Abstract
The molecular mechanisms by which differentiated cells combat cell death and injury have remained unclear. In the current issue, it has been shown in neurons that cell differentiation is accompanied by a decrease in Apaf-1 and the activity of the apoptosome with an increased ability of the inhibitor of apoptosis proteins (IAPs) to sustain survival (Wright et al., 2004). These results, together with earlier ones, deepen our understanding of how cell death and the apoptosome are regulated during differentiation and in tumor cells.
Apoptosis and its dysfunction play a crucial role in different human diseases, such as cancer and neurological degenerative disorders. Cell suicide is regulated by both extra- and intracellular factors that activate conserved cellular processes. The intrinsic pathway involving mitochondria is especially interesting with the participation of molecules both upstream and downstream of the organelle. The complexity of this pathway allows for the strict regulation of cell death and provides the possibility for intervention both at the pre- and post-mitochondrial level.
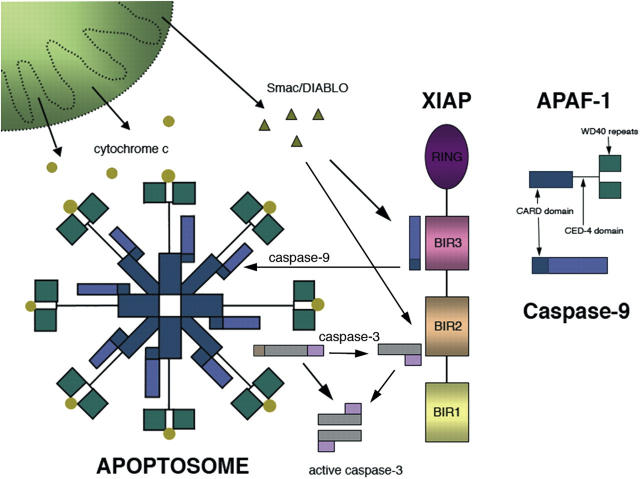
Proteins of the Bcl-2 family are major determinants of cell viability, controlling the mitochondrial membrane pore formation induced by different death signals converging on the organelle (Danial and Korsmeyer, 2004). Following an increase in the permeability of the outer membrane, cytochrome c and other pro-apoptotic proteins are released from the intermembrane space triggering cell demise (Lindholm et al., 2004). In the cytosol, a complex known as the apoptosome is formed from apoptotic protease activating factor-1 (Apaf-1), procaspase-9, and cytochrome c/dATP. Apaf-1 resembles the C. elegans protein CED-4 that is required for apoptosis in the worm (Li et al., 1997; Zhou et al., 1997). Apaf-1 has a caspase recruitment domain (CARD) that allows it to bind to the CARD in caspase 9, a central P loop motif that binds ATP, and at the COOH terminus are WD40 repeats that bind to cytochrome c (Fig. 1). Oligomerization of Apaf-1 leads to autoactivation of procaspase-9 that in turn cleaves caspase-3, ultimately causing cell death (Acehan et al., 2002; Hill et al., 2004). The structure of the apoptosome thus constitutes a cellular “death wheel” (Fig. 1).
Figure 1.
The apoptosome death wheel. Apaf-1 together with procaspase-9 form the wheel-shaped apoptosome complex. Cytochrome c binds to Apaf-1 and drives cell death through the activation of caspase 3. The IAPs, such as XIAP, can counteract caspase activation, but this brake on cell death is diminished by Smac/Diablo released from the mitochondria. The steady-state levels of the various interacting proteins may vary between cell types and with cell differentiation. As well as the apoptosome, other cellular pathways can be activated and probably interact during cell demise.
Wright et al. (2004) show that the activity of the apoptosome changes with neuronal differentiation with alterations in both Apaf-1 and the efficacy of the IAPs. IAPs contain one to three well-conserved domains, the so called baculovirus IAP repeats (BIR). Via its BIRs, X chromosome–linked IAP (XIAP) can bind processed caspase-9 and -3, and can in turn be inhibited by proteins, such as Smac/Diablo released from the mitochondria during cell death (Du et al., 2000; Fig. 1). Overexpression of XIAP can protect cells against different death stimuli (Perrelet et al., 2002; Trapp et al., 2003), but the role of endogenous IAPs is unclear as neither gene deletion of XIAP or Smac/Diablo showed any abnormality in apoptosis (Harlin et al., 2001; Okada et al., 2002).
The biology of the apoptosome has been studied mainly in cell-free systems, and less in living cells. Wright et al. (2004) now demonstrate that during differentiation of PC12 cells or primary sympathetic neurons, the level of Apaf-1 is reduced and the protective activities of IAPs are increased. This explains earlier observations that cytoplasmic cytochrome c was not able to trigger death of sympathetic neurons, although it was sufficient to kill nonneuronal cells (Deshmukh et al., 2002; Potts et al., 2003). The levels of Apaf-1 are high in nondifferentiated PC-12 cells and sympathetic neurons, but drop dramatically during neuronal differentiation, and this reduction renders the mature neurons resistant to cytochrome c–triggered apoptosis. The authors also show that reduction in the level of Apaf-1 is accompanied by increased protection by the IAPs. Although there was no change in the amounts of IAPs or caspase-9, Apaf-1 levels and apoptosome activity were reduced, allowing for more effective regulation by the IAPs. Consistent with this outcome, Vyas et al. (2004) recently showed that differentiated PC12 cells have increased resistance to cell death that is IAP dependent.
Does this only apply to neuronal cells? Wright et al. (2004) show that cytochrome c induces rapid caspase activation in the lysates of differentiated primary fibroblasts, as is seen in undifferentiated nerve cells. Thus, in dividing nonneuronal cells, the high Apaf-1 levels keep the activity of the IAPs low. Also, regulation of cell death by the IAPs seems blunted in many mitotic compared with postmitotic cells. In Apaf-1 heterozygous fibroblasts, however, the lowered levels of Apaf-1 enhanced the activity of IAPs, as in the differentiated neurons.
Although the findings refer to postmitotic normal cells, they may have implications for abnormal cells. Small molecular compounds targeting XIAP can lead to activation of caspases and induction of cell death of tumors (Schimmer et al., 2004). In many cancer cells, the brake exerted by the IAPs is strong enough to counteract cell death. In view of the present findings of a strict correlation between Apaf-1 and the action of XIAP in cell differentiation, it would be interesting to study whether Apaf-1 might be an additional target to consider in cancer therapies. In this case, Apaf-1 may be elevated by enhancing transcription of the gene or possibly by influencing the stability/post-transcriptional modifications of the protein.
Apart from Apaf-1/IAPs, other molecules may influence cell death at the level of the apoptosome. In this respect, a novel factor was recently cloned, named Apaf-1 interacting protein (AIP). It is expressed in brain tissue and is able to protect neurons after ischemia/stroke (Cao et al., 2004). AIP contains only the NH2-terminal CARD domain and constitutes a dominant-negative inhibitor of the activity of the Apaf-1–caspase-9 complex. Changes in expression of AIP by cell differentiation or in tumor cells have so far not been reported.
Although there is a partial requirement for Apaf-1 for developmental neuronal apoptosis (Cecconi et al., 1998; Yoshida et al., 1998; Cozzolino et al., 2004), loss of Apaf-1 does not prevent all types of cell death, as is evident in lymphoid cells lacking both Apaf-1 and caspase-9 (Marsden et al., 2002). In the absence of the apoptosome, apoptosis did not occur in interleukin-3–dependent myeloid cells but the number of cells that eventually died following death stimuli was unaltered (Ekert et al., 2004). Deprivation of neurons of trophic factors usually triggers the mitochondrial death pathway and activation of the apoptosome. Differentiated neurons with low levels of Apaf-1 and reduced apoptosome activity die also when challenged by different factors or in neurodegenerative diseases. Whether this still involves Apaf-1–mediated cell death or contributions from other factors is unknown. Cellular pathways, not primarily involving mitochondria and the apoptosome complex, are probably also activated in differentiated brain cells. Thus, sympathetic neurons die through a nonmitochondrial, but still caspase-dependent, pathway in the absence of glial cell line–derived neurotrophic factor (Yu et al., 2003).
In conclusion, Wright et al. (2004) reveal many important aspects of neuronal apoptosis, but also raise further questions. It would be interesting to know what makes the IAPs more efficient protectors in the differentiated neurons. Is this achieved by the mere reduction in Apaf-1 levels, or is there a closer association between these proteins in the cell involving some feedback mechanisms? The work nicely connects the regulation of Apaf-1 and the functional state of the IAPs with cell differentiation. However, there is certainly more to be learned about how cell death is regulated in normal, differentiated, and highly proliferating tumor cells. In view of this, the wheel to more insights is still spinning.
Acknowledgments
We thank Dr. Laura Korhonen for artwork.
The authors are supported by Uppsala University, Cancerfonden, Sigrid Juselius, Minerva Foundation, Finnish Academy, and Institute of Biotechnology.
Abbreviations used in this paper: AIP, Apaf-1 interacting protein; Apaf-1, apoptotic protease activating factor-1; BIR, baculovirus IAP repeat; CARD, caspase recruitment domain; IAP, inhibitor of apoptosis protein; XIAP, X chromosome–linked IAP.
References
- Acehan, D., X. Jiang, D. Gene Morgan, J.E. Heuser, X. Wang, and C.W. Akey. 2002. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell. 9:423–432. [DOI] [PubMed] [Google Scholar]
- Cao, G., M. Xiao, F. Sun, X. Xiao, W. Pei, J. Li, S.H. Graham, R.P. Simon, and J. Chen. 2004. Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J. Neurosci. 24:6189–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi, F., G. Alvarez-Bolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. [DOI] [PubMed] [Google Scholar]
- Cozzolino, M., E. Ferraro, A. Ferri, D. Rigamonti, F. Quondamatteo, H. Ding, Z.S. Xu, F. Ferrari, D.F. Angelini, G. Rotilio, et al. 2004. Apoptosome inactivation rescues proneural and neural cells from neurodegeneration. Cell Death Differ. In press. [DOI] [PubMed] [Google Scholar]
- Danial, N., and S.J. Korsmeyer. 2004. Cell death: critical control points. Cell. 116:205–219. [DOI] [PubMed] [Google Scholar]
- Deshmukh, M., C. Du, X. Wang, and E.M. Johnson Jr. 2002. Exogenous Smac induces competence and permits caspase activation in sympathetic neurons. J. Neurosci. 22:8018–8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 102:33–42. [DOI] [PubMed] [Google Scholar]
- Ekert, P.G., S.H. Read, J. Silke, V.S. Marsden, H. Kaufmann, C.J. Hawkins, R. Gerl, S. Kumar, and D.L. Vaux. 2004. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J. Cell Biol. 165:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin, H., S.B. Reffey, C.S. Duckett, T. Lindsten, and C.B. Thompson. 2001. Characterization of XIAP-deficient mice. Mol. Cell. Biol. 21:3604–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, M.M., C. Adrain, P.J. Duriez, E.M. Creagh, and S. Martin. 2004. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 23:2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. [DOI] [PubMed] [Google Scholar]
- Lindholm, D., O. Eriksson, and L. Korhonen. 2004. Mitochondrial proteins in neuronal degeneration. Biochem. Biophys. Res. Commun. 321:753–758. [DOI] [PubMed] [Google Scholar]
- Marsden, V.S., L. O'Connor, L.A. O'Reilly, J. Silke, D. Metcalf, P.G. Ekert, D.C. Huang, F. Cecconi, K. Kuida, K.J. Tomaselli, et al. 2002. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 419:634–637. [DOI] [PubMed] [Google Scholar]
- Okada, H., W.K. Suh, J. Jin, M. Woo, C. Du, A. Elia, G.S. Duncan, A. Wakeham, A. Itie, S.W. Lowe, et al. 2002. Generation and characterization of Smac/DIABLO-deficient mice. Mol. Cell. Biol. 22:3509–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrelet, D., A. Ferri, P. Liston, P. Muzzin, R.G. Korneluk, and A.C. Kato. 2002. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat. Cell Biol. 4:175–179. [DOI] [PubMed] [Google Scholar]
- Potts, P.R., S. Singh, M. Knezek, C.B. Thompson, and M. Deshmukh. 2003. Critical function of endogenousXIAP in regulating caspase activation during sympathetic neuronal apoptosis. J. Cell Biol. 163:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer, A., K. Welsh, C. Pinilla, Z. Wang, M. Krajewska, M.-J. Bonneau, I.M. Pedersen, S. Kitada, F.L. Scott, B. Bailly-Maitre, et al. 2004. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 5:25–35. [DOI] [PubMed] [Google Scholar]
- Trapp, T., L. Korhonen, M. Besselmann, R. Martinez, E.A. Mercer, and D. Lindholm. 2003. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol. Cell. Neurosci. 23:302–313. [DOI] [PubMed] [Google Scholar]
- Vyas, S., P. Juin, D. Hancock, Y. Suzuki, R. Takahashi, A. Triller, and G. Evan. 2004. Differentiation dependent sensitivity to apoptogenic factors in PC12 cells. J. Biol. Chem. 279:30983–30993. [DOI] [PubMed] [Google Scholar]
- Wright, K.M., M.W. Linhoff, P. Ryan Potts, and M. Deshmukh. 2004. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J. Cell Biol. 167:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H., Y.Y. Kong, R. Yoshida, A.J. Elia, A. Hakem, R. Hakem, J.M. Penninger, and T.W. Mak. 1998. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 94:739–750. [DOI] [PubMed] [Google Scholar]
- Yu, L.-Y., E. Jokitalo, Y.-F. Sun, P. Mehlen, D. Lindholm, M. Saarma, and U. Arumae. 2003. GDNF-deprived sympathetic neurons die via a novel nonmitochondrial pathway. J. Cell Biol. 163:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., W.J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 90:405–413. [DOI] [PubMed] [Google Scholar]