Abstract
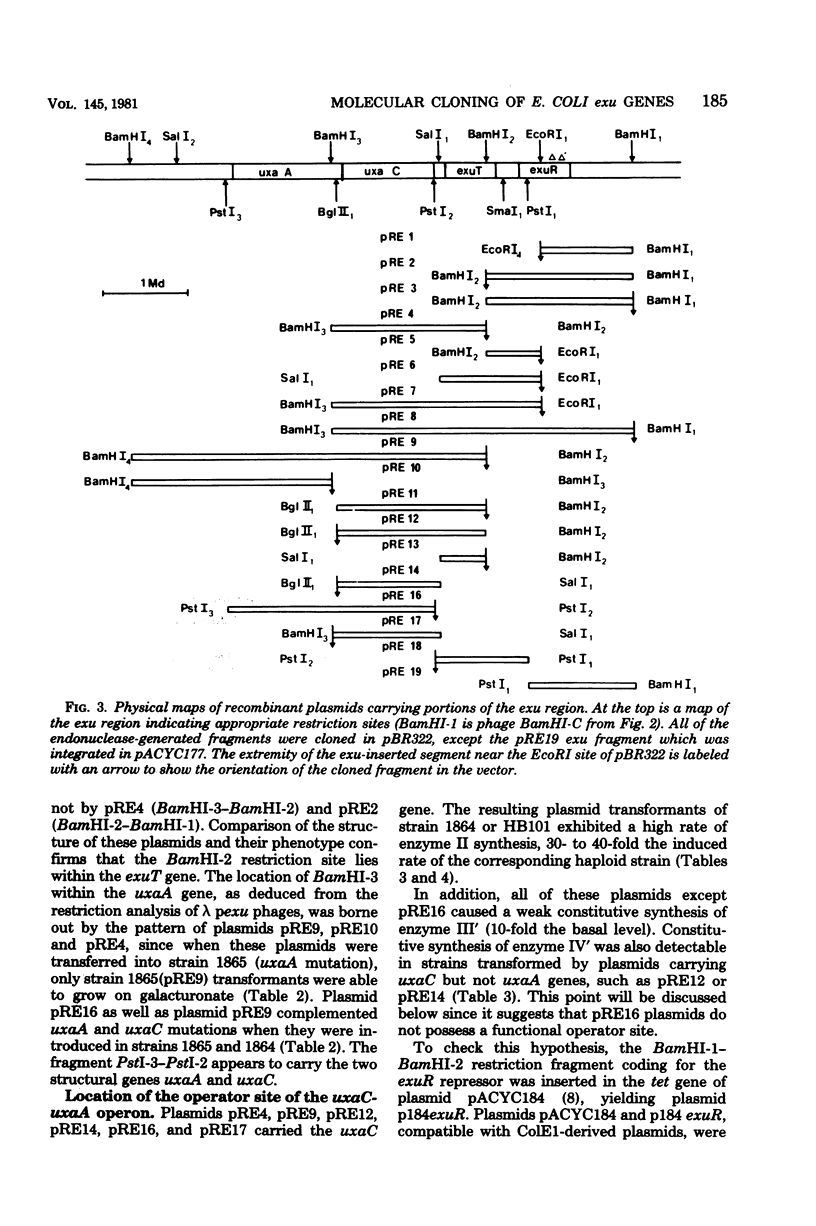
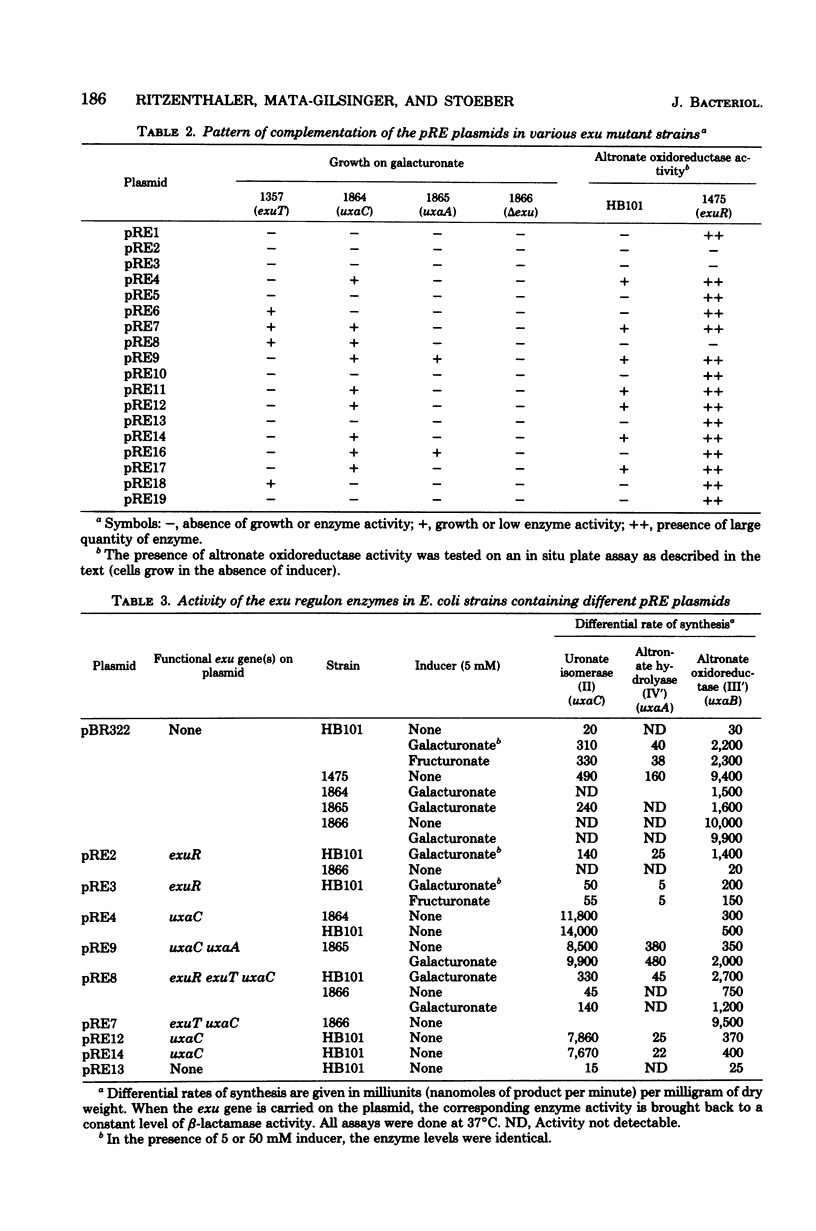
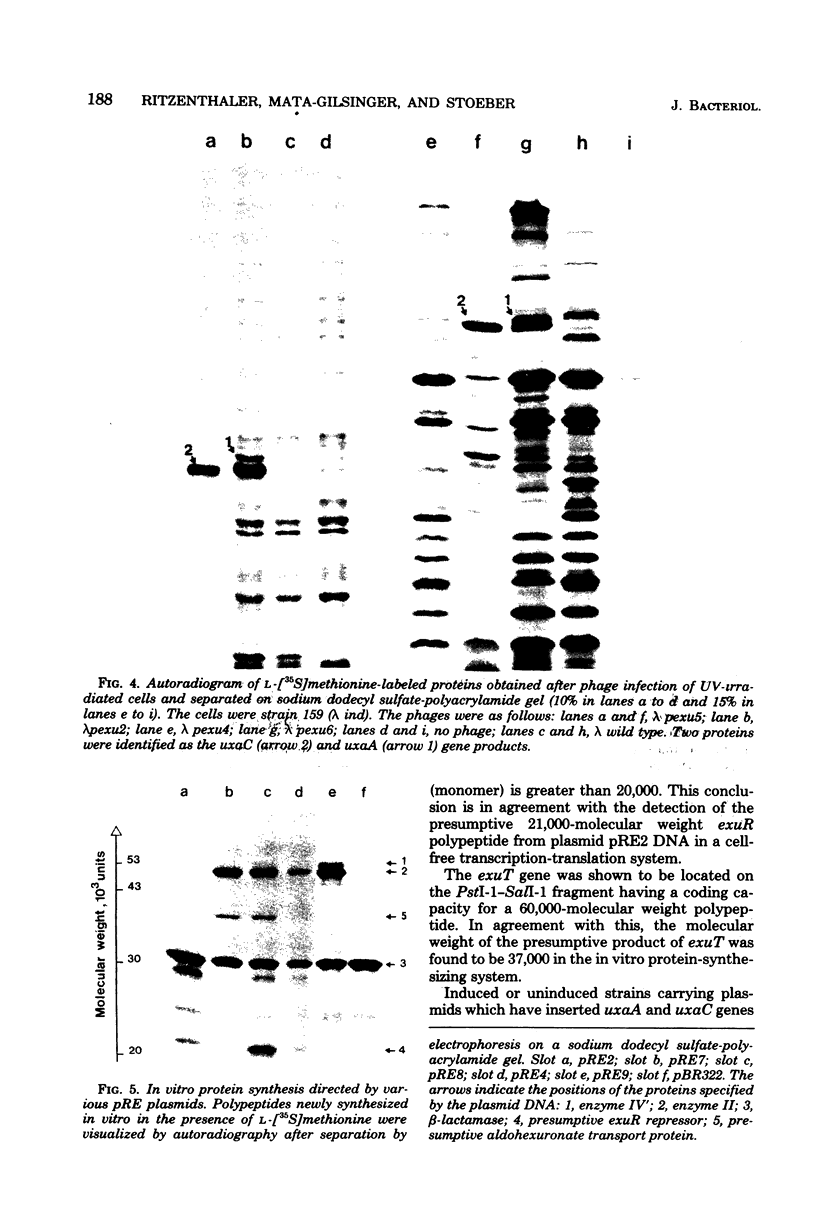
Lambda transducing bacteriophages carrying the exu region (min 66) of Escherichia coli K-12 (lambda pexu) were previously isolated. A restriction map of these phages is presented. Starting from the lambda pexu phage deoxyribonucleic acid, various endonuclease-generated exu fragments were subcloned into multicopy plasmid vectors, using in vitro recombination techniques. The precise location of the exu genes, relative to the endonuclease sites, was determined. Plasmids carrying uxaC and uxaA genes overproduced the corresponding enzymes 30- to 40-fold. When these plasmids were expressed in an in vitro protein-synthesizing system, two polypeptides of 50,500 and 53,000 molecular weights appeared and were identified as the uxaC and uxaA gene products. A 2.6-kilobase-pair deoxyribonucleic acid fragment was shown to code for a functional exuR repressor which controls the expression of the exu region. Plasmids containing this fragment overproduced the regulatory protein. It was possible to localize the operator region, uxaCo, which overlapped a PstI endonuclease site, and to confirm the transcriptional direction of the uxaC-uxaA operon from uxaC to uxaA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Katagiri K. J., Gesteland R. F. Characterization of polypeptides made in vitro from bacteriophage lambda DNA. J Mol Biol. 1973 Aug 25;78(4):589–600. doi: 10.1016/0022-2836(73)90281-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mata M., Delstanche M., Robert-Baudouy J. Isolation of specialized transducing bacteriophages carrying the structural genes of the hexuronate system in Escherichia coli K-12: exu region. J Bacteriol. 1978 Feb;133(2):549–557. doi: 10.1128/jb.133.2.549-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoz G., Robert-Baudouy J., Stoeber F. Physiological and genetic regulation of the aldohexuronate transport system in Escherichia coli. J Bacteriol. 1976 Aug;127(2):706–718. doi: 10.1128/jb.127.2.706-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M. Tight-binding repressors of the lac operon: selection system and in vitro analysis. J Bacteriol. 1979 Jan;137(1):137–145. doi: 10.1128/jb.137.1.137-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. Dosages colorimétriques des oxydoréductases aldoniques d'Escherichia coli K 12: applications. Biochim Biophys Acta. 1972 Nov 10;289(1):19–27. doi: 10.1016/0005-2744(72)90103-9. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. La D-altronate: NAD-oxydoréductase d'Escherichia coli K12. Purification, propriétés et individualité. Eur J Biochem. 1972 Mar 15;26(1):50–61. doi: 10.1111/j.1432-1033.1972.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Portalier R., Robert-Baudouy J., Stoeber F. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J Bacteriol. 1980 Sep;143(3):1095–1107. doi: 10.1128/jb.143.3.1095-1107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M., Stoeber F. Construction and expression of hybrid plasmids containing Escherichia coli K-12 uxu genes. J Bacteriol. 1980 Sep;143(3):1116–1126. doi: 10.1128/jb.143.3.1116-1126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Régulation due métabolisme des hexuronates chez Escherichia coli K12. Modalités de l'induction des enzymes du système hexuronate. Eur J Biochem. 1974 Mar 15;43(1):1–15. doi: 10.1111/j.1432-1033.1974.tb03378.x. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Hennecke H. Specialized transducing phage for the initiation factor 3 gene in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3970–3974. doi: 10.1073/pnas.74.9.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]