Abstract
β-Catenin plays essential roles in both cell–cell adhesion and Wnt signal transduction, but what precisely controls β-catenin targeting to cadherin adhesive complexes, or T-cell factor (TCF)-transcriptional complexes is less well understood. We show that during Wnt signaling, a form of β-catenin is generated that binds TCF but not the cadherin cytoplasmic domain. The Wnt-stimulated, TCF-selective form is monomeric and is regulated by the COOH terminus of β-catenin, which selectively competes cadherin binding through an intramolecular fold-back mechanism. Phosphorylation of the cadherin reverses the TCF binding selectivity, suggesting another potential layer of regulation. In contrast, the main cadherin-binding form of β-catenin is a β-catenin–α-catenin dimer, indicating that there is a distinct molecular form of β-catenin that can interact with both the cadherin and α-catenin. We propose that participation of β-catenin in adhesion or Wnt signaling is dictated by the regulation of distinct molecular forms of β-catenin with different binding properties, rather than simple competition between cadherins and TCFs for a single constitutive form. This model explains how cells can control whether β-catenin is used independently in cell adhesion and nuclear signaling, or competitively so that the two processes are coordinated and interrelated.
Introduction
β-Catenin is a cytoplasmic protein that plays essential roles in two different cellular processes: calcium-dependent intercellular adhesion and Wnt-mediated transcriptional activation (for review see Gottardi et al., 2002). For cell–cell adhesion, β-catenin binds the cytoplasmic domain of cadherin adhesion receptors along with the actin binding protein, α-catenin, to bridge the extracellular adhesive activity of cadherins with the underlying actin cytoskeleton (Rimm et al., 1995). This cadherin-bound pool of β-catenin ultimately serves to link the cytoskeletal networks of adjacent cells, which is considered essential for normal tissue architecture and morphogenesis (for review see Gumbiner, 2000). For nuclear signaling, β-catenin binds the DNA binding factor, T-cell factor (TCF)–Lef, and together this complex recruits components of the general transcriptional machinery (e.g., TATA binding protein), as well as proteins involved in chromatin modification and remodeling (e.g., the histone acetyltransferase, p300/CBP and the ATPase, Brg-1) to coordinate the activation of gene targets (Hecht et al., 1999, 2000; Barker et al., 2001; Tutter et al., 2001). How β-catenin binding to cell surface cadherins or DNA binding proteins is regulated, and the relationship between these two functions, remain to be explored.
Why might the cell use a single protein for both cell–cell adhesion and nuclear signaling? One possibility is that signaling and adhesion are tightly coordinated through competition for a common pool of β-catenin. Indeed, some evidence suggests that the signaling and adhesive pools of β-catenin are interrelated in this way. Overexpression of cadherins in Xenopus and other systems can antagonize β-catenin signaling activity (Heasman et al., 1994; Fagotto et al., 1996; Sanson et al., 1996; Orsulic et al., 1999), whereas reduction in the expression of cadherins in Drosophila embryos can enhance β-catenin signaling (Cox et al., 1996). Although experimental manipulation of cadherin levels can affect β-catenin nuclear signaling, it is not yet clear whether β-catenin signaling in vivo is actually regulated by changes in endogenous cadherin levels or function. Moreover, there is also evidence that these two processes can occur largely independently of each other. For example, cadherin loss-of-function is not often associated with enhanced β-catenin signaling (Caca et al., 1999; Vasioukhin et al., 2001), and Wnt activation does not typically alter cell–cell adhesion, although enhanced adhesive activity has been reported previously (Bradley et al., 1993; Hinck et al., 1994). Thus, how the roles of β-catenin in signaling and adhesion are controlled to be either coordinated or independent remains to be elucidated.
In the cases where expression of cadherins antagonizes β-catenin nuclear signaling activity, inhibition occurs by direct binding of β-catenin to the cytoplasmic domain of cadherins, and sequestration from the nuclear compartment (Fagotto et al., 1996; Orsulic et al., 1999; Shtutman et al., 1999; Gottardi et al., 2001). Recent evidence argues that the signaling and adhesive forms of β-catenin may share molecularly similarities. For example, comparison of β-catenin–cadherin and β-catenin–TCF cocrystal structures revealed that both β-catenin ligands bind to extensively overlapping regions along β-catenin, and in some regions engage identical residues (for review see Gottardi and Gumbiner, 2001). Given such similarity in binding mechanism between β-catenin–cadherin and β-catenin–TCF complexes, we proposed that the cadherin is such a favorable inhibitor of β-catenin nuclear signaling, not only because it sequesters β-catenin away from the nucleus, but also because it can compete directly with TCF for β-catenin binding (Gottardi and Gumbiner, 2001).
Nonetheless, the functions of β-catenin can be molecularly distinct. For example, C. elegans uses three different β-catenin gene products for adhesion and signaling functions (Korswagen et al., 2000). BAR-1 mediates Wnt signaling by forming a transcription complex with the TCF homologue, POP-1, whereas HMP-2 interacts exclusively with the cadherin gene product, HMR-1. WRM-1 is involved in a divergent Wnt pathway where it regulates POP-1 indirectly. Although this simple organism has evolved three distinct genes to segregate adhesive from signaling forms of β-catenin, there is no evidence for multiple β-catenin gene products in vertebrates. A splice variant of β-catenin lacking the COOH-terminal transactivation domain has been identified in Drosophila, however, which seems to function only in adhesion (Loureiro and Peifer, 1998). Vertebrates, therefore, must somehow be able to rely on a single β-catenin gene product for both adhesion and signaling functions.
The notion of a single molecular form of β-catenin that participates identically in cell adhesion and gene expression seems to be over simplified. In SW480 tumor cells, a large fraction of β-catenin was found to be refractory to both cadherin- and TCF-binding in vitro (Gottardi et al., 2001). This is due, at least in part, to the interaction of β-catenin with a 9-kD polypeptide, ICAT (Tago et al., 2000), which can prevent β-catenin binding to both TCF and cadherin proteins (Gottardi and Gumbiner, 2004). The small fraction of β-catenin that can bind to TCF also can interact with the cadherin, which explains how cadherin expression inhibits β-catenin–TCF signaling and cell growth in this cell line (Gottardi et al., 2001). Thus, cells contain at least two distinct forms of β-catenin, raising the possibility that the binding properties of β-catenin may be regulated. These distinct forms were identified, however, in a tumor cell line harboring a mutation in the adenomatous polyposis coli (APC) tumor suppressor gene product, which is known to deregulate the normal degradation of β-catenin in cells. Therefore, we sought to examine the regulation of β-catenin binding properties by its physiological regulator, Wnt.
Results
Wnts regulate β-catenin binding specificity
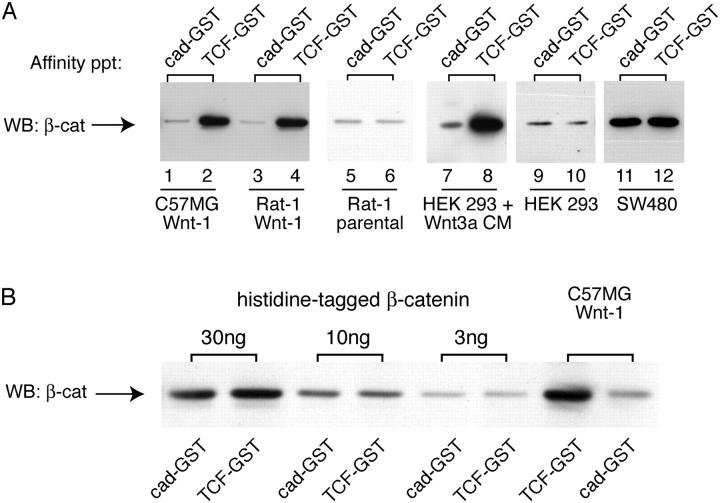
We sought to determine the binding properties of β-catenin generated by Wnt signaling. Using an in vitro pull-down assay, we find that cytosolic β-catenin from cells stably expressing Wnt1 shows preferential binding to TCF-GST compared with a cadherin cytoplasmic domain-GST fusion protein (Fig. 1 A, lanes 1–4). The selective binding activity of β-catenin does not require stable, long-term expression of Wnt in cells, as similar binding properties are observed in human embryonic kidney (HEK) 293T cells incubated with Wnt3a-conditioned media for short periods (Fig. 1 A, lanes 7 and 8). Importantly, this difference in binding is not observed in the untreated or parental cell lines (Fig. 1, lanes 5, 6, 9, and 10), with recombinant β-catenin purified from SF9 cells (Fig. 1 B), or in a cell line that contains elevated levels of β-catenin due to loss of the APC tumor suppressor (Fig. 1, lanes 11 and 12). These findings demonstrate that the differential binding activity of β-catenin is actually induced by Wnts, and is not simply due to binding differences between cad-GST and TCF-GST recombinant proteins, nor to the accumulation of high levels of cytosolic β-catenin. Thus, Wnts may activate β-catenin signaling by generating a molecular form of β-catenin that selectively binds to the downstream transcription factor, TCF, as well as by raising the overall cytosolic levels of β-catenin.
Figure 1.
Wnt signaling generates a form of β-catenin that binds preferentially to TCF-GST compared with cadherin-GST. (A) Detergent-free supernatants were prepared from C57MG and Rat1 cells stably expressing Wnt-1, and HEK293T cells incubated overnight ± Wnt3a-conditioned media (CM). Samples were affinity precipitated using equimolar amounts of cad-GST or TCF-GST fusion proteins. GST gives no binding and is not depicted. A fivefold excess of parental cell lysates was required to detect a signal in lanes 5 and 6. Cytosolic β-catenin from C57MG parentals binds cad-GST and TCF-GST proteins equivalently, like the Rat1 and HEK293 controls (not depicted). The blot was probed with a pAb to β-catenin. (B) Preferential binding of β-catenin to TCF-GST over cadherin-GST is not observed with purified, recombinant β-catenin. Recombinant, purified Xenopus β-catenin (Suh and Gumbiner, 2003) and β-catenin from a C57MG/Wnt cytosolic fraction were affinity precipitated with cad-GST and TCF-GST proteins, and blotted with an antibody to β-catenin.
β-Catenin binding selectivity is determined by its COOH-terminal region
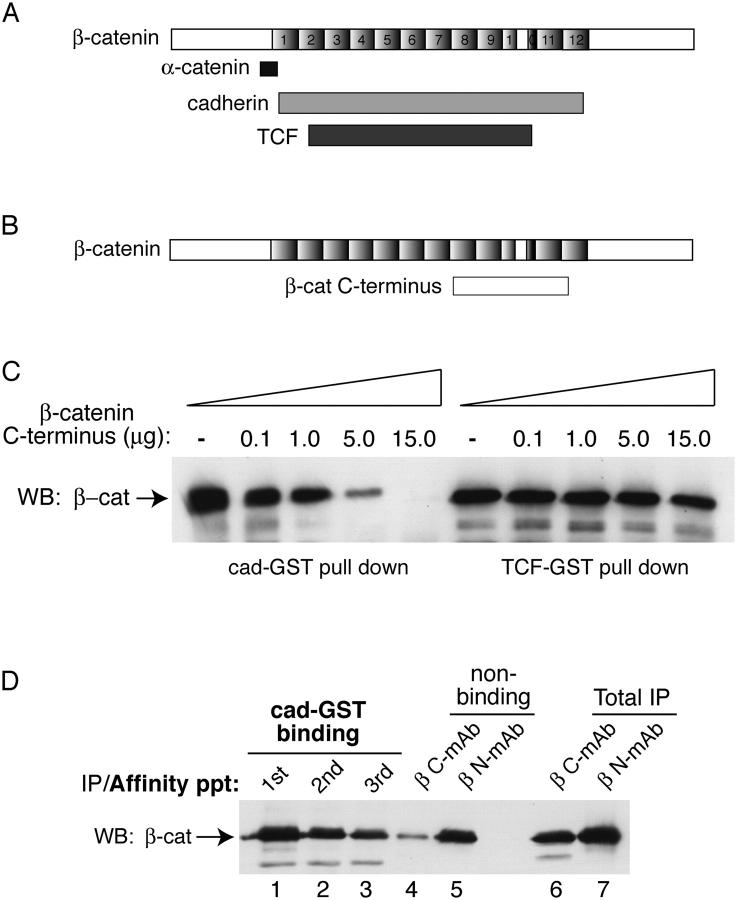
Several findings influenced our investigation of a mechanism that could generate a form of β-catenin that binds selectively to TCF. The COOH terminus of β-catenin can interact with the armadillo repeat region of β-catenin (Cox et al., 1999; Piedra et al., 2001) and compete with β-catenin binding to the cadherin cytoplasmic domain in vitro (Castano et al., 2002). These observations raised the possibility that conformational changes in the COOH terminus of β-catenin, and in particular, a “closed” conformation, might be incompatible with cadherin binding. Because Wnt signaling alters β-catenin binding to the cadherin and TCF differently (Fig. 1), we sought to determine whether the COOH terminus of β-catenin might contribute to this binding selectivity. Indeed, although the COOH terminus of β-catenin can compete β-catenin binding to the cadherin, as demonstrated previously (Castano et al., 2002), its interaction with TCF is not competed (Fig. 2 C). Thus, if a COOH-terminal conformational change giving rise to a closed form of β-catenin were to occur during Wnt signaling, it could alter β-catenin ligand interactions selectively.
Figure 2.
The COOH terminus of β-catenin restricts binding to cadherin. COOH terminus of β-catenin competes cadherin, but not TCF binding. (A) Schematic shows where α-catenin, cadherin, and TCF interact with β-catenin (Huber et al., 1997; Graham et al., 2000; Pokutta and Weis, 2000; Huber and Weis, 2001). (B) The COOH terminus of β-catenin binds the arm repeat region of β-catenin in yeast-two hybrid (Cox et al., 1999) and recombinant protein assays (Piedra et al., 2001). (C) COOH-terminal region of β-catenin competes β-catenin binding to cad-GST, but not to TCF-GST fusion protein. Recombinant β-catenin (1.5 μg) purified from baculovirus (Suh and Gumbiner, 2003) was incubated with cadherin-GST (2 μg) or TCF-GST (2.4 μg) coupled agarose beads in the presence of increasing amounts of β-catenin COOH-terminal peptide (amino acids 695–781). Affinity precipitates were analyzed by SDS-PAGE and Western blotting with an antibody to β-catenin. (D) Cadherin-GST preferentially depletes the fraction of β-catenin recognized by a COOH-terminal mAb (M5.2). A cytosolic fraction from Rat1/Wnt cells was affinity precipitated (×3) with cadherin-GST (lanes 1–3). The cad-GST nonbinding pool (lanes 4 and 5) was divided in two and immunoprecipitated with either an mAb that recognizes a COOH-terminal β-catenin epitope (βC-mAb (M5.2), lane 4) or an NH2-terminal β-catenin epitope (βN-mAb (1.1), lane 5). As a control, these antibodies were used to immunoprecipitate β-catenin from the total starting material (not previously depleted with cad-GST; lanes 6 and 7).
To determine whether the COOH-terminal region of endogenous β-catenin exhibits a different conformation as a result of Wnt signaling, we examined whether epitopes are masked in the TCF-selective, closed form. The ability of anti–NH2- and COOH-terminal–directed mAbs to immunoprecipitate β-catenin after affinity depletion by cad-GST was examined (Fig. 2 D). Although β-catenin is immunoprecipitated similarly by the NH2- and COOH-terminal antibodies from the total starting cytosol (lanes 6 and 7), when the cadherin binding fraction is first depleted by the cadherin-GST, the remaining β-catenin is poorly immunoprecipitated by the COOH-terminal antibody compared with the NH2-terminal antibody (compare lanes 4 and 5). These antibodies, therefore, recognize distinct forms of β-catenin (see Fig. 5 C), which may differ as a result of a conformational change in the COOH terminus of β-catenin that alters β-catenin binding to cadherins. Thus, the COOH-terminal region of β-catenin is more accessible in the cadherin-binding fraction of β-catenin, and much less accessible in the nonbinding fraction.
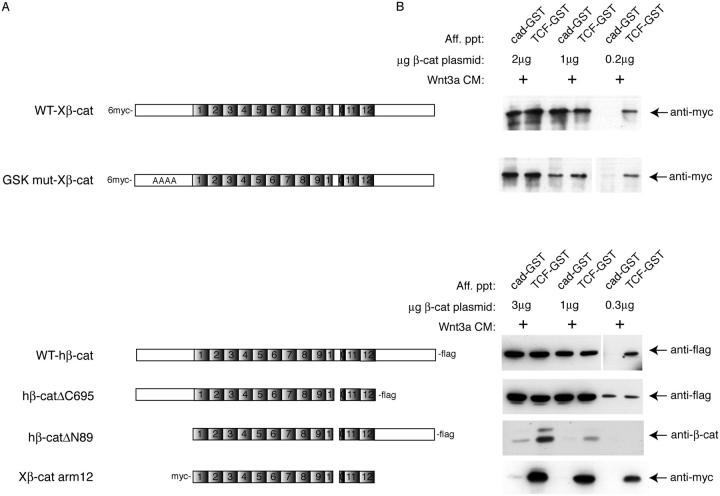
To further determine the regions of β-catenin control this binding selectivity, we also examined the binding properties of a series of β-catenin deletion mutants expressed in cells. Full-length NH2-terminal myc-, or COOH-terminal flag-tagged β-catenin exhibits preferential binding to TCF-GST relative to cad-GST when transfected into HEK293 cells incubated with Wnt3a-conditioned media (Fig. 3). Importantly, the level of exogenous β-catenin expression needed to be kept low in order to detect β-catenin binding selectivity (compare 0.2 μg with 2 μg plasmid), suggesting that the cellular machinery responsible for generating binding selectivity may be easily saturable. As a control, simply diluting the sample transfected with 2 μg plasmid by 10-fold, so that the β-catenin levels were similar to those extracts transfected with 0.2 μg plasmid, did not result in differential binding (unpublished data), indicating that binding selectivity is due to an active cellular process. A construct bearing a deletion of the COOH terminus (hβ-catΔC695) shows equivalent binding to both cadherin and TCF, consistent with a role for the COOH terminus in regulating binding selectivity. Curiously, however, deletion of both NH2- and COOH-terminal domains generates a protein that binds TCF significantly better than the cadherin, even at relatively high levels of expression (e.g., compare 3 μg plasmid for Xβ-cat arm 12 with WTX-β-cat 2 μg). It was recently proposed that the NH2-terminal region of β-catenin may be required for efficient cadherin binding, as a GST-β-catenin fusion protein missing the first 119 aa showed little cadherin binding activity in vitro (Castano et al., 2002). We also find that Δ89β-catenin binds poorly to the cadherin compared with TCF, even at our highest expression levels (Fig. 3). Thus, we suggest that the NH2-terminal region of β-catenin is required for cadherin but not TCF binding, which gives rise to apparent binding selectivity. When β-catenin is able to bind the cadherin (i.e., when the NH2 terminus is present), however, the COOH terminus is required for generating β-catenin binding selectivity.
Figure 3.
Differential binding activity of recombinant β-catenin as revealed by deletion analysis. (A) Schematic representation of β-catenin constructs. WT-myc-Xenopus β-catenin and GSK3β mutant (S/T>A residues 33, 37, 41, and 45) β-catenin were described previously by Guger and Gumbiner (2000). WT-human β-catenin-flag, ΔC695-flag and ΔN89-flag constructs were described in Kolligs et al. (1999). The myc-tagged, Xenopus β-catenin construct encoding only the arm repeat region of β-catenin was described previously (Funayama et al., 1995). (B) Recombinant β-catenin binding to cad-GST versus TCF-GST proteins. HEK293T cells were transfected with decreasing amounts of β-catenin plasmid and incubated in the presence (+) of Wnt3a conditioned media (CM). Cytosolic fractions were affinity precipitated and immunoblotted with anti-myc, -flag, or β-catenin antibodies. Input amounts of wild-type β-catenin, −ΔC695, and arm 12 constructs were the same in accordance with similar expression levels (not depicted).
Cadherin preferentially binds β-catenin–α-catenin complexes
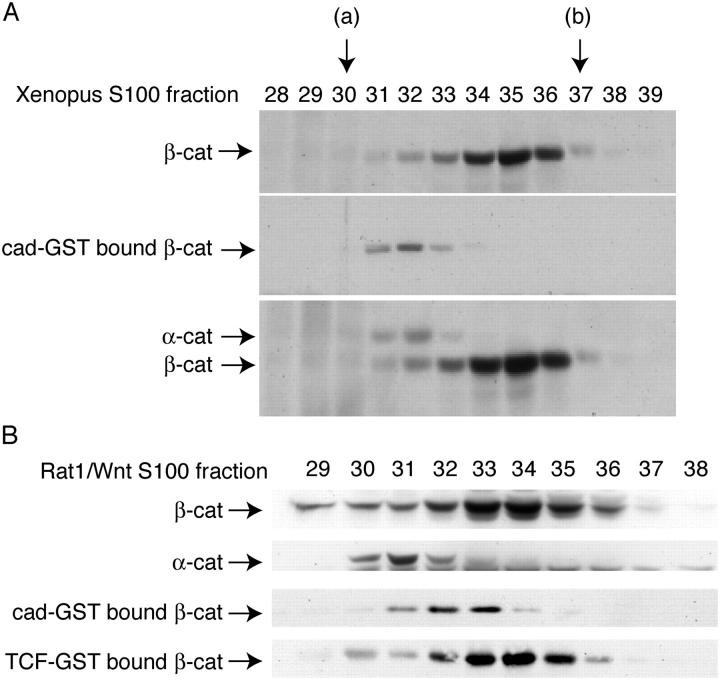
To better characterize the molecular forms of β-catenin that bind to cad-GST and TCF-GST fusion proteins, we examined cytosolic fractions by gel filtration chromatography (Fig. 4). We began our analysis with Xenopus embryos, because it was easier to obtain a large amount of cytosolic β-catenin from cell lysates (Fig. 4 A). One half of each column fraction was precipitated with TCA to show the total profile of β-catenin (Fig. 4 A, top blot); the other half was subjected to cadherin-GST affinity precipitation. β-Catenin eluted across a number of fractions extending from 66 kD (BSA standard, peak fraction 37) to 232 kD (catalase standard, peak fraction 30). The major peak corresponded to ∼100 kD (fraction 35), suggesting that most of the cytosolic β-catenin is monomeric. Importantly, only fractions from the higher molecular mass “shoulder” of the broad β-catenin profile were able to interact with the cadherin cytoplasmic domain (Fig. 4 A, middle blot), whereas TCF could also bind the lower molecular sized fractions corresponding to the major peak (Fig. 4 B below and not depicted). The high molecular weight cadherin-binding fractions cofractionated with α-catenin (Fig. 4 A, bottom blot), suggesting that they contain β-catenin–α-catenin dimers. A similar binding profile was observed with a cytosolic lysate from Wnt-expressing Rat1 cells (Fig. 4 B). However unlike in Xenopus, the cadherin-binding fractions did not perfectly cofractionate with the α-catenin-containing fractions (Fig. 4 B). This small difference in fractionation profiles may be due to the presence of α-catenin homodimers, which may fractionate differently than β-catenin–α-catenin dimers during gel filtration (Koslov et al., 1997). Nevertheless, the cadherin preferentially binds a form of β-catenin that cofractionates with α-catenin, rather than the smaller molecular size fractions, which can bind TCF. Furthermore, the preferential binding of β-catenin to TCF compared with the cadherin observed in Fig. 1 appears to be due to an ability of TCF, but not the cadherin, to bind the monomeric form of β-catenin.
Figure 4.
Larger molecular size, α-catenin–containing fractions of β-catenin show preferential binding to cad-GST. (A) A cytosolic fraction from stage 12 Xenopus embryos was applied to a Sephacryl 300 gel filtration column, and fractions 28–39 were divided in two: one half of each sample was TCA-precipitated (top blot), whereas the other half was precipitated with cad-GST (middle blot). The top blot was reprobed with an antibody to α-catenin and is shown below. (B) Same as A except that starting material is an S100 fraction from Rat1/Wnt cells. Arrows refer to elution volumes of standard proteins with known molecular weight: (a) catalase (Mr = 232,000); (b) BSA (Mr = 66,000), purified mouse IgG (150 kD) eluted in fractions 31–33.
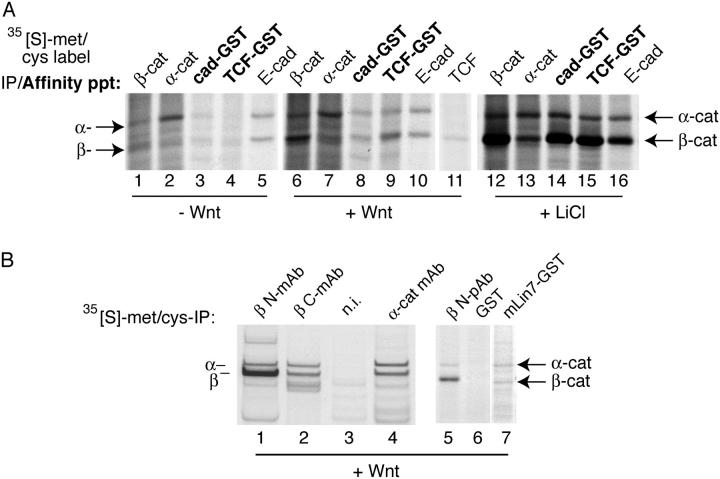
The higher molecular size fractions of β-catenin could be due to its association with α-catenin or other possible proteins. To see what proteins associate with β-catenin and bind to cad-GST and TCF-GST fusion proteins, we performed the binding assay using cytosol prepared from [35S]methionine and cysteine-labeling cells. Metabolic labeling of Wnt ± expressing cells reveals α-catenin as the major binding partner of cytosolic β-catenin (Fig. 5 A, lanes 6 and 7). No other major bands were detected between the 10–200 kD molecular mass region by [35S]methionine/cysteine labeling or Coomassie staining (unpublished data). The cadherin-GST appears to affinity precipitate β-catenin and α-catenin bands at a ratio ∼1:1, whereas the TCF-GST precipitates much more β-catenin than α-catenin (band ratios of ∼3:1; Fig. 5 A, lanes 8 and 9). Given that α-catenin and β-catenin contain nearly identical numbers of cysteine and methionine residues (40 and 41, respectively), and that the labeling time is long (13 h) compared with the half lives of both proteins (7 and 5 h, respectively; unpublished data), it appears that the cadherin binds a stoichiometric complex of β-catenin–α-catenin. We conclude, therefore, that the cadherin preferentially binds β-catenin that is associated with α-catenin, whereas TCF can bind monomeric β-catenin in addition to β-catenin–α-catenin dimers.
Figure 5.
The α-catenin–free, monomeric form of β-catenin exhibits preferential binding to TCF compared with cadherin in Wnt cells. (A) Rat1 cells were labeled to steady-state with [35S]methionine/cysteine, and a cytosolic fraction was prepared from each condition (−Wnt, +Wnt, 10 mM LiCl, 12 h) and immunoprecipitated with the designated antibodies or affinity precipitated with GST proteins. Note that immunoprecipitation of endogenous E-cadherin (from the 100,000 g membrane pellet, lanes 5, 10, and 16) and TCF (lane 11) are also shown. Non-specific bands were not seen with a GST control (not depicted). Overnight incubation with LiCl (10 mM) allows the α-catenin–free pool of β-catenin to bind cad-GST, TCF-GST, and the endogenous E-cadherin (lanes 14–16), equivalently. (B). COOH-terminal epitopes of β-catenin are masked in the α-catenin–free fraction of β-catenin. Equivalent amounts of an S100 fraction from [35S]methionine/cysteine steady-state–labeled Rat1+Wnt cells were immunoprecipitated with the following antibodies: anti–β-catenin NH2-terminal mAb (1.1.1; lane 1), anti–β-catenin COOH-terminal mAb (M5.2; lane 2), anti–α-catenin mAb (lane 4), and a nonimmune control (lane 3). (Lanes 5–7) PDZ protein, mLin7, preferentially binds to β-catenin–α-catenin dimer: metabolically labeled Rat1+Wnt lysates were affinity precipitated with (lane 5) anti–β-catenin pAb, (lane 6) control GST, and (lane 7) mLin7-GST.
We determined in Fig. 2 D that a COOH-terminal epitope of β-catenin was inaccessible in the fraction of β-catenin that could not interact with the cadherin. Because our experiments in Figs. 4 and 5 A showed that β-catenin–α-catenin dimers preferentially interact with the cadherin, we asked whether this COOH-terminal epitope of β-catenin was accessible in the β-catenin–α-catenin dimer fraction, and masked in the form of β-catenin that preferentially binds to TCF. To do so, we immunoprecipitated β-catenin from metabolically labeled, Wnt1-expressing cells using the NH2- and COOH-terminal monospecific antibodies previously shown to recognize distinct forms of β-catenin (Fig. 2 D). The antibody that recognizes the COOH terminus of β-catenin appears to preferentially immunoprecipitate β-catenin and α-catenin bands at a ratio of ∼1:1, whereas the antibody that recognizes the NH2 terminus of β-catenin immunoprecipitates significantly more β-catenin than α-catenin (Fig. 5 B, lanes 1–4). Thus, the NH2-terminal antibody can immunoprecipitate β-catenin monomers as well as β-catenin–α-catenin dimers. Because the COOH-terminal antibody preferentially immunoprecipitates β-catenin–α-catenin dimers, the COOH terminus in most of the monomeric fraction of β-catenin is masked. To determine whether this inaccessibility of the COOH-terminal epitope was due to a more general masking of the COOH terminus, we examined the binding of β-catenin to the PDZ-containing protein, Lin7. The extreme COOH terminus of β-catenin contains a PDZ interaction motif, and has been shown previously to bind Lin 7 (Perego et al., 2000). Similar to the COOH-terminal antibody findings, Lin7 affinity precipitates β-catenin and α-catenin bands at a ratio of ∼1:1, suggesting that it preferentially interacts with β-catenin–α-catenin dimers. Together, these results show that the COOH-terminal region of β-catenin is accessible in the β-catenin–α-catenin dimer fraction, and is masked in the monomeric fraction, which is enriched in the TCF-selective form of β-catenin.
Effect of APC mutations, inhibition of GSK-3β activity, NH2-terminal phosphorylation of β-catenin, and cadherin phosphorylation on β-catenin binding specificity
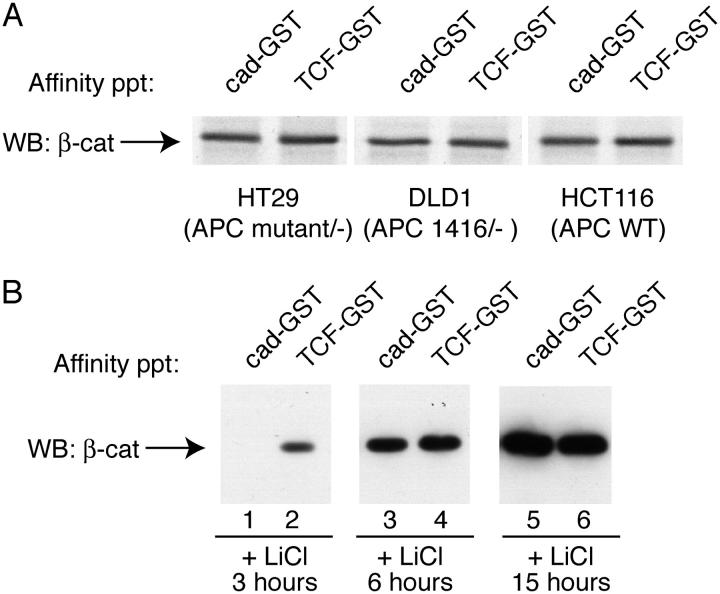
To explore the signaling pathways that might control these forms of β-catenin, we sought to examine the role of the APC tumor suppressor gene product and glycogen synthase kinase (GSK)-3β, two key components in the Wnt pathway, as well as the role of known phosphorylations of β-catenin in response to Wnts. We examined several colon carcinoma cell lines that contain either wild-type or mutant forms of APC (Fig. 6 A). All of these cell lines manifest constitutive β-catenin signaling due to inactivating mutations in APC (HT29, DLD1), or activating mutations within the GSK-3β regulatory region of β-catenin (HCT116). No differences in β-catenin binding to cadherin- and TCF-GST fusion proteins were observed, suggesting that β-catenin binding selectivity is not simply due to inhibition of APC-mediated destruction of β-catenin. Interestingly, the role of GSK-3β is more complex. Short-term inhibition of GSK3β by lithium chloride (LiCl; under 4 h at 10 mM) mimics the Wnt effect on β-catenin, i.e., β-catenin preferentially binds TCF-GST greater than cad-GST (Fig. 6 B, lanes 1 and 2). Therefore, inhibition of GSK3β activity alone is sufficient to mimic the effect of Wnt on β-catenin binding activities. Curiously, however, long-term inhibition of GSK3β by LiCl (over 6 h at 10 mM) generates a pool of β-catenin that binds TCF and cadherin-GST proteins equally well (Fig. 6 B, lanes 3–6). Thus, more potent effects of LiCl do not mimic Wnt signaling, but instead result in the accumulation of high levels of β-catenin with no binding specificity, similar to tumor cells with APC mutations. One interpretation of these two types of effects is that GSK might have multiple targets besides the NH2 terminus of β-catenin (e.g., APC, Rubinfeld et al., 1996; or Axin, Jho et al., 1999). Alternatively, long-term incubation with LiCl could have pleiotropic effects on cell signaling pathways, or the cellular machinery that regulates β-catenin binding to TCF versus cadherin may be easily saturable, so that differential binding is not observed when β-catenin levels rise to unphysiological levels. This explanation is consistent with findings that total cytosolic levels of β-catenin appear to increase substantially with the duration of LiCl treatment (Fig. 6 B, compare lanes 2, 4, and 6), and because expression levels via transfection give similar results (Fig. 3).
Figure 6.
β-Catenin binding selectivity as a function of APC mutant status or GSK inhibition by LiCl. (A) A cytosolic fraction was prepared from colon carcinoma cell lines containing wild-type (HCT116) or mutant (HT29 and DLD1) forms of APC. (B) Selective binding activity of β-catenin in response to short-term, but not long-term treatment with LiCl. HEK293T cells were treated with 10 mM LiCl for 3, 6, and 15 h, after which cytosolic fractions were affinity precipitated as described above.
Several well-characterized NH2-terminal GSK phosphorylation sites are known to target β-catenin for degradation by an SCF-E3-ligase complex (Winston et al., 1999), and recent work has shown that Wnt signaling is specifically mediated through forms of β-catenin that remain unphosphorylated at these NH2-terminal sites S-33, -37, and T-41 (Staal et al., 2002). We therefore asked whether the state of phosphorylation at these sites is responsible for generating the form of β-catenin selective for TCF-binding. A pAb that recognizes NH2-terminal, unphosphorylated β-catenin was shown to stain cell nuclei, whereas cell–cell contact staining was conspicuously absent (Staal et al., 2002), raising the possibility that unphosphorylated forms of β-catenin might preferentially bind to TCF in the nucleus, and be unable to bind cadherins at the plasma membrane. Contrary to this suggestion, however, we find that β-catenin that is not phosphorylated at residues 33 and 37 can interact with the cad-GST in vitro (Fig. 7 A), associate with endogenous cadherin proteins in cell lysates (not depicted), and localize to sites of cell–cell contact (not the nucleus, as originally observed in Staal et al., 2002; Fig. 7 B). Moreover, cells transfected with a form of β-catenin that cannot be phosphorylated by GSK3β and casein kinase (CK1; S/T>A point mutants at residues 33, 37, 41 and 45) exhibit binding selectivity similar to cells transfected with wild-type β-catenin (Fig. 3). Thus, the preferential binding of β-catenin to TCF over cadherin is not simply due to NH2-terminal phosphorylation status.
Figure 7.
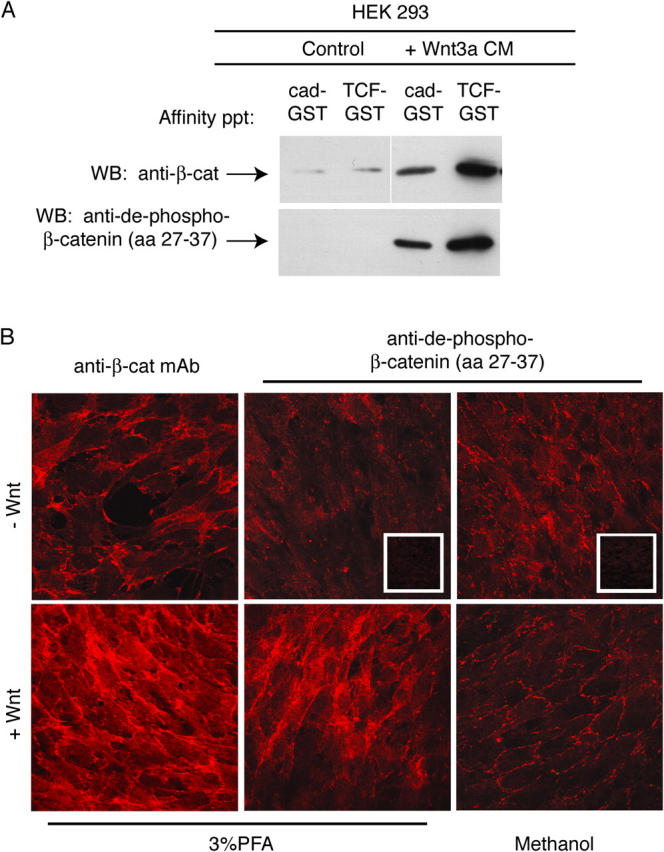
β-Catenin not phosphorylated at NH 2 -terminal GSK-3β sites binds to cadherin. (A) Cytosolic fraction from HEK293 cells ± Wnt3a was affinity precipitated with cad-GST and TCF-GST, and blotted with pAbs to β-catenin (top blot) or NH2-terminal unphosphorylated–β-catenin (amino acids 27–37, bottom blot). (B) NH2-terminal unphospho–β-catenin localizes to sites of cell–cell contact in Wnt-expressing cells. Rat1 fibroblasts ± Wnt were fixed and processed for immunofluorescence using standard protocols. Images were captured with the Axioplan 2 microscope and AxioVision2.0 software (Carl Zeiss MicroImaging, Inc.). Note that membrane staining of the unphospho-β-catenin (Cy3) is more readily detected under methanol, rather than PFA fixation conditions, perhaps accounting for the apparent differences observed between our study and Staal et al. (2002).
We also asked whether modification of the cadherin could affect β-catenin binding selectivity. A previous study showed that the serine-rich, β-catenin binding region of the cadherin is phosphorylated in vivo (Stappert and Kemler, 1994), and this phosphorylation can enhance β-catenin binding to the cadherin (Lickert et al., 2000; Huber and Weis, 2001). We therefore wished to explore whether β-catenin binding selectivity for TCF in Wnt stimulated cells would be altered by cadherin phosphorylation in our binding assay. Phosphorylation of the cadherin greatly enhances binding to β-catenin compared with unphosphorylated cadherin (Fig. 8 A and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200402153/DC1). Indeed, phospho-cadherin is able to binding the same fraction of β-catenin that binds TCF (Fig. 8, B and C), suggesting that cadherin phosphorylation allows the monomeric, closed form of β-catenin to bind the cadherin. Thus, although Wnt signaling generates a form of β-catenin that exhibits preferential binding to TCF over the cadherin, this mechanism can be overridden by extensive phosphorylation of the cadherin.
Figure 8.
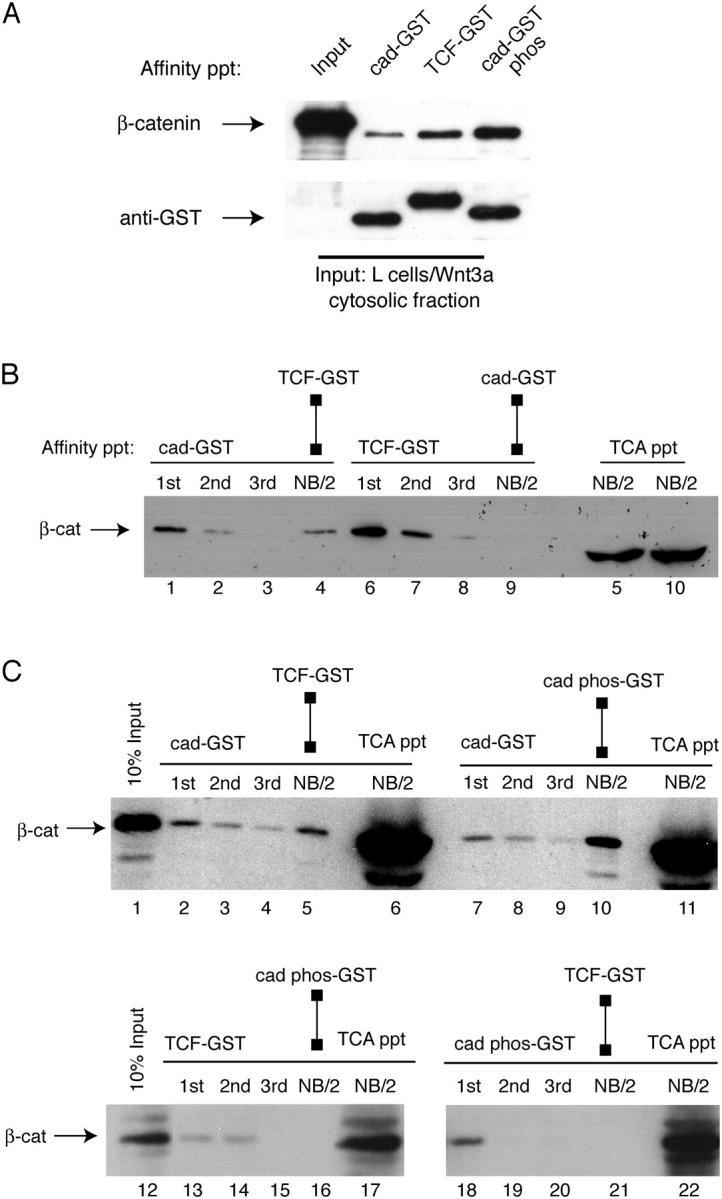
Cadherin phosphorylation reverses β-catenin binding selectivity during Wnt signaling. (A) Phosphorylation of cad-GST increases β-catenin binding to cadherin compared with TCF. A cytosolic fraction from L cells transfected with Wnt3a were incubated with equimolar amounts of cad-GST, TCF-GST, and CK2-P-cad-GST-glutathione–coupled beads for 1 h at 4°C (see Fig. S1 for characterization of GST fusion proteins). The resulting anti–β-catenin and anti-GST immunoblots are shown. (B) Fraction of β-catenin that binds cadherin is a subset of fraction of β-catenin that binds TCF. Cytosolic fraction of Wnt cells was sequentially affinity precipitated with cad-GST (lanes 1–3) or TCF-GST (lanes 6–8) proteins. After cad-GST depletion (lanes 1–3), half of the cad-GST non-binding fraction (NB/2) was precipitated with TCF-GST (lane 4); the other half was precipitated with TCA to show amount remaining (lane 5, far right). After TCF-GST depletion (lanes 6–8), half of the TCF-GST non-binding fraction (NB/2, lane 9) was precipitated with cad-GST, whereas the other half was precipitated with TCA to show amount remaining (lane 10, far right). Lanes 5 and 10 reveal a fraction of β-catenin that binds neither TCF nor cadherin. This fraction is likely due to β-catenin already complexed with partners such as ICAT (Gottardi and Gumbiner, 2004). (C) Phosphorylated cadherin-GST and TCF-GST bind the same pool of β-catenin in Wnt-activated cells. Cytosolic fraction was precipitated with cad-GST (top blot), TCF-GST (bottom left) or P-cadherin-GST (bottom right) fusion proteins. After cad-GST depletion (lanes 2–4 and 7–9), there is a fraction of β-catenin that binds TCF-GST (lane 5) and P-cadherin-GST (lane 10). Note that after TCF-GST depletion (lanes 13–15), there is no β-catenin remaining to bind P-cadherin-GST (lane 16). After P-cadherin-GST depletion (lanes 18–20), there is no β-catenin remaining to bind TCF-GST (lane 21). Reciprocal depletions suggest that P-cadherin-GST and TCF-GST bind the same form of β-catenin.
Discussion
We show that Wnt signaling generates a monomeric form of β-catenin that binds TCF selectively compared with the cadherin. In contrast, the cadherin preferentially binds a β-catenin–α-catenin dimer. This selective targeting of distinct molecular forms of β-catenin provides a mechanism by which cells could potentially separate the adhesion and signaling functions of β-catenin. We propose that segregation of these functions of β-catenin may be necessary for two reasons. First, selective targeting of β-catenin to transcriptional complexes would prevent the cadherin from competing with Wnt signaling activity, which may be important during low or transient Wnt activation where signaling may need to be especially efficient. Second, such a mechanism would ensure that cell–cell adhesion is maintained during Wnt inductions throughout development. Loading the cadherin with β-catenin monomers generated by strong Wnt signals might have undesired consequences for cell adhesion, because cadherins bound to β-catenin without α-catenin would be unable to contribute to adhesion. Thus, generation and targeting of distinct molecular forms of β-catenin could ensure that adhesion and signaling are not always coupled, and when necessary, can be regulated independently of one another.
We provide evidence that the Wnt-stimulated, TCF-binding selectivity of β-catenin is mediated by the COOH-terminal region of β-catenin. First, COOH-terminal epitopes of β-catenin are masked in the fraction of β-catenin that is unable bind the cadherin. Second, a COOH-terminal peptide of β-catenin can compete β-catenin binding to cadherin, but not to TCF. Third, deletion of the COOH terminus of β-catenin results in a loss of binding selectivity. Together with previously published data showing that the COOH-terminal region of β-catenin can bind directly to the armadillo repeat region of β-catenin (Cox et al., 1999; Piedra et al., 2001) and restrict cadherin binding in vitro (Castano et al., 2002), we propose that in vivo, the COOH terminus of β-catenin adopts a folded-over conformation which controls β-catenin binding selectivity by restricting cadherin but not TCF binding. Thus, Wnts may activate β-catenin signaling not only by increasing its cytosolic levels, but by regulating the conformation of its COOH terminus.
The existence of a form of β-catenin that distinguishes between cadherins and TCF was not anticipated, given the overall structural similarity between the β-catenin–cadherin and β-catenin–TCF binding interfaces revealed by X-ray crystallography (Graham et al., 2000; Huber and Weis, 2001). Upon closer examination, however, the β-catenin–TCF binding interface is less extensive than the β-catenin–cadherin binding interface, spanning arm repeats 3–10 compared with all 12 armadillo repeats for the cadherin. Thus, it is possible that the COOH-terminal region of β-catenin may fold-back over the last two armadillo repeats of β-catenin, which could have consequences for cadherin but not TCF binding. Indeed, alteration of a single residue in the 12th arm repeat of β-catenin decreases β-catenin binding to the cadherin by a factor of four (Roura et al., 1999), further arguing that small perturbations in the β-catenin–cadherin interface can have significant consequences for binding.
It has also been shown that phosphorylation of E-cadherin increases cadherin–β-catenin complex formation (Lickert et al., 2000). In crystal structures, this phosphorylation results in interactions with β-catenin that appear to mimic TCF binding (Huber and Weis, 2001). Indeed, we find that cadherin phosphorylation allows the cadherin to bind the monomeric, closed form of β-catenin that otherwise would be TCF selective. The fact that cadherin phosphorylation can reverse Wnt-mediated β-catenin binding selectivity suggests a mechanism by which cadherins compete for the Wnt-activated form of β-catenin. It will be important, therefore, to determine when and where cadherin modification occurs to better understand the relationship between adhesion and signaling.
Our observation that the cadherin binds preferentially to β-catenin–α-catenin dimers compared with β-catenin monomers raises the possibility that α-catenin plays a positive role in β-catenin binding to cadherin. Indeed, one study showed that preassociation of recombinant α-catenin with β-catenin increases β-catenin binding to cadherin, suggesting that α-catenin induces an open conformation of β-catenin (Castano et al., 2002). Other evidence, however, argues that α-catenin is not required for β-catenin binding to cadherin. For example, recombinant cadherin–β-catenin complexes are readily formed in vitro (Huber et al., 2001), and cells lacking α-catenin still form cadherin–β-catenin complexes (Bullions et al., 1997; Vasioukhin et al., 2001). We suggest that the form of β-catenin that binds preferentially to cadherin, also binds α-catenin.
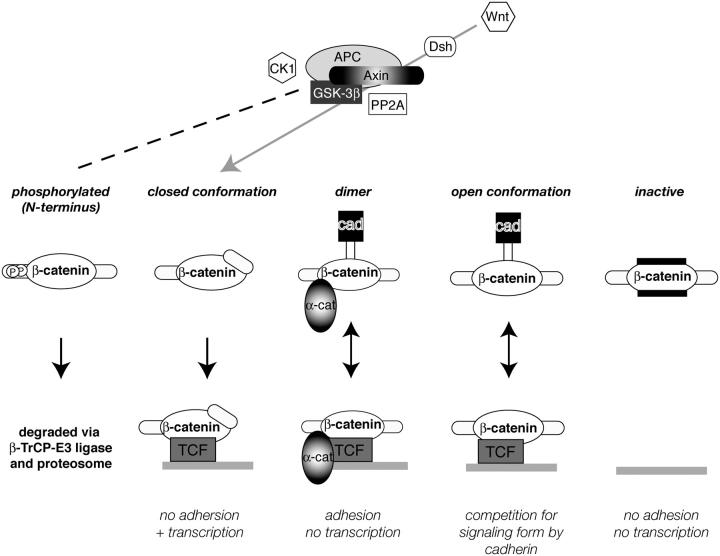
Based on our findings from this and previous reports, we propose that cells contain a number of distinct molecular forms of β-catenin (Fig. 9). Thus, although an organism like C. elegans controls the adhesive and signaling functions of β-catenin through expression of a multi-gene family, vertebrates regulate β-catenin functions by generating distinct molecular forms at the protein level. First, there is the well-known form of β-catenin that is phosphorylated at the NH2 terminus and is targeted for degradation (Fig. 9, phosphorylated; for review see Polakis, 1999). We reported previously a large pool of β-catenin in the SW480 tumor cell line that cannot bind to either TCF or cadherin, and provided evidence that this was an “inactive” form for both adhesion and signaling (Fig. 9; Gottardi et al., 2001). This form may be due, at least in part, to ICAT, a small 9-kD polypeptide that inhibits β-catenin binding to both TCF and cadherin (Gottardi and Gumbiner, 2004; Tago et al., 2000). Here, we provide evidence for a TCF-selective form of β-catenin that is targeted to transcription complexes (closed conformation), and a form that can target to adhesive complexes (β-catenin–α-catenin dimer). Although the latter form can interact with both the cadherin and TCF, there is evidence that α-catenin inhibits the transcriptional activity of β-catenin in the nucleus (Giannini et al., 2000), suggesting that this form is specific for adhesion functions. Finally, we postulate that cells can contain a form of β-catenin that is competent for both signaling and cadherin binding (open conformation) which is observed, for example, under long-term LiCl treatment (Fig. 5 A, lanes 14 and 15), and would explain the many cases in which cadherin expression inhibits the transcriptional activity of β-catenin (Heasman et al., 1994; Fagotto et al., 1996; Sanson et al., 1996; Orsulic et al., 1999; Shtutman et al., 1999; Gottardi et al., 2001).
Figure 9.
Multiple forms of β-catenin exist in cells. NH2-terminal phospho-β-catenin is well characterized and generated by the APC-Axin-GSK3β-CK1 complex (dashed line). Closed form of β-catenin is generated by Wnt signaling, perhaps through some of the same machinery (gray arrow). β-Catenin–α-catenin dimer is active for adhesion but not signaling. Open form binds both cadherin and TCF, and could explain how cadherin antagonizes β-catenin signaling in overexpression systems. The inactive form cannot participate in adhesion or signaling.
Other than the targeting of β-catenin for degradation, the modifications and machinery that regulate these various forms of β-catenin are presently unknown. It is not clear, for example, whether the formation of the inactive ICAT complex is simply controlled by levels of ICAT expression, or is regulated in another way (Gottardi and Gumbiner, 2004). Also, it is not understood what controls the formation of the β-catenin–α-catenin dimer, although it is clear that β-catenin monomers and α-catenin can coexist in the cytosol without forming complexes, even though they readily bind with high affinity in vitro (Koslov et al., 1997). Nor is the time and place of cadherin phosphorylation known. We show that the TCF-selective, closed form of β-catenin is regulated by the Wnt pathway, and that the binding selectivity observed after short-term LiCl treatment suggests that GSK3β may be involved. However, the mechanism must be distinct from the pathway that regulates β-catenin levels in the cytosol, because the absence of GSK3β-dependent NH2-terminal phosphorylation does not account for its binding properties, and long-term LiCl treatments and APC mutations lead to β-catenin accumulation without generating the TCF-selective form. It is tempting to speculate that the APC-axin-GSK3β–containing complex regulates the generation of these various forms of β-catenin by post-translational modifications, in addition to the targeting of β-catenin for degradation. Indeed APC mutations have been found to affect adhesive functions of β-catenin as well as Wnt signaling in Drosophila (Hamada and Bienz, 2002).
If we propose that Wnt signaling generates a TCF-selective form of β-catenin that is resistant to cadherin binding, how do we explain the fact that cadherin expression has been found to antagonize Wnt signaling in numerous model systems (Heasman et al., 1994; Fagotto et al., 1996; Gottardi et al., 2001)? One possibility is that cadherin overexpression of cadherin drives the formation of complexes that do not occur under normal physiological conditions. Although the various molecular forms of β-catenin seem fairly stable in our experiments, it is possible that they are more interconvertible in the cell, or that one form is an intermediate for the other, and can be depleted during its generation. A more interesting possibility is that the relationship between cadherins and Wnt signaling may depend on the specific situation faced by each cell responding to a Wnt signal. For example, our finding that cadherin phosphorylation increases β-catenin binding to cadherin, reversing the differential binding activity observed during Wnt signaling, suggests that variations in cadherin phosphorylation may alter the extent to which adhesion and signaling are coupled. We also hypothesize that the cell can potentially generate either the open form of β-catenin, which binds to both cadherin and TCF, or the closed, TCF-selective form, and the relative proportion of these two forms may differ between different cells responding to Wnt signaling, or different strengths of Wnt signaling.
Indeed, consideration of the various findings suggests a model in which the extent of coupling between the adhesive and nuclear signaling functions of β-catenin is regulated differentially in different cell types, depending on the biological needs of the cells and tissues responding to a Wnt signal. Elucidating the cellular and biochemical mechanisms regulating the generation of the different forms of β-catenin and determining when and where they occur should provide insight into the relationship between the adhesive and signaling functions of β-catenin.
Materials and methods
Cell culture
C57MG and Rat1 parental and Wnt1-expressing cell lines were provided by J. Kitajewski (Columbia University, New York, NY). Wnt3a-expressing L cells were provided by L. Schweizer and H. Varmus (Sloan-Kettering Institute, New York, NY). HEK293T, SW480, HCT116, DLD1, and HT29 cell lines were purchased from American Type Culture Collection.
Antibody and plasmid reagents
Rabbit pAbs to α-catenin, and the NH2-terminal region of β-catenin have been described elsewhere (McCrea et al., 1993); 1:5,000 WB; 1:500 IP). The NH2-terminal and COOH-terminal monoclonal anti–β-catenin antibodies (1.1.1 and M5.2, respectively) were provided by N. Gruel, V. Choumet, and J. Luc Teillard (Pasteur Institute, Paris, France). Other antibodies used in this study: anti–NH2-terminal dephospho–β-catenin pAb (8E4; A.G. Scientific), anti–β-catenin COOH-terminal mAb (C19220; Transduction Laboratories), anti–α-catenin mAb (BD Transduction Laboratories), anti–human E-cadherin (HECD-1 mAb; Zymed Laboratories; 1:500 IP), anti–TCF-4 (1:500 IP, 6H5-3; Upstate Biotechnology), anti-FLAG epitope (1:5,000 WB, M2 mAb, Sigma-Aldrich) and anti-Myc (1:5,000 WB, 9E10 epitope). The mouse Lin7 GST fusion construct was provided by S. Straight and B. Margolis (University of Michigan, Ann Arbor, MI). Xenopus C-cadherin cytoplasmic domain (cad-GST) and Xenopus TCF-3 β-catenin binding region (TCF-GST) fusion proteins have been described previously (Gottardi et al., 2001).
Affinity precipitation experiments
Cells were grown to in 14-cm tissue culture dishes and a detergent-free, cytosolic fraction was generated by centrifugation at 100,000 g according to Gottardi et al. (2001). Metabolic labeling of proteins with [35S]methionine/cysteine was done according to Gottardi and Gumbiner (2004). Affinity precipitations were performed with recombinant GST-cad, GST-TCF and GST-mLin7 fusion proteins. For cadherin/TCF-GST binding experiments, 100–500 μg of a cytosolic fraction was subjected to affinity precipitation with ∼40 pmol of cad-GST or TCF-GST prebound as a 50% suspension of glutathione-coupled agarose beads (Sigma-Aldrich) for 60′ at 4°C. Each precipitation was washed in a detergent buffer (50 mM Tris, pH 7.5, 2 mM EDTA, 150 mM NaCl and 0.1% NP-40), and bound protein complexes were analyzed by standard Western analysis. For in vitro competition of the β-cat COOH-terminal peptide (amino acids 696–781; provided by A. Garcia de Herreros, Universitat Pompeu Fabra, Barcelona; Castano et al., 2002), the β-catenin COOH terminus was cleaved from GST with PreScission protease (Amersham Biosciences). β-Catenin (∼17 pmol, produced by the baculovirus system), cadherin-GST (51 pmol), or TCF-GST (51 pmol) were incubated in 150 ml of buffer (10 mM Tris, pH 8.0, 140 mM NaCl, 1 mM EDTA, 0.1% NP-40, 10 μg/ml leupeptin and aprotinin) for 60' at 4°C with shaking in the presence or absence of increasing amounts of the β-catenin COOH-terminal peptide (0, 0.1, 1.0, 5.0, and 15.0 μg [1.6 nmol peptide]). β-Catenin that was affinity precipitated by cadherin-GST and TCF-GST immobilized to glutathione-coupled agarose was washed and subjected to SDS-PAGE and Western analysis.
Gel filtration chromatography
The cytosolic fraction was separated on a Hi Prep 16/60 Sephacryl S-300 sizing column (Amersham Biosciences; High Resolution Code 17–1167-01, 10–1500 kD inclusion range) equilibrated with buffer containing 30 mM Hepes, pH 7.5, and 150 mM KCl and developed at 0.4 ml/min. 75 2.0-ml fractions were collected.
Online supplemental material
Fig. S1 depicts in vitro phosphorylation of cadherin-GST. (A) Purification of cad-GST and TCF-GST proteins and detection by Coomassie. (B and C) In vitro phosphorylation of cad-GST by CK-2. Cad-GST (3 μg) was incubated with or without 50 U of recombinant human CK-2 (New England Biolabs, Inc.) and 200 or 600 μM ATP using conditions suggested by the manufacturer. Cad-GST input was evaluated with an antibody to GST; evidence for phosphorylation was detected by immunoblotting with an anti–P-serine antibody (Sigma-Aldrich). (C) Efficiency of cad-GST phosphorylation: the first and sixth lanes in B were resolved on a long gel to detect the mobility shift of phospho-cad-GST. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200402153/DC1.
Acknowledgments
We thank Nadege Gruel, Valerie Choumet, and Jean Luc Teillard for NH2- and COOH-terminal specific β-catenin mAbs, Drs. Antonio García de Herreros and Mireia Duñach (Universitat Pompeu Fabra, Barcelona, Spain), for the β-catenin COOH terminus-GST, and Alin Vonica (The Rockefeller University, New York, NY) and Mungo Marsden (University of Waterloo, Ontario, Canada) for Xenopus embryos. We thank Martin Wiedmann for discussions and Filippo Giancotti for support throughout the project.
This work was supported by National Institutes of Health grant R37 GM374432 awarded to B.M. Gumbiner.
Abbreviations used in this paper: APC, adenomatous polyposis coli; CK1, casein kinase 1; GSK, glycogen synthase kinase; HEK, human embryonic kidney; LiCl, lithium chloride; TCF, T-cell factor.
References
- Barker, N., A. Hurlstone, H. Musisi, A. Miles, M. Bienz, and H. Clevers. 2001. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 20:4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, R.S., P. Cowin, and A.M. Brown. 1993. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J. Cell Biol. 123:1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullions, L.C., D.A. Notterman, L.S. Chung, and A.J. Levine. 1997. Expression of wild-type α-catenin protein in cells with a mutant α-catenin gene restores both growth regulation and tumor suppressor activities. Mol. Cell. Biol. 17:4501–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caca, K., F.T. Kolligs, X. Ji, M. Hayes, J. Qian, A. Yahanda, D.L. Rimm, J. Costa, and E.R. Fearon. 1999. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 10:369–376. [PubMed] [Google Scholar]
- Castano, J., I. Raurell, J.A. Piedra, S. Miravet, M. Dunach, and A. Garcia de Herreros. 2002. β-catenin N- and C-terminal tails modulate the coordinated binding of adherens junction proteins to β-catenin. J. Biol. Chem. 277:31541–31550. [DOI] [PubMed] [Google Scholar]
- Cox, R.T., C. Kirkpatrick, and M. Peifer. 1996. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 134:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R.T., L.M. Pai, C. Kirkpatrick, J. Stein, and M. Peifer. 1999. Roles of the C terminus of Armadillo in Wingless signaling in Drosophila. Genetics. 153:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto, F., N. Funayama, U. Gluck, and B. Gumbiner. 1996. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J. Cell Biol. 132:1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama, N., F. Fagotto, P. McCrea, and B. Gumbiner. 1995. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J. Cell Biol. 128:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini, A.L., M. Vivanco, and R.M. Kypta. 2000. Alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J. Biol. Chem. 275:21883–21888. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., and B.M. Gumbiner. 2001. Adhesion signaling: how beta-catenin interacts with its partners. Curr. Biol. 11:R792–R794. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., and B.M. Gumbiner. 2004. Role for ICAT (inhibitor of {beta}-catenin and TCF-4) in {beta}-catenin-dependent nuclear signaling and cadherin functions. Am. J. Physiol. Cell Physiol. 286:C747–C756. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., C.M. Niessen, and B.M. Gumbiner. 2002. The adherens junction. Cell Adhesion. Oxford University Press, Oxford, UK. 259–287.
- Gottardi, C.J., E. Wong, and B.M. Gumbiner. 2001. E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J. Cell Biol. 153:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, T.A., C. Weaver, F. Mao, D. Kimelman, and W. Xu. 2000. Crystal structure of a beta-catenin/Tcf complex. Cell. 103:885–896. [DOI] [PubMed] [Google Scholar]
- Guger, K.A., and B.M. Gumbiner. 2000. A mode of regulation of beta-catenin signaling activity in Xenopus embryos independent of its levels. Dev. Biol. 223:441–448. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2000. Regulation of cadherin adhesive activity. J. Cell Biol. 148:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, F., and M. Bienz. 2002. A Drosophila APC tumour suppressor homologue functions in cellular adhesion. Nat. Cell Biol. 4:208–213. [DOI] [PubMed] [Google Scholar]
- Heasman, J., A. Crawford, K. Goldstone, P. Garner-Hamrick, B. Gumbiner, P. McCrea, C. Kintner, C. Noro, and C. Wylie. 1994. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 79:791–803. [DOI] [PubMed] [Google Scholar]
- Hecht, A., C.M. Litterst, O. Huber, and R. Kemler. 1999. Functional characterization of multiple transactivating elements in beta-catenin, some of which interact with the TATA-binding protein in vitro. J. Biol. Chem. 274:18017–18025. [DOI] [PubMed] [Google Scholar]
- Hecht, A., K. Vleminckx, M.P. Stemmler, F. van Roy, and R. Kemler. 2000. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 19:1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck, L., W.J. Nelson, and J. Papkoff. 1994. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 124:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, A.H., D.B. Stewart, D.V. Laurents, W.J. Nelson, and W.I. Weis. 2001. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 276:12301–12309. [DOI] [PubMed] [Google Scholar]
- Huber, A.H., and W.I. Weis. 2001. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 105:391–402. [DOI] [PubMed] [Google Scholar]
- Huber, O., M. Krohn, and R. Kemler. 1997. A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J. Cell Sci. 110:1759–1765. [DOI] [PubMed] [Google Scholar]
- Jho, E., S. Lomvardas, and F. Costantini. 1999. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem. Biophys. Res. Commun. 266:28–35. [DOI] [PubMed] [Google Scholar]
- Kolligs, F.T., G. Hu, C.V. Dang, and E.R. Fearon. 1999. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 19:5696–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korswagen, H.C., M.A. Herman, and H.C. Clevers. 2000. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature. 406:527–532. [DOI] [PubMed] [Google Scholar]
- Koslov, E.R., P. Maupin, D. Pradhan, J.S. Morrow, and D.L. Rimm. 1997. Alpha-catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with beta-catenin. J. Biol. Chem. 272:27301–27306. [DOI] [PubMed] [Google Scholar]
- Lickert, H., A. Bauer, R. Kemler, and J. Stappert. 2000. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J. Biol. Chem. 275:5090–5095. [DOI] [PubMed] [Google Scholar]
- Loureiro, J., and M. Peifer. 1998. Roles of Armadillo, a Drosophila catenin, during central nervous system development. Curr. Biol. 8:622–632. [DOI] [PubMed] [Google Scholar]
- McCrea, P.D., W.M. Brieher, and B.M. Gumbiner. 1993. Induction of a secondary body axis in Xenopus by antibodies to β-catenin. J. Cell Biol. 123:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic, S., O. Huber, H. Aberle, S. Arnold, and R. Kemler. 1999. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 112:1237–1245. [DOI] [PubMed] [Google Scholar]
- Perego, C., C. Vanoni, S. Massari, R. Longhi, and G. Pietrini. 2000. Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 19:3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra, J., D. Martinez, J. Castano, S. Miravet, M. Dunach, and A.G. de Herreros. 2001. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 276:20436–20443. [DOI] [PubMed] [Google Scholar]
- Pokutta, S., and W.I. Weis. 2000. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol. Cell. 5:533–543. [DOI] [PubMed] [Google Scholar]
- Polakis, P. 1999. The oncogenic activation of beta-catenin. Curr. Opin. Genet. Dev. 9:15–21. [DOI] [PubMed] [Google Scholar]
- Rimm, D.L., E.R. Koslov, P. Kebriaei, C.D. Cianci, and J.S. Morrow. 1995. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA. 92:8813–8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura, S., S. Miravet, J. Piedra, A. Garcia de Herreros, and M. Dunach. 1999. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734–36740. [DOI] [PubMed] [Google Scholar]
- Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 272:1023–1026. [DOI] [PubMed] [Google Scholar]
- Sanson, B., P. White, and J.P. Vincent. 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 383:627–630. [DOI] [PubMed] [Google Scholar]
- Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA. 96:5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal, F.J., M. Noort Mv, G.J. Strous, and H.C. Clevers. 2002. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 3:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert, J., and R. Kemler. 1994. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes. Commun. 2:319–327. [DOI] [PubMed] [Google Scholar]
- Suh, E.K., and B.M. Gumbiner. 2003. Translocation of beta-catenin into the nucleus independent of interactions with FG-rich nucleoporins. Exp. Cell Res. 290:447–456. [DOI] [PubMed] [Google Scholar]
- Tago, K., T. Nakamura, M. Nishita, J. Hyodo, S. Nagai, Y. Murata, S. Adachi, S. Ohwada, Y. Morishita, H. Shibuya, and T. Akiyama. 2000. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- Tutter, A.V., C.J. Fryer, and K.A. Jones. 2001. Chromatin-specific regulation of LEF-1-beta-catenin transcription activation and inhibition in vitro. Genes Dev. 15:3342–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin, V., C. Bauer, L. Degenstein, B. Wise, and E. Fuchs. 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 104:605–617. [DOI] [PubMed] [Google Scholar]
- Winston, J.T., P. Strack, P. Beer-Romero, C.Y. Chu, S.J. Elledge, and J.W. Harper. 1999. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]