Abstract
Specific integrins expressed on oligodendrocytes, the myelin-forming cells of the central nervous system, promote either differentiation and survival or proliferation by amplification of growth factor signaling. Here, we report that the Src family kinases (SFKs) Fyn and Lyn regulate each of these distinct integrin-driven behaviors. Fyn associates with α6β1 and is required to amplify platelet-derived growth factor survival signaling, to promote myelin membrane formation, and to switch neuregulin signaling from a phosphatidylinositol 3-kinase to a mitogen-activated protein kinase pathway (thereby changing the response from proliferation to differentiation). However, earlier in the lineage Lyn, not Fyn, is required to drive αVβ3-dependent progenitor proliferation. The two SFKs respond to integrin ligation by different mechanisms: Lyn, by increased autophosphorylation of a catalytic tyrosine; and Fyn, by reduced Csk phosphorylation of the inhibitory COOH-terminal tyrosine. These findings illustrate how different SFKs can act as effectors for specific cell responses during development within a single cell lineage, and, furthermore, provide a molecular mechanism to explain similar region-specific hypomyelination in laminin- and Fyn-deficient mice.
Introduction
The development of complex tissues requires that cell migration, proliferation, differentiation, and survival be precisely regulated so that each occurs only at appropriate stages within each cell lineage. This regulation is achieved by the integration of multiple extracellular cues that act in concert with intrinsic developmental gene expression programs to control cell behavior, but the mechanisms of integration remain poorly understood. We have recently described a novel integrative mechanism for growth factor and ECM signaling in oligodendrocytes, the myelinating cells of the central nervous system. At physiological concentrations of PDGF, two orders of magnitude less than typically used for culture studies, the PDGFα receptor (PDGFαR) expressed on oligodendroglial cells does not stimulate proliferation directly, but rather activates associated αVβ3; this high affinity integrin subsequently triggers downstream signaling pathways after ECM binding (Baron et al., 2002). A similar mechanism contributes to the survival of newly formed oligodendrocytes: α6β1 integrin is required for the amplification of growth factor survival signaling that is necessary for the oligodendrocytes' survival at physiological PDGF concentrations (Colognato et al., 2002). Thus, growth factor signaling is spatially controlled by the location of ECM ligands such as α2 chain–containing laminins found within axon tracts, thereby providing a mechanism for target-dependent survival of oligodendrocytes during development (Colognato et al., 2002). Similarly, the switch observed in the integrin associated with PDGFαR, from αVβ3 to α6β1, may contribute to the temporal regulation of growth factor signaling by changing the cell's response from proliferation to survival (Baron et al., 2002, 2003).
However, a key prediction is that each integrin would be able to trigger associated signaling molecules whose effects are specific for one component of cell behavior. Our current understanding of integrin signaling within developing systems has not yet confirmed the identity of signaling molecules with the required specificity. The Src family kinases (SFKs) are nonreceptor tyrosine kinases that integrate external signals received through both integrin and growth factor receptors and, thus, are good candidates to transduce signals that regulate both integrin- and growth factor–driven phases of oligodendrocyte development. One of these, Fyn, is expressed throughout the brain, in both neurons and glia, but the peak of its activity during development can be correlated with myelination (Umemori et al., 1992; Osterhout et al., 1999). Fyn may be involved in the oligodendrocyte differentiation process, because transgenic mice lacking Fyn activity are hypomyelinated (Umemori et al., 1994; Sperber et al., 2001) and cultured oligodendrocytes from Fyn −/− mice or those expressing dominant-negative Fyn show defects in the numbers of newly formed oligodendrocytes, as well as in the formation of complex branches of myelin membrane (Osterhout et al., 1999; Sperber and McMorris, 2001). Furthermore, mice deficient in the laminin α2 chain have a similar region-specific hypomyelination, suggesting that Fyn and laminins may operate in the same signaling pathway, and that integrin receptors may contribute to this pathway (Chun et al., 2003). Another SFK, Lyn, is also expressed in oligodendrocytes, but no function has been described. However, in the hematopoietic lineage, β1 integrin has been found in a complex with Lyn after fibronectin (FN)-mediated adhesion (Miller et al., 1999), and it was recently shown that β1 and Src can be directly associated, indicating that different cell types may regulate unique combinations of SFK–integrin associations based on the cell functions required (Arias-Salgado et al., 2003).
Distinct roles for Fyn and Lyn could provide a mechanism that enables each integrin to have distinct functions in oligodendrocyte development. Here, we tested this hypothesis by determining the associations among integrins, growth factor receptors, and the two SFKs, using siRNAs to knock down each SFK and examine the effect in developing oligodendrocyte cultures freshly isolated from the brain, and by examining the activation of each SFK by integrin signaling. We found that Lyn and Fyn were associated with αVβ3 and α6β1, respectively, and that Lyn, but not Fyn, was required for PDGF-stimulated proliferation of oligodendrocyte progenitors. However, at later stages of differentiation Fyn, but not Lyn, was associated with α6β1 and was required for laminin-mediated amplification of growth factor–mediated survival and for differentiation with enhancement of myelin membrane formation. These results suggest a model in which integrins determine the consequences of growth factor signaling in oligodendrocytes via an associated SFK, and demonstrate how different SFKs can act within a single cell lineage as effectors that are specific for individual aspects of cell behavior and are able to integrate multiple upstream signaling cues.
Results
Generating SFK-deficient oligodendrocytes using small interfering RNA
Oligodendrocytes have been shown previously to express three different SFKs: Fyn, Lyn, and Src (Umemori et al., 1992; Sperber et al., 2001). We confirmed that primary rat oligodendrocytes expressed these kinases, and that their expression was regulated in differentiating cells (Fig. 1 A). Progenitor cells were withdrawn at day 0 from growth factors that promote their division and prevent differentiation, and lysates were prepared from cells grown in conditions that promote oligodendrocyte differentiation, at days 1, 2, 4, or 6. Fyn and Lyn showed the highest levels of expression at days 2 and 4 of differentiation, correlating with the transition from oligodendrocyte progenitors to newly formed oligodendrocytes. Src expression was relatively difficult to detect, except at earlier stages (days 1 and 2), and was undetectable by day 6.
Figure 1.
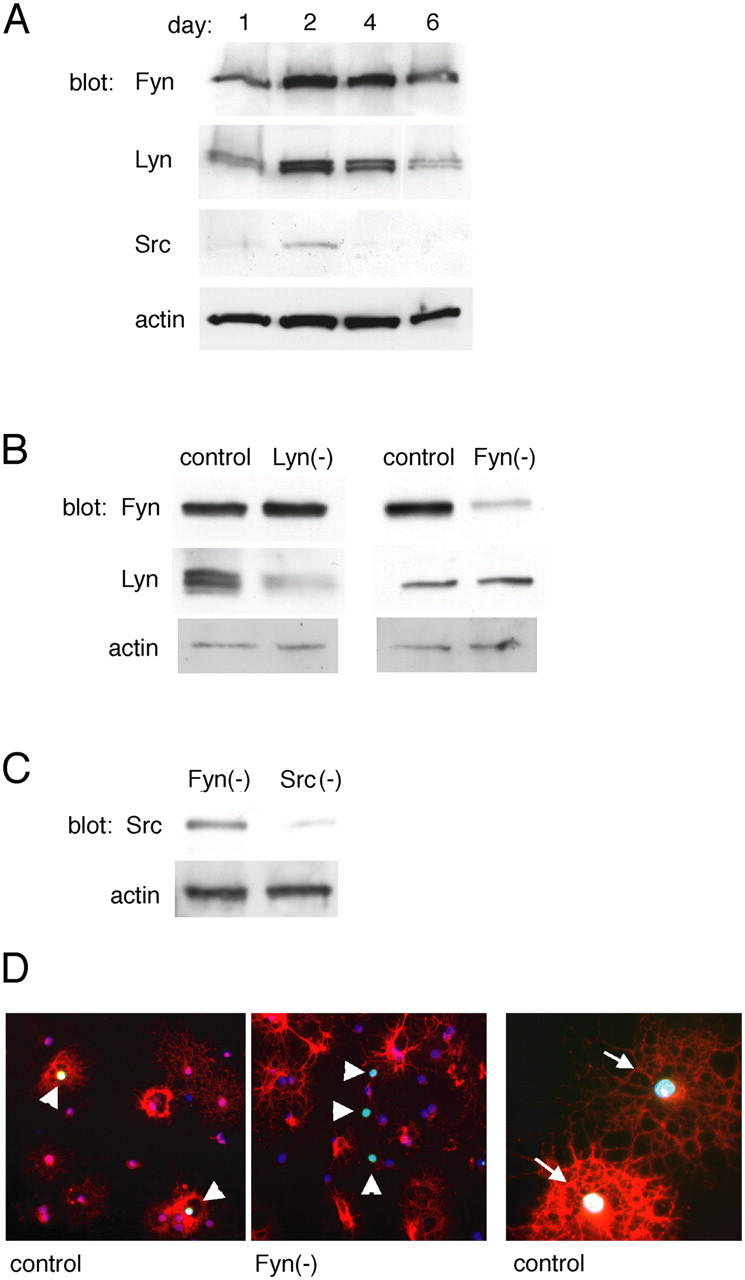
siRNA depletes Src family kinases in primary oligodendrocytes. (A) Src family kinase (SFK) protein expression in oligodendrocytes differentiated for 1, 2, 4, or 6 d after growth factor withdrawal. (B) SFK Western blots of G418-selected progenitors transfected with Fyn and Lyn depletion constructs. (C) Western blots of oligodendrocyte progenitors transfected with Src depletion construct. (A–C) Blots were also probed with actin antibodies as protein loading controls. (D) Immunostaining to visualize Fyn protein in a mixed population containing YFP+-transfected cells. Control (left) and Fyn(−) (middle) micrographs depict newly differentiated oligodendrocytes labeled with antibodies against Fyn (red) and GFP (green) (arrowheads). Double-labeled cells do not appear in the Fyn(−)-transfected population. (right) Control cells expressing late differentiation markers MBP (red) and YFP (green) (arrows).
To investigate the roles of individual SFKs, we used pSUPER to drive expression of small interfering RNA (siRNA). We performed Western blots on lysates of G418-resistant cells to evaluate protein levels (Fig. 1, B and C). Blots using Fyn-specific antibodies showed that cells transfected with a Fyn-targeting construct, Fyn(−), had a decrease in Fyn protein (59 kD), whereas cells transfected with the Lyn-targeting construct, Lyn(−), had no change in Fyn expression. Conversely, blots using Lyn-specific antibodies showed a decrease in Lyn protein (53 and 56 kD) in Lyn(−)-transfected cells but not in Fyn(−) cells. Transfection with control vectors had no effect on Fyn or Lyn protein expression. Western blots using Src-specific antibodies showed that Src(−) cells had reduced levels of Src protein, whereas Src protein levels in Fyn(−) cells remained unchanged.
Loss of protein expression was also confirmed using immunohistochemistry. Because isolated progenitors expanded in PDGF and FGF respond to growth factors and ECM differently from the way progenitors that are freshly isolated from cortical cultures respond, we avoided expansion in experiments examining cell behavior; instead, we evaluated a mixed population in which the subset of transfected cells was identified by YFP expression. Shown in Fig. 1 D are two cell populations that have been transfected with different vectors: one expressed YFP alone (control, left and right), and one expressed YFP and Fyn siRNA (Fyn(−), middle). In controls (Fig. 1 D, left), many cells expressed both Fyn and YFP (arrowheads), whereas in Fyn(−) cells, the transfected cell population (YFP) and the Fyn-expressing population were mutually exclusive. Control-transfected cells (Fig. 1 D, right) had normal morphology and differentiated identically to nontransfected oligodendrocytes, as illustrated by cells labeled with myelin basic protein (MBP) and GFP antibodies (arrows).
Lyn regulates integrin-specific proliferation, but not migration
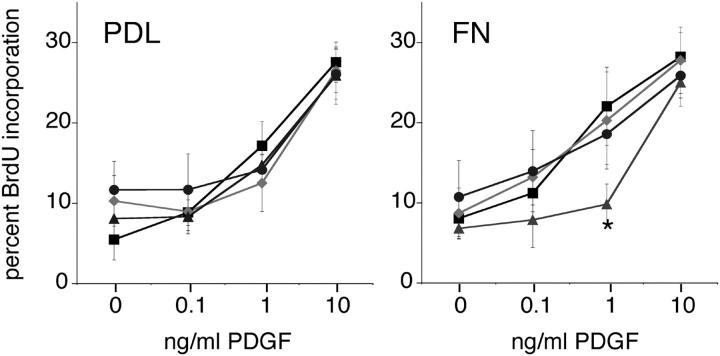
To determine whether SFKs play a role in the responses of oligodendrocyte progenitors, we evaluated two essential functions of progenitors, proliferation and migration, using cells in which individual SFKs were depleted. PDGF is a mitogen for oligodendrocyte progenitors, and the proliferative response seen at physiological PDGF concentrations (0.1–1.0 ng/ml) can be enhanced by αVβ3 integrin engagement (Baron et al., 2002). We evaluated proliferation of SFK-depleted progenitors in the presence of increasing amounts of PDGF and in the presence or absence of the αVβ3 ligand FN (Fig. 2). With increasing PDGF concentrations, proliferation increased equally well in control and Fyn-, Lyn-, and Src-depleted progenitors grown on non-integrin substrate poly-d-lysine (PDL; Fig. 2, left). However, proliferation of Lyn-depleted cells on the αVβ3 ligand FN was reduced in response to physiological levels of PDGF (Fig. 2, right; *, P < 0.05). In contrast, depletion of Fyn or Src did not reduce PDGF-mediated proliferation on either substrate. Proliferation of progenitors grown on the α6β1 ligand laminin-2 (Lm2) also increased with increasing PDGF; however, SFK depletion had no effect (not depicted).
Figure 2.
Progenitors require Lyn, but not Fyn or Src, for PDGF-mediated proliferation on the αVβ3 ligand. Oligodendrocyte progenitors exposed to 0, 0.1, 1, or 10 ng/ml PDGF for 24 h were evaluated for the percentage of cells that incorporated BrdU. Profiles of control (black squares), Fyn-depleted (gray diamonds), Lyn-depleted (gray triangles), and Src-depleted (gray circles) cells are shown. On poly-d-lysine (PDL), all progenitors showed a similar dose-dependent increase in BrdU incorporation. In contrast, Lyn-depleted progenitors grown on the αVβ3 ligand fibronectin (FN) showed reduced BrdU incorporation (*, P < 0.05). Error bars represent SD.
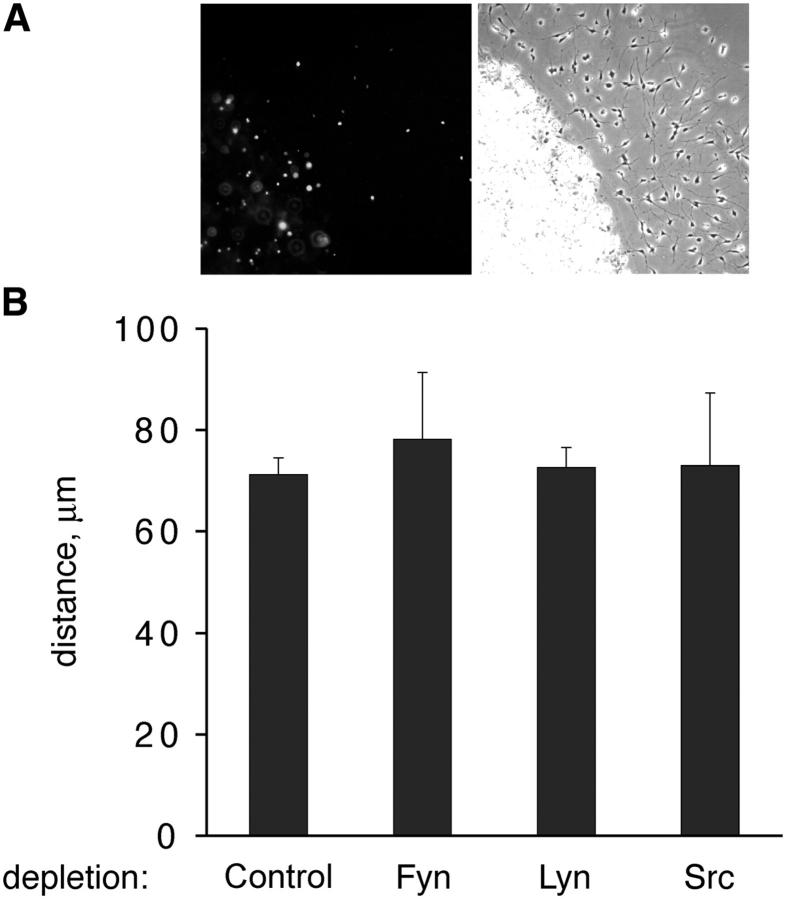
Next, we tested whether progenitor migration in response to PDGF also involves a role for Lyn. The migration of SFK-depleted progenitor cells on ECM ligands was measured in the presence of varying amounts of PDGF. Progenitors were concentrated in a drop of low melting temperature agarose to establish a defined starting point, and the distance between the agarose boundary and each YFP+ cell was measured (Fig. 3). A drop that contains Lyn-depleted cells migrating on FN in the presence of 1 ng/ml PDGF is shown in Fig. 3 A. Unlike proliferation, no difference in PDGF-mediated migration was observed after the removal of Fyn, Lyn, or Src. The mean distances (micrometers) of migration on FN in response to 1 ng/ml PDGF were 71.2 ± 3.2 (control), 78.2 ± 13.2 (Fyn(−)), 72.6 ± 3.9 (Lyn(−)), and 73.0 ± 14.3 (Src(−)) (Fig. 3 B). Cells migrated further in response to 10 ng/ml PDGF; however, no significant difference among different SFK-depleted cells was observed: the mean migration distances (micrometers) were 117.9 ± 5.6 (control), 126.2 ± 16.8 (Fyn(−)), 130.3 ± 2.2 (Lyn(−)), and 120.2 ± 14.2 (Src(−)). Progenitors on PDL or Lm2 migrated less than cells on FN, but also exhibited no difference in their ability to migrate after SFK depletion (not depicted). Thus, oligodendrocyte progenitors have different requirements for Lyn during migration and proliferation in response to the same growth factor (PDGF) and ECM stimulus (FN).
Figure 3.
Progenitors do not require Lyn for PDGF-mediated migration. Oligodendrocyte progenitors were concentrated in agarose drops and stimulated to migrate using PDGF. (A) Micrograph depicting Lyn-deficient cells (YFP+) migrating on FN in response to 1 ng/ml PDGF. (left) Anti-GFP; (right) phase. (B) Migration of SFK-deficient cells on FN in response to 1 ng/ml PDGF at day 2. Each bar depicts the mean migration distance of all YFP+ cells that have exited drops. Error bars represent SD.
Newly formed oligodendrocytes require Fyn for laminin amplification of survival
As oligodendrocyte progenitors exit the cell cycle and differentiate, they have an increased dependency on survival factors such as PDGF and neuregulin (NRG) (Barres et al., 1993; Canoll et al., 1996; Calver et al., 1998; Fernandez et al., 2000). Laminins that are found in myelinating axon tracts can potentiate the effects of these soluble survival factors (Frost et al., 1999; Colognato et al., 2002). We evaluated the ability of newly formed oligodendrocytes (defined by expression of the lineage marker galactocerebroside [GalC]) to respond to survival factors in the presence of Lm2 after depletion of individual SFKs. Cells were differentiated for 4 d with increasing doses of PDGF or NRG, and were evaluated for survival by TUNEL assays on double-labeled GFP/GalC-positive cells (Fig. 4).
Figure 4.
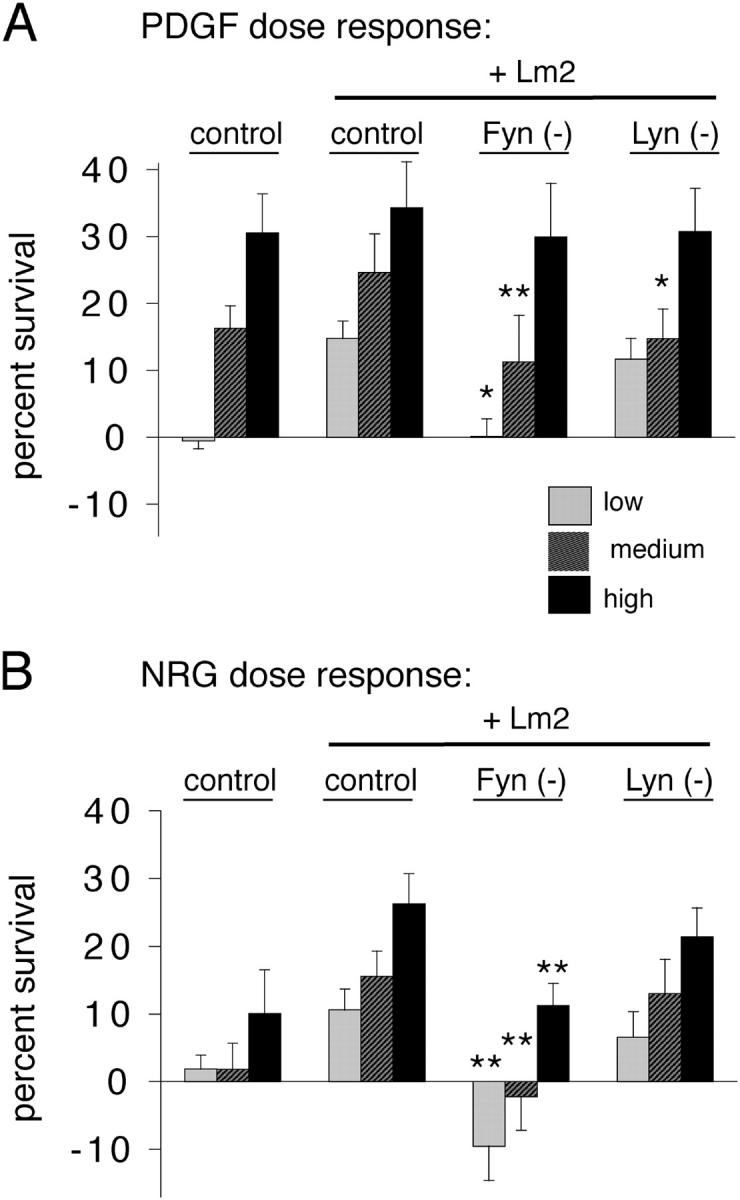
Newly formed oligodendrocytes require Fyn for laminin-mediated amplification of survival. SFK-depleted progenitors were differentiated on PDL or laminin-2 (Lm2) for 4 d with increasing amounts of the soluble growth factors PDGF or neuregulin (NRG). Survival was evaluated using TUNEL on a YFP/GalC double-positive cell population (transfected, newly formed oligodendrocytes). Low, medium, and high amounts of growth factors are depicted by light gray, dark gray, and black bars, respectively. Error bars represent SD. (A) Fyn depletion caused a significant shift in the PDGF dose–response curve when cells were differentiated on Lm2 (low, *, P < 0.05; medium, **, P < 0.01). (B) NRG-mediated survival was significantly reduced in Fyn-depleted cells differentiated on Lm2 (**, P < 0.01 for low, medium, and high concentrations of NRG), whereas no significant reduction in survival occurred in Lyn-depleted cells.
Newly formed oligodendrocytes grown on Lm2 amplified the survival-promoting effects of PDGF (Fig. 4 A, control). In contrast, Fyn-depleted cells grown on Lm2 did not shift the dose–response to PDGF, such that survival in response to 0.1 or 1.0 ng/ml PDGF (physiological levels) was significantly lower than in control cells grown on Lm2 (Fig. 4 A, *, P < 0.05 and **, P < 0.01). As described in Materials and methods, these assays were performed at a density of 20,000 cells/well, but the same trend in response to Fyn depletion was observed at lower plating densities of 2,500 and 10,000 cells/well (unpublished data). Lyn depletion had a smaller effect on Lm2 amplification of PDGF survival, with a reduction in survival only observed at 1.0 ng/ml PDGF (Fig. 4 A, *, P < 0.05). No reduction in survival was observed at high, nonphysiological levels of PDGF (10 ng/ml) that are not amplified by Lm2. Src depletion did not affect survival on either substrate.
A laminin-mediated switch in NRG survival signaling requires Fyn
NRG-mediated survival is minimal when freshly isolated cells are grown on PDL or FN; however, Lm2 amplifies NRG's ability to mediate survival (Colognato et al., 2002). We observed a robust amplification of NRG-mediated survival when control-transfected newly formed oligodendrocytes were grown on Lm2 (Fig. 4 B, control). In contrast, Fyn-depleted cells on Lm2 did not amplify NRG-mediated survival, and a significant reduction in survival (Fig. 4 B, **, P < 0.01) was observed at all NRG concentrations. However, Lyn-depleted cells grown on Lm2 retained the ability to amplify NRG-mediated survival, and, as with PDGF, depletion of Src had no effect. In cells grown on PDL, Fyn and Lyn depletion had no significant effect on NRG-mediated survival (not depicted), indicating that, as with PDGF, the SFK plays a role in amplification of survival by the ECM substrate rather than by the growth factor signal alone.
Laminin switches the preferred signaling pathways activated during NRG-mediated survival (Colognato et al., 2002). Thus, on nonlaminin substrates, survival is sensitive to PI3K inhibition but insensitive to MAPK inhibition. This pattern is reversed by Lm2, such that survival is insensitive to PI3K inhibition but sensitive to MAPK inhibition (Fig. 5 A). Here, we observed that the laminin-driven switch in NRG signaling did not occur in Fyn-deficient cells, whereas Lyn-deficient cells remained able to switch. Wortmannin treatment of Fyn(−) cells grown with NRG on Lm2 significantly reduced survival (**, P < 0.01) compared with control and Lyn-depleted cells grown on Lm2 (Fig. 5 A). Furthermore, Fyn depletion caused the cells to become less sensitive to the MEK inhibitor PD098059 (Fig. 5 A). In addition, cells grown on Lm2 and treated with NRG show enhanced phosphorylation of extracellular signal–related kinase (ERK), yet do not amplify phosphorylation of Akt. Using a modified electroporation technique to obtain a high percentage of siRNA-positive cells (∼50%), Western blots of oligodendrocyte lysates revealed that Fyn-depleted cells treated with NRG were unable to amplify ERK phosphorylation (Fig. 5 B).
Figure 5.
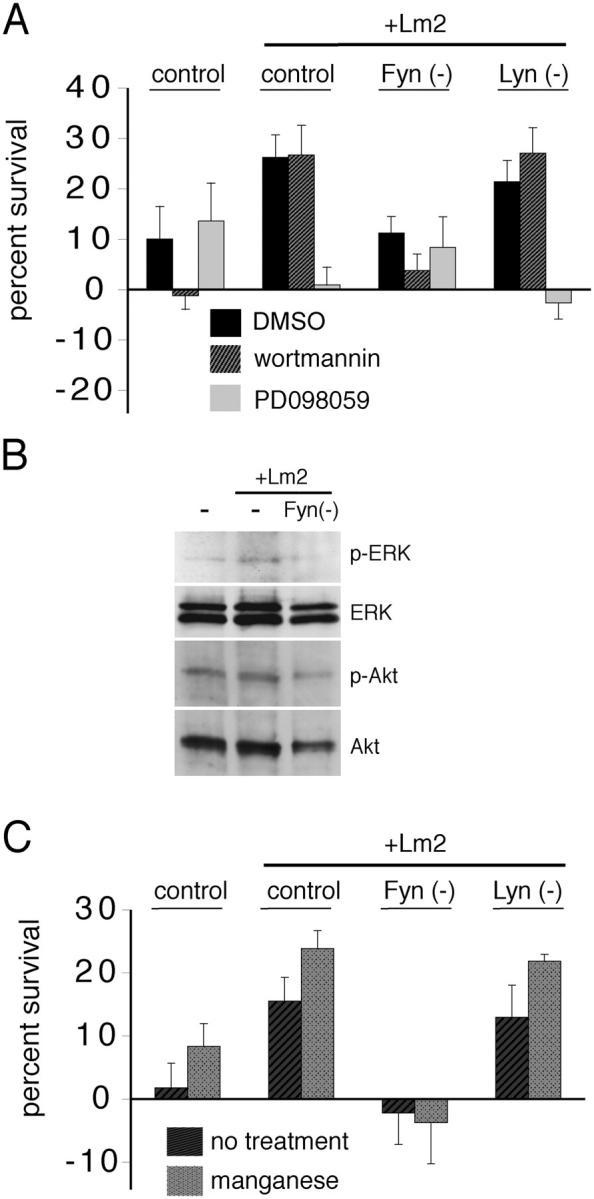
Fyn is required for a laminin-mediated switch in NRG signaling and for integrin activation to increase survival. (A) Survival of newly formed SFK-depleted oligodendrocytes in the presence of the PI3K pathway signaling inhibitor wortmannin (hatched bars), the MAPK pathway signaling inhibitor PD098059 (light gray bars), or DMSO control (black bars). Lm2 switches NRG-mediated survival from PI3K-sensitive to PI3K-insensitive, and Fyn depletion, but not Lyn depletion, abolishes this effect. Error bars represent SD. (B) Oligodendrocytes treated for 30 min with NRG. PhosphoERK is enhanced by Lm2 in control cells, but not in Fyn(−) cells. (C) Survival of newly formed SFK-depleted oligodendrocytes in the presence (light gray bars) or absence (dark gray bars) of integrin-activating manganese. Integrin activation using manganese increased NRG-mediated survival, and this increase was lost in the absence of Fyn, but not of other SFKs. Error bars represent SD.
We showed previously that activation of αVβ3 integrin using manganese is sufficient to amplify PDGF-driven proliferation in progenitors, and we have recently shown that manganese can also induce an amplification of PDGF-mediated survival similar to that seen using the α6β1 ligand Lm2 (Baron et al., 2002; Decker and ffrench-Constant, 2004). Activation is a process in which the integrin subunits undergo conformational changes that lead to an increase in affinity for ECM ligands and/or to alterations in receptor clustering, both of which can enhance associations with effector molecules and promote integrin signaling (Travis et al., 2003). We determined whether integrin activation altered the requirement for SFKs during survival signaling; first, by evaluating whether activation altered the survival of newly formed oligodendrocytes in the presence of limiting concentrations of NRG, and second, by evaluating whether depletion of SFKs could affect survival in the presence of manganese (Fig. 5 C). With manganese, NRG-mediated survival increased in control (grown on PDL or Lm2) and Lyn-depleted oligodendrocytes. However, Fyn-depleted oligodendrocytes treated with manganese did not increase survival in response to NRG. This observation strengthens our conclusion that Fyn, but not Lyn or Src, is required for integrin-mediated amplification of survival signaling, and suggests that Fyn either acts downstream of or helps to maintain integrin activation during oligodendrocyte survival.
Differentiation on laminin requires Fyn
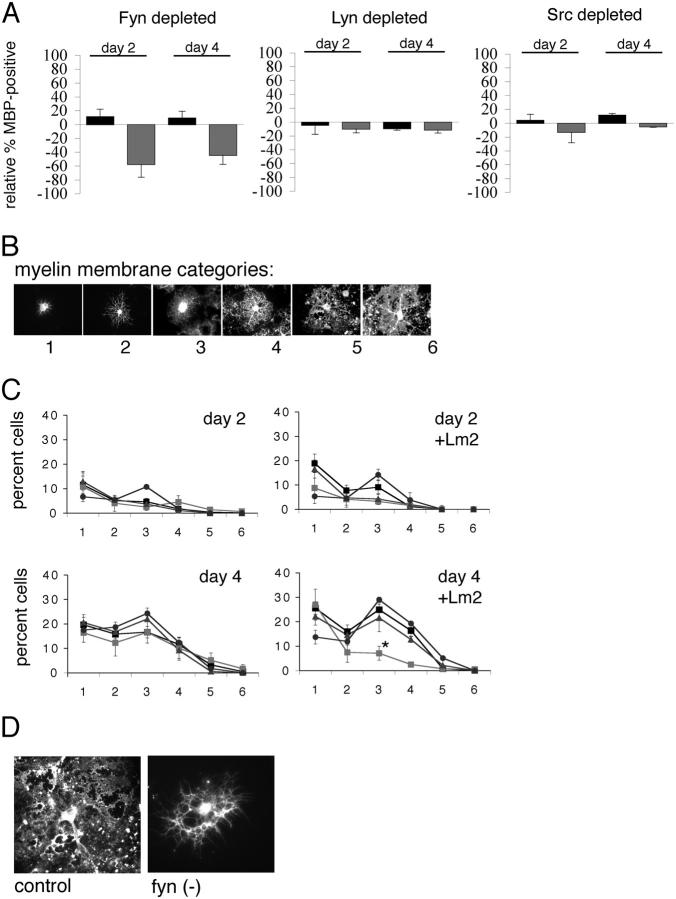
The same factors that are critical for newly formed oligodendrocytes to survive also regulate entry into the myelin-forming stage of differentiation. To investigate whether SFKs regulate the ability of the ECM to alter oligodendrocyte differentiation, we evaluated MBP expression in the presence of integrin ligands in SFK-depleted cells. The percentage of MBP-expressing cells was determined, and the relative change between SFK-deficient cells and control cells is shown in Fig. 6 A. In cells differentiating on PDL, depletion of Fyn, Lyn, or Src had no effect on the percentage of cells that acquired MBP expression by days 2 or 4 (Fig. 6 A, black bars). In contrast, Fyn-depleted cells differentiated on Lm2 showed a large reduction in the percentage of cells expressing MBP, at both days 2 and 4 (Fig. 6 A, gray bars). However, Lyn- or Src-depleted cells showed no change in differentiation on either substrate.
Figure 6.
Differentiation in response to laminin requires Fyn. (A) Decreased MBP expression in cells grown on laminin in the absence of Fyn. The percentage of SFK-depleted cells expressing the late stage differentiation marker MBP was expressed relative to the percentage of MBP+ in control cells. SFK-depleted cells grown on PDL (black bars) and on Lm2 (gray bars) were compared at days 2 and 4 after growth factor withdrawal. Error bars represent SD. (B) Myelin membrane classification scheme. Examples of MBP-expressing cells are shown. Stages 1, 2, and 3 show increasing levels of process outgrowth and branching, without myelin membrane, whereas stages 4, 5, and 6 show increasing levels of complexity and myelin membrane. (C) Fyn-depleted cells have less myelin membrane acquisition and complexity on Lm2 substrates. The percentage of cells within each category is shown for SFK-depleted YFP/MBP double-positive cells. Oligodendrocytes differentiated on PDL or Lm2 (control), black squares; Fyn(−), light gray squares; Lyn(−), gray triangles; and Src(−), dark gray circles (*, P < 0.050). Error bars represent SD. (D) Typical MBP-expressing (control) and Fyn-depleted oligodendrocytes grown on Lm2.
To further evaluate the cells that acquired MBP expression, we used a classification scheme to describe the morphology and degree of myelin membrane formation in MBP+ cells (Fig. 6 B). Categories 1, 2, and 3 were used to describe MBP+ cells with increasing degrees of complex branched processes in the absence of myelin membrane (low, medium, and high, respectively), whereas categories 4, 5, and 6 were used to describe cells with increasing levels of myelin membrane (low, medium, and high, respectively). Cells double-labeled with MBP and GFP antibodies were assessed at days 2 and 4 and assigned to a myelin category (Fig. 6 C). A reduction in branching complexity and myelin membrane acquisition was seen in Fyn-depleted cells (Fig. 6 C, light gray squares; *, P < 0.05) differentiated on Lm2 for 4 d. A typical Fyn-depleted, MBP+ cell is shown in Fig. 6 D. In contrast, Lyn- and Src-depleted cells formed complex myelin membranes as normal. None of the SFK-depleted cells showed significant differences in myelin membrane formation when grown on the non-integrin substrate PDL (Fig. 6 C) or on FN (not depicted).
Fyn associates with α6β1, whereas Lyn associates with PDGFαR and αVβ3 integrin
Having shown that Lyn and Fyn regulate proliferation and survival/differentiation, respectively, we asked whether each SFK was associated with the integrin–growth factor receptor complexes responsible for these different stages of oligodendroglial development. Using immunofluorescence microscopy, we detected α6β1 and Fyn in newly formed oligodendrocytes in an overlapping distribution (Fig. 7 A). Next, detergent lysates of newly formed oligodendrocytes grown on PDL, Lm2, and FN in the presence or absence of growth factors were evaluated by immunoprecipitation for the formation of protein complexes (Fig. 7, B–E). Antibodies specific for the α6 integrin subunit isolated complexes containing Fyn, but not Lyn, and the α6β1–Fyn association was independent of substrate or growth factor stimuli (Fig. 7 B). Immunoprecipitations using Fyn antibodies also revealed a potential association between Fyn and the ErbB4 NRG receptor subunit (Fig. 7 C). This association was difficult to detect but, interestingly, was only observed in cells treated with NRG on non-integrin substrates. PDGFαR immunoprecipitations revealed an association between PDGFαR and Lyn that, in the absence of PDGF, was most robust on FN but, in the presence of PDGF, was also observed in cells grown on PDL or Lm2 (Fig. 7 D). No association between Fyn and PDGFαR was observed (unpublished data). Lyn immunoprecipitations revealed an association between Lyn, but not Fyn, and the αVβ3 integrin that was also enhanced by FN but, in contrast to the Lyn–PDGFαR association, was not detected after PDGF treatment (Fig. 7 E).
Figure 7.
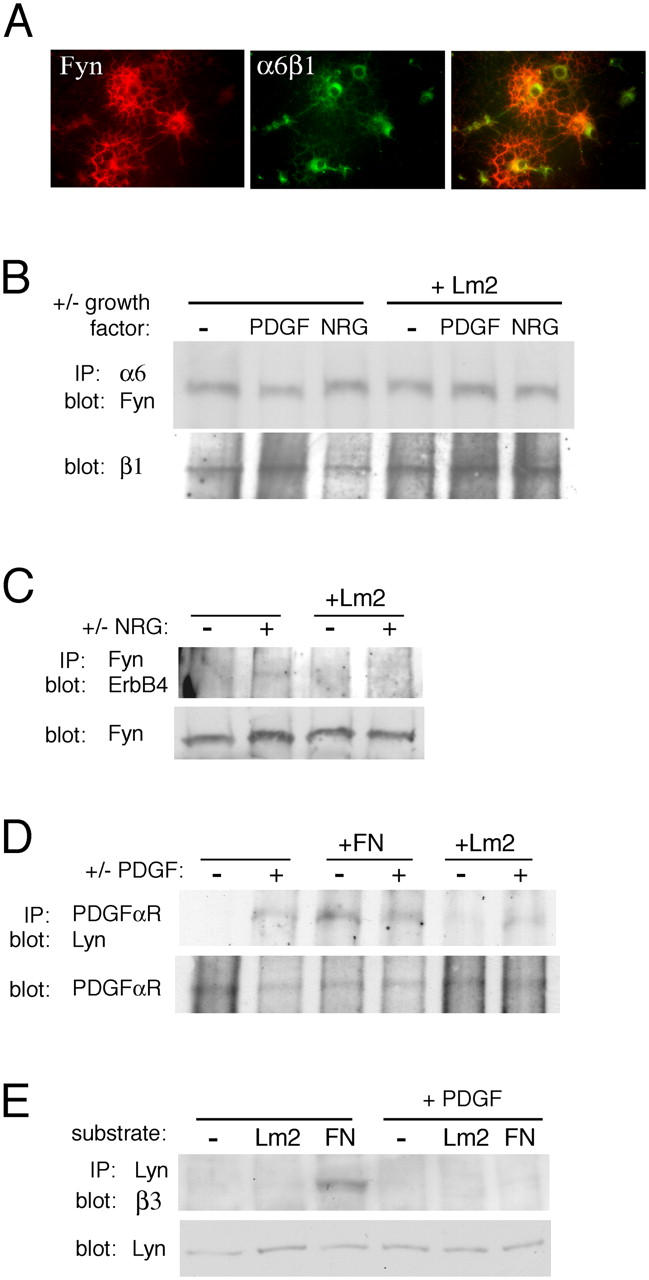
SFK associations with integrins and growth factors. (A) Newly formed oligodendrocytes immunostained with antibodies against Fyn (red) and α6β1 integrin (green). Merged panel is shown on the right. (B) Oligodendrocyte lysates from cells differentiated on ECM substrates in the presence or absence of PDGF or NRG. Western blot on α6 integrin antibody immunoprecipitation complexes to detect Fyn. (C) Western blot on Fyn antibody immunoprecipitation complexes to detect ErbB4 NRG receptor subunit. (D) Western blot on PDGFαR antibody immunoprecipitation complexes to detect Lyn. (E) Western blots on Lyn antibody immunoprecipitation complexes to detect the β3 integrin subunit.
Distinct mechanisms of Fyn and Lyn activation by α6β1 and αVβ3 ligands
We asked whether ligand binding by each integrin specifically altered associated SFK activity. We tested this using antibodies specific for two phosphorylated tyrosines within Src that also recognize the following conserved tyrosine (Y) sites in Fyn and Lyn: (1) Y418 (Y420 and Y397 in rat Fyn and Lyn, respectively), which is an autophosphorylation site; and (2) Y527 (Y531 and Y508 in rat Fyn and Lyn, respectively), which is phosphorylated by COOH-terminal Src kinase (Csk) to inactivate the SFK. First, we used the antibody against Src phosphoY418 to examine the phosphorylation of Fyn or Lyn by oligodendrocyte precursors grown on PDL, Lm2, or FN (Fig. 8 A). We found that the catalytic tyrosine was phosphorylated on Fyn, independent of substrate. In contrast, on Lyn, phosphorylation of Y397 was only seen in cells grown on the αVβ3 ligand FN, suggesting that this SFK was activated by αVβ3 and not by α6β1.
Figure 8.
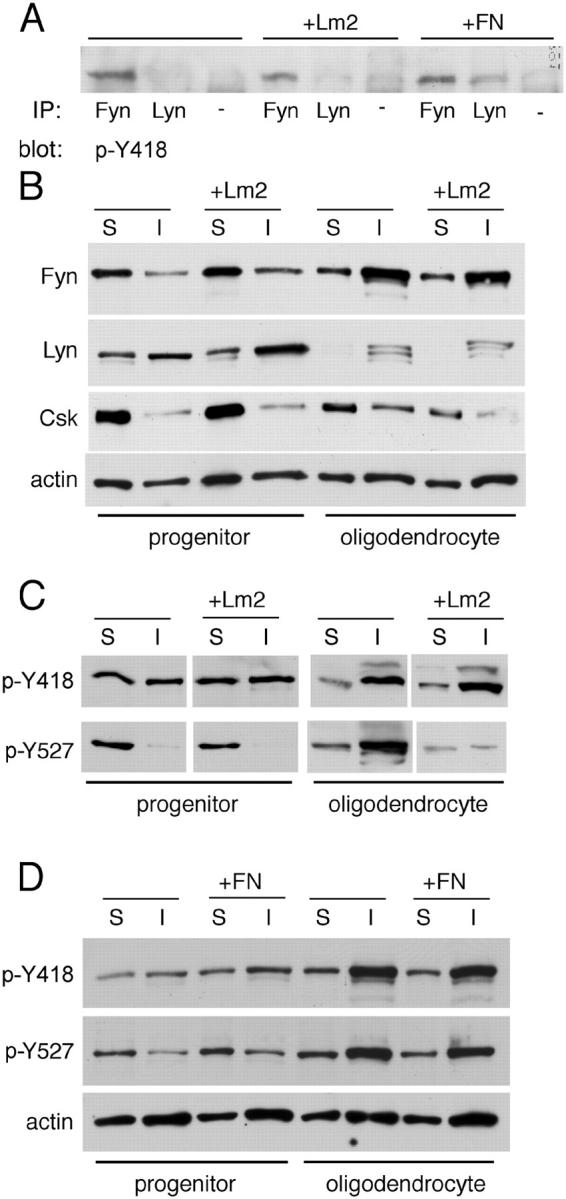
SFK activity is regulated by laminin in differentiating oligodendrocytes, not progenitors. (A) Active Lyn is detected in response to the αVβ3 ligand FN. Immunoprecipitation complexes using antibodies against Fyn, Lyn, or control mouse IgG (−) were evaluated by Western blot for the presence of autophosphorylated SFK phosphoY418. (B) Expression and solubility of Fyn, Lyn, and COOH-terminal Src kinase (Csk) change during oligodendrocyte lineage progression and in response to Lm2. Western blots were performed using antibodies specific for SFKs and SFK regulatory kinase Csk. S, Triton X-100–soluble protein; I, Triton X-100–insoluble protein. Blots were also probed with actin antibodies as protein loading controls. (C) Phosphorylation of the SFK negative regulatory site is reduced by laminin in oligodendrocytes, not progenitors. The same lysates as in B were used, but Western blots were analyzed using antibodies against two SFK sites: phosphoY418 (catalytic) and phosphoY527 (COOH-terminal negative regulatory). (D) FN does not alter phosphorylation of the SFK negative regulatory site. Lysates of progenitors and oligodendrocytes grown on control substrate PDL or on the αVβ3 ligand FN. Western blots were performed using SFK antibodies specific for phosphoY418 (catalytic) and phosphoY527 (COOH-terminal negative regulatory). Blots were also probed with actin antibodies as protein loading controls. (A–D) In the absence of ECM ligands, cells were grown on non-integrin substrate PDL.
Next, we examined SFK activity in newly differentiated oligodendrocytes. Fyn and Lyn are enriched in detergent-insoluble lipid raft domains in oligodendrocytes, so we examined both Triton X-100–insoluble and Triton X-100–soluble fractions in both differentiated cells and progenitors. We confirmed that Fyn was highly enriched in the insoluble pool at the oligodendrocyte stage and additionally found that treatment with Lm2 had no effect on the ratios of Fyn and Lyn found in the insoluble pools (Fig. 8 B). We observed that in freshly isolated progenitors grown in PDGF, substantially less Fyn was found in the insoluble pool compared with Lyn (Fig. 8 B), indicating that in proliferating cells, less Fyn may be raft-associated than in more differentiated cells. A high level of phosphorylation detected with the antibody against Src phosphoY418 was observed within this insoluble fraction in both progenitors and oligodendrocytes, and Lm2 had no effect on this phosphorylation (Fig. 8 C). However, Lm2 substrates did reduce the phosphorylation detected by antibodies against phosphorylated negative regulatory Src Y527 in the insoluble fraction of oligodendrocytes.
We found that oligodendrocytes express Csk, the kinase that phosphorylates the COOH-terminal SFK negative regulatory site, and that levels of Csk within the SFK-containing insoluble fractions were decreased in cells grown on Lm2, but only in oligodendrocytes, not in progenitors (Fig. 8 B). Therefore, we conclude that the α6β1 ligand Lm2 alters Fyn activity by regulating the dephosphorylation of the inhibitory COOH-terminal Y531, at least in part by down-regulation of Csk, with the catalytic tyrosine (Y420) being unaffected by Lm2 engagement. We also evaluated SFK phosphorylation in cells grown on FN (Fig. 8 D). In contrast with our observations in cells grown on Lm2, we detected no change in phosphoY527 immunoreactivity.
Two findings make it unlikely that a significant level of the immunoreactivity detected by the antibody against phosphoY527 can be attributed to phosphorylation of the homologous inhibitory Y508 in Lyn. First, comparison of the soluble/insoluble fractions in the precursors shows Lyn to be enriched in the insoluble fraction, whereas phosphoY527 immunoreactivity was detected entirely in the soluble fraction, where Fyn and Csk are highly expressed. Second, we did not detect immunoreactivity using the antibody against Src phosphoY527 after Lyn-specific pulldowns (unpublished data). We conclude that, in contrast with Fyn, Lyn is activated by αVβ3 ligands, resulting in the autophosphorylation of the catalytic Y397, whereas the inhibitory Y508 remains unphosphorylated and does not contribute to the regulation of Lyn.
Discussion
Myelinating oligodendrocytes provide a model system for understanding how ECM and growth factors regulate distinct aspects of cell behavior during development. We have shown previously that changing interactions between integrins and growth factor receptors within the membrane provide a mechanism for temporal and spatial specificity of growth factor signaling. Here, we identify the SFKs Fyn and Lyn as key effector molecules within these integrin–growth factor receptor complexes that selectively promote either proliferation or differentiation/survival. As such, they serve both to integrate the extracellular signals provided by ECM and growth factors and to translate these signals into specific cell behaviors during development. The illustration in Fig. 9 summarizes how these different integrins regulate distinct phases of the oligodendrocyte lineage by using distinct SFK partners and thereby ensuring correct timing and location for oligodendrocyte proliferation, survival, and, ultimately, myelin formation. In this model, the key association necessary for the specificity of each SFK is with the integrin, rather than with the growth factor receptor. In support of this, a role for SFKs in integrin signaling is well established, whereas a link between SFK signaling and PDGFαR signaling remains less clear. Triple gene knockouts revealed that Src, Yes, and Fyn were dispensable for PDGF-mediated signaling in fibroblasts, but necessary for several ECM-mediated functions (Klinghoffer et al., 1999). And, although PDGF deficiency leads to hypomyelination during development, this defect occurs in regions of the central nervous system that are unaffected in Fyn −/− mice (Fruttiger et al., 1999). Together, these findings are consistent with a model in which growth factor stimulation in the absence of integrin ligation is not sufficient to activate a requirement for SFK.
Figure 9.
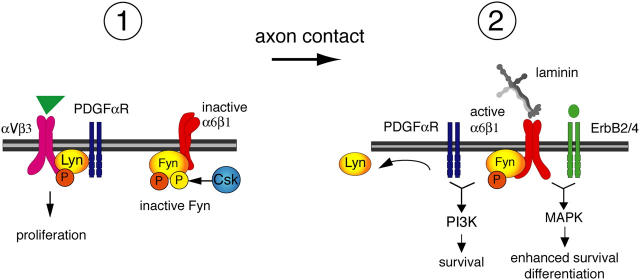
Model for integrin regulation of SFK activity during oligodendrocyte lineage progression. In oligodendrocyte progenitors, Lyn is associated with the PDGFαR–αVβ3 integrin complex and contributes to proliferation signaling. Catalytic Y397 of Lyn (orange) is phosphorylated after αVβ3 integrin ligation. Fyn is maintained in the inactive state by Csk phosphorylation of Fyn-inhibitory COOH-terminal Y531 (yellow). After axonal contact and ligation by α6β1 of the α2 chain laminins expressed in myelinating axon tracts, Lyn dissociates from the integrin–growth factor complex and Csk is downregulated, reducing Fyn phosphorylation at Y531 and promoting Fyn activity. Active Fyn–α6β1 complexes can then trigger PI3K and MAPK signaling, depending on the ligand binding of PDGFαR and ErbB2/4 receptors, respectively, thereby promoting oligodendrocyte survival, differentiation, and myelin formation.
An important component of the proposed model is the selectivity for particular cell functions of two or more SFKs expressed in a given cell type. Previous work in T cells has shown how two SFKs expressed in these cells, Lck and Fyn, both contribute to development. Lck −/− mice show a failure of early thymocyte differentiation, whereas Fyn −/− mice are normal, with Fyn being able to compensate partially for the loss of Lck. In mature T cells, both Lck and Fyn are associated with the T cell receptor, and phosphorylation induced by T cell receptor signaling is mediated by either SFK, depending on the targets. However, a high (but not complete) degree of compensation in the T cell lineage is observed in SFK knockout mice. In oligodendrocytes, each SFK appears to have a more unique and distinct role than in mature T cells and is associated with different membrane signaling complexes. However, in knockout mice compensatory mechanisms may develop, as is seen in Fyn null mice where Src is up-regulated; therefore, we feel that shorter-term knockdowns using siRNA are valuable for examining the requirement of Fyn in the absence of compensation (Sperber et al., 2001).
We found that, in addition to having distinct integrin associations, Fyn and Lyn are activated by different molecular mechanisms. We observed that oligodendrocytes express Csk, a kinase that negatively regulates the function of SFKs (Schmedt et al., 1998). Csk expression was highest in progenitors, and its down-regulation in oligodendrocytes grown on Lm2 correlated with reduced phosphorylation of the negative regulatory tyrosine in Fyn. Laminin may influence the activity of Csk directly, such as by reducing Csk levels or Csk accessibility, or indirectly by accelerating the differentiation process during which Csk activity is modulated. Preliminary data suggest that oligodendrocytes express Csk binding protein, a transmembrane molecule that has been shown in T cells to target Csk to the plasma membrane and direct a negative feedback loop for SFK signaling (Kawabuchi et al., 2000). Developmentally regulated changes in Csk binding protein levels could, therefore, alter Csk activity. Another possible mechanism for an integrin-dependent change in SFK activity would be regulation of the availability or activity of phosphatases such as receptor protein tyrosine phosphatase, which has been shown to regulate SFKs in an integrin-dependent manner in fibroblasts (von Wichert et al., 2003).
The loss of phosphorylation of Fyn tyrosine 531 triggered by laminin was associated with a switch from PI3K to MAPK NRG survival signaling. This switch in signaling has significant consequences for the development of the oligodendrocyte lineage, in which NRG signaling alone has been shown to stimulate PI3K signaling and keep oligodendrocytes in a differentiation-arrested state (Canoll et al., 1999). We have shown previously that integrin-mediated contact with axons is an important regulatory mechanism for triggering oligodendrocytes to survive and to complete the differentiation program (Colognato et al., 2002). Csk may play an instructive role in this switch, because Csk null fibroblasts grown on laminin-10 show increased MAPK signaling, whereas PI3K signaling is decreased under these circumstances (Gu et al., 2003). Preliminary data using Csk siRNA indicate that when Csk is decreased, Fyn Y531 phosphorylation is reduced and newly formed oligodendrocytes survive better than they do in control cells. Therefore, a prediction from the current study is that reducing Csk expression or activity may also help trigger myelination in the stalled preoligodendrocytes that have been observed in demyelinated multiple sclerosis lesions (Chang et al., 2002).
In contrast, we did not observe phosphorylation of the negative regulatory tyrosine in Lyn, indicating that Csk may not regulate Lyn activity in oligodendrocytes. Instead, we observed that phosphorylation of a catalytic tyrosine was increased when cells were grown on αVβ3 substrates. The ability of Csk to regulate Fyn but not Lyn may result from the distribution of Csk and from the SFKs within lipid raft membrane microdomains. Both Fyn and Lyn are associated with lipid rafts in oligodendrocytes (Kramer et al., 1999). Lipid microdomains are more ordered regions of the plasma membrane that are highly enriched in cholesterol and glycosphingolipids and, thus, are insoluble in many detergents. These microdomains are thought to act as signaling platforms that can sequester or segregate signaling molecules, including integrin receptors. Indeed, laminin causes a redistribution of integrin α6β1 to rafts in newly formed oligodendrocytes, in which PDGF-mediated survival signaling becomes dependent on the integrity of lipid raft domains (Baron et al., 2003). We confirmed that Fyn and Lyn are detergent insoluble in differentiated oligodendrocytes, but found that Fyn was predominantly detergent soluble in oligodendrocyte progenitors stimulated with PDGF. This indicates that Fyn may be excluded from rafts in proliferating cells. A differential lipid raft association for Fyn and Lyn would provide a mechanism for ensuring that Csk, which is also found in the soluble, nonraft pool, is available to inactivate Fyn, but not Lyn, in proliferating progenitors.
Previous studies have shown that mice lacking Fyn or Fyn kinase activity are hypomyelinated in the brain (Umemori et al., 1994; Sperber et al., 2001). This is believed to be an oligodendrocyte-intrinsic defect because oligodendrocytes with altered or absent Fyn activity differentiate defectively in culture (Osterhout et al., 1999; Umemori et al., 1999; Sperber and McMorris, 2001; Liang et al., 2004). Expression of dominant-negative Fyn resulted in morphological defects such as reduced process outgrowth, whereas Fyn null cells had a reduced propensity to differentiate but exhibited normal morphology. Fyn has been shown to activate p190RhoGAP, and expression of dominant-negative or constitutively active forms of the RhoGTPases Rho, Rac1, and cdc42 perturbs oligodendrocyte process formation, suggesting that these GTPases are one set of downstream targets for Fyn in the oligodendrocyte lineage (Wolf et al., 2001; Liang et al., 2004). Mice deficient in the laminin α2 subunit also have a hypomyelination defect, similar in regional specificity to that in Fyn null mice (Chun et al., 2003). Laminin- and Fyn-deficient mice are both hypomyelinated in the forebrain and optic nerve, but have normal-appearing myelin in the spinal cord. Furthermore, the expression of laminin associated with axon tracts correlates with and peaks with myelination, as does Fyn activity (Umemori et al., 1994; Powell et al., 1998; Farwell and Dubord-Tomasetti, 1999; Osterhout et al., 1999; Colognato et al., 2002). These similarities in phenotype and expression pattern can be explained by our current findings showing that the laminin receptor α6β1 integrin is constitutively associated with Fyn, and that Fyn is required for laminin to amplify differentiation and survival in response to PDGF and NRG.
Several molecules have been proposed to regulate Fyn in oligodendrocytes. Antibody-mediated clustering of the cell surface molecule myelin-associated glycoprotein (MAG) was found to increase Fyn kinase activity (Umemori et al., 1994). However, MAG null mice do not have gross myelination defects but instead are characterized by subtle defects in myelin structure and periaxonal organization (Montag et al., 1994; Li et al., 1998). MAG/Fyn double knockout mice are more severely hypomyelinated than Fyn knockout mice, suggesting that the relationship between MAG and Fyn is complex and that the two molecules may operate in different signaling pathways (Biffiger et al., 2000). The GPI-anchored IgG superfamily molecule F3/contactin has also been proposed to regulate Fyn, because antibody-mediated clustering of F3/contactin in an oligodendrocyte cell line, Oli-neu, increased Fyn kinase activity (Kramer et al., 1999). However, like MAG null mice, F3 null mice do not have a hypomyelination phenotype similar to that of the Fyn null mice (Berglund et al., 1999). Therefore, Fyn may be able to activate multiple signaling pathways within the oligodendrocyte. An important question for further studies is to identify the mechanism by which adaptor and other molecules regulate the specific patterns of SFK association with the different receptors.
Materials and methods
siRNA
siRNAs were expressed using a modified version of the pSUPER plasmid, provided by Jonathan Pines and Claire Aquaviva (University of Cambridge, Cambridge, UK), in which YFP expression was driven from a CMV promoter (Brummelkamp et al., 2002). A neomycin resistance cassette (CLONTECH Laboratories, Inc.) was inserted to allow G418 selection of transfected cells. Several different 19-nucleotide target sequences within rat Fyn, Lyn, and Src cDNA were chosen according to the guidelines of AA(N19)TT, or AA(N19)XX as a second choice (Tuschl, 2002). The following target sequences were used: Fyn, 5′-GCAGGACAGAAGATGACCT-3′; Lyn, 5′-GCCTGGACAATGGTGGTTA-3′; and Src, 5′-TTCAACAGCCTGCAGCAGC-3′. Oligonucleotide pairs 5′-GATCCCC(N19)TTCAAGAGA(N19-rev-comp)TTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAA(N19)-TCTCTTGAA(N19-rev-comp)GGG-3′ were annealed and phosphorylated, and then they were ligated into pSUPER/YFP digested with BglII and HindIII. Plasmids containing the correct insert were identified using an EcoRI and HindIII restriction digest, and were sequenced. After selection of transfected cells, Western blot analysis on protein levels was used to evaluate constructs, and the most effective construct was subsequently used to deplete each mRNA. Controls used included empty vector, nonfunctional targeting vectors, and scrambled targets.
Survival assay
8-well chamber slides (Nunc) were coated for 4 h at 37°C with PDL (Sigma-Aldrich), FN, or Lm2. Each well received 20,000 progenitors suspended in SATO's medium. 1 h after attachment, the indicated growth factors were added and the cells were differentiated for 4 d. For PDGF assays, 0.1, 1.0, or 10 ng/ml PDGF was included, and for NRG assays, 1, 10, or 100 ng/ml NRG was included. Immunostaining using mouse anti-GFP and rabbit anti-GalC antibodies was used to identify transfected, newly formed oligodendrocytes. TUNEL using indirect immunofluorescence was used to visualize nicked DNA according to the manufacturer's instructions (Apoptag). In each well, a minimum of 100 GFP/GalC double-positive cells were scored for TUNEL. Cell survival was determined by the percentage of TUNEL-negative cells in the GFP/GalC double-positive population. To compare different experiments, the percent change in cell survival above or below the internal control (survival on PDL with no treatment or growth factors) was calculated. Experiments were performed a minimum of three times and the mean percent changes and SDs were calculated. Statistical significance was determined using the paired t test.
Migration assay
Transfected progenitors were suspended in 10 μl SATO defined medium plus PDGF and FGF. The volume of the cell suspension was measured and one third of the volume of 1% low melting temperature agarose (prepared in sterile PBS and equilibrated to 37°C) was added. 1.5-μl drops of cell–agarose suspension were added to the center of PDL-coated 8-well chamber slides. The drops were incubated at 4°C for 15 min to solidify agarose, and then flooded with 0.2 ml SATO medium with 0, 1, or 10 ng/ml PDGF and 10 ng/ml FN or Lm2. At day 2, cells were fixed with methanol and immunostained with GFP antibodies to detect transfected cells. The migration distance from the agarose drop boundary was measured on captured images using OpenLab image software. For each experiment, all transfected cells within four drops were measured and averaged for each growth factor/ECM condition. Each experiment was performed at least three times and a representative experiment is shown.
Proliferation assay
8-well Permanox chamber slides were coated for 4 h at 37°C with PDL, FN, or Lm2. Slides were blocked in PBS containing 0.5% heat-inactivated BSA for 30 min and washed with PBS. Progenitors (20,000 per well) in DME were allowed to attach for 1 h, and then an equal volume of DME containing BrdU and PDGF was added. Final concentrations were 0, 0.1, 1, and 10 ng/ml for PDGF and 10 μM for BrdU. At 24 h, cells were washed and fixed in methanol, immunofluorescence was performed with antibodies to GFP to visualize YFP+-transfected cells and to BrdU to visualize cells that have entered S phase, and cells were stained with Hoechst to visualize nuclei. BrdU incorporation was defined as the percentage of healthy GFP+ cells that were positive for BrdU. Each experiment was repeated at least three times, with individual conditions performed in duplicate.
Cell culture
Disassociated rat neonatal cortices were cultured (at 37°C in 7.5% CO2) in DME with 10% FCS on PDL-coated flasks. By day 10, mixed glial cultures were obtained, consisting of oligodendrocyte precursor cells and microglia growing on an astrocyte monolayer. Purified oligodendrocyte precursor cells were isolated using a modification of the mechanical dissociation and differential adhesion method described by McCarthy and de Vellis (1980).
Transfection
Overnight incubation with lipid carrier FUGENE (Roche) was used to introduce 5 μg plasmid DNA per 75-cm2 flask of mixed glial cultures. Next, oligodendrocyte progenitors were purified by mechanical dissociation, and then they were selected in 400 μg/ml G418 with 10 ng/ml PDGF and FGF to prevent differentiation and maintain proliferation. In functional assays (survival, proliferation, migration, and differentiation), progenitors were not selected and were plated directly in 8-well chamber slides for analysis with no previous growth factor treatment. Transfected cells (typically ∼10%) were visualized by YFP fluorescence. Cells prepared for biochemistry were transfected using Nucleofector electroporation system according to the manufacturer's instructions with efficiencies of ∼50% (Amaxa).
Protein analysis
Cells were washed with ice-cold PBS and lysed in 1% Triton X-100, 10 mM Tris, pH 7.4, 5 mM EDTA, and 150 mM NaCl on ice. Cells were scraped and transferred to microfuge tubes and placed on ice for 15 min. Lysates were centrifuged at 14,000 rpm to separate detergent-insoluble and -soluble material. The Triton-insoluble pellet was solubilized in 10 mM Tris and 1% SDS by trituration through a 21-gauge needle. Before immunoprecipitations, lysates were prepared as above but incubated at 37°C to solubilize lipid raft-associated proteins. Protein concentration was determined by protein assay (Bio-Rad Laboratories) and lysates were boiled for 5 min in Laemmli solubilizing buffer and 3% β-mercaptoethanol. Proteins were separated by SDS-PAGE using 7.5% or 10% acrylamide minigels (Bio-Rad Laboratories) and blotted onto 0.45 μm nitrocellulose. Membranes were blocked for 1 h in 0.1% Tween 20 and TBS (TBS-T) containing either 5% milk or 5% BSA, and then in primary antibodies in blocking buffer overnight at 4°C. Membranes were washed in TBS-T, incubated for 1 h in HRP-conjugated secondary antibodies (Amersham Biosciences), washed again in TBS-T, and developed using chemiluminescence. For immunoprecipitation of protein complexes, lysates were incubated with antibodies and protein A/G beads (Santa Cruz Biotechnology, Inc.) at 4°C overnight on a rotating wheel. Bead immune complexes were washed four times and prepared for electrophoresis and Western blotting.
Immunocytochemistry and image acquisition
To detect YFP in fixed cells, we incubated the cells for 10 min in PBS containing 3% PFA and 2% sucrose, and then performed immunocytochemistry with GFP antibodies (Molecular Probes) in PBS containing 0.4% BSA and 0.1% Triton X-100. For double immunofluorescence, additional primary antibodies were included to detect MBP, GalC, CNPase, or Fyn, followed by FITC- and TRITC-labeled donkey secondary antibodies. To visualize Fyn and the α6 integrin subunit, we fixed cells with methanol for 5 min at −20°C. Slides were mounted in Immunofluor (ICN Biomedicals) and evaluated at room temperature using an Axioplan fluorescence microscope (Carl Zeiss MicroImaging, Inc.) fitted with 10× eyepiece magnification using 20× (0.5 NA) and 40× (0.75 NA) objectives. Images were acquired using a digital camera (model C4742-95; Hamamatsu) and imaging software (OpenLab) and were exported as TIFF files to Adobe Photoshop.
Analysis of myelin membrane morphology
Oligodendrocytes transfected with siRNA constructs were differentiated for 2 or 4 d in Sato's medium with 0.5% FCS (differentiation medium). YFP-positive cells were evaluated for the expression of MBP using immunocytochemistry and graded according to morphological characteristics and degree of myelin membrane formation (Results).
Reagents
Reagents were obtained from Sigma-Aldrich unless otherwise indicated.
Antibodies.
The following rabbit polyclonal antibodies were used: GalC, Fyn, PDGFαR, and ErbB4 NRG receptor subunit (Santa Cruz Biotechnology, Inc.); SFK phosphoY527 and phosphoY418 (Biosource International); GFP (Molecular Probes); total ERK (New England Biolabs, Inc.); and β3 integrin subunit (CHEMICON International). The following mouse monoclonals were used: GFP; Fyn, and Csk (Transduction); pp60src (Oncogene Research Products); a rat mAb against MBP (Serotec); a hamster IgM against β1 integrin (BD Biosciences); and FITC or TRITC donkey secondary antibodies (Jackson ImmunoResearch Laboratories). For triple immunofluorescence, aminomethylcoumarin-streptavidin (Vector Laboratories) was used to detect biotin-conjugated secondary antibodies.
Proteins.
Human recombinant PDGF-A and FGF-2 (PeproTech) were used at 10 ng/ml, except where otherwise indicated. The active component of NRG-1 was a recombinant protein comprising the EGF-like domain (Neomarkers). PDL, FN, and human placental laminin, a mixture of laminins that is primarily Lm2, were used at 10 μg/ml.
Other chemicals.
Inhibitors (Calbiochem) were suspended in DMSO and used at 50 nM (wortmannin) and at 25 μM (PD098059). In control wells, the equivalent volume of DMSO was added. Manganese was used at 50 μM.
Acknowledgments
We thank Claire Aquaviva and Jonathan Pines for their gift of modified pSUPER, and members of the ffrench-Constant lab for many helpful discussions.
This work was funded by a National Multiple Sclerosis Society Career Transition Fellowship and a National Institutes of Health Ruth L. Kirschstein National Research Service Award postdoctoral fellowship (NS11035; both to H. Colognato) and a Wellcome Trust Research Leave Fellowship (to C. ffrench-Constant).
H. Colognato's present address is Dept. of Pharmacology, State University of New York at Stony Brook, Stony Brook, NY 11794.
Abbreviations used in this paper: Csk, COOH-terminal Src kinase; ERK, extracellular signal–related kinase; FN, fibronectin; GalC, galactocerebroside; Lm2, laminin-2; MAG, myelin-associated glycoprotein; MBP, myelin basic protein; NRG, neuregulin; PDGFαR, PDGFα receptor; PDL, poly-d-lysine; SFK, Src family kinase; siRNA, small interfering RNA.
References
- Arias-Salgado, E.G., S. Lizano, S. Sarkar, J.S. Brugge, M.H. Ginsberg, and S.J. Shattil. 2003. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA. 100:13298–13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, W., S.J. Shattil, and C. ffrench-Constant. 2002. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 21:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, W., L. Decker, H. Colognato, and C. ffrench-Constant. 2003. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13:151–155. [DOI] [PubMed] [Google Scholar]
- Barres, B.A., R. Schmid, M. Sendnter, and M.C. Raff. 1993. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 118:283–295. [DOI] [PubMed] [Google Scholar]
- Berglund, E.O., K.K. Murai, B. Fredette, G. Sekerkova, B. Marturano, L. Weber, E. Mugnaini, and B. Ranscht. 1999. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 24:739–750. [DOI] [PubMed] [Google Scholar]
- Biffiger, K., S. Bartsch, D. Montag, A. Aguzzi, M. Schachner, and U. Bartsch. 2000. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and fyn tyrosine kinase. J. Neurosci. 20:7430–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp, T.R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science. 296:550–553. [DOI] [PubMed] [Google Scholar]
- Calver, A.R., A.C. Hall, W.P. Yu, F.S. Walsh, J.K. Heath, C. Betsholtz, and W.D. Richardson. 1998. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 20:869–882. [DOI] [PubMed] [Google Scholar]
- Canoll, P.D., J.M. Musacchio, R. Hardy, R. Reynolds, M.A. Marchionni, and J.L. Salzer. 1996. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 17:229–243. [DOI] [PubMed] [Google Scholar]
- Canoll, P.D., R. Kraemer, K.K. Teng, M.A. Marchionni, and J.L. Salzer. 1999. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol. Cell. Neurosci. 13:79–94. [DOI] [PubMed] [Google Scholar]
- Chang, A., W.W. Tourtellotte, R. Rudick, and B.D. Trapp. 2002. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 346:165–173. [DOI] [PubMed] [Google Scholar]
- Chun, S.J., M.N. Rasband, R.L. Sidman, A.A. Habib, and T. Vartanian. 2003. Integrin-linked kinase is required for laminin-2–induced oligodendrocyte cell spreading and CNS myelination. J. Cell Biol. 163:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., W. Baron, V. Avellana-Adalid, J.B. Relvas, A. Baron-Van Evercooren, E. Georges-Labouesse, and C. ffrench-Constant. 2002. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4:833–841. [DOI] [PubMed] [Google Scholar]
- Decker, L., and C. ffrench-Constant. 2004. Lipid rafts and integrin activation regulate oligodendrocyte survival. J. Neurosci. 24:3816–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell, A.P., and S.A. Dubord-Tomasetti. 1999. Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology. 140:4221–4227. [DOI] [PubMed] [Google Scholar]
- Fernandez, P.A., D.G. Tang, L. Cheng, A. Prochiantz, A.W. Mudge, and M.C. Raff. 2000. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 28:81–90. [DOI] [PubMed] [Google Scholar]
- Frost, E.E., P.C. Buttery, R. Milner, and C. ffrench-Constant. 1999. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr. Biol. 9:1251–1254. [DOI] [PubMed] [Google Scholar]
- Fruttiger, M., L. Karlsson, A.C. Hall, A. Abramsson, A.R. Calver, H. Bostrom, K. Willetts, C.H. Bertold, J.K. Heath, C. Betsholtz, and W.D. Richardson. 1999. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 126:457–467. [DOI] [PubMed] [Google Scholar]
- Gu, J., S. Nada, M. Okada, and K. Sekiguchi. 2003. Csk regulates integrin-mediated signals: involvement of differential activation of ERK and Akt. Biochem. Biophys. Res. Commun. 303:973–977. [DOI] [PubMed] [Google Scholar]
- Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 404:999–1003. [DOI] [PubMed] [Google Scholar]
- Klinghoffer, R.A., C. Sachsenmaier, J.A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, E.M., C. Klein, T. Koch, M. Boytinck, and J. Trotter. 1999. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 274:29042–29049. [DOI] [PubMed] [Google Scholar]
- Li, C., B. Trapp, S. Ludwin, A. Peterson, and J. Roder. 1998. Myelin associated glycoprotein modulates glia-axon contact in vivo. J. Neurosci. Res. 51:210–217. [DOI] [PubMed] [Google Scholar]
- Liang, X., N.A. Draghi, and M.D. Resh. 2004. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J. Neurosci. 24:7140–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, K.D., and J. de Vellis. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, L.A., J.J. Hong, M.S. Kinch, M.L. Harrison, and R.L. Geahlen. 1999. The engagement of beta1 integrins on promonocytic cells promotes phosphorylation of Syk and formation of a protein complex containing Lyn and beta1 integrin. Eur. J. Immunol. 29:1426–1434. [DOI] [PubMed] [Google Scholar]
- Montag, D., K.P. Giese, U. Bartsch, R. Martini, Y. Lang, H. Bluthmann, J. Karthigasan, D.A. Kirschner, E.S. Wintergerst, K.A. Nave, et al. 1994. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 13:229–246. [DOI] [PubMed] [Google Scholar]
- Osterhout, D.J., A. Wolven, R.M. Wolf, M.D. Resh, and M.V. Chao. 1999. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J. Cell Biol. 145:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, S.K., C.C. Williams, M. Nomizu, Y. Yamada, and H.K. Kleinman. 1998. Laminin-like proteins are differentially regulated during cerebellar development and stimulate granule cell neurite outgrowth in vitro. J. Neurosci. Res. 54:233–247. [DOI] [PubMed] [Google Scholar]
- Schmedt, C., K. Saijo, T. Niidome, R. Kuhn, S. Aizawa, and A. Tarakhovsky. 1998. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 394:901–904. [DOI] [PubMed] [Google Scholar]
- Sperber, B.R., and F.A. McMorris. 2001. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J. Neurosci. Res. 63:303–312. [DOI] [PubMed] [Google Scholar]
- Sperber, B.R., E.A. Boyle-Walsh, M.J. Engleka, P. Gadue, A.C. Peterson, P.L. Stein, S.S. Scherer, and F.A. McMorris. 2001. A unique role for Fyn in CNS myelination. J. Neurosci. 21:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis, M.A., J.D. Humphries, and M.J. Humphries. 2003. An unraveling tale of how integrins are activated from within. Trends Pharmacol. Sci. 24:192–197. [DOI] [PubMed] [Google Scholar]
- Tuschl, T. 2002. Expanding small RNA interference. Nat. Biotechnol. 20:446–448. [DOI] [PubMed] [Google Scholar]
- Umemori, H., A. Wanaka, H. Kato, M. Takeuchi, M. Tohyama, and T. Yamamoto. 1992. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res. Mol. Brain Res. 16:303–310. [DOI] [PubMed] [Google Scholar]
- Umemori, H., S. Sato, T. Yagi, S. Aizawa, and T. Yamamoto. 1994. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 367:572–576. [DOI] [PubMed] [Google Scholar]
- Umemori, H., Y. Kadowaki, K. Hirosawa, Y. Yoshida, K. Hironaka, H. Okano, and T. Yamamoto. 1999. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J. Neurosci. 19:1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert, G., G. Jiang, A. Kostic, K. De Vos, J. Sap, and M.P. Sheetz. 2003. RPTP-α acts as a transducer of mechanical force on αv/β3-integrin–cytoskeleton linkages. J. Cell Biol. 161:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, R.M., J.J. Wilkes, M.V. Chao, and M.D. Resh. 2001. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J. Neurobiol. 49:62–78. [DOI] [PubMed] [Google Scholar]