Abstract
Cell migration is essential for proper development of numerous structures derived from embryonic neural crest cells (NCCs). Although the migratory pathways of NCCs have been determined, the molecular mechanisms regulating NCC motility remain unclear. NCC migration is integrin dependent, and recent work has shown that surface expression levels of particular integrin α subunits are important determinants of NCC motility in vitro. Here, we provide evidence that rapid cranial NCC motility on laminin requires integrin recycling. NCCs showed both ligand- and receptor-specific integrin regulation in vitro. On laminin, NCCs accumulated internalized laminin but not fibronectin receptors over 20 min, whereas on fibronectin neither type of receptor accumulated internally beyond 2 min. Internalized laminin receptors colocalized with receptor recycling vesicles and were subsequently recycled back to the cell surface. Blocking receptor recycling with bafilomycin A inhibited NCC motility on laminin, indicating that substratum-dependent integrin recycling is essential for rapid cranial neural crest migration.
Introduction
The neural crest is a transient cell population that arises on the dorsal side of the neural tube and migrates extensively throughout the developing vertebrate embryo. These cells generate a diverse array of derivatives, including the neurons and glia of the peripheral and autonomic nervous systems, craniofacial connective tissue and bone, pigment cells, and adrenomedullary cells, as well as the outflow tract of the heart (for review see Bronner-Fraser, 1993a; Anderson, 1997; Le Douarin and Kalcheim, 1999; Christiansen et al., 2000; Dorsky et al., 2000; Gammill and Bronner-Fraser, 2003). Numerous reports have documented severe perturbation of neural crest cell (NCC) migration after manipulations of integrin function both in vitro and in vivo (Kil et al., 1996; Testaz and Duband, 2001; Alfandari et al., 2003; Tucker, 2004), but the molecular and cellular basis of this flawed motility remain unclear.
Integrins are a major metazoan family of cell adhesion receptors and play key roles in development, immune response, and cancer metastasis (for review see De Arcangelis and Georges-Labouesse, 2000; van der Flier and Sonnenberg, 2001). These heterodimeric transmembrane receptors, composed of an α and a β subunit, bind the ECM and convey signals intracellularly. During vertebrate development, integrins are required at numerous stages for proper cell migration, proliferation, survival, and differentiation of many embryonic cell populations, including the neural crest.
To migrate long distances through diverse tissues in vivo, NCCs must be able to adapt to changing extracellular environments. We have previously shown that embryonic sensory neurons and their immediate embryonic precursors, NCCs, are able to migrate across at least a 10-fold range of ECM protein concentrations in vitro (Condic and Letourneau, 1997; Condic, 2001; Strachan and Condic, 2003). NCCs attain optimal adhesion for sustained motility over a wide range of ECM concentrations by altering surface integrin expression in order to match their adhesion receptor levels to the concentration of ligand. In contrast, many other motile cell types appear unable to modulate surface integrin levels and therefore only migrate on a limited range of ECM concentrations (Goodman et al., 1989; Buettner and Pittman, 1991; Duband et al., 1991; Arroyo et al., 1992; DiMilla et al., 1993; Palecek et al., 1997). These results suggest that rapid NCC motility over a wide range of substratum concentrations is dependent on continuous monitoring of and response to the extracellular environment.
The response of NCCs to the ECM varies along the rostrocaudal axis of the embryo. The neural crest can be divided into four subpopulations (cranial, vagal, truncal, and sacral), each of which occupies its own segment of the neural tube and gives rise to distinct derivatives (Bronner-Fraser, 1993b). We have shown that different crest populations have distinct motility and integrin regulation in culture. For example, cranial and trunk neural crest have similar migratory properties on low concentrations of laminin. Yet, on high concentrations of laminin, cranial NCCs migrate nearly twice as fast as trunk NCCs. Correspondingly, cranial NCCs regulate surface levels of integrin α6 (a laminin receptor) to a greater extent than do trunk NCCs. When integrin α6 is overexpressed in cranial NCCs, their velocity slows to that of trunk NCCs, suggesting that low surface integrin levels are required for rapid motility (Strachan and Condic, 2003). Thus, we focused here on the mechanism cranial NCCs use to modulate their surface integrin levels, thereby promoting rapid cell migration.
One mechanism by which cells can modulate their surface integrin levels is via the clathrin-mediated receptor recycling pathway (Bretscher, 1992; Fabbri et al., 1999; Pierini et al., 2000; Long et al., 2001). Clathrin-mediated endocytosis modulates signal transduction both by controlling the levels of surface signaling receptors and by mediating the rapid clearance and down-regulation of activated signaling receptors. For motile cells, receptor recycling also provides an efficient way to transport receptors from the tailing edge, where the cell is releasing from the substratum, to the leading edge, where new adhesions are being formed (Roberts et al., 2001; Rappoport and Simon, 2003; Powelka et al., 2004). Receptors may either be returned to the surface nearby the site of internalization via fast recycling vesicles, or can be trafficked through the cell via the slower receptor recycling compartment (Sonnichsen et al., 2000). Each endocytic compartment is characterized by the expression of specific rab GTPases (Mellman, 1996; Sheff et al., 1999; Qualmann and Mellor, 2003). Because cranial NCCs down-regulate surface levels of integrin α6 in response to the same conditions under which they migrate relatively quickly (i.e., high laminin concentrations), we questioned whether laminin receptors were being transported through the receptor recycling pathway in rapidly moving NCCs.
In the present work we demonstrate that cranial NCCs regulate their surface integrin expression in a substratum-dependent manner. Using a modified, short-term, biochemical assay we followed the internalization kinetics of α5 (a fibronectin receptor) and α6 (a laminin receptor) integrins in cranial NCCs cultured on fibronectin and laminin. We find that in cells cultured on laminin, but not fibronectin, surface integrin α6 is internalized and intracellular pools of internalized receptor accumulate over a 20-min time period. Internalized laminin receptors colocalize in rab4+ and rab11+ recycling vesicles, indicating that they are transported via the receptor recycling pathway. By labeling surface receptors immunohistochemically, we also demonstrate that internalized receptors are recycled back to the cell surface. Finally, using an acute time-lapse assay we show that inhibiting receptor recycling with bafilomycin A (BafA) significantly slows cranial neural crest migration on high laminin but not on low laminin, suggesting that this pathway is critical for regulating adhesion and supporting rapid motility on this substrata. We conclude that receptor recycling is an important mechanism underlying rapid cranial neural crest migration.
Results
Surface integrin expression is substratum dependent
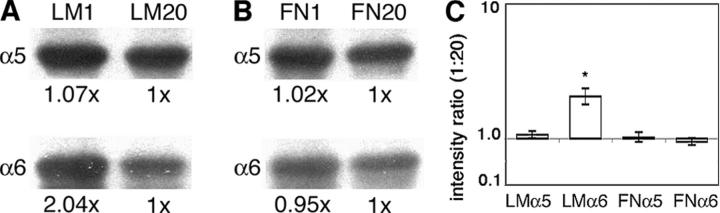
To determine whether cranial NCCs modulate their surface integrin levels in a substratum-dependent manner, we examined the steady-state surface expression of two integrin receptors, α6 (a laminin receptor) and α5 (a fibronectin receptor), in cranial NCCs cultured on two different concentrations of laminin and fibronectin. Mesencephalic neural tubes were cultured on glass coated with low (1 μg/ml) and high (20 μg/ml) concentrations of either laminin or fibronectin. Application of laminin at 1 and 20 μg/ml resulted in a density of 45 and 430 ng/cm2 bound laminin, respectively, and application of fibronectin at 1 and 20 μg/ml resulted in 35 and 700 ng/cm2 bound fibronectin, respectively (Strachan and Condic, 2003). After labeling surface proteins with biotin, integrins α5 and α6 were immunoprecipitated, and the surface pools were detected with avidin on Western blots. As we previously observed (Strachan and Condic, 2003), cranial NCCs cultured on varying concentrations of laminin regulated surface levels of the laminin receptor, integrin α6, but not the fibronectin receptor, integrin α5 (Fig. 1, A and C). On low concentrations of laminin (LM1), cells expressed greater amounts of integrin α6 on their surface compared with cells cultured on high laminin concentrations (LM20). Quantification of band intensities from both laminin concentrations indicates an average twofold increase in surface α6 protein on LM1 relative to LM20 (n = 6; P < 0.002, Mann-Whitney U-test). On both concentrations of laminin, surface levels of the fibronectin receptor, integrin α5, were equivalent, suggesting that integrin regulation is receptor specific (Fig. 1, A and C). In contrast to cells cultured on laminin, cranial NCCs cultured on varying concentrations of fibronectin did not modulate surface levels of either integrin α5 or α6 (Fig. 1, B and C).
Figure 1.
Cranial neural crest modulate surface integrin levels in a substratum-dependent manner. Cell surface–labeled integrins were immunoprecipitated and analyzed on Western blot. (A and B) Average band intensities (relative to the high substratum condition) are given below each lane. (A) Cranial neural crest down-regulate surface levels of the laminin receptor integrin α6, but not the fibronectin receptor integrin α5, when cultured on high (LM20) compared with low (LM1) concentrations of laminin. (B) Surface levels of both integrin α5 and α6 are equivalent in cranial neural crest cultured on low (FN1) and high (FN20) concentrations of fibronectin. (C) The average relative band intensity ratio (LM1:LM20 or FN1:FN20) for both integrin α5 and α6; y-axis is a log scale. Means and 95% confidence intervals from at least five independent experiments are as follows: LMα5 1.07x (1.01–1.13), LMα6 2.04x (1.79–2.34), FNα5 1.02x (0.95–1.11), and FNα6 0.95x (0.90–1.01). Asterisk indicates that the average band intensity of integrin α6 on LM1 is significantly greater than on LM20 (P < 0.002; Mann-Whitney U-test). Average band intensities for all other conditions did not significantly differ. Equal protein amounts were used for immunoprecipitations.
Modulating surface integrin levels (0.17- to 3-fold) has been shown to affect motility in a substratum-dependent manner (Palecek et al., 1997; Condic et al., 1999; Condic, 2001; Strachan and Condic, 2003). Based on our previous finding that cranial neural crest on high concentrations of laminin compared with low both down-regulated surface integrin α6 levels and had greater velocities (Strachan and Condic, 2003), we speculated that integrin internalization on high substratum concentrations was related to the rapid motility of these cells. An alternative explanation is that low substratum concentrations stabilize receptor expression at the surface; however, that does not seem to be the case either for integrin α5 on low laminin, or for integrin α5 and α6 on low fibronectin concentrations. These results suggest that down-regulation of relevant (i.e., ligand-binding) surface receptors in response to high laminin concentrations is unique to this substratum. Thus, we decided to further investigate the substratum-related differences in cranial neural crest surface integrin regulation.
Integrin internalization kinetics are substratum dependent
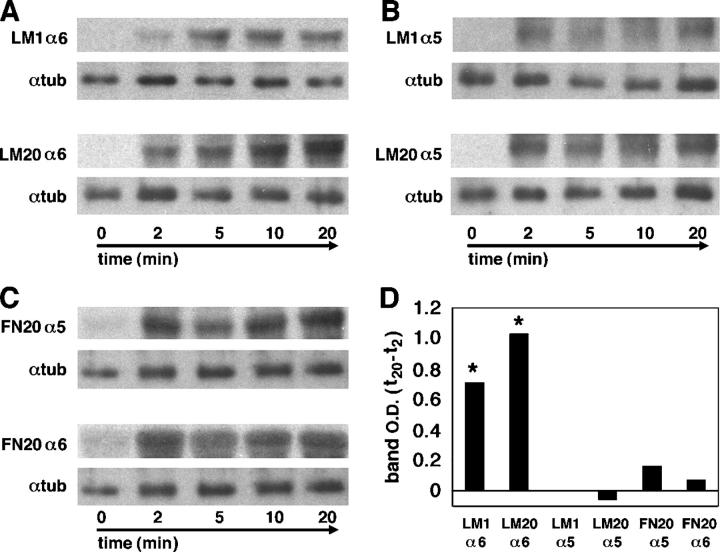
To analyze the kinetics of surface integrin regulation in cranial NCCs, we followed the internalization of labeled receptors during a short time period. We found a difference between the accumulation of internalized integrin α6 and integrin α5 (Fig. 2). On both low and high laminin concentrations, internalized integrin α6 reached steady-state levels by 20 min; i.e., there was no greater accumulation after 30 or 60 min (unpublished data). In contrast to integrin α6, low levels of integrin α5 accumulated rapidly (within 2 min) in cells cultured on both laminin concentrations, and the internalized pool did not increase over time (Fig. 2, B and D). Thus, cranial NCCs on laminin internalize and accumulate laminin, but not fibronectin receptors.
Figure 2.
Cranial neural crest accumulate internalized surface integrin receptors in a substratum-dependent manner. Surface receptors were labeled with biotin, cells were incubated at 37°C for the indicated time points, then cooled to 25°C and treated with the reducing agent MesNa to remove biotin from receptors remaining on the surface. Cells were lysed, the receptors were immunoprecipitated, and the internalized portion revealed on Western blots with HRP-conjugated streptavidin. Bottom panels show internal loading control (α-tubulin). (A) On both low (LM1) and high (LM20) concentrations of laminin, there is an accumulation of internalized integrin α6 over 20 min. (B) On both LM1 and LM20, accumulation of internalized integrin α5 reaches steady-state levels by 2 min. (C) On high (FN20) fibronectin concentrations there is no accumulation of internalized integrin α5 or integrin α6 above the level observed at 2 min. (D) The average increase in band intensity from 2 to 20 min for each condition from three independent experiments is shown. Asterisks indicate significant increases in internalized α6 over time on both LM1 and LM20 (P < 0.05; Wilcoxon signed-rank test).
To determine whether the lack of integrin α5 internal accumulation on laminin was due to its inability to bind laminin, we analyzed the internalization of integrin α5 and α6 on fibronectin. When cranial NCCs were cultured on high concentrations of fibronectin, they rapidly internalized both integrin α5 and integrin α6, but there was no differential accumulation of either receptor inside the cell beyond the steady-state level reached after 2 min (Fig. 2, C and D). Thus, the internal accumulation of integrins in cranial NCCs depends both on the receptor (i.e., α5 vs. α6) and on the substratum. These results indicate that cranial NCCs respond to high concentrations of laminin differently than they do to fibronectin, suggesting that differences in receptor trafficking may underlie differences in motility on dissimilar substrata.
Internalized integrins colocalize with receptor recycling pathways
To determine in which intracellular pathway internalized integrins were being trafficked, we investigated the colocalization of receptors with markers of the receptor recycling pathway. Internalized integrins could be targeted for destruction or sequestered in receptor recycling compartments; however, the latter possibility is more likely because synthesis of new receptor molecules is generally incompatible with rapid motility (Bretscher and Aguado-Velasco, 1998). Cranial NCCs were cultured on high concentrations of laminin or fibronectin and colabeled for either integrin α5 or integrin α6, and rab4 and rab11, markers of both the fast and slow recycling pathways. To quantify the colocalization events we used Volocity (Improvision), a program that reconstructs confocal z-series of cells three dimensionally to allow XZ and YZ reslicing in order to verify true colocalization events.
On high laminin concentrations, both integrin α5 and integrin α6 colocalized with vesicles positive for either rab4 or rab11, or double-positive for both (rab+ vesicles), but a significantly greater percentage of rab+ vesicles contained integrin α6 (40%) compared with integrin α5 (21%) (Fig. 3, A and B; Table I). Integrin α5 appeared to be largely localized in intracellular compartments that were neither rab 4 nor rab11 positive (rab− vesicles), suggesting that the majority of α5 integrin does not participate in receptor recycling. On high fibronectin concentrations, both integrin α5+ and integrin α6+ vesicles colocalized with rab+ vesicles to similar extents (18 and 17%, respectively) (Fig. 3 B; Table I). Thus, in cranial NCCs cultured on high concentrations of laminin, twice as many recycling vesicles contain the relevant receptor, integrin α6, as the nonrelevant receptor, integrin α5. Furthermore, in cranial NCCs cultured on high concentrations of fibronectin, neither receptor appears to be selectively targeted to the receptor recycling pathway (Table I).
Figure 3.
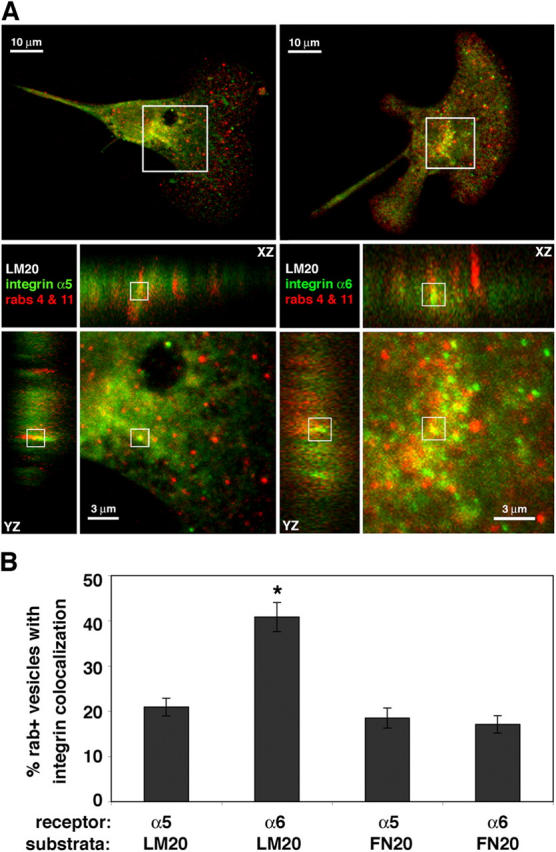
Internalized integrins colocalize with receptor recycling pathway markers in a substratum-dependent manner. Cranial NCCs were cultured on high concentrations of laminin (LM20) and stained for rabs 4 and 11 (red), and either integrin α5 or integrin α6 (green). (A) Confocal z-sections were obtained and the area indicated (white box) zoomed below. Although the whole-cell image represents a compressed z-series, the zoomed portion is one 0.2-μm section. Three-dimensional reconstructions show XZ and YZ cross sections of the white-boxed area within the zoomed portion to aid in determining true colocalization events. (B) Quantification of the amount of rab+ vesicles with integrin colocalization. Integrin α5 is only found in 21% ± 2.0 (mean ± SEM) of rab+ vesicles on LM20, and 18% ± 2.2 of rab+ vesicles on FN20. In contrast, integrin α6 is found in 41% ± 3.2 of rab+ vesicles on LM20, compared with only 17% ± 1.9 of rab+ vesicles on FN20. These results represent four independent experiments (total cells analyzed: n > 10 for each condition). Asterisk indicates significant difference from the other conditions (P < 0.001; t test).
Table I. Distribution of rab+ and integrin+ vesicles.
| Condition (# of cells) |
Labeled vesicles
|
Double labeled
|
Percentage of total labeled vesicles |
Percentage of Int+ vesicles
|
Percentage of Rab+ vesicles
|
|||
|---|---|---|---|---|---|---|---|---|
| Rab+ | Int+ | (Rab/Int)+ | Rab+ | Int+ | (Rab/Int)+ | (Rab/Int)+ | (Rab+/Int)+ | |
| CL20 α5 (n = 12) | 215 ± 25 | 147 ± 16 | 42 ± 4 | 58 ± 4 | 42 ± 4 | 12 ± 1 | 31 ± 4 | 21 ± 2 |
| CL20 α6 (n = 11) | 273 ± 31 | 171 ± 18 | 102 ± 10a | 60 ± 4 | 40 ± 4 | 23 ± 1a | 66 ± 11a | 40 ± 3a |
| CF20 α5 (n = 10) | 288 ± 37 | 184 ± 34 | 53 ± 10 | 62 ± 6 | 38 ± 6 | 11 ± 2 | 36 ± 7 | 18 ± 2 |
| CF20 α6 (n = 10) | 222 ± 25 | 202 ± 42 | 41 ± 9 | 55 ± 5 | 45 ± 5 | 10 ± 1 | 27 ± 7 | 17 ± 2 |
Average number of labeled vesicles per cell (± SEM) for each condition from at least three independent experiments.
Conditions that are statistically different from all others (P < 0.001; t test).
Interestingly, in the conditions in which we saw less colocalization (i.e., α5 and α6 on fibronectin; α5 on laminin), the observed colocalization was similar for all receptors (∼20%), suggesting that one fifth of the rab+ vesicles are occupied by internalized integrins regardless of their substratum relevance (Fig. 3 B, Table I). The fact that integrin α5 does not colocalize with rab+ vesicles to any greater degree on fibronectin compared with laminin indicates that unlike integrin α6, this receptor does not colocalize with the recycling pathway in a substratum-dependent manner. Thus, in cranial neural crest the intracellular trafficking of surface receptors is different when cells are cultured on laminin compared with fibronectin, suggesting cranial NCCs use different mechanisms to support efficient motility on these two ECM molecules.
Internalized integrins are recycled back to the cell surface
Next, we investigated the fate of internalized surface integrin α6 in cranial NCCs cultured on high laminin concentrations. In other fast-moving cells and growth cones, internalized receptors are recycled back to the leading edge rather than being degraded (Lawson and Maxfield, 1995; Kamiguchi and Lemmon, 2000). Using an adapted immunohistochemical technique based on that of Kamiguchi and Lemmon (2000) we examined the recycling of integrin α6 in cranial NCCs. Surface α6 was labeled with anti-α6-Fab while cells were allowed to endocytose and traffic receptors for 30 min at 37°C. Cells were cooled to room temperature to prevent further trafficking and Fab remaining at the surface was blocked with unconjugated secondary antibodies. Subsequently, cells were allowed to traffic internalized receptors at 37°C for 20 or 90 min, and any integrin α6-Fab complexes that returned to the surface were detected with labeled secondary antibodies. Intentionally permeabilized cells (Fig. 4 A, first column; Fig. 4 B) could be distinguished from unpermeabilized cells by significantly higher integrin α6 and actin fluorescence. In the 0-min condition, cells fixed at the beginning of the recycling period and reincubated for 90 min had low fluorescence of both integrin α6 and actin (Fig. 4 C).
Figure 4.
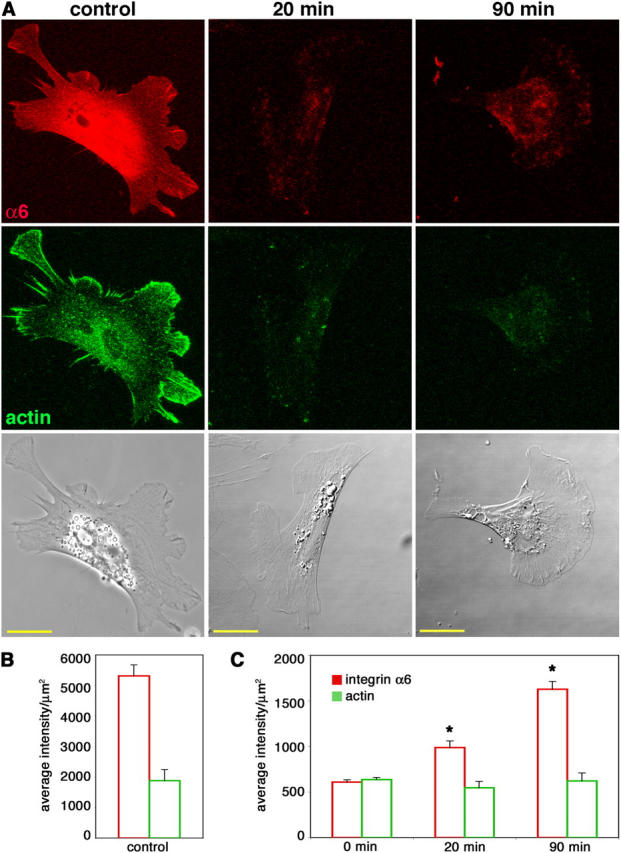
Cranial NCCs recycle internalized integrin α6 back to the cell surface. Cells cultured on high laminin concentrations were allowed to internalize anti-integrin α6 Fab bound to surface receptors for 30 min. The remaining cell surface Fab was blocked and cells were reincubated at 37°C for 20 or 90 min to allow for exocytosis of the integrin α6–Fab complex. (A) Recycled integrin α6 was detected by labeling the unblocked Fab that had reappeared on the surface (red). Cells were also stained for actin (green) as a permeabilization control. Control cells were intentionally permeabilized to show total integrin α6 and actin staining. Bars, 20 μm. (B and C) Average intensities per unit area + SEM (arbitrary units) of integrin α6 and actin fluorescence were determined for at least 20 cells from three independent experiments for each condition. (B) Measurements from permeabilized control cells, as shown in the first column in A, have significantly higher integrin α6 and actin fluorescence than unpermeabilized 0-min controls, as shown in C (P < 0.001; t test). (C) In unpermeabilized cells, actin fluorescence is constant, whereas integrin α6 fluorescence increases at both 20 and 90 min. Asterisk indicates significant difference from 0-min levels (P < 0.0001; t test).
Our results demonstrate that internalized integrin α6 is returned to the surface (Fig. 4). After 20 min, a small amount of recycled receptors could be detected on the cell surface, and by 90 min the levels of recycled receptors on the surface had further increased (Fig. 4 C). Levels of integrin α6 fluorescence at both 20 min (988 ± 71 average intensity units/μm2) and 90 min (1,627 ± 87) were significantly different from 0-min (608 ± 25) levels (P < 0.0001; t test), reflecting a 39 and 63% increase from the 0-min time point, respectively (Fig. 4 C). Recycled receptors were observed near the cell center and the leading edges of filopodia and lamellipodia, which is consistent with the observations of recycled transferrin receptors in migrating fibroblasts (Hopkins et al., 1994), and recycled L1 protein in neuronal growth cones (Kamiguchi and Lemmon, 2000). Thus, in cranial NCCs cultured on high concentrations of laminin, integrin α6 receptors are rapidly internalized, transported intracellularly, and then recycled back to the cell surface.
Inhibiting receptor trafficking slows cranial neural crest motility
Because cranial NCCs down-regulate surface levels of integrin α6 (Fig. 1), internalize this receptor only on laminin (Fig. 2 and Fig. 3), and recycle it back to the cell surface when cultured on high concentrations of laminin (Fig. 4), we postulated that rapidly moving cranial NCCs cultured on high concentrations of laminin would be more dependent on receptor trafficking than the same cells on low levels of laminin where they migrate more slowly. To determine whether substratum-dependent receptor recycling affects cranial neural crest motility, we inhibited receptor trafficking using a pharmacologic approach.
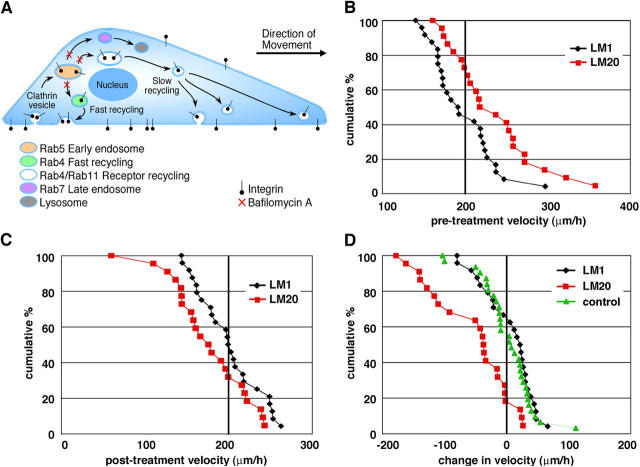
Bafilomycin A is a specific inhibitor of the vacuolar type H+-ATPase found in all animal cells, plant cells, and microorganisms. BafA does not prevent endocytosis, yet it prevents the acidification of endocytic structures, which impairs the transport of vesicles out of the early endosome (Thomsen et al., 1999). Because internalized receptors destined for both the fast and slow receptor recycling pathways originate in the early endosome, treatment with BafA allows cells to internalize surface receptors but impairs receptor recycling back to the surface (Fig. 5 A). Previous work has shown that BafA treatment retards the rate of transferrin receptor recycling twofold in CHO cells (Presley et al., 1997). We controlled for any nonspecific effects of BafA treatment by comparing the same cell type cultured on two different concentrations of laminin.
Figure 5.
Inhibiting receptor trafficking slows cranial NCC motility on high concentrations of laminin. (A) Internalized cell surface receptors are endocytosed via clathrin-dependent mechanisms to the early endosome. Cargo is then transported either to the late endosome, to the receptor recycling compartments, or rapidly recycled back to the cell surface through a fast recycling pathway. Distinct areas of the pathway are characterized by specific Rab GTPases. The vacuolar proton pump inhibitor bafilomycin A prevents acidification of endocytic structures, thereby impairing transport out of the early endosome. (B) Cumulative distribution plots of cell velocity before drug treatment are shown where each point represents the pre-treatment velocity of a single cell. Cumulative percent on the y-axis refers to the percentage of the population traveling that speed or faster. The average velocity of cranial NCCs before treatment is 232 μm/h ± 11 (SEM; n = 22) on LM20 (red squares), compared with 196 μm/h ± 8 (n = 24) on LM1 (black diamonds). The difference between the two conditions is statistically significant (P < 0.01; t test). (C) Cumulative distribution plots of cell velocity after drug treatment are shown where each point represents the post-treatment velocity of a single cell. Cumulative percent on the y-axis refers to the percentage of the population traveling that speed or faster. The average velocity of cranial NCCs post-treatment is 174 μm/h ± 10 (SEM; n = 22) on LM20 (red squares), compared with 199 μm/h ± 8 (n = 24) on LM1 (black diamonds). The difference between the two conditions is statistically significant (P < 0.05; t test). (D) Cumulative distribution plots of the change in cell velocity after BafA (100 nM) treatment. Each point represents the change in velocity of a single cell after BafA treatment. Cells cultured on LM20 (red squares) and treated with BafA slowed an average of 58 μm/h ± 14 (n = 22) (post-treatment velocities were statistically different from pre-treatment velocities; P < 0.001; paired t test). In contrast, cells cultured on LM1 and treated with BafA (black diamonds; n = 24), and controls, cells cultured on either laminin concentration and treated with vehicle (green triangles; n = 31), did not slow significantly (pre- and post-treatment velocities were not statistically different; P > 0.05, paired t test). Post-treatment velocities of control cells were significantly different from post-treatment velocities of BafA-treated cells on high laminin (P < 0.05; t test). Specific points in each graph are not directly comparable to corresponding points in other graphs, as each point merely reflects a cell's velocity, or change in velocity, in relation to the whole population.
As we have previously reported, cranial NCCs migrate more rapidly on high compared with low concentrations of laminin (Strachan and Condic, 2003). Using time-lapse videomicroscopy, we established a pre-treatment velocity for each cell and graphed these data in a cumulative distribution plot (see Materials and methods for generation of these plots) to show the range of velocities in this heterogeneous cell population (Fig. 5 B). We found that ∼70% of cranial NCCs cultured on high laminin concentrations traveled faster than 200 μm/h, compared with only 45% of the same cell type cultured on low laminin concentrations (Fig. 5 B). After following each cell for 30 min, 100 nM BafA was applied and allowed to diffuse for 15 min. Each cell was then followed for another 30 min to determine a post-treatment velocity (Fig. 5 C). Cumulative distribution plots of the change in each cell's velocity after BafA treatment show that nearly 80% of cranial NCCs cultured on high laminin concentrations slowed when receptor recycling was inhibited (Fig. 5 D); post-treatment velocities were significantly different from pre-treatment velocities (P < 0.001; paired t test). In contrast, there was no change in velocity for control cells (on both laminin concentrations) or cells on low laminin treated with BafA; for all conditions, pre- and post-treatment velocities were not significantly different from each other (P > 0.05; paired t test). Moreover, post-treatment velocities of cells in control conditions and cells on low laminin treated with BafA were significantly different from post-treatment velocities of BafA-treated cells on high laminin (P < 0.05; t test; Fig. 5 D). These results indicate that inhibiting receptor recycling only affects the motility of rapidly migrating cranial NCCs, whose surface receptor expression levels are low and tightly regulated.
Discussion
In this work we have investigated whether cranial NCCs use receptor recycling as a mechanism to maintain rapid motility in different extracellular environments. We show that cranial NCCs internalize and recycle cell surface integrins in a substratum-dependent manner in vitro. On high concentrations of laminin, cranial NCCs rapidly internalize and recycle the laminin receptor, integrin α6, whereas on low laminin they retain higher levels of integrin α6 on the surface. In contrast, on high concentrations of fibronectin, cranial NCCs do not differentially accumulate either fibronectin or laminin receptors internally. Furthermore, internalized integrin α6 colocalizes with receptor recycling pathway markers to a greater degree when the cells are cultured on the ligand for this receptor (i.e., laminin), indicating that receptor trafficking in these cells is also substratum dependent. In contrast, integrin α5 colocalization with receptor recycling vesicles is not affected by the substratum, indicating that different integrins are distinctly regulated in cranial NCCs. Finally, we demonstrate that blocking receptor trafficking out of the endosome slows cranial neural crest motility on laminin in a concentration-dependent manner, indicating that in addition to substratum composition, substratum density plays an important role in regulated NCC motility.
To date, only a handful of investigations have examined receptor recycling (Kamiguchi and Lemmon, 2000) and its role in cell motility (Fabbri et al., 1999; Pierini et al., 2000). Previous work in CHO cells and neutrophils has shown that the recycling of integrins to the leading edge of the cell is essential for efficient motility (Fabbri et al., 1999; Pierini et al., 2000). However, these studies were performed using transformed cell lines or adult primary cell populations where large numbers of cells can be easily obtained (Lawson and Maxfield, 1995; Fabbri et al., 1999; Kamiguchi and Lemmon, 2000). In contrast, the single neural tube explant used here will produce, at most, 100,000 NCCs, a limitation that has significantly hampered quantitative assessments of integrin trafficking in this system. The approach described here allows us to gain insight into the molecular mechanisms this developmentally important cell population uses for migration. It is likely that these techniques can be used to examine receptor trafficking and motility in other primary embryonic cell populations.
Integrin recycling and receptor specificity
The first observation that integrins differentially participate in the endocytic pathway was published over a decade ago (Bretscher, 1992). In this paper, Bretscher showed that in CHO cells grown in suspension, integrins α5β1 and α6β4 circulate, but α3β1, α4β1, and αLβ2 do not. Although it was initially postulated that the ability to participate in endocytic trafficking may be a property of the receptor itself, other groups have since shown that the substratum onto which the cells are adhered also greatly influences this process. When neutrophils are cultured on vitronectin, the vitronectin receptor mediating their migration (integrin αVβ3) is recycled in a polarized manner (Lawson and Maxfield, 1995). In contrast, when the same cells are cultured on fibronectin, integrin αVβ3 is no longer distributed in a polarized manner, and instead integrin α5β1 (a fibronectin receptor) is internalized into endocytic recycling compartments (Pierini et al., 2000). These studies suggest that cells may preferentially recycle the receptors that are mediating their migration, and this is consistent with our current demonstration of substratum-dependent integrin recycling in cranial NCCs.
Our finding that, in contrast to neutrophils, integrin α5 participates predominantly in short time-frame recycling in cranial NCCs cultured on fibronectin suggests that distinct cell types can regulate the same receptor in different ways. How NCCs modulate adhesion to maintain motility on fibronectin is unknown, but could involve functional modulation of integrin receptors; a possibility we are currently investigating.
Substratum-specific receptor internalization has been shown for nonintegrin receptors as well. For example, insulin receptor internalization was inhibited 40–60% in CHO cells adhered to galectin-8 (an ECM protein and an integrin ligand) when compared with the same cells adhered to fibronectin, collagen, or laminin (Boura-Halfon et al., 2003). Other studies have shown that in embryonic dorsal root ganglia neurons, L1, a homophilic cell adhesion receptor that promotes axon growth along preexisting axon bundles, is recycled in a spatially regulated manner within growth cones cultured on L1 but not laminin (Kamiguchi and Lemmon, 2000). It is likely that the internalization of many receptors may depend on the extracellular environment of the cell. In this way, intracellular signaling of downstream events such as migration, differentiation, and apoptosis could be regulated in a region specific manner throughout the embryo.
Recycling kinetics and substratum density
Receptor recycling has not been previously investigated in a single cell population cultured on different concentrations of the same substrata. Our findings suggest that not only is substratum composition a key regulator of receptor recycling, but also that the density of ligands on the substratum can affect the importance of receptor internalization for motility. BafA treatment has a greater effect on cranial neural crest motility when the cells are cultured on high concentrations of laminin compared with low. Thus, when cranial NCCs are cultured on low concentrations of laminin, their dependence on receptor recycling for efficient motility is decreased in comparison to the same cells cultured on high laminin concentrations. These results clearly demonstrate that receptor recycling makes a stronger contribution to NCC velocity on high laminin concentrations, where surface receptor expression levels are low.
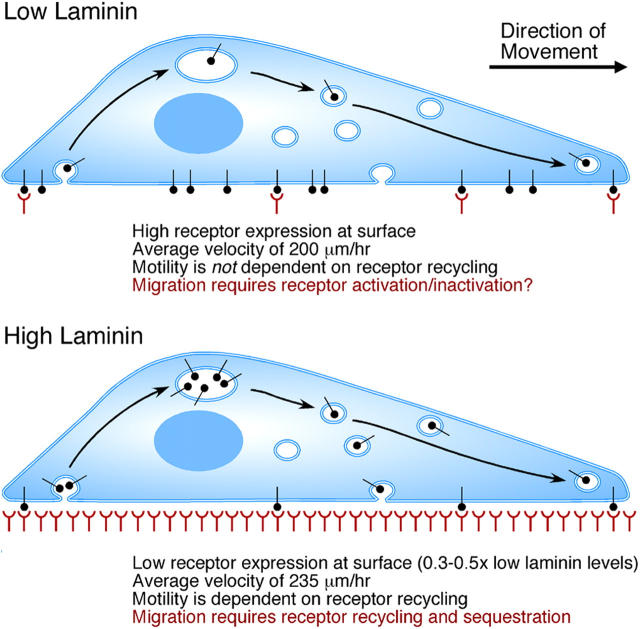
These factors suggest a model for NCC migration on laminin (Fig. 6). Our model proposes that efficient motility on laminin is largely regulated by the appropriate matching of cell surface adhesion receptor number to ligand availability. On low concentrations of laminin, there is very little ligand available (calculations from our current measurements of radiolabeled laminin binding indicate that on low laminin only ∼300 molecules of laminin are bound per μm2) so efficient motility requires a large number of receptors on the cell surface to increase the probability of ligand binding events. In contrast, on high concentrations of laminin (10-fold higher density), ligand is more readily available and fewer receptors are needed on the surface to achieve the same probability of ligand binding. In fact, on high substratum concentrations, high surface receptor number would increase adhesion and concomitantly decrease motility. Thus, on high ligand concentrations, motile NCCs rely on recycling of internal receptor pools to promote ligand binding events at the leading edge of the cell, whereas on low ligand concentrations the receptors are already at the cell surface.
Figure 6.
Summary of cranial NCC migration on laminin. On low concentrations of laminin there is high receptor expression at the surface, cell velocities are slower, and motility does not depend on receptor recycling. On high concentrations of laminin there is low receptor expression at the surface, cell velocities are higher, and rapid motility depends on recycling of receptors from internal pools.
Efficient motility and cell fate
Although the lessons learned from investigating cell motility in vitro have established a strong model for integrin-mediated migration, it is the application of these lessons that provides insight into the importance of efficient motility in a specific biological context (in this case, the developing embryo). During development, NCCs migrate along defined pathways in the embryo and differentiate into distinct cell types. For example, in the trunk, NCCs initially migrate either dorsolaterally along a laminin-rich basal lamina, or ventromedially through tissue rich in fibronectin. Cells that follow the dorsolateral pathway are destined to become melanoblasts, whereas those which migrate ventromedially will largely become neurons and glia (Bronner-Fraser, 1993a). In comparison, in the head, NCC migration proceeds through the mesoderm lateral to the neural tube and then ventrally toward the branchial arches, areas rich in ECM proteins including both laminin and fibronectin. Although the relative amounts of ECM proteins that these cells encounter while migrating have not been determined, our results suggest the intriguing possibility that differences in migratory speed may influence NCC differentiation. The ability of cranial NCCs to migrate so quickly on high laminin concentrations may reflect a difference in the capability of these cells to reach certain targets within a critical time period and thereby be available to receive short-lived, target-derived differentiation cues. Alternatively, if NCC differentiation affects cell motility, the high speed of cranial NCCs on high laminin may reflect early cell fate specification.
In summary, we have shown that substratum-dependent receptor recycling is an important molecular mechanism used by cranial NCCs to promote rapid motility on laminin. We have also demonstrated that the same cell population can respond differently to the same substratum, depending on its concentration. Our data suggest that cranial NCCs tightly regulate the cellular mechanisms that support motility in response to the specific conditions the cells encounter. The investigation of receptor recycling in a primary embryonic cell population is both novel and fundamental to the understanding of cell migration during development.
Materials and methods
Substratum preparation and cell culture
Glass coverslips (Goldseal; Fisher Scientific) were acid washed, rinsed in dH2O, and baked at 350°C for 12 h. 13-mm holes were drilled in the bottom of 35-mm tissue culture dishes (Fisher Scientific) and the prepared coverslips were affixed to the dish with Sylgard (Dow Corning). Bovine plasma fibronectin (FN; GIBCO BRL) and natural mouse laminin (LM; GIBCO BRL) were diluted to high (20 μg/ml) and low (1 μg/ml) concentrations in PBS, and 300 μl of the protein solution was added to the coverslips, incubated for 3 h at RT, and then rinsed once with PBS. As previously published (Strachan and Condic, 2003), absolute protein concentration on the coverslips was determined by measuring the amount of titrated laminin and fibronectin bound to glass as described in de Curtis et al. (1991). Application of laminin at 1 and 20 μg/ml resulted in a density of 45 and 430 ng/cm2 bound laminin, respectively. Application of fibronectin at 1 and 20 μg/ml resulted in 35 and 700 ng/cm2 bound fibronectin, respectively.
White Leghorn chicken eggs (supplied by Utah State University, Logan, UT) were incubated at 38°C until the embryos reached stage 8 (Hamburger, 1992). All animal studies were approved by the University of Utah Institutional Review Board. Neural tubes from the mesencephalic level were dissected away from the surrounding tissue with tungsten needles. Neural tubes were cultured at 37°C with 5% CO2 for 48–72 h in 250 μl neurobasal medium (GIBCO BRL) supplemented with 25 μM glutamic acid (Sigma-Aldrich), 500 μM l-glutamine (Sigma-Aldrich), 1× B-27 and N-2 (GIBCO BRL), 10 ng/ml NT3 (CHEMICON International), 100 ng/ml EGF (Upstate Biotechnology), 10 ng/ml FGF (Upstate Biotechnology), and 50 ng/ml NGF (R&D Systems). Neurobasal medium without the supplements and buffered with N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (Hepes; USBiological) was used for the time-lapse assays.
Reagents and antibodies
Antibodies against the following proteins were used in these experiments: integrin α5 (D71E2; DSHB, and AB1928; CHEMICON International), integrin α6 (P2C62C4;DSHB, and SC10370; Santa Cruz Biotechnology, Inc.), rab4 (610888; BD Biosciences), rab11 (610656; BD Biosciences), actin (AAN01; Cytoskeleton, Inc.), and α-tubulin (T5168; Sigma-Aldrich). For the integrin recycling experiments a Fab fragment of a monoclonal integrin α6 antibody (MAB13444; CHEMICON International) was generated. Bafilomycin A1 (B1793; Sigma-Aldrich) was dissolved in DMSO (D8418; Sigma-Aldrich). Sodium 2-mercaptoethanesulfonate (MesNa) was purchased from Sigma-Aldrich (M1511) and dissolved immediately before use in Hepes-buffered neurobasal medium.
Immunoprecipitations
Cell surface receptors were labeled with NHS-SS-biotin (Pierce Chemical Co.) on ice and the cells were then lysed on ice in a solution containing 0.1% SDS, 1% Triton X-100, and protease inhibitors. Protein concentration in each cell lysate was determined with a BCA protein assay (Pierce Chemical Co.) and equalized across conditions. Integrins were immunoprecipitated by using standard protocols (de Curtis et al., 1991), and immunoprecipitated proteins were size fractionated under nonreducing conditions on acrylamide gels and electrophoretically transferred to nitrocellulose membranes. Biotinylated proteins were detected by using Strep-Avidin conjugated to HRP and a chemoluminescent reagent (Pierce Chemical Co.) followed by exposure to film. For the internalization assays (Fig. 2), where reducing agents interfere with protein concentration assays, 15 μl of lysate was removed before immunoprecipitation as a protein loading normalization control, size fractionated on an acrylamide gel, transferred to nitrocellulose, and revealed with an anti-α-tubulin antibody (T5168; Sigma-Aldrich). Using nonreduced lysates, BCA assays were performed alongside α-tubulin Western blots to ensure that α-tubulin expression was not different in cells cultured on LM1, LM20, or FN20. Comparisons of protein concentrations (BCA) and α-tubulin band intensities as a measure of protein loading give identical results. Exposures of blots that were in the linear range were quantified using GelDoc and QuantityOne (Bio-Rad Laboratories).
Internalization assay
For the analysis of receptor internalization, we adapted a biochemical assay (Fabbri et al., 1999) for NCCs. Cranial neural crest cultures were grown for 48 h, the neural tubes were removed, and the remaining crest cells were scraped off the coverslip (Bronner-Fraser, 1996). This procedure yields relatively pure NCC populations as determined by HNK-1 staining (Bronner-Fraser, 1986). The cells were spun, resuspended in neurobasal medium with supplements, and plated (equal number of cells per dish) as dissociated cells overnight or until the cultures reached ∼70% confluency. Cell surface receptors were labeled with biotin at 37°C for 0, 2, 5, 10, or 20 min. For the 0-min controls, cells were gently rinsed once with biotin. After biotinylation, the cells were placed on ice, rinsed gently, and treated with a noncell-permeant reducing agent (MesNa) to remove the biotin from the receptors that remained on the cell surface. After MesNa treatment, cells were lysed on ice and immunoprecipitations and analysis were then performed as detailed above.
Recycling assay
For the analysis of recycling we adapted an immunohistochemical assay based on that of Kamiguchi and Lemmon (2000). Cranial neural crest cultures were grown on high laminin concentrations for 72 h. Live cells were incubated with anti-integrin α6 Fab (0.5 μg/ml) for 30 min at 37°C to allow for optimal internalization of the integrin α6–Fab complex. To avoid internalization induced by cross-linking caused with bivalent antibodies, Fab fragments are routinely used to follow receptor internalization and trafficking. Although it is possible these fragments may induce receptor internalization through an unknown mechanism, and that ligand binding could reverse this effect, it is unlikely. The cells were then cooled to RT to stop further endocytic trafficking and were incubated with unlabeled anti–mouse IgG (1:100; Jackson ImmunoResearch Laboratories) for 30 min at RT to block the Fab remaining at the cell surface. After gentle washing, the cells were then reincubated at 37°C for 20 or 90 min in neurobasal medium to allow for trafficking of endocytosed integrin α6–Fab complexes. The cells were fixed with 2% PFA with 30% sucrose for 20 min at RT, and were incubated with an anti-actin antibody to reveal whether the cells had been permeabilized during fixation. Cells treated with 0.1% Triton X-100 (22686; USBiological) were used as permeabilization controls (Fig. 4 A, first column). Cells fixed but not permeabilized before the reincubation period were used as 0-min time points (Fig. 4 C). The recycled integrin α6 on the cell surface was then detected by visualizing the anti-integrin α6 Fab that had not been blocked with the unconjugated secondary. The unpermeabilized cells were incubated with Alexa-conjugated secondary antibodies (1:500; Molecular Probes, Inc.) for 1 h at RT.
To confirm that the detection of recycled receptors was not due to the loss of unconjugated secondary antibody over time, we performed the following control experiment. Cells were labeled live with anti-α6 Fab, fixed, incubated with unconjugated secondary antibodies, allowed to sit for up to 2 h, and then incubated with labeled secondary antibodies. After 2 h we still did not detect any α6 staining, indicating that the unconjugated secondary antibody remained complexed with the Fab antibody over the time course of our assay. Images were acquired from all conditions of a single experiment on the same day and were collected with a confocal laser microscope as detailed below. Average intensity per cell area was determined using MetaMorph 5.0 (AG Heinz) software. In brief, cell outlines were traced from phase images and applied to corresponding fluorescent images, and average pixel intensity was determined as a function of cell area.
Immunohistochemistry and colocalization analysis
For the analysis of integrin and rab colocalization, neural crest cultures were grown for 48 h and then were fixed with 2% PFA (Sigma-Aldrich) for 1 h at RT. The cultures were then rinsed and incubated in blocking buffer (PBS with 0.1% Triton X-100, 1% BSA, and 5% normal goat serum) for 1 h at RT. Antibodies were diluted (1:100) in blocking buffer and added to the cells for 1 h at RT. After rinsing, Alexa-conjugated secondary antibodies (Molecular Probes, Inc.) were diluted (1:500) in blocking buffer and were added to the cells for 1 h at RT. Images were acquired with an Olympus IX70 confocal laser microscope (Fluoview 4.3) using an argon or HeNe laser (excitation lines, 488 and 543 nm) and a 60× Plan-APO (NA 1.15) water immersion objective. All images that were compared were acquired in the same session using the identical PMT, laser power, pinhole, gain, and offset settings. For the colocalization analysis, rab+ and integrin+ vesicles were selected independently using an intensity-based classifier to apply segmentation to the image (Volocity 2.5; Improvision). Anything smaller than 10 voxels was eliminated from the analysis. The number of rab+ vesicles that overlapped with integrin+ vesicles was divided by the total number of rab+ vesicles to determine the percentage of rab+ vesicles with integrin colocalization. XZ and YZ reslicing was performed to show the position of these vesicles along the apical–basal axis of the cell.
BafA treatment
Cranial neural crest were cultured for 48–72 h. The neurobasal media was replaced with Hepes-buffered neurobasal media for filming. Independent cells at the outer edge of the explant were videographed at 10× for 30 min to establish the pre-treatment velocity. Phase images were acquired (Metamorph 5.0) every 150 s. BafA (100 nM) or DMSO alone (vehicle) was then added, and after 15 min the post-treatment velocity was determined by filming the cells for an additional 60 min. Cell velocity was determined by tracking the position of the nucleus over time (Metamorph 5.0). Cumulative distribution graphs were generated by sorting each cell's velocity from highest to lowest speed, and determining the percentage of cells (in the same condition) that traveled slower than that cell. The speed of the cell was plotted against the percentage of cells traveling slower in order to show the velocity of each cell in relation to the whole population. Specific points in each graph are not directly comparable to corresponding points in other graphs, as each point merely reflects a cell's speed in relation to the whole population.
Acknowledgments
We thank Drs. R. Ash, M. Beckerle, and R.I. Dorsky for comments on the manuscript, and Dr. C. Rodesch for help with the confocal imaging and Volocity analysis. Antibodies were provided by the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA).
This work was supported by a Basil O'Connor Award from the March of Dimes to M.L. Condic and by a National Institutes of Health grant (NIH-F31-NS43849-01) to L.R. Strachan.
Abbreviations used in this paper: BafA, bafilomycin A; FN1, fibronectin applied at 1 μg/ml (low fibronectin); FN20, fibronectin applied at 20 μg/ml (high fibronectin; LM1, laminin applied at 1 μg/ml (low laminin); LM20, laminin applied at 20 μg/ml (high laminin); MesNa, sodium 2-mercaptoethanesulfonate; NCC, neural crest cell.
References
- Alfandari, D., H. Cousin, A. Gaultier, B.G. Hoffstrom, and D.W. DeSimone. 2003. Integrin α5β1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev. Biol. 260:449–464. [DOI] [PubMed] [Google Scholar]
- Anderson, D.J. 1997. Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 13:276–280. [DOI] [PubMed] [Google Scholar]
- Arroyo, A.G., P. Sanchez-Mateos, M.R. Campanero, I. Martin-Padura, E. Dejana, and F. Sanchez-Madrid. 1992. Regulation of the VLA integrin-ligand interactions through the β1 subunit. J. Cell Biol. 117:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boura-Halfon, S., H. Voliovitch, R. Feinstein, K. Paz, and Y. Zick. 2003. Extracellular matrix proteins modulate endocytosis of the insulin receptor. J. Biol. Chem. 278:16397–16404. [DOI] [PubMed] [Google Scholar]
- Bretscher, M.S. 1992. Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 11:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, M.S., and C. Aguado-Velasco. 1998. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10:537–541. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. 1986. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev. Biol. 115:44–55. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. 1993. a. Mechanisms of neural crest cell migration. Bioessays. 15:221–230. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. 1993. b. Environmental influences on neural crest cell migration. J. Neurobiol. 24:233–247. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser, M. 1996. Methods in Avian Embryology. Academic Press, Inc., San Diego, CA. 369 pp.
- Buettner, H.M., and R.N. Pittman. 1991. Quantitative effects of laminin concentration on neurite outgrowth in vitro. Dev. Biol. 145:266–276. [DOI] [PubMed] [Google Scholar]
- Christiansen, J.H., E.G. Coles, and D.G. Wilkinson. 2000. Molecular control of neural crest formation, migration and differentiation. Curr. Opin. Cell Biol. 12:719–724. [DOI] [PubMed] [Google Scholar]
- Condic, M.L. 2001. Adult neuronal regeneration induced by transgenic integrin expression. J. Neurosci. 21:4782–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic, M.L., and P.C. Letourneau. 1997. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 389:852–856. [DOI] [PubMed] [Google Scholar]
- Condic, M.L., D.M. Snow, and P.C. Letourneau. 1999. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J. Neurosci. 19:10036–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis, A., and E. Georges-Labouesse. 2000. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 16:389–395. [DOI] [PubMed] [Google Scholar]
- de Curtis, I., V. Quaranta, R.N. Tamura, and L.F. Reichardt. 1991. Laminin receptors in the retina: sequence analysis of the chick integrin α6 subunit. Evidence for transcriptional and posttranslational regulation. J. Cell Biol. 113:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla, P.A., J.A. Stone, J.A. Quinn, S.M. Albelda, and D.A. Lauffenburger. 1993. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 122:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky, R.I., R.T. Moon, and D.W. Raible. 2000. Environmental signals and cell fate specification in premigratory neural crest. Bioessays. 22:708–716. [DOI] [PubMed] [Google Scholar]
- Duband, J.L., S. Dufour, S.S. Yamada, K.M. Yamada, and J.P. Thiery. 1991. Neural crest cell locomotion induced by antibodies to β1 integrins. A tool for studying the roles of substratum molecular avidity and density in migration. J. Cell Sci. 98:517–532. [DOI] [PubMed] [Google Scholar]
- Fabbri, M., L. Fumagalli, G. Bossi, E. Bianchi, J.R. Bender, and R. Pardi. 1999. A tyrosine-based sorting signal in the β2 integrin cytoplasmic domain mediates its recycling to the plasma membrane and is required for ligand-supported migration. EMBO J. 18:4915–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill, L.S., and M. Bronner-Fraser. 2003. Neural crest specification: migrating into genomics. Nat. Rev. Neurosci. 4:795–805. [DOI] [PubMed] [Google Scholar]
- Goodman, S.L., G. Risse, and K. von der Mark. 1989. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J. Cell Biol. 109:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger, V. 1992. The stage series of the chick embryo. Dev. Dyn. 195:273–275. [DOI] [PubMed] [Google Scholar]
- Hopkins, C.R., A. Gibson, M. Shipman, D.K. Strickland, and I.S. Trowbridge. 1994. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 125:1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi, H., and V. Lemmon. 2000. Recycling of the cell adhesion molecule L1 in axonal growth cones. J. Neurosci. 20:3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil, S.H., T. Lallier, and M. Bronner-Fraser. 1996. Inhibition of cranial neural crest adhesion in vitro and migration in vivo using integrin antisense oligonucleotides. Dev. Biol. 179:91–101. [DOI] [PubMed] [Google Scholar]
- Lawson, M.A., and F.R. Maxfield. 1995. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 377:75–79. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N.M., and C. Kalcheim. 1999. The Neural Crest. Cambridge University Press, Cambridge, UK. 445 pp.
- Long, K.E., H. Asou, M.D. Snider, and V. Lemmon. 2001. The role of endocytosis in regulating L1-mediated adhesion. J. Biol. Chem. 276:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625. [DOI] [PubMed] [Google Scholar]
- Palecek, S.P., J.C. Loftus, M.H. Ginsberg, D.A. Lauffenburger, and A.F. Horwitz. 1997. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 385:537–540. [DOI] [PubMed] [Google Scholar]
- Pierini, L.M., M.A. Lawson, R.J. Eddy, B. Hendey, and F.R. Maxfield. 2000. Oriented endocytic recycling of α5β1 in motile neutrophils. Blood. 95:2471–2480. [PubMed] [Google Scholar]
- Powelka, A.M., J. Sun, J. Li, M. Gao, L.M. Shaw, A. Sonnenberg, and V.W. Hsu. 2004. Stimulation-dependent recycling of integrin β1 regulated by ARF6 and Rab11. Traffic. 5:20–36. [DOI] [PubMed] [Google Scholar]
- Presley, J.F., S. Mayor, T.E. McGraw, K.W. Dunn, and F.R. Maxfield. 1997. Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J. Biol. Chem. 272:13929–13936. [DOI] [PubMed] [Google Scholar]
- Qualmann, B., and H. Mellor. 2003. Regulation of endocytic traffic by Rho GTPases. Biochem. J. 371:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport, J.Z., and S.M. Simon. 2003. Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116:847–855. [DOI] [PubMed] [Google Scholar]
- Roberts, M., S. Barry, A. Woods, P. van der Sluijs, and J. Norman. 2001. PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11:1392–1402. [DOI] [PubMed] [Google Scholar]
- Sheff, D.R., E.A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149:901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan, L.R., and M.L. Condic. 2003. Neural crest motility and integrin regulation are distinct in cranial and trunk populations. Dev. Biol. 259:288–302. [DOI] [PubMed] [Google Scholar]
- Testaz, S., and J.L. Duband. 2001. Central role of the α4β1 integrin in the coordination of avian truncal neural crest cell adhesion, migration, and survival. Dev. Dyn. 222:127–140. [DOI] [PubMed] [Google Scholar]
- Thomsen, P., O. Rudenko, V. Berezin, and B. Norrild. 1999. The HPV-16 E5 oncogene and bafilomycin A1 influence cell motility. Biochim. Biophys. Acta. 1452:285–295. [DOI] [PubMed] [Google Scholar]
- Tucker, R.P. 2004. Antisense knockdown of the β1 integrin subunit in the chicken embryo results in abnormal neural crest cell development. Int. J. Biochem. Cell Biol. 36:1135–1139. [DOI] [PubMed] [Google Scholar]
- van der Flier, A., and A. Sonnenberg. 2001. Function and interactions of integrins. Cell Tissue Res. 305:285–298. [DOI] [PubMed] [Google Scholar]