Abstract
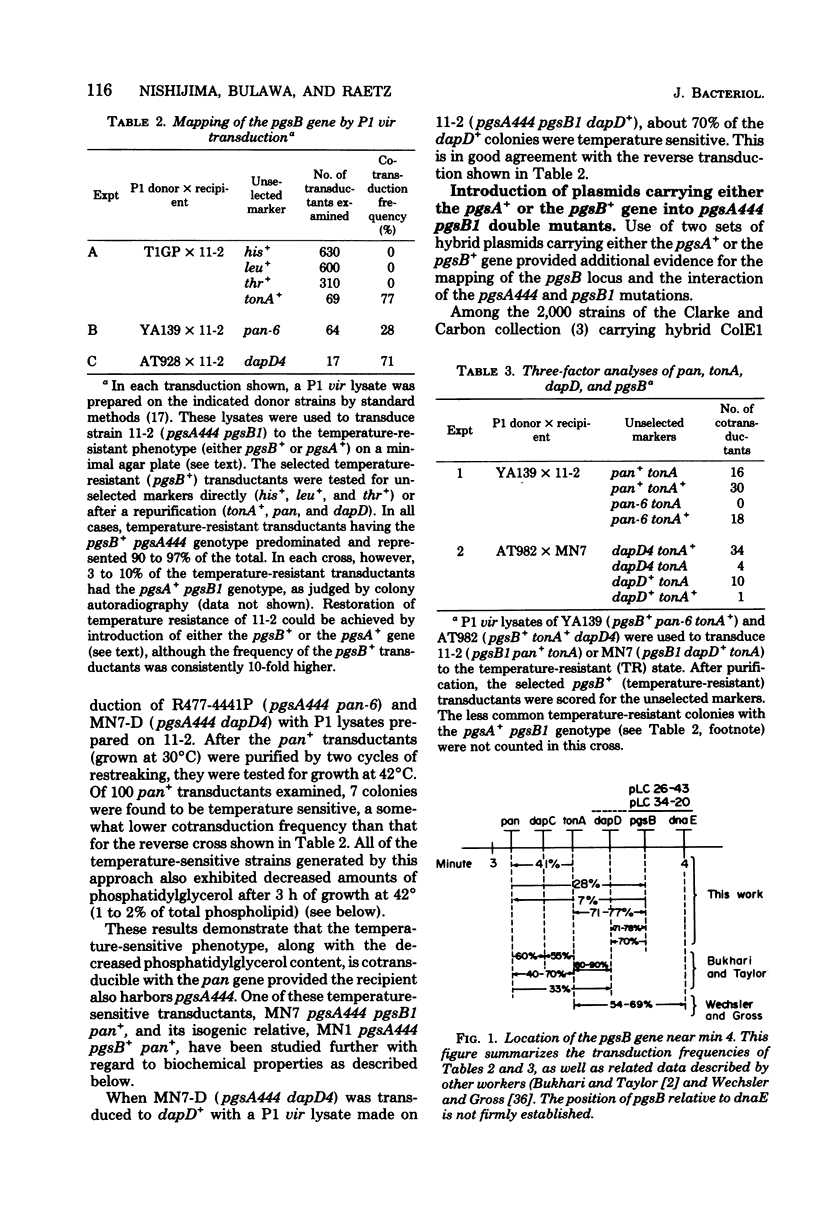
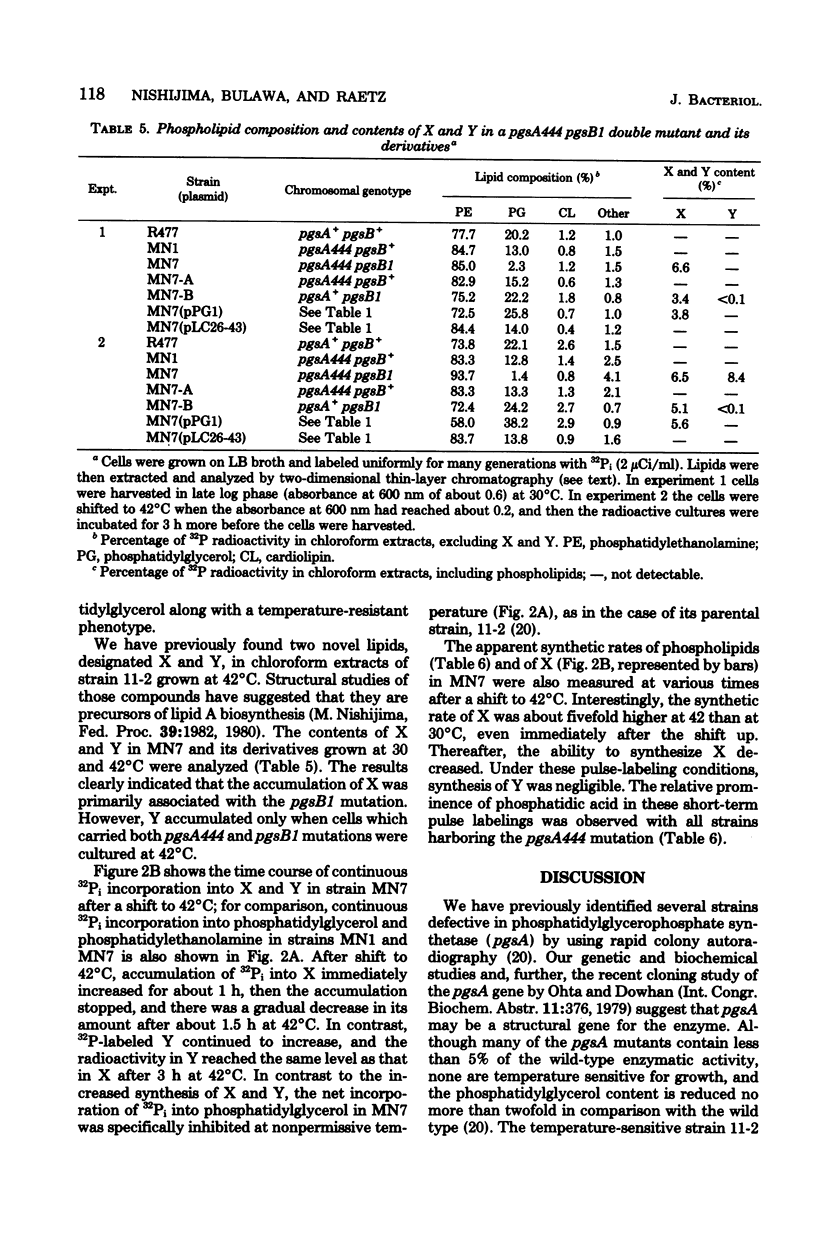
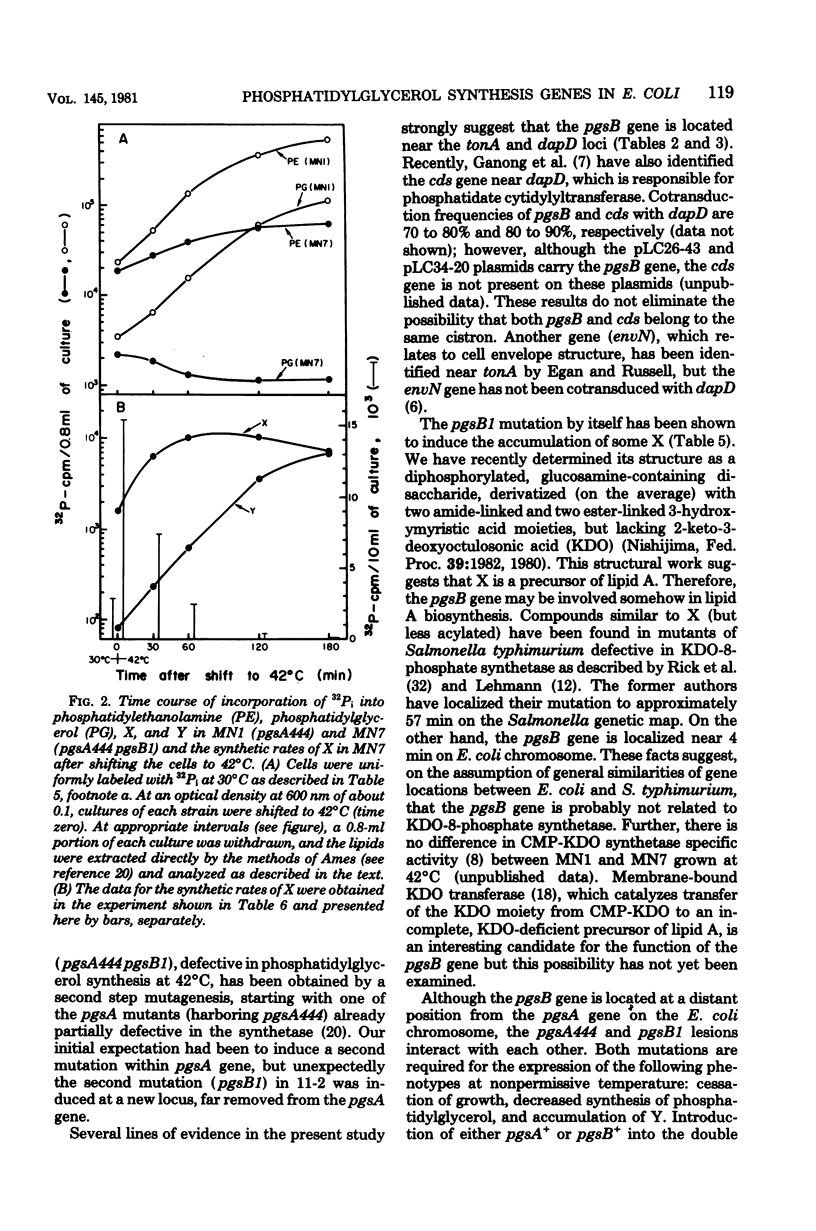
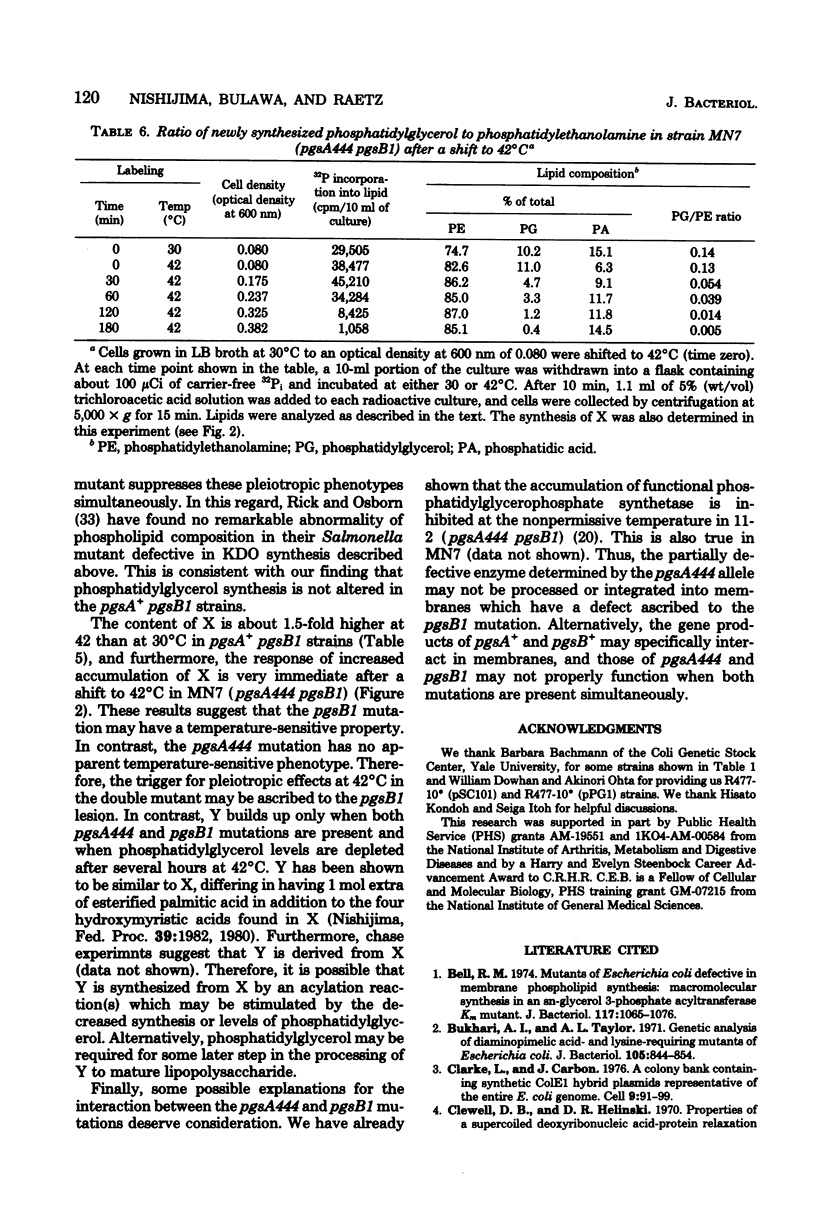
A conditionally lethal mutant of Escherichia coli lacking phosphatidylglycerol in vivo at 42 degrees C has been previously isolated by two-stage mutagenesis (M. Nishijima and C. R. H. Raetz, J. Biol. Chem. 254:7837-7844, 1979). In the first step (designated pgsA444) the phosphatidylglycerophosphate synthetase is partially inactivated, but the resulting strain continues to make about two-thirds of the normal level of phosphatidylglycerol and is not temperature sensitive. The second lesion, termed pgsB1, causes temperature-sensitive growth and phosphatidylglycerol synthesis in strains harboring pgsA444. The pgsA locus appears to be the structural gene for the synthetase and maps near min 42. In the present study we mapped the pgsB1 mutation and characterized its interaction with pgsA444 by genetic and biochemical methods. Unexpectedly, pgsB1 was not a second lesion in the pgsA structural gene, but rather mapped at a distinct site near minute 4. P1 vir-mediated contransduction suggested the gene order pantonA-dapD-pgsB-dnaE (clockwise). Independent evidence for the genetic mapping was provided by the identification of two hybrid ColE1 plasmids (pLC26-43 and pLC34-20. L. Clarke and J. Carbon, Cell 9:91-99, 1976) which both carry pgsB+ and dnaE+. Introduction of either the pgsA+ or the pgsB+ gene (via episomes, hybrid plasmids or P1 vir transduction) suppressed the temperature sensitivity of the double mutant (pgsA444 pgsB1) and restored normal levels of phosphatidylglycerol at 42 degrees C. In addition, strains with the pgsA+ pgsB1 genotype produced a novel lipid (X) at all temperatures, whereas the double mutant (pgsA444 pgsB1) contained two unusual lipids (X and Y) after 3 h at 42 degrees C. Both X and Y are precursors of lipopolysaccharide, and introduction of pgsB+ into the double mutant caused the disappearance of X and Y. Although the biochemical basis of the pgsB1 lesion is unknown, its existence suggests a previously unrecognized link between lipopolysaccharide and phosphatidylglycerol syntheses in E. coli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Egan A. F., Russell R. R. Conditional mutations affecting the cell envelope of Escherichia coli K-12. Genet Res. 1973 Apr;21(2):139–152. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Leonard J. M., Raetz C. R. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980 Feb 25;255(4):1623–1629. [PubMed] [Google Scholar]
- Ghalambor M. A., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. IV. Purification and properties of cytidine monophosphate 3-deoxy-d-manno-octulosonate synthetase. J Biol Chem. 1966 Jul 10;241(13):3216–3221. [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Phospholipid composition and membrane function in phosphatidylserine decarboxylase mutants of Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8213–8220. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson T. J., Lightner V. A., Green P. R., Modrich P., Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Identification of the sn-glycerol-3-phosphate acyltransferase polypeptide as the plsB gene product. J Biol Chem. 1980 Oct 10;255(19):9421–9426. [PubMed] [Google Scholar]
- Lehmann V. Isolation, purification and properties of an intermediate in 3-deoxy-D-manno-octulosonic acid--lipid A biosynthesis. Eur J Biochem. 1977 May 2;75(1):257–266. doi: 10.1111/j.1432-1033.1977.tb11525.x. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Larson T. J., Tailleur P., Kantor G. D., Raetz C. R., Bell R. M., Modrich P. Membrane phospholipid synthesis in Escherichia coli. Cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyl/transferase. J Biol Chem. 1980 Oct 10;255(19):9413–9420. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Rasmussen N. S., Osborn M. J. Biosynthesis of lipid A. Enzymatic incorporation of 3-deoxy-D-mannooctulosonate into a precursor of lipid A in Salmonella typhimurium. J Biol Chem. 1978 Mar 10;253(5):1503–1511. [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Ohta A., Shibuya I. Membrane phospholipid synthesis and phenotypic correlation of an Escherichia coli pss mutant. J Bacteriol. 1977 Nov;132(2):434–443. doi: 10.1128/jb.132.2.434-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol. 1979 Feb;137(2):860–868. doi: 10.1128/jb.137.2.860-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem. 1978 Jun 10;253(11):3882–3887. [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Tyhach R. J., Hawrot E., Satre M., Kennedy E. P. Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. Altered association of enzyme with the membrane. J Biol Chem. 1979 Feb 10;254(3):627–633. [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]



