to the leaflet surface of each mitochondrial membrane, it is very likely that DGDG is not only located at the surface of mitochondria, but also should be present in the inner leaflet of the outer membrane and in the inner membrane bilayer. Moreover, it is possible that Pi deprivation induces some development of the inner membrane because we observed that the amount of DPG, a specific lipid of the inner membrane of mitochondria, increases by ∼45% in cells and in isolated mitochondria. Altogether, this indicates that DGDG is likely to be present in both mitochondrial membranes.
All types of phospholipids (with the notable exception of DPG) seem to be replaced by DGDG in mitochondria during Pi deprivation. Our data show that this is not only the case of PC, but also of PE and PG. Although DGDG and PC are both bilayer-forming neutral lipids, PE and PG are negative lipids and, in addition, PE preferentially organizes in hexagonal-II phase. Therefore, the lipid changes induced by Pi deprivation in mitochondria mean a strong modification of the structure and of the charge of the lipid bilayer subsequently altering the environment of membrane proteins. Our results primarily question the possible role of DGDG in mitochondria membranes. In thylakoids, it has been shown that DGDG is required for structure and stability of LHCII in vitro (Reinsberg et al., 2000). A preservation of the mitochondrial electron transport machinery by changing phospholipids for DGDG when Pi is on shortage is a possibility. Indeed, isolated mitochondria were preserved in their respiratory activity (this paper and Rébeillé et al., 1984). Whether DGDG can take the place of phospholipids in the fine structures of the plant respiratory complexes is an open question. One should have expected that the level of DPG, being a Pi-rich phospholipid, can be decreased during Pi deprivation. Obviously this is not the case, and DPG appears to be quite protected. One possible explanation is that DPG fulfils a key function that is maintained or even enhanced in the Pi-deprived cells, and consequently preserved. In support to this hypothesis, several reports indicate that DPG is required for structure and stability of respiratory chain complexes in animal cells (Zhang et al., 2002; Pfeiffer et al., 2003).
The second intriguing question is the origin of mitochondrial DGDG. To date, all the DGDG synthase enzymes, galactolipid–galactolipid galactosyltransferase, DGD1, and DGD2, were reported to be localized in chloroplast envelope membranes (Dorne et al., 1982; Froehlich et al., 2001; Kelly et al., 2003). Our NMR analyses indicated that the structure of mitochondrial DGDG, 1,2-diacyl-3-O-(α-d-galactopyranosyl-(1→6)-O-β-d-galactopyranosyl)-sn-glycerol, is consistent with a synthesis via a DGD type enzyme and not via the galactolipid–galactolipid galactosyltransferase. Indeed, working on dgd1 dgd2 double mutants, Kelly et al. (2003) have shown that both DGD1 and DGD2 contribute to DGDG synthesis during Pi deprivation and only them. The fatty acid composition and positional distribution of mitochondrial DGDG can give some indications about which of the DGD1 and DGD2 enzymes is involved in its synthesis. Considering analyses of dgd1 and dgd2 mutants (Härtel et al., 2000; Klaus et al., 2002; Kelly et al., 2003), the slight increase in 16:0 in DGDG compared with control cell DGDG suggests the involvement of DGD2, whereas the strong amount of 18:3 is indicative of the DGD1 functioning. Therefore, mitochondrial DGDG likely results from both DGD1 and DGD2 enzyme activities present in the chloroplast envelope. Eventually, the presence in DGDG of high proportion of 18:3, which was shown not to depend on ER FAD3 desaturation (Klaus et al., 2002), is an additional indication of the synthesis of mitochondrial DGDG in chloroplast envelope membranes. As additional evidence, we verified that the level of galactolipid synthesis in fractions enriched in mitochondria was strictly related to the level of mitochondria cross-contamination by envelope membranes.
Several models were proposed to explain net transfers of lipid between membranes and organelles (for review see Voelker, 2003). Particularly, lipid movements can occur via specialized zones of apposition between subcellular membranes. For example, in yeast and in mammalian cells, a movement of phosphatidylserine (PS) from ER to mitochondria inner membrane was clearly demonstrated to occur through specialized region of ER referred to as mitochondria-associated membranes (MAM; Vance, 1990; Ardail et al., 1991; Achleitner et al., 1999). To investigate how DGDG transfers from chloroplast envelope to mitochondria membranes, we surveyed cell structures during the course of adaptation to Pi deprivation. We failed to observe any formation of vesicles. Rather, we noticed numerous tight appositions of membranes from envelope and mitochondria during early phases of Pi deprivation that could sustain contact-favored transfer. In the case of PS transfer through MAM, physical interactions between mitochondria and ER were not easily disrupted (Voelker, 1990). Similarly, we observed that mitochondria were more difficult to separate from envelope after 12 h of Pi deprivation than after 3 d. Together, our data indicate that DGDG uptake by mitochondria seems to require a physical contact between mitochondria and chloroplast envelope membranes. Although we could not detect a transfer of DGDG from isolated chloroplasts toward isolated mitochondria, we did observe an in vitro transfer of DGDG from mitochondria-associated envelope membranes to mitochondria. In addition, the transfer was selective for DGDG compared with MGDG or TriGDG. The association of DGDG synthesis with the transfer process is possible, but could not be investigated. The same kind of association was considered for PS transfer to mitochondria to encounter the fact that a selected pool of PS was transferred (Vance, 1990).
In conclusion, this work provides evidence that in plant cells starved for Pi, DGDG is present in several specific membranes outside plastids and becomes rapidly highly abundant in mitochondria membranes. A future challenge includes elucidation of the molecular mechanisms involved in the synthesis and transfer of DGDG from plastid envelope to mitochondria membranes. The high stability observed in lipid composition of mitochondrial membranes under standard situation, even when comparing different plants and the triggering of the DGDG transfer upon Pi deprivation, will have to be considered. A last challenging question is the role of DGDG in mitochondria, specifically whether this role extends further than building up primary membrane blocks.
Materials and methods
Cultivation of Arabidopsis cell lines
A photosynthetic A. thaliana cell suspension (Axelos et al., 1992) was cultured as described in Jouhet et al. (2003).
Preparation of cell fractions
About 70 g of Arabidopsis cells were filtered and resuspended in 50 ml ice-cold grinding buffer (0.3 M mannitol, 15 mM MOPS, 2 mM EGTA, 0.6% [wt/vol] polyvinylpyrrolidone 25, 0.5% BSA, 10 mM DTT, 1 mM PMSF, 5 mM α-aminocaproic acid, and 1 mM benzamidine, pH 8.0). The cells were disrupted by adding 10 ml of sand and by crushing with a mortar. Cell extract was collected as a supernatant after centrifugation at 150 g for 10 min.
Mitochondria were enriched by a three-step centrifugation from cell extract: two centrifugations at 3,000 g for 5 min and a centrifugation at 18,000 g for 15 min. The mitochondrial fraction was resuspended in washing buffer (0.3 M mannitol, 10 mM MOPS, 1 mM PMSF, 5 mM α-aminocaproic acid, and 1 mM benzamidine, pH 7.4) and layered on top of a three-layer Percoll (Amersham Biosciences) gradient (18% [vol/vol], 23% [vol/vol], and 40% [vol/vol] Percoll in 0.3 M mannitol, 10 mM MOPS, 1 mM EGTA, and 0.1% BSA, pH 7.2). After centrifugation for 45 min at 70,000 g the mitochondria were isolated from the 23%/40% interphase. To remove Percoll, the mitochondria were centrifuged twice in washing buffer for 15 min at 17,000 g. The crude mitochondria fraction was resuspended in washing buffer and layered on top of continuous 28% Percoll gradients in 0.3 M mannitol, 10 mM MOPS, 1 mM EGTA, and 0.1% BSA, pH 7.2. After centrifugation for 1 h at 40,000 g the mitochondria were isolated in the medium of the gradient. To remove Percoll, the purified mitochondria were centrifuged three times in washing buffer for 15 min at 17,000 g. Purified mitochondria pellet was resuspended in washing buffer and frozen at −80°C.
Chloroplasts were purified from cell extract by centrifugation at 3,000 g for 5 min. The chloroplast fraction was resuspended in washing buffer, and 6 ml was layered on top of a two-step Percoll gradient (33% [vol/vol] and 50% [vol/vol] Percoll in 0.3 M mannitol, 10 mM MOPS, 1 mM EGTA, and 0.1% BSA, pH 7.2). After centrifugation for 15 min at 3,000 g chloroplasts were collected at the 33%/50% interphase. To remove Percoll, the purified chloroplasts were centrifuged twice in washing buffer for 5 min at 2,000 g. Purified chloroplast pellet was resuspended in a minimum volume of washing buffer and conserved at −80°C.
Mitochondria respiration measurement
O2 electrode (Oxygraph Hansatech) was calibrated in water and equilibrated for 5 min in 1 ml of 0.3 M mannitol, 20 mM MOPS, 10 mM Pi, 10 mM KCl, 5 mM MgCl2, and 0.1% BSA, pH 7.5. O2 consumption was measured after addition of 10 μl of mitochondria fraction, 10 μl succinic acid 1 M, and 10 μl ADP 100 mM. 10 μl KCN 100 mM was added to measure level of sensitivity to cyanide.
Western blotting and immunoblotting
Protein quantification was done by the Lowry method (Lowry et al., 1951). Proteins were solubilized in 0.1 M Tris, pH 7.8, 10% glycerol (vol/vol), 2% SDS (wt/vol), 25 mM DTT, and 0.1% bromophenol blue (wt/vol). Samples were analyzed by SDS–PAGE (Broglie et al., 1980). After electrophoresis, proteins were stained in isopropanol/acetic acid (3:1, vol/vol) or Coomassie brillant blue (R-250; Sigma-Aldrich) 0.25% (wt/vol), or electrophoretically transferred to nitrocellulose membrane for immunoblotting. The proteins were visualized with a rabbit primary antibody against a specific protein and an anti–rabbit IgG-HRP conjugate and the ECL Plus Western blotting detection system (Amersham Biosciences). The anti-OEP21 antibodies were prepared by rabbit immunization using the recombinant protein corresponding to the full-length sequence At1g76405.2.
Antibodies against Nad9 were provided by Dr. Grienenberger (IBMP-CNRS, Strasbourg, France), antibodies against TOM20 and TOM40 by Dr. Braun (Universität Hannover, Hannover, Germany), antibodies against LHCII by Dr. Vallon (IBPC, Saclay, France), antibodies against BCCP1 by Dr. Alban (INRA, Grenoble, France), and antibodies against HPPK by Dr. Ravanel (Université Grenoble, Grenoble, France).
Lipid analysis
Lipids were extracted from 2 g of harvested cells, according to Folch et al. (1957) or from mitochondria fraction, according to Bligh and Dyer (1959). They were analyzed as described in Jouhet et al. (2003).
Galactolipid synthesis in organelle fraction
Organelle fractions were incubated at 25°C in washing buffer with 1 mM DTT, 1 mM MgCl2, and 10 mM UDP-[14C]galactose, 10,000 dpm/nmol. Reaction was stopped by a Bligh and Dyer lipid extraction (Bligh and Dyer, 1959). A sample was taken after 30 min and after 1 h to verify reaction linearity. We verified that addition of DAG in the incubation buffer and sonication did not increase galactose incorporation into lipids. Lipids were separated by 2D-TLC as described before, scraped of the plate, and radioactivity in each lipid was counted by scintillation. Activity was determined in dpm incorporated into lipids/h and mg of protein and converted in nmol.h−1.mg−1.
Agglutination assays
Antibodies raised against plastid envelope, mitochondria outer membrane polypeptides, and DGDG were used to probe the outer surface of isolated intact mitochondria or chloroplasts. For agglutination assays, mitochondria or chloroplast suspension corresponding to 15 nmol O2/min respiration or 18 μg chlorophyll, respectively, were incubated 10 min on a glass slide with 4 μl of the washing buffer (0.3 M mannitol, 10 mM MOPS, 1 mM PMSF, 5 mM α-aminocaproic acid, and 1 mM benzamidine, pH 7.4) and 6 μl antibodies. The suspensions were then examined at RT under light microscopy (Axioplan 2, Carl Zeiss MicroImaging, Inc.) using an immersion 100× objective and a CCD camera (Hamamatsu Corporation) to follow agglutination.
Epifluorescence
Arabidopsis cells were fixed 20 min by 5% methanol-free formaldehyde (Polyscience) in TBS (1.3 M NaCl, 13 mM KCl, and 15 mM Tris-HCl, pH 7.4), washed in TBS, permeabilized 15 min with 0.002% saponin in TBS, and saturated 1 h with TBS, 5% FBS, and 5% goat serum. After incubation for 1 h with 1/10 rabbit serum against DGDG (Maréchal et al., 2002) and 1/100 guinea pig serum against BCCP1 or HPPK in TBS, 1% FBS, three washes of 10 min in TBS, 1% FBS, incubation for 1 h with 1/100 anti–rabbit IgG-goat BODIPY conjugate and 1/20 anti–guinea pig IgG-goat Alexa 594 (Molecular Probes, Inc.) in TBS, 1% FBS in the dark, three washes of 10 min in the dark in TBS, 1% FBS, and one in TBS, cells were deposed on glass slide with ProLong AntiFade kit (Molecular Probes, Inc.) and stored in the dark. Before fixation, some cells were incubated for 1 h in the presence of MitoTracker orange CMTMRos (Molecular Probes, Inc.) and washed twice in TBS. Slides were observed with an immersion 40× objective at RT the following day by confocal laser scanning microscopy using a TCS-SP2 operating system (Leica). BODIPY, Alexa 594, and MitoTracker were excited and collected sequentially (400 Hz, line by line) using the 488-nm line of an argon laser for BODIPY and the 543-nm line of a He-Ne laser for Alexa 594 and MitoTracker. Fluorescences were collected between 498 and 533 nm, 580 and 650 nm, and 553 and 607 nm for BODIPY, Alexa 594, and MitoTracker, respectively.
Electron microscopy
Samples were fixed overnight in 1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 6.9). Next, they were post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 2 h. After this double fixation, samples were exposed for 30 min to a solution of 1% tannic acid in the same buffer at RT, dehydrated in a graded series of ethanol and propylene oxide, and finally embedded in an Epon-Araldite mixture. Thin sections were cut with an ultramicrotome (Ultracut, Reichert-Jung), post-stained with lead citrate, and examined with a transmission electron microscope (model 300; Hitachi) operating at 75 kV.
1H-NMR spectroscopy
DGDG was isolated from TLC as described before and was extracted from silica by addition of 1.5 ml chloroform/methanol, 2:1, and 400 μl of NaCl 10 g/l. Extracts were dried under argon and dissolved in 440 μl deuterated chloroform and 220 μl deuterated methanol (Merck). 1H-NMR spectra were obtained on a Varian Unity+ 500 MHz spectrometer (Bruker) at 10°C with a 5-mm indirect detection probe. The methanol resonance was used as a lock signal. Acquisition parameters were as follows: 90° pulse, repetition time 3 s, spectral width 4993 Hz, number of scans = 64. Free induction decays were collected as 30-K data points, zero filled to 65 K, and processed with a 0.18-Hz exponential line broadening. A 1-s recycling time was used to obtain fully relaxed spectra.
Online supplemental material
Fig. S1 shows immunofluorescence localization of DGDG in A. thaliana cells grown with Pi. Analysis of galactolipid composition of mitochondria fraction is presented in Table S1, where the lipid contamination attributable to chloroplast envelope is compared with mitochondrial lipid data. Analysis of DGDG content in mitochondria fractions prepared from control A. thaliana cells subsequent to an extra purification step of mitochondria is presented in Table S2. Experiment was done as in Table III, except that mitochondria were purified from control cells. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200407022/DC1.
Acknowledgments
The authors are grateful to Dr. Mercier for teaching epifluorescence techniques, to Dr. Gout and Gennaro for NMR analyses, and to R. Douce for critical reading of the manuscript. We sincerely acknowledge Dr. Grunwald (DRDC-CIS, CEA-Grenoble) for his expert management of the confocal microscopy facility.
Abbreviations used in this paper: BCCP, biotin carboxyl carrier protein; DGDG, digalactosyldiacylglycerol; DPG, diphosphatidylglycerol; HPPK, dihydropterin pyrophosphokinase; MGDG, monogalactosyldiacylglycerol; NMR, nuclear magnetic resonance; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; SQDG, sulfoquinovosyldiacylglycerol; TriGDG, trigalactosyldiacylglycerol.
References
- Achleitner, G., B. Gaigg, A. Krasser, E. Kainersdorfer, S.D. Kohlwein, A. Perktold, G. Zellnig, and G. Daum. 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 264:545–553. [DOI] [PubMed] [Google Scholar]
- Alban, C., P. Baldet, and R. Douce. 1994. Localization and characterization of two structurally different forms of acetyl-CoA carboxylase in young pea leaves, of which one is sensitive to aryloxyphenoxypropionate herbicides. Biochem. J. 300:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, M.X., M.H. Stridh, K.E. Larsson, C. Liljenberg, and A.S. Sandelius. 2003. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537:128–132. [DOI] [PubMed] [Google Scholar]
- Ardail, D., F. Lerme, and P. Louisot. 1991. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266:7978–7981. [PubMed] [Google Scholar]
- Awai, K., E. Maréchal, M.A. Block, D. Brun, T. Masuda, H. Shimada, K. Takamiya, H. Ohta, and J. Joyard. 2001. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 98:10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelos, M., C. Curie, L. Mazzolini, C. Bardet, and B. Lescure. 1992. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol. Biochem. 30:123–128. [Google Scholar]
- Bligh, E.G., and W.J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. [DOI] [PubMed] [Google Scholar]
- Block, M.A., A.J. Dorne, J. Joyard, and R. Douce. 1983. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J. Biol. Chem. 258:13281–13286. [PubMed] [Google Scholar]
- Bolter, B., J. Soll, K. Hill, R. Hemmler, and R. Wagner. 1999. A rectifying ATP-regulated solute channel in the chloroplastic outer envelope from pea. EMBO J. 18:5505–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie, R.M., C.N. Hunter, P. Delepelaire, R.A. Niederman, N.H. Chua, and R.K. Clayton. 1980. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA. 77:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, H.E., R.H. McCluer, and E.D. Slifer. 1956. Lipids of wheat flour. I. Characterization of galactosylglycerol components. J. Am. Chem. Soc. 78:3735–3738. [Google Scholar]
- Dorne, A.J., M.A. Block, J. Joyard, and R. Douce. 1982. The galactolipid: galactolipid galactosyltransferase is located on the outer surface of the outer chloroplast envelope. FEBS Lett. 145:30–34. [Google Scholar]
- Dorne, A.J., J. Joyard, M.A. Block, and R. Douce. 1985. Localization of phosphatidylcholine in outer envelope membrane of spinach chloroplasts. J. Cell Biol. 100:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce, R. 1974. Site of galactolipid synthesis in spinach chloroplasts. Science. 183:852–853. [DOI] [PubMed] [Google Scholar]
- Douce, R. 1985. Mitochondria in Higher Plants: Structure, Function and Biogenesis. Academic Press Inc., NY. 322 pp.
- Essigmann, B., S. Güler, R.A. Narang, D. Linke, and C. Benning. 1998. Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 95:1950–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch, J., M. Lees, and G.A. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Froehlich, J.E., C. Benning, and P. Dormann. 2001. The digalactosyldiacylglycerol (DGDG) synthase DGD1 is inserted into the outer envelope membrane of chloroplasts in a manner independent of the general import pathway and does not depend on direct interaction with monogalactosyldiacylglycerol synthase for DGDG biosynthesis. J. Biol. Chem. 276:31806–31812. [DOI] [PubMed] [Google Scholar]
- Härtel, H., and C. Benning. 2000. Can digalactosyldiacylglycerol substitute for phosphatidylcholine upon phosphate deprivation in leaves and roots of Arabidopsis? Biochem. Soc. Trans. 28:729–732. [PubMed] [Google Scholar]
- Härtel, H., B. Essigmann, H. Lokstein, S. Hoffmann-Benning, M. Peters-Kottig, and C. Benning. 1998. The phospholipid-deficient pho1 mutant of Arabidopsis thaliana is affected in the organization, but not in the light acclimation, of the thylakoid membrane. Biochim. Biophys. Acta. 1415:205–218. [DOI] [PubMed] [Google Scholar]
- Härtel, H., P. Dormann, and C. Benning. 2000. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 97:10649–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood, J.L. 1987. Phosphoglycerides of mitochondrial membranes. Methods in Enzymology. Vol. 148. L. Packer and R. Douce, editors. Academic Press, Inc., London. 475–485.
- Haschke, H.-P., G. Kaiser, E. Martinoia, U. Hammer, T. Teucher, A.J. Dorne, and E. Heinz. 1990. Lipid profiles of leaf tonoplasts from plants with different CO2-fixation mechanisms. Bot. Acta. 103:32–38. [Google Scholar]
- Jouhet, J., E. Maréchal, R. Bligny, J. Joyard, and M.A. Block. 2003. Transient increase of phosphatidylcholine in plant cells in response to phosphate deprivation. FEBS Lett. 544:63–68. [DOI] [PubMed] [Google Scholar]
- Joyard, J., E. Maréchal, M.A. Block, and R. Douce. 1996. Plant galactolipids and sulfolipid. Structure, distribution and biosynthesis. Membranes: Specialized Function in Plants. M. Smallwood, P. Knox, and D.J. Bowles, editors. BIOS Scientific Publishers, Oxford, UK. 179–194.
- Kelly, A.A., and P. Dormann. 2002. DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J. Biol. Chem. 277:1166–1173. [DOI] [PubMed] [Google Scholar]
- Kelly, A.A., J.E. Froehlich, and P. Dormann. 2003. Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell. 15:2694–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus, D., H. Hartel, L.M. Fitzpatrick, J.E. Froehlich, J. Hubert, C. Benning, and P. Dormann. 2002. Digalactosyldiacylglycerol synthesis in chloroplasts of the Arabidopsis dgd1 mutant. Plant Physiol. 128:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, M., K. Seki, M. Ohnishi, S. Ito, and Y. Fujino. 1990. Structure of novel glyceroglycolipids in Adzuki bean (Vigna angularis) seeds. Biochem. Cell Biol. 68:59–64. [PubMed] [Google Scholar]
- Lamattina, L., D. Gonzalez, J. Gualberto, and J.M. Grienenberger. 1993. Higher plant mitochondria encode an homologue of the nuclear-encoded 30-kDa subunit of bovine mitochondrial complex I. Eur. J. Biochem. 217:831–838. [DOI] [PubMed] [Google Scholar]
- Lowry, H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- Maréchal, E., N. Azzouz, C.S. de Macedo, M.A. Block, J.E. Feagin, R.T. Schwarz, and J. Joyard. 2002. Synthesis of chloroplast galactolipids in apicomplexan parasites. Eukaryot. Cell. 1:653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillon, J.M., S. Ravanel, R. Douce, and F. Rébeillé. 2002. Folate synthesis in higher-plant mitochondria: coupling between the dihydropterin pyrophosphokinase and the dihydropteroate synthase activities. Biochem. J. 363:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger, M., E.P. Journet, R. Bligny, J.P. Carde, and R. Douce. 1982. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch. Biochem. Biophys. 217:312–323. [DOI] [PubMed] [Google Scholar]
- Norberg, P., and C. Liljenberg. 1991. Lipids of plasma membranes prepared from oat root cells. Plant Physiol. 96:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, K., V. Gohil, R.A. Stuart, C. Hunte, U. Brandt, M.L. Greenberg, and H. Schagger. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278:52873–52880. [DOI] [PubMed] [Google Scholar]
- Raghothama, K.G. 1999. Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:665–693. [DOI] [PubMed] [Google Scholar]
- Raghothama, K.G. 2000. Phosphate transport and signaling. Curr. Opin. Plant Biol. 3:182–187. [PubMed] [Google Scholar]
- Rébeillé, F., R. Bligny, and R. Douce. 1984. Is the cytosolic Pi concentration a limiting factor for plant cell respiration? Plant Physiol. 74:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsberg, D., P.J. Booth, C. Jegerschold, B.J. Khoo, and H. Paulsen. 2000. Folding, assembly, and stability of the major light-harvesting complex of higher plants, LHCII, in the presence of native lipids. Biochemistry. 39:14305–14313. [DOI] [PubMed] [Google Scholar]
- Roughan, G. 1970. Turnover of the glycerolipids of pumpkin leaves. The importance of phosphatidylcholine. Biochem. J. 117:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier, E., M.A. Block, R. Douce, and J. Joyard. 1996. Is E37, a major polypeptide of the inner membrane from plastid envelope, an S-adenosyl methionine-dependent methyltransferase? Plant J. 10:903–912. [DOI] [PubMed] [Google Scholar]
- Vallon, O., F.-A. Wollman, and J. Olive. 1986. Lateral distribution of the main protein complexes of the photosynthetic apparatus in Chlamydomonas reinhardtii and in spinach: an immunocytochemical study using intact thylakoid membranes and a PSII enriched membrane. Photobiochem. Photobiophys. 12:203–220. [Google Scholar]
- van Besouw, A., and J.F. Wintermans. 1978. Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim. Biophys. Acta. 529:44–53. [DOI] [PubMed] [Google Scholar]
- Vance, J.E. 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265:7248–7256. [PubMed] [Google Scholar]
- Voelker, D.R. 1990. Characterization of phosphatidylserine synthesis and translocation in permeabilized animal cells. J. Biol. Chem. 265:14340–14346. [PubMed] [Google Scholar]
- Voelker, D.R. 2003. New perspectives on the regulation of intermembrane glycerophospholipid traffic. J. Lipid Res. 44:441–449. [DOI] [PubMed] [Google Scholar]
- Werhahn, W., A. Niemeyer, L. Jansch, V. Kruft, U.K. Schmitz, and H. Braun. 2001. Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis. Identification of multiple forms of TOM20. Plant Physiol. 125:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J.P., V. Imperial, M.U. Khan, and J.N. Hodson. 2000. The role of phosphatidylcholine in fatty acid exchange and desaturation in Brassica napus L. leaves. Biochem. J. 349:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C.C., J.L. Fan, W. Riekhof, J.E. Froehlich, and C. Benning. 2003. A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J. 22:2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., E. Mileykovskaya, and W. Dowhan. 2002. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 277:43553–43556. [DOI] [PubMed] [Google Scholar]