Abstract
Regulation of actin polymerization is essential for cell functioning. Here, we predict a novel phenomenon—the force-driven polymerization of actin filaments mediated by proteins of the formin family. Formins localize to the barbed ends of actin filaments, but, in contrast to the standard capping proteins, allow for actin polymerization in the barbed direction. First, we show that the mechanism of such “leaky capping” can be understood in terms of the elasticity of the formin molecules. Second, we demonstrate that if a pulling force acts on the filament end via the leaky cap, the elastic stresses can drive actin polymerization. We estimate that a moderate pulling force of ∼3.4 pN is sufficient to reduce the critical actin concentration required for barbed end polymerization by an order of magnitude. Furthermore, the pulling force increases the polymerization rate. The suggested mechanism of force-driven polymerization could be a key element in a variety of cellular mechanosensing devices.
Introduction
Actin polymerization plays a pivotal role in fundamental cellular processes such as locomotion, cytokinesis, and adhesion. It has been predicted theoretically (Mogilner and Oster, 1996) and recently demonstrated in single filament experiments (Kovar and Pollard, 2004) that actin polymerization produces mechanical forces. These forces are apparently responsible for different forms of cell motility and, in particular, extension of cell protrusions (Mogilner and Oster, 2003; Pollard and Borisy, 2003). The principles of thermodynamics predict not only that polymer growth can produce a force but also that an external force can control polymerization (Hill and Kirschner, 1982; Hill, 1987).
Pulling forces applied to actin filaments can be developed by myosin-type molecular motors (Howard, 2001). Force-enhanced actin polymerization could be involved in a large spectrum of cellular mechanisms related to mechanosensitivity such as stress fiber and focal adhesion formation driven by myosin II–mediated contractility or by externally applied forces (Burridge and Chrzanowska-Wodnicka, 1996; Galbraith and Sheetz, 1998; Geiger and Bershadsky, 2002; Bershadsky et al., 2003). However, effects of pulling forces on actin polymerization have never been studied.
A major challenge is to understand the specific mechanisms by which a force can drive actin polymerization in the cell. A variety of actin-binding proteins are known to regulate actin assembly (Higgs and Pollard, 2001; Pantaloni et al., 2001). Recently, the novel and important family of formin homology proteins was recognized to control actin polymerization (Pollard, 2004; Zigmond, 2004). Notably, one member of this family, diaphanous-related formin mDia1, has been proposed to mediate the force-dependent assembly of focal adhesions (Riveline et al., 2001).
The present study suggests a mechanism for force-driven actin polymerization, in which a crucial role is played by formins.
Formins are processive cappers
The multidomain formin proteins exhibit features of both nucleators and cappers of actin filaments (Wallar and Alberts, 2003; Zigmond, 2004). Formins nucleate actin polymerization and remain persistently bound to the barbed ends of the growing filaments (Pruyne et al., 2002; Pring et al., 2003; Zigmond et al., 2003; Kovar and Pollard, 2004; Romero et al., 2004) walking with them during the course of polymerization (Higashida et al., 2004). Based on these observations, formins are considered to be “processive” cappers (Zigmond et al., 2003), which, in contrast to the usual capping proteins, allow the actin monomers to join the filaments.
All formins contain the highly conserved homology domains 1 (FH1) and 2 (FH2). The FH2 domain binds actin, whereas the FH1 domain mediates formin interaction with another actin-binding protein, profilin (Watanabe et al., 1997). The relative role of the two homology domains in the formin processive capping activity is a subject of the ongoing discussion (Copeland et al., 2004; Kovar and Pollard, 2004; Romero et al., 2004; Zigmond, 2004). Functioning of formins as processive cappers can be divided into a passive “leaky” capping (Zigmond et al., 2003) and the ATP-dependent processive motor activity (Romero et al., 2004). The leaky cappers do not use any energy sources and slow down actin polymerization by several tens of percents. The processive motors use the energy of ATP hydrolysis (Dickinson et al., 2004; Romero et al., 2004) and can induce up to a 15-fold acceleration of filament growth (Romero et al., 2004). According to recent papers, the FH2 domains of the majority of formins studied to date (Bni1p, mDia1, mDia2, and FRLa) (Pruyne et al., 2002; Li and Higgs, 2003; Pring et al., 2003; Copeland et al., 2004; Higashida et al., 2004) and FH1FH2 domains of Bni1 in the absence of profilin (Kovar and Pollard, 2004) can act as leaky cappers. The processive motor activity requires profilin, and, hence, involves FH1FH2 domains (Higashida et al., 2004; Romero et al., 2004). The model presented here relies only on the leaky capping properties of formins. At the same time, the predicted effects are applicable also to the processive motors.
The key event necessary for formins to behave as leaky cappers is dimerization (or, perhaps, higher order oligomerization) of their FH2 homology domains (Li and Higgs, 2003; Zigmond et al., 2003; Copeland et al., 2004; Moseley et al., 2004; Shimada et al., 2004; Xu et al., 2004). An FH2 dimer can be regarded as consisting of two hemidimers, each of which can bind to a single actin subunit at the filament barbed end (Xu et al., 2004). The monomeric FH2 domains inhibit polymerization similarly to regular capping proteins (Shimada et al., 2004; Xu et al., 2004), whereas the FH2 dimers exhibit properties of leaky cappers.
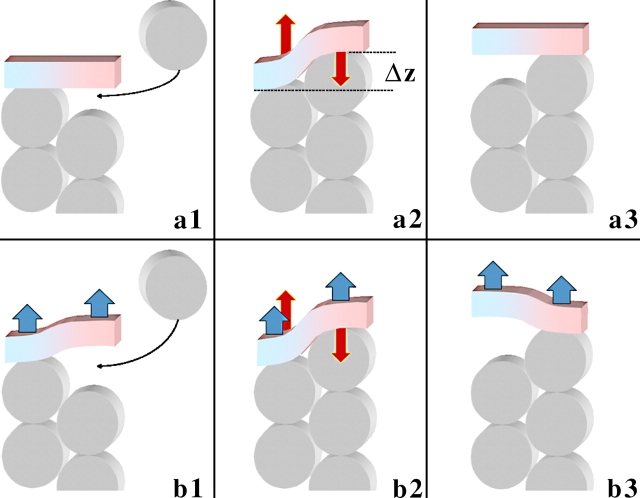
A plausible scenario proposed for the leaky capping is based on the “stair-stepping behavior” of an FH2 dimer associated with an elongating actin filament (Xu et al., 2004). While one of the FH2 hemidimers is bound to the protruding actin subunit, the second one detaches from the recessed (penultimate) subunit, thereby producing a vacancy for a next actin monomer to join the filament (see Fig. 1). In the next step, the two FH2 hemidimers exchange their roles and polymerization proceeds in a stair-stepping manner.
Figure 1.
Stages of leaky capping driven by pulling force and elasticity of formin dimer. Actin subunits are represented by gray discs. The formin dimer is shown as a bar consisting of blue and pink halves (hemidimers). (a) Actin polymerization in the absence of pulling force. (a1) Formin dimer is in a nondeformed state; it is bound to the protruding but not to the recessed actin subunit, thereby leaving a vacancy for a new actin monomer to insert. (a2) An actin monomer inserts into the existing vacancy and binds to the formin hemidimer and the recessed actin subunit. The two binding events are energetically favorable and accompanied by release of energies ɛAB and ɛAF, respectively. At the same time, the formin dimer and, probably, the terminal actin subunits involved in interaction with formin undergo deformation, resulting in a relative shift of the formin hemidimers by distance Δz ≈ 2.75 nm, characterizing the helix periodicity of an actin filament (Lorenz et al., 1993). This results in accumulation of elastic energy ɛEL and development of elastic force f EL (red arrows) tending to restore the initial relative position of the formin subunits. (a3) Elastically driven detachment of formin from the recessed actin subunit accompanied by relaxation of the elastic energy, ɛEL → 0, and elastic force, f EL → 0, at the expense of the actin-formin binding energy. This results in creation of a new vacancy for the next actin monomer. (b) Actin polymerization in the presence of pulling force (blue arrows). Insertion of the new actin monomer (b2) and detachment of formin from the recessed subunit (b3) are facilitated by the pulling force, which reduces the energy of each of these stages by fpull · Δz. As a result, the critical actin concentration required for polymerization at the barbed end is reduced dramatically compared with the critical concentration in the absence of the force.
The simplest form of the stair-stepping scenario requires rotation of the formin cap with respect to the filament axis resulting from the helical structure of actin filament (Kovar and Pollard, 2004; Pollard, 2004). In case the formin cap is immobilized, this should result in rotation of the whole actin filament, which has not been detected in recent experiments (Kovar and Pollard, 2004). Although this issue remains not completely clear, modifications of the stair-stepping mechanism will be, probably, required. Specifically, a “shaft in a bearing”-like connection between actin helix and the FH2 hemidimer (Kovar and Pollard, 2004) can enable the leaky capping without formin rotation. In addition, it is not unambiguously established that FH2 binds exactly at the barbed end, as opposed to near the barbed end, and a possibility remains that an FH2 hemidimer binds to more than two actin subunits.
The common feature of all models for leaky capping irrespective of their detail is that the formin cap moves together with the filament end along the filament axis. This movement requires an alternating binding of the two FH2 hemidimers to the barbed end in such a way that at each step of polymerization the FH2 dimer dissociates from the recessed subunit of the barbed end, hence, preparing conditions for the next step.
Essence of this work
Here, we predict a phenomenon of force-driven actin polymerization based on the phenomenon of leaky capping of actin filaments by formins. Our model is equally applicable for the cases where leaky capping is performed by FH2 dimers or FH1FH2 dimers. We will not distinguish between these cases and refer to the leaky capping domains simply as formins. First, we propose that the mechanism of leaky capping is based on the elasticity of the formin dimer. This simple idea accounts for all the requirements of leaky capping, including the alternating binding of the formin hemidimers to the barbed end subunits. Moreover, predictions of the model agree with the existing data on the critical actin concentrations of polymerization of the free and formin-capped filament ends. Second, we show that if a pulling force is applied to the formin-dimer capping the filament end, the elastic mechanism drives filament growth. Specifically, the critical actin concentration of the barbed end is dramatically reduced compared with that of an uncapped filament, and the rate of polymerization increases.
Results and discussion
Hypothesis of formin dimer elasticity
Model
First, we consider the formin dimer as an elastic molecule, which can undergo deformations accompanied by accumulation of elastic energy. In the initial nondeformed conformation, the two hemidimers of the formin dimer lie in the same plane, as found for isolated FH2 hemidimers (Xu et al., 2004) and represented schematically in Fig. 1 (a1). While binding the new actin monomer, the formin dimer deforms in such a way that the hemidimers are shifted with respect to each other, as illustrated in Fig.1 (a2). Although binding of formin to the barbed end is shown for simplicity as attachment to the tops of the recessed and protruding subunits (Fig. 1, a2), it may involve multiple interaction sites at more than two actin subunits. Deformation of formin and, perhaps, the related elastic deformations of the terminal actin subunits result in the elastic energy ɛEL. The exact character of the molecular deformations resulting from capping of the barbed end by formin dimer (Fig. 1, a2) is not critical for the qualitative essence of the model. In the following sections, we refer to the complex deformation of formin and the barbed end as an effective deformation of the formin dimer. Generally, the elastic energy of the formin dimer deformation ɛEL(Fig. 1, a2) can be approximated by the quadratic law
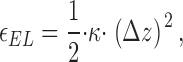 |
(1) |
where κ is the effective rigidity of the formin dimer and Δz is the length of the mutual shift of the formin hemidimers (Fig. 1, a2), which, based on the known structure of actin filaments, should be close to 2.75 nm (Lorenz et al., 1993). The deformation generates an elastic stress, f EL, within the formin dimer (Fig. 1, a2). This stress tends to return the dimer to its initial shape with hemidimers lying in the same plane (Fig. 1, a3) and is determined by the rigidity κ and the deformation Δz,
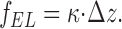 |
(2) |
Formin-dimer elasticity explains leaky capping
We next consider the energy changes accompanying each intermediate step of actin polymerization according to the leaky capping scenario. Our consideration depends only on the shift of the formin hemidimers along the filament axis, which is common for all possible mechanisms of the leaky capping.
In the state where one formin hemidimer is bound to the protruding subunit of the filament end, while the second formin hemidimer is unbound, the formin dimer is in its relaxed nondeformed conformation (Fig. 1, a1).
At the next stage, an actin monomer inserts into the existing vacancy between the recessed subunit of the barbed end and the unbound formin hemidimer (Fig. 1, a2). This event gives rise to energy release, due to binding of the actin monomer to the barbed end and to the formin hemidimer. The related changes of the energy will be denoted as ɛAB and ɛAF, respectively, the two values being negative as the two binding steps are favorable and result in a decrease of the system's energy. In case multiple binding sites exist for a formin hemidimer at the barbed end, the energy ɛAF accounts for all of them.
Insertion of the new actin monomer also results in deformation of the formin dimer (Fig. 1, a2). This generates the elastic stress f EL and leads to accumulation of positive elastic energy ɛEL (Eq. 1). In addition, we must take into account that the actin monomers are dissolved at a concentration c A in the aqueous solution surrounding the actin filament. By joining the barbed end, an actin monomer loses its translational entropy so that the related free energy changes by
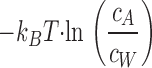 |
(see e.g., Hill, 1987), where k B T ≅ 4 · 10−21 J ≈ 0.6 kcal/M is the product of the Boltzmann constant and the absolute temperature, and c W ≈ 55 M is the concentration of water molecules. Thus, the total energy change of the actin monomer insertion (Fig. 1, a2) is
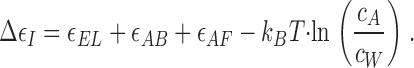 |
(3) |
The elastic stress, f EL, generated at this step within the formin cap acts on its hemidimers. It presses one of the hemidimers against the protruding subunit of the barbed end (Fig. 1, a2), hence, reinforcing binding between them. At the same time, it pulls the second hemidimer attempting to detach it from the recessed subunit of the barbed end (Fig. 1, a2) thereby weakening the connection between them. This means that the elastic stress f EL fulfills the major requirement of the leaky capping model. It produces an effective asymmetric interaction between the two binding sites in such a way that the hemidimer attached to the recessed subunit of the barbed end tends to detach, whereas the one bound to the protruding subunit fastens even more strongly, and hence maintains the connection of the dimer with the actin filament.
In the following step, the formin hemidimer detaches from the recessed subunit of the barbed end under the action of the elastic stress f EL (Fig. 1, a3). This destroys the favorable actin-formin bonds, but allows the elastic energy to relax. The resulting energy change accompanying the detachment is
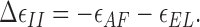 |
(4) |
Comparison with experimental results.
Our model accounts for the quantitative data accumulated on formin-mediated polymerization. First, it explains the observation that the FH2 dimers of Bni1 formin do not alter the critical concentration of actin 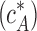 required for barbed end polymerization (Pring et al., 2003). The total energy of one polymerization cycle consisting of the two intermediate steps,
required for barbed end polymerization (Pring et al., 2003). The total energy of one polymerization cycle consisting of the two intermediate steps, 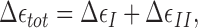 is (according to Eqs. 3 and 4):
is (according to Eqs. 3 and 4):
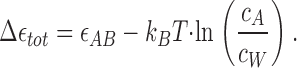 |
(5) |
If this energy change is negative (Δɛtot < 0) polymerization proceeds spontaneously, whereas in the opposite case of Δɛtot > 0 polymerization is energetically unfavorable. A total energy change of zero (Δɛtot = 0) defines the conditions where no net polymerization proceeds. This means that the existing actin filaments do not change their lengths on average over time. The corresponding actin concentration is the critical concentration 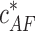 required for polymerization at the barbed end in the presence of the bound formin dimer
required for polymerization at the barbed end in the presence of the bound formin dimer
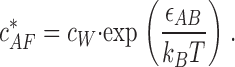 |
(6) |
The critical concentration (Eq. 6) is determined only by the actin-barbed end binding energy ɛAB and is independent of the fact that the barbed end is capped by formin. As a result, 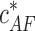 has the same value as the critical concentration for free actin filaments
has the same value as the critical concentration for free actin filaments 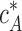 :
:
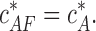 |
(7) |
This is an expected result because a formin molecule attached to the barbed end influences only the boundary subunits of an actin polymer and, consequently, cannot change the thermodynamic characteristics of the system as a whole, such as the critical concentration.
Second, the model accounts for the fact that formins are known to reduce the rate of actin polymerization at the barbed end by several tens of percents (Kovar et al., 2003; Harris et al., 2004; Pollard, 2004; Shimada et al., 2004; Xu et al., 2004; Zigmond, 2004). It has been recognized that in the absence of any capping protein, the on-rate of the barbed end polymerization (k on) is largely controlled by diffusion of actin monomers (Drenckhahn and Pollard, 1986; Pollard and Borisy, 2003). The fact that formins are able to considerably slow down polymerization says that, in this case, the on-rate of the reaction is not limited by diffusion alone but is also determined by the activation energy ɛactiv of insertion of actin monomers into the barbed end capped by formin (Berg and von Hippel, 1985; Hanngi et al., 1990). We suggest that this activation energy arises from the stage of deformation of the formin dimer in the course of insertion of a new actin monomer (Fig. 1, a2) and/or the stage of detachment of the formin hemidimer from the recessed actin subunit (Fig. 1, a3). A thorough analysis of polymerization kinetics in the presence of an elastic leaky capper including the dynamics of formin exchange at the barbed end, and the kinetic effects of actin monomer concentration requires a detailed mathematical approach and will be published elsewhere. On a qualitative level, this analysis shows that the activation energy has to be of the order of several k B T and results from an interplay between the formin-actin binding energy ɛAF, the formin dimer elastic energy ɛEL, and the work performed by the elastic stress f EL in the course of the formin monomer detachment from the recessed subunit of the barbed end. In a simplest scenario of leaky capping, the stage of the formin hemidimer detachment accompanied by relaxation of its elastic energy (Fig. 1, a2) and the following stage of insertion of a new actin monomer requiring formin deformation (Fig.1, a3) provide equal contributions to the effective activation energy. This assumption, along with the requirement that formin slows down actin polymerization by ∼50%, implies that the rates of formin detachment from the recessed subunit, actin monomer supply by diffusion, and formin attachment to the new actin subunit have similar values (unpublished data). In this case, the elastic energy has to possess a value close to that of the actin-formin binding energy, ɛEL≈ ɛAF, where the latter can be estimated based of the dissociation constant of the actin-FH2 complex of Bni1 (Pruyne et al., 2002) as ɛAF≈ −22 · k B T. According to Eqs. 1 and 2, the elastic stress developed in the FH2 dimer and corresponding to the elastic energy of ɛAF≈ −22 · k B T is
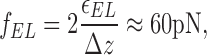
whereas the dimer rigidity can be estimated as
κ*=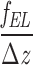 ≈0.02
≈0.02 .
.
The rigidity 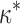 can be related to an effective flexural rigidity k
FORM of the formin dimer by k
FORM ≈
can be related to an effective flexural rigidity k
FORM of the formin dimer by k
FORM ≈ 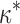 · L
3, where L ≈ 10 nm is the linear dimension of the dimer (Xu et al., 2004). Estimation gives k
FORM ≈ 10−26 J · m2 what is of the same order of magnitude as the flexural rigidity of an actin filament k
A = 6 · 10−26 J · m2 (Yasuda et al., 1996). This may mean that the terminal actin subunits contribute considerably to the effective rigidity of formin dimer. In addition, it is worth noting that, although we have assumed a simplest model for the formin cap elasticity expressed by Eqs. 1 and 2, the qualitative predictions of the model will also hold for a more general case of a nonlinear formin elasticity.
· L
3, where L ≈ 10 nm is the linear dimension of the dimer (Xu et al., 2004). Estimation gives k
FORM ≈ 10−26 J · m2 what is of the same order of magnitude as the flexural rigidity of an actin filament k
A = 6 · 10−26 J · m2 (Yasuda et al., 1996). This may mean that the terminal actin subunits contribute considerably to the effective rigidity of formin dimer. In addition, it is worth noting that, although we have assumed a simplest model for the formin cap elasticity expressed by Eqs. 1 and 2, the qualitative predictions of the model will also hold for a more general case of a nonlinear formin elasticity.
The elastic model provides specific predictions about the dependence of leaky capping on the effective rigidity κ of the formin dimer. Increase or decrease of κ with respect to the optimal value, 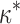 , must shift the formin dimer properties toward those of a regular rather than leaky capper. Indeed, an excessive stiffening of formin dimer, κ ≫
, must shift the formin dimer properties toward those of a regular rather than leaky capper. Indeed, an excessive stiffening of formin dimer, κ ≫ 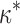 , should eliminate the leaky capping by disfavoring the formin dimer deformation at the stage of insertion of a new actin monomer into the existing vacancy (Fig. 1, a2), and, hence, producing a high energy barrier of the reaction. In case the formin dimer is too flexible, κ ≪
, should eliminate the leaky capping by disfavoring the formin dimer deformation at the stage of insertion of a new actin monomer into the existing vacancy (Fig. 1, a2), and, hence, producing a high energy barrier of the reaction. In case the formin dimer is too flexible, κ ≪ 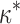 , the developed elastic force f
EL will be insufficiently strong to detach the formin hemidimer from the recessed actin subunit of the barbed end (Fig. 1, a3) within a relevant time scale. As a result, both the recessed and the protruding subunits will be blocked practically irreversibly. The predicted limited range of formin elasticity enabling the leaky capping can be verified experimentally provided a possibility is found to engineer systematically the elastic properties of formin molecules.
, the developed elastic force f
EL will be insufficiently strong to detach the formin hemidimer from the recessed actin subunit of the barbed end (Fig. 1, a3) within a relevant time scale. As a result, both the recessed and the protruding subunits will be blocked practically irreversibly. The predicted limited range of formin elasticity enabling the leaky capping can be verified experimentally provided a possibility is found to engineer systematically the elastic properties of formin molecules.
Importantly, as it follows from the detailed analysis, within the elastic model, the on-rate of the reaction (k on) is very sensitive to the formin dimer rigidity κ because, according to the Arrhenius law, the on-rate depends exponentially on the activation energy
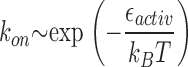 |
(Hanngi et al., 1990), which, in turn, depends linearly on the rigidity ɛactiv ∼ κ. Such strong dependence may explain the considerable difference in the observed slowing down of polymerization by different formins (Kovar et al., 2003; Zigmond et al., 2003; Harris et al., 2004), which may be characterized by different values of the rigidity κ. Structural analysis in conjunction with single molecules measurements is needed to verify agreement of this prediction with the known differences in structures and actin polymerization activities of different formins.
Pulling force can drive actin polymerization
Let us now consider the process of addition of a new actin monomer to the barbed filament end capped by a formin dimer, which is subject to a pulling force. For convenience, we denote by f pull the force pulling one hemidimer, as illustrated in Fig. 1 (b1–b3) by blue arrows, so that the total pulling force equals 2f pull. Similarly to the scenario presented in the previous section (Fig. 1 a), the cycle consists of attachment of the actin monomer generating elastic deformation of the formin dimer and subsequent detachment of formin hemidimer from the recessed subunit of the barbed end producing a vacancy for the next actin monomer (Fig. 1 b). The pulling force facilitates each of the intermediate stages of this process.
Deformation of the formin dimer at the first stage is related to shifting one of the formin hemidimers with respect to the other by Δz = 2.75 nm (Fig. 1, a2). The force f pull pulling the formin hemidimer in the direction of the shifting (Fig. 1, b1 and b2) reduces the energy of this event by ɛpull= −f pull · Δz. As a result, this step becomes more favorable energetically, its energy being
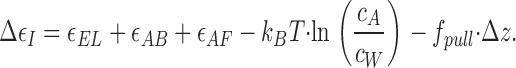 |
(8) |
Also, the subsequent step of detachment of the formin hemidimer from the recessed part of the barbed end becomes energetically more favorable. It is accompanied by a shift of the detaching subunit of about the same distance (Δz = 2.75 nm) along the direction of the force application (Fig. 1, b3). The energy change accompanying this step accounting for the effect of the pulling force is
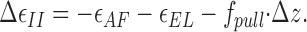 |
(9) |
The total energy change of the polymerization cycle, Δɛtot = ΔɛI + ΔɛII, is
 |
(10) |
Based on Eq. 10, the critical actin concentration 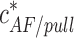 corresponding to Δɛtot = 0 upon action of the pulling force becomes exponentially small as compared with the critical concentration in the absence of the force,
corresponding to Δɛtot = 0 upon action of the pulling force becomes exponentially small as compared with the critical concentration in the absence of the force, 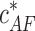 , which is equal to the critical concentration of a pure barbed end,
, which is equal to the critical concentration of a pure barbed end, 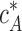 . Accounting for Eqs. 6, 7, and 10, it is given by
. Accounting for Eqs. 6, 7, and 10, it is given by
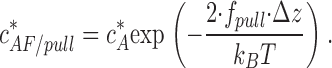 |
(11) |
Based Eq. 11, a total pulling force, which is as little as 2f pull ≈ 3.4 pN, is predicted to reduce the critical concentration by 10-fold.
The generality of the result (Eq. 11), according to which the critical concentration decreases exponentially with the pulling force, should go beyond any particular model for the formin dimer. Consideration based on general thermodynamics (Hill, 1987) predicts that a pulling force stretching a regular polymer decreases the chemical potential of the polymerized monomers and, consequently, decreases the critical concentration according to an equation similar to Eq. 11.
According to Eq.11, polymerization can occur at very low actin concentrations, provided an appropriately strong pulling force is applied to the filament end. However, it is worth noting that in this case diffusion limitations will significantly slow down the force-driven filament growth. Furthermore, at low actin concentrations, additional factors, such as occasional filament severing, which have not been taken into account by the present model, may limit actin polymerization.
It is also essential that the pulling force should not be too large; otherwise, in addition to promoting polymerization, the force may detach the entire formin dimer from the actin filament. According to our estimations presented in the previous section, an elastic force of ∼60 pN would result in fast separation between the formin hemidimer and the recessed part of the barbed end. If a pulling force of the same order of magnitude is applied to the formin hemidimer attached to the protruding part, the entire connection between FH2 dimer and the barbed end is expected to break within a short time scale. Based on these considerations, we predict that the pulling force per formin hemidimer generated in the system and able to accelerate the growth of the actin filament must be in the piconewton range but smaller than several tens of piconewtons.
Our discussion was limited to the effects of the pulling force on the critical concentration characterizing the equilibrium properties of the system. However, it is worth noting that the pulling force can, to some extent, also influence the kinetics of polymerization by reducing the activation energy of this process. As has been suggested, the activation energy ɛactiv results from the formin cap and limits the rate of actin monomer insertion. Upon the application of force, this activation energy becomes
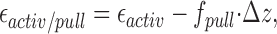 |
(12) |
so that a small pulling force is sufficient to eliminate it, and, hence, to speed the polymerization process. Estimating ɛactiv in the absence of the force as few k B T, we obtain that a pulling force of several piconewtons is sufficient to remove the reduction of the polymerization rate imposed by formin and to accelerate polymerization as compared with the case where the filament ends are capped by the formin dimers.
Recently, other mechanisms facilitating actin polymerization have been suggested (Dickinson et al., 2004; Romero et al., 2004). In a theoretical work by Dickinson et al. (2004), it has been proposed that hypothetical filament end-tracking proteins can work as molecular motors, which use the energy of actin driven ATP hydrolysis for enhancement of actin polymerization. In line with this theoretical idea, the experimental study by Romero et al. (2004) shows that the barbed end polymerization is dramatically accelerated by a complex of FH1FH2 and profilin, which speeds up the actin-ATP hydrolysis. The mechanism of the force-driven actin polymerization predicted by our model is independent of the ATP hydrolysis and must produce new effects as well as contribute additively to the action of the ATP-driven motors. Although the mDia1 FH1FH2 domains with profilin do not change the critical concentration of actin polymerization (Romero et al., 2004), the pulling force is predicted to considerably reduce this value. The effects of the pulling force on the polymerization rate have to reinforce the ATP hydrolysis–driven effects. However, it is worth noting that the pulling force effects predicted in this work do not require profilin and, therefore, could be especially pronounced in cell compartments lacking this protein.
Conclusions
The major result of this work is the proposal of a molecular model predicting force-driven actin polymerization mediated by formin dimers capping the filament ends.
We envision several lines of possible experimental verification of this prediction. First, such force could be applied to the formin caps of actin filaments growing in vitro. This can be done by immobilizing the formin complex and creating a pulling force affecting the growing filaments using laser tweezers or hydrodynamic flow. Individual myosin molecules could also produce forces sufficient for significant stimulation of formin-mediated actin polymerization (Howard, 2001), which can serve as a basis for another kind of in vitro setup. The second approach is probing the elastic properties of formins in single molecule experiments such as atomic force microscopy. Finally, the third direction is to attempt to identify force-driven polymerization in natural cell processes involving actin and formins. In general, this phenomenon can drive processes where a pulling force is produced by myosin interacting with the growing actin filament, provided that the formin complex capping the filament end is anchored at cell cortex or membrane. In such a system, tension generated in the filament is transmitted to the formin cap and pulls it away from the membrane. A counter force is then developed by the membrane, which may deform, but does not move. This counter force pulls the barbed end via the formin dimer. The proper association of formin with cortex/membrane is crucial for the feasibility of the proposed mechanism.
Force-driven polymerization involving only two kinds of proteins could be a key element in a variety of cellular mechanosensing devices including focal adhesions. A similar sort of mechanism could, in principle, explain the force-enhanced self-assembly of other cytoskeletal polymers, e.g., microtubules. Moreover, one can think about chimeric capping proteins containing built-in elastic elements, which may behave according to the predictions of the present model and, hence, mediate force-enhanced actin polymerization. This hypothesis opens an avenue for engineering molecular nano-devices for the local control of filament polymerization using applied force.
Acknowledgments
We are grateful to Tom Shemesh for productive discussions and critical reading of the manuscript.
The work of M.M. Kozlov is supported by the Human Frontier Science Program Organization, the Israel Science Foundation (ISF), and the Binational USA-Israel Science Foundation (BSF). A.D. Bershadsky holds the Joseph Moss Chair of Biomedical Research and his work is supported by ISF, BSF, and the Minerva Foundation (Munich, Germany).
References
- Berg, O.G., and P.H. von Hippel. 1985. Diffusion-controlled macromolecular interactions. Annu. Rev. Biophys. Biophys. Chem. 14:131–160. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A.D., N.Q. Balaban, and B. Geiger. 2003. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and M. Chrzanowska-Wodnicka. 1996. Focal adhesions, contractility and signaling. Annu. Rev. Cell Dev. Biol. 12:463–518. [DOI] [PubMed] [Google Scholar]
- Copeland, J.W., S.J. Copeland, and R. Treisman. 2004. Homo-oligomerisation is essential for F-actin assembly by the formin family FH2 domain. J Biol Chem. 27950250–50256; 10.1074/jbc.M404429200. [DOI] [PubMed]
- Dickinson, R.B., L. Caro, and D.L. Purich. 2004. Force generation by cytoskeletal filament end-tracking motors. Biophys. J. 87:2838–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn, D., and T.D. Pollard. 1986. Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J. Biol. Chem. 261:12754–12758. [PubMed] [Google Scholar]
- Galbraith, C.G., and M.P. Sheetz. 1998. Forces on adhesive contacts affect cell function. Curr. Opin. Cell Biol. 10:566–571. [DOI] [PubMed] [Google Scholar]
- Geiger, B., and A. Bershadsky. 2002. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 110:139–142. [DOI] [PubMed] [Google Scholar]
- Hanngi, P., P. Talkner, and M. Borkovec. 1990. Reaction-rate theory: fifty years after Kramers. Reviews of Modern Physics. 62:251–341. [Google Scholar]
- Harris, E.S., F. Li, and H.N. Higgs. 2004. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 279:20076–20087. [DOI] [PubMed] [Google Scholar]
- Higashida, C., T. Miyoshi, A. Fujita, F. Oceguera-Yanez, J. Monypenny, Y. Andou, S. Narumiya, and N. Watanabe. 2004. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 303:2007–2010. [DOI] [PubMed] [Google Scholar]
- Higgs, H.N., and T.D. Pollard. 2001. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70:649–676. [DOI] [PubMed] [Google Scholar]
- Hill, T.L. 1987. Linear Aggregation Theory in Cell Biology. Springer Verlag, New York. 305 pp.
- Hill, T.L., and M.W. Kirschner. 1982. Bioenergetics and kinetics of microtubule and actin filament assembly-disassembly. Int. Rev. Cytol. 78:1–125. [PubMed] [Google Scholar]
- Howard, J. 2001. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer, Sunderland, MA. 384 pp.
- Kovar, D.R., and T.D. Pollard. 2004. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc Natl Acad Sci USA. 101:14725–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., J.R. Kuhn, A.L. Tichy, and T.D. Pollard. 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., and H.N. Higgs. 2003. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13:1335–1340. [DOI] [PubMed] [Google Scholar]
- Lorenz, M., D. Popp, and K.C. Holmes. 1993. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J. Mol. Biol. 234:826–836. [DOI] [PubMed] [Google Scholar]
- Mogilner, A., and G. Oster. 1996. Cell motility driven by actin polymerization. Biophys. J. 71:3030–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner, A., and G. Oster. 2003. Polymer motors: pushing out the front and pulling up the back. Curr. Biol. 13:R721–R733. [DOI] [PubMed] [Google Scholar]
- Moseley, J.B., I. Sagot, A.L. Manning, Y. Xu, M.J. Eck, D. Pellman, and B.L. Goode. 2004. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell. 15:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni, D., C. Le Clainche, and M.F. Carlier. 2001. Mechanism of actin-based motility. Science. 292:1502–1506. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D. 2004. Formins coming into focus. Dev. Cell. 6:312–314. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., and G.G. Borisy. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. [DOI] [PubMed] [Google Scholar]
- Pring, M., M. Evangelista, C. Boone, C. Yang, and S.H. Zigmond. 2003. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 42:486–496. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond, A. Bretscher, and C. Boone. 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science. 297:612–615. [DOI] [PubMed] [Google Scholar]
- Riveline, D., E. Zamir, N.Q. Balaban, U.S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A.D. Bershadsky. 2001. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, S., C. Le Clainche, D. Didry, C. Egile, D. Pantaloni, and M.F. Carlier. 2004. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 119:419–429. [DOI] [PubMed] [Google Scholar]
- Shimada, A., M. Nyitrai, I.R. Vetter, D. Kuhlmann, B. Bugyi, S. Narumiya, M.A. Geeves, and A. Wittinghofer. 2004. The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol. Cell. 13:511–522. [DOI] [PubMed] [Google Scholar]
- Wallar, B.J., and A.S. Alberts. 2003. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13:435–446. [DOI] [PubMed] [Google Scholar]
- Watanabe, N., P. Madaule, T. Reid, T. Ishizaki, G. Watanabe, A. Kakizuka, Y. Saito, K. Nakao, B. Jokusch, and S. Narumiya. 1997. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and a ligand for profilin. EMBO J. 16:3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., J.B. Moseley, I. Sagot, F. Poy, D. Pellman, B.L. Goode, and M.J. Eck. 2004. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 116:711–723. [DOI] [PubMed] [Google Scholar]
- Yasuda, R., H. Miyata, and K. Kinosita Jr. 1996. Direct measurement of the torsional rigidity of single actin filaments. J. Mol. Biol. 263:227–236. [DOI] [PubMed] [Google Scholar]
- Zigmond, S.H. 2004. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 16:99–105. [DOI] [PubMed] [Google Scholar]
- Zigmond, S.H., M. Evangelista, C. Boone, C. Yang, A.C. Dar, F. Sicheri, J. Forkey, and M. Pring. 2003. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13:1820–1823. [DOI] [PubMed] [Google Scholar]