Abstract
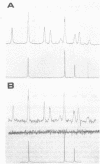
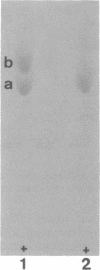
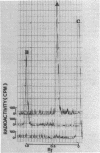
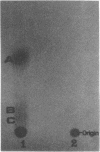
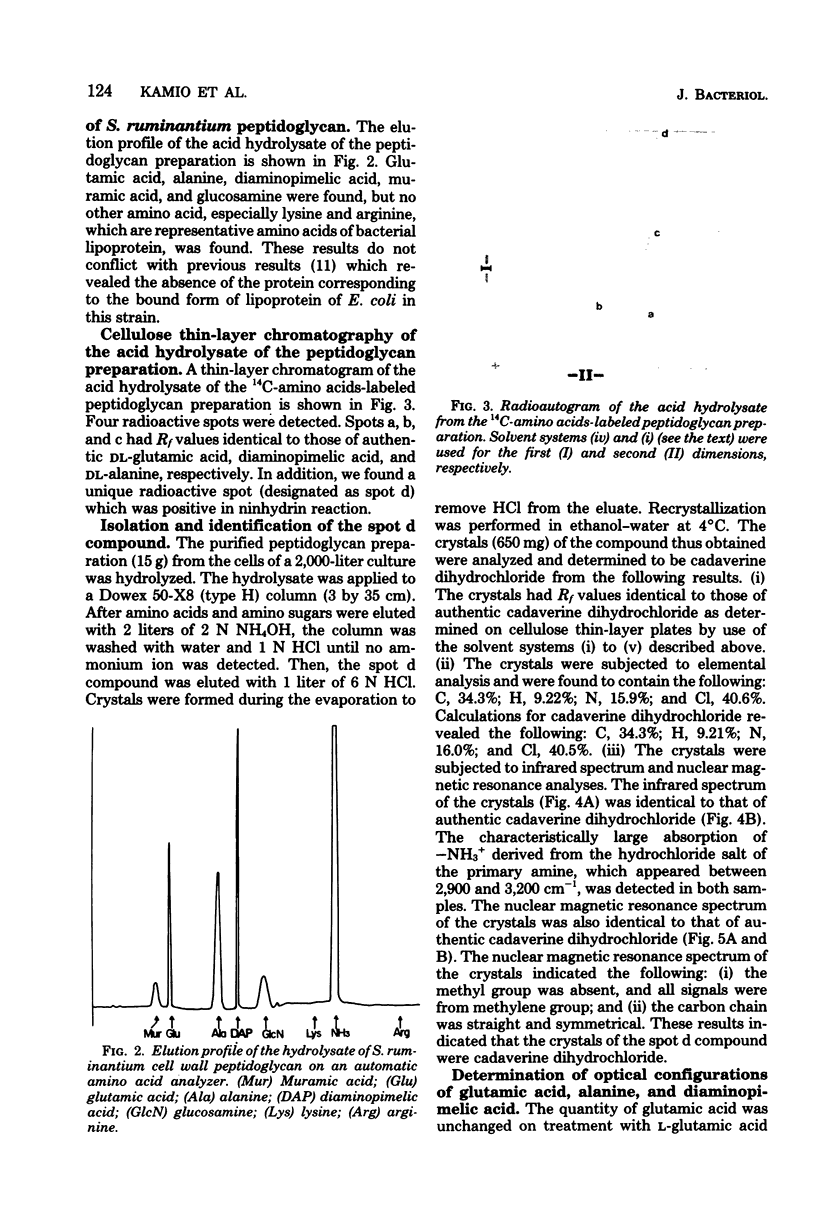
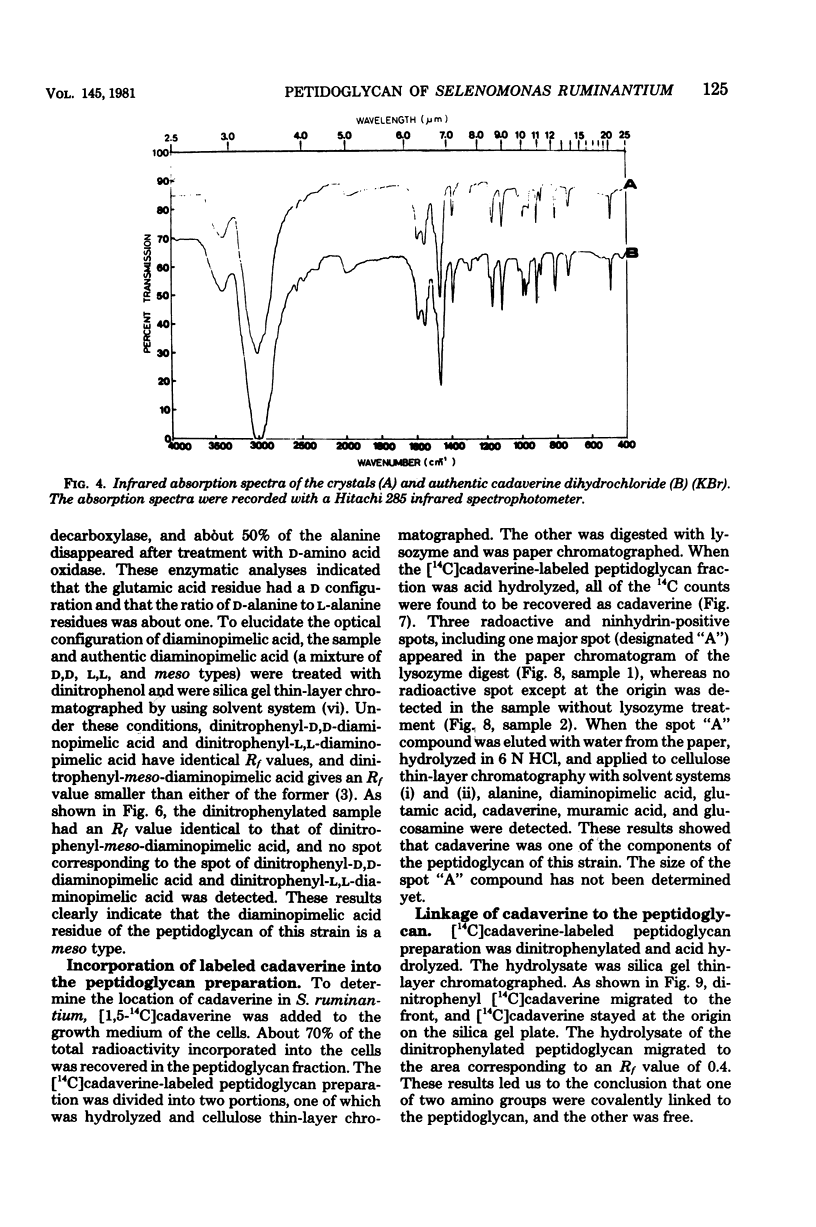
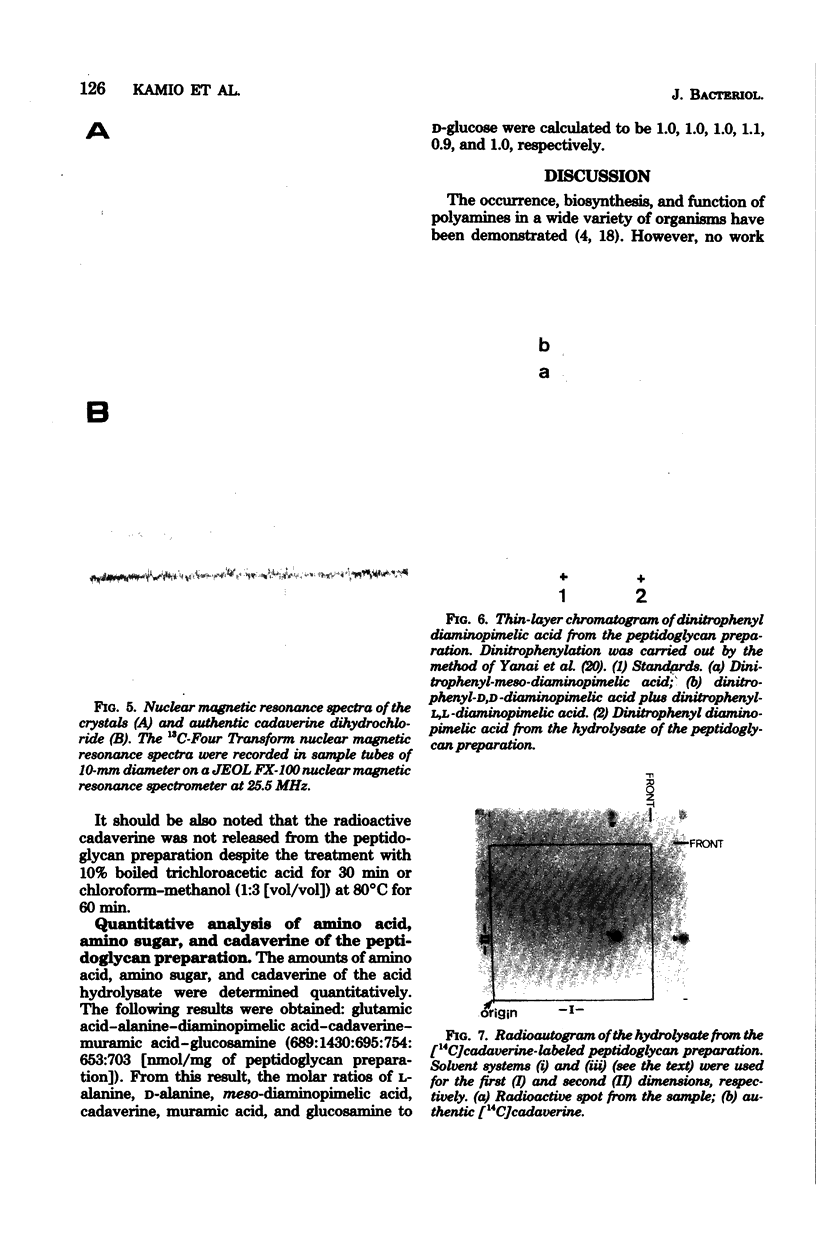
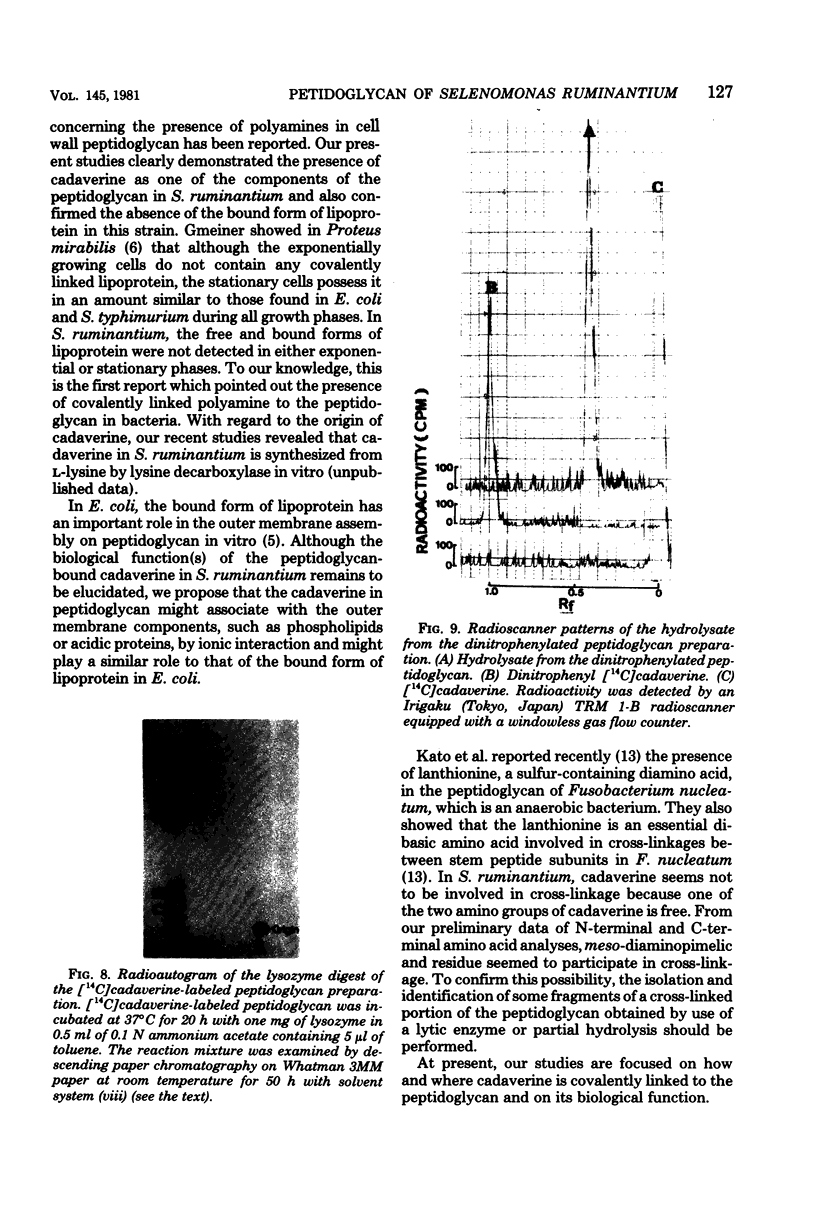
Cadaverine was found to exist as a component of cell wall peptidoglycan of Selenomonas ruminantium, a strictly anaerobic bacterium. [14C]cadaverine added to the growth medium was incorporated into the cells, and about 70% of the total radioactivity incorporated was found in the peptidoglycan fraction. When the [14C]cadaverine-labeled peptidoglycan preparation was acid hydrolyzed, all of the 14C counts were recovered as cadaverine. The [14C]cadaverine-labeled peptidoglycan preparation was digested with lysozyme into three small fragments which were radioactive and were positive in ninhydrin reaction. One major spot, a compound of the fragments, was composed of alanine, glutamic acid, diaminopimelic acid, cadaverine, muramic acid, and glucosamine. One of the two amino groups of cadaverine was covalently linked to the peptidoglycan, and the other was free. The chemical composition of the peptidoglycan preparation of this strain was determined to be as follows: L-alanine-D-alanine-D-glutamic acid-meso-diaminopimelic acid-cadaverine-muramic acid-glucosamine (1.0:1.0:1.0:1.0:1.1:0.9:1.0).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Bricas E., Ghuysen J. M., Dezélée P. The cell wall peptidoglycan of Bacillus megaterium KM. I. Studies on the stereochemistry of alpha, alpha'-diaminopimelic acid. Biochemistry. 1967 Aug;6(8):2598–2607. doi: 10.1021/bi00860a043. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Furukawa H., Yamada H., Mizushima S. Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):1071–1080. doi: 10.1128/jb.140.3.1071-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J. Covalent linkage of lipoprotein to peptidoglycan is not essential for outer membrane stability in Proteus mirabilis. Arch Microbiol. 1979 May;121(2):177–180. doi: 10.1007/BF00689983. [DOI] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Johnson K., Duez C., Frère J. M., Ghuysen J. M. Beta-lactamases (Actinomycetes species). Methods Enzymol. 1975;43:687–698. doi: 10.1016/0076-6879(75)43134-2. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Isolation and characterization of outer and inner membranes of Selenomonas ruminantium: lipid compositions. J Bacteriol. 1980 Feb;141(2):888–898. doi: 10.1128/jb.141.2.888-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Outer membrane proteins and cell surface structure of Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):899–907. doi: 10.1128/jb.141.2.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegasaki S., Takahashi H. Function of growth factors for rumen microorganisms. I. Nutritional characteristics of Selenomonas ruminantium. J Bacteriol. 1967 Jan;93(1):456–463. doi: 10.1128/jb.93.1.456-463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Yanagida I., Kato K., Matsuda T. Studies on peptides, glycopeptides and antigenic polysaccharide-glycopeptide complexes isolated from an L-11 enzyme lysate of the cell walls of Mycobacterium tuberculosis strain H37Rv. Biken J. 1970 Dec;13(4):249–275. [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Shepherd S. T., Chase H. A., Reynolds P. E. The separation and properties of two penicillin-binding proteins from Salmonella typhimurium. Eur J Biochem. 1977 Sep;78(2):521–523. doi: 10.1111/j.1432-1033.1977.tb11765.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Wallace R. J. Cytoplasmic reserve polysaccharide of Selenomonas ruminantium. Appl Environ Microbiol. 1980 Mar;39(3):630–634. doi: 10.1128/aem.39.3.630-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978 Mar;133(3):1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]