Abstract
We have used a modified, dual pipette assay to quantify the strength of cadherin-dependent cell–cell adhesion. The force required to separate E-cadherin–expressing paired cells in suspension was measured as an index of intercellular adhesion. Separation force depended on the homophilic interaction of functional cadherins at the cell surface, increasing with the duration of contact and with cadherin levels. Severing the link between cadherin and the actin cytoskeleton or disrupting actin polymerization did not affect initiation of cadherin-mediated adhesion, but prevented it from developing and becoming stronger over time. Rac and Cdc42, the Rho-like small GTPases, were activated when E-cadherin–expressing cells formed aggregates in suspension. Overproduction of the dominant negative form of Rac or Cdc42 permitted initial E-cadherin–based adhesion but affected its later development; the dominant active forms prevented cell adhesion outright. Our findings highlight the crucial roles played by Rac, Cdc42, and actin cytoskeleton dynamics in the development and regulation of strong cell adhesion, defined in terms of mechanical forces.
Introduction
Prominent among the transmembrane adhesion molecules, cadherins play a key role in establishing and maintaining intercellular adhesion. Cadherin-mediated adhesion is thought to develop by several discrete, sequential steps (Braga, 2002; Jamora and Fuchs, 2002). E-cadherin initiates intercellular contacts by homophilic ligation in the presence of calcium. This triggers association of the cytoplasmic domain of cadherin with the actin cytoskeletal network via α-catenin (α-cat) and β-catenin (β-cat; Vestweber and Kemler, 1985; Kemler, 1993). In epithelial cells, the recruitment of E-cadherin and actin to regions of intercellular contact is essential for the formation and stabilization of adherens junctions (Yonemura et al., 1995; Adams et al., 1996; Yap et al., 1997).
In addition to promote cell adhesion, cadherins often function as ligand-activated cell surface receptors, triggering signals that regulate cell shape, migration, proliferation, differentiation, and survival. These two functions show considerable interdependence, with the regulatory processes exercising feedback control over cell adhesion, often through inside-out signaling (for review see Gumbiner, 2000). GTPases of the Rho family—Rho, Cdc42 and Rac—are known to mediate cadherin-actin signaling and actin reorganization (Braga et al., 1997, Braga, 2002; Yap and Kovacs, 2003). Rho family GTPase activity is also involved in the formation and development of cadherin-dependent cell–cell contacts (Kim et al., 2000; Vasioukhin et al., 2000; Noren et al., 2001; Ehrlich et al., 2002). However, details on the initiation, progressive organization and regulation of E-cadherin–based adhesion remain unclear.
In recent years, several high resolution techniques (e.g., flow chamber assay, atomic force microscopy, and surface force analysis) have been used to investigate aspects of cadherin–cadherin interactions at the level of individual molecules (Baumgartner et al., 2000; Sivasankar et al., 2001; Perret et al., 2002); nevertheless, analysis of the mechanical aspects of cadherin-mediated adhesion at the cellular level has proven more difficult. Various assays used in multiple studies of cadherin-dependent intercellular adhesion (Nose et al., 1988; Friendlander et al., 1989; Steinberg and Takeichi, 1994; Angres et al., 1996; Niessen and Gumbiner, 2002; Duguay et al., 2003) have yielded a basic understanding of the underlying processes. However, these assays typically analyze the behavior of large populations of cells, providing little insight into adhesion at the level of individual cells.
We used a dual pipette assay for measuring the forces required to separate two adherent cells (Daoudi et al., 2004) maintained in suspension to avoid the complicating impact of cell–matrix adhesion and signaling (Monier-Gavelle and Duband, 1997; Gimond et al., 1999). The assay can be used for simultaneous measurement of separation force (SF), a quantitative estimate of cell adhesiveness, and detection of fluorescent proteins involved in adhesion. In this study, we used this assay to quantify intercellular adhesion in terms of mechanical forces at the cellular level and to investigate the mechanisms of adhesion specifically regulated by E-cadherin and the actin cytoskeleton.
Results
Characterization of E-cadherin–expressing cells and measurement of SF between cells by a dual pipette assay
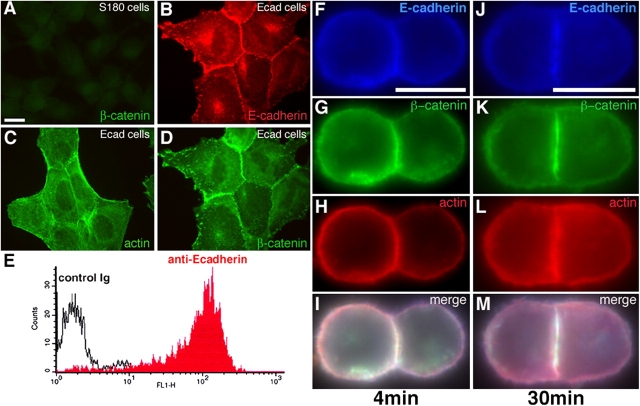
S180 cells contain no detectable β-cat (Fig. 1 A) or cadherins (not depicted) and display minimal cell–cell adhesion in tissue culture (Friendlander et al., 1989; Dufour et al., 1999). By contrast, S180 cells stably transfected to express E-cadherin (Ecad clone) displayed characteristic intercellular adhesion in culture, with E-cadherin, β-cat and actin all detected concentrated at sites of cell–cell adhesion (Fig. 1, B–D). Ecad cells that had been dissociated by trypsin-calcium (TC) treatment (see Materials and methods) expressed E-cadherin on the cell surface (Fig. 1 E) and readily formed doublets or aggregates in suspension. Cell adhesion sites matured over time, becoming enriched in E-cadherin, β-cat and actin, and increasing in area (Fig. 1, F–I vs. J–M). In doublets of S180 cells transiently transfected with pEcad-GFP, E-cadherin–GFP molecules were concentrated at cell–cell interface (Video 3, frames 1–5, available at http://www.jcb.org/cgi/content/full/jcb.200403043/DC1) but were redistributed uniformly in the membrane after separation of the adherent cells (see next paragraph; Video 3, frames 9–12).
Figure 1.
Adhesive properties of Ecad cells. Immunodetection of β-cat (A and D), E-cadherin (B), and actin (C) in S180 cells (A) and Ecad cells (B–D). E, FACS analysis on isolated Ecad cells in suspension, after TC treatment, with an antibody directed against the extracellular domain of E-cadherin. Immunodetection of E-cadherin (F and J), β-cat (G and K), and actin (H and L) in doublets formed in suspension for 4- (F–I) or 30-min (J–M). Merged images are shown in I and M. Bars: (A) 20 μm; (F and J) 10 μm.
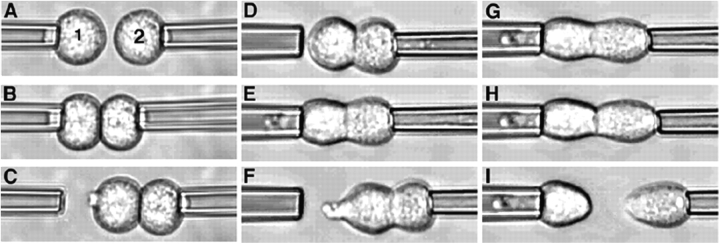
The micromanipulation assay was used to quantify the force required to separate pairs of adherent cells. Cadherin expressing cells held by gentle aspiration at the tips of two micropipettes (Fig. 2 A) were first brought gently into contact and held for a predetermined time (Fig. 2, B and Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200403043/DC1). Fig. 2 D illustrates an example of a doublet of Ecad cells obtained after 4 min of contact (a 4-min doublet), the right pipette withdrawn to visualize the resulting adhesion (Fig. 2 C). Such a doublet was cyclically brought back into contact with the left pipette and then withdrawn to the right, each time after a step-wise increase in the strength of aspiration by the left pipette, until the cells were separated (see Materials and methods; Fig. 2, D–I; Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200403043/DC1). The SF was defined as the aspiration force required to separate the doublet, such that one cell remained in each pipette when the right pipette was withdrawn (Fig. 2 I). SF was considered to be zero for pairs of cells that did not form adherent doublets in this assay.
Figure 2.
Dual micropipette assay. (A) Two cells in suspension (1 and 2) are held under weak aspiration by micropipettes, and placed in contact (B; Video 1). The formation of contact is checked (C) after displacement of the right pipette. (D) Second cell is held by the micropipette under strong aspiration. (E–I) First cell is held by the micropipette and the aspiration applied is increased as the right micropipette displaced, step by step, until the adherent cells are separated (I; Video 2).
Dependence of SF on cadherin's homophilic interaction and its activity
We measured SF for pairs of Ecad cells after different times of contact (Fig. 3 A). Adhesion was initiated rapidly, with cells adhering to each other after only a few seconds of contact (not depicted), but measurements for contact periods of <30 s were not reproducible. At 30 s of contact, a mean force of 20 nN was required to separate adherent cells. From 30 s to 30 min of contact (30-s doublets and 30-min doublets, respectively), the force required to separate the cells increased rapidly. It stabilized at ∼200 nN after 1 h of contact (60-min doublets). Anti–E-cadherin significantly reduced the SF of 4-min doublets (Fig. 3 D), and S180 cells lacking cadherins displayed no detectable adhesion after 4 min (Fig. 3 D) or 30 min of contact (not depicted), both results clearly indicating that the doublet formation was E-cadherin dependent.
Figure 3.
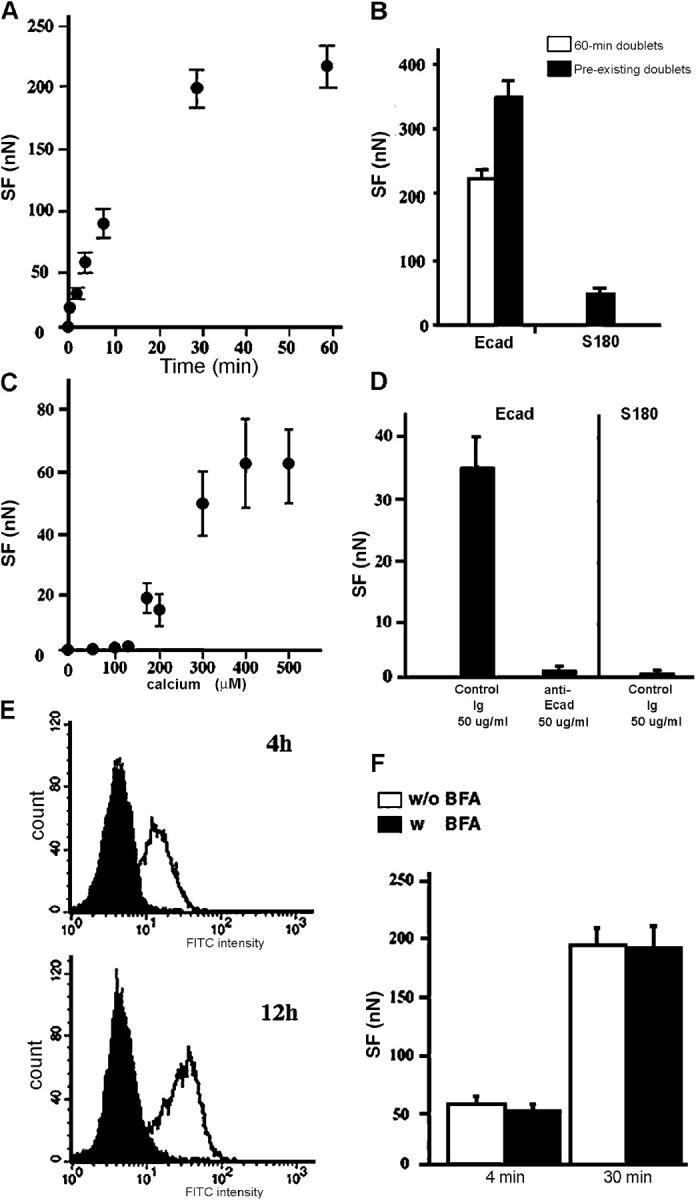
Characterization of Ecad cell adhesion. (A) SF measurements for Ecad cells held in contact for 0.5–60 min. (B) SF required to separate 60-min doublets (white bar) and preexisting doublets (black bars), selected as described in Materials and methods. (C) Dose-response curve of force measurements for 4-min doublets in various concentrations of calcium. (D) The effect of a control or anti–E-cadherin antibody on SF in Ecad or S180 cells. (E) FACS analysis of E-cadherin expression on the surface of Ecad cells treated with 10 μM BFA (black peaks) for 4 and 12 h or untreated (white peaks). (F) The mean SFs measured for 4- or 30-min Ecad doublets treated with 10 μM BFA (black bars) for 1 h or untreated doublets (white bars).
We determined the “maximal” SF using doublets not separated during the dissociation procedure (see Materials and methods). The mean SF for such Ecad “preexisting doublets” (Fig. 3 B) was much higher than that for 60-min doublets (350 nN vs. 200 nN). By contrast, an SF of only 50 nN was obtained for preexisting doublets of S180 cells.
Homophilic interaction is thought to be key to cadherin functions. In our assay, cells expressing similar levels of either E- or N-cadherin (unpublished data) readily formed homotypic doublets and rapidly developed strong adhesion, but the SF displayed by Ecad cells was considerably higher than that of Ncad cells (Table 1). Heterotypic interaction was not detected. Ecad-Ncad pairs held together for times up to 30 min were separated immediately upon withdrawal of the right pipette therefore no SF could be measured.
Table I. Homophilic and heterophilic interactions between Ecad and Ncad cells.
| Cell pair | Ecad-Ecad (homophilic) |
Ncad-Ncad (homophilic) |
Ecad-Ncad (heterophilic) |
|---|---|---|---|
| Formation of doublets | Yes | Yes | No |
| SF at 4 min (nN) | 52.6 ± 7.0 | 7.7 ± 1.4 | 0 |
| SF at 30 min (nN) | 194 ± 14.9 | 46.9 ± 7.8 | 0 |
Calcium dependence is a characteristic feature of E-cadherin–mediated adhesion so we assessed the calcium requirement of Ecad cell adhesion in our assay (Fig. 3 C). For 4-min doublets, no SF could be measured below 100 μM calcium (CaCl2) and by 400 μM calcium, the SF reached a maximum equivalent to that obtained in the control buffer (containing 2 mM CaCl2; Fig. 3 A).
Ecad cells dissociated by TE treatment to degrade surface cadherins progressively recovered cadherins at the cell surface, as shown by FACS analysis performed 4 and 12 h after TE treatment (Fig. 3 E, white peaks). Treatment of cells with 10 μM brefeldin A (BFA; a vesicular transport blocking agent; Misumi et al., 1986) abolished the recovery of E-cadherin at the surface, demonstrating that the drug effectively blocked cadherin export from the cytoplasmic and other newly synthesized pools (Fig. 3 E, black peaks). However, preincubation of TC-treated Ecad cells (retaining their cadherins at the surface) with 10 μM BFA for 1 h had no effect upon the measured SF at 30 min (Fig. 3 F). This indicates that adhesion between the cells of a doublet is mediated mainly by E-cadherins already present at the cell surface, and that export of cadherins from the cytoplasmic pool plays only a minor role at times shorter than 30 min.
Thus, in our experimental system, the SF of paired cells is a function of the type of cadherin expressed, the functional state of cadherin at the cell surface and the duration of contact between cells.
Modulation of SF by E-cadherin expression level
To test the effect of cadherin concentration on cell adhesion, we generated various stably transfected S180 clones differing in the amount of E-cadherin expressed at the cell surface. Clones were selected by FACS, on the basis of homogeneous cadherin expression in all cells of the population. Western blot analyses with anti–E-cadherin and anti–β-cat were used to quantify the levels of these two proteins in each clone. The highest value obtained, that of the Ecad clone, was set at 100% and was treated as the reference clone in the analysis (E100). Clones were renamed based on their E-cadherin levels on Western blots. Clones E2, E14, E38, E41, and E58 were selected for further analysis; their total cadherin levels relative to the Ecad (100%) were 2%, 14%, 38%, 41%, and 58%, respectively (Fig. 4 A). The relative levels of β-cat were similar to those of cadherins, indicating that β-cat content could be used to estimate cadherin content in cells. By contrast, α-cat and p120 levels, and the pattern of tyrosine phosphorylation, were similar in all the clones studied (unpublished data). The results obtained by flow cytometry analysis (Fig. 4 B) were similar to those obtained by Western blotting.
Figure 4.
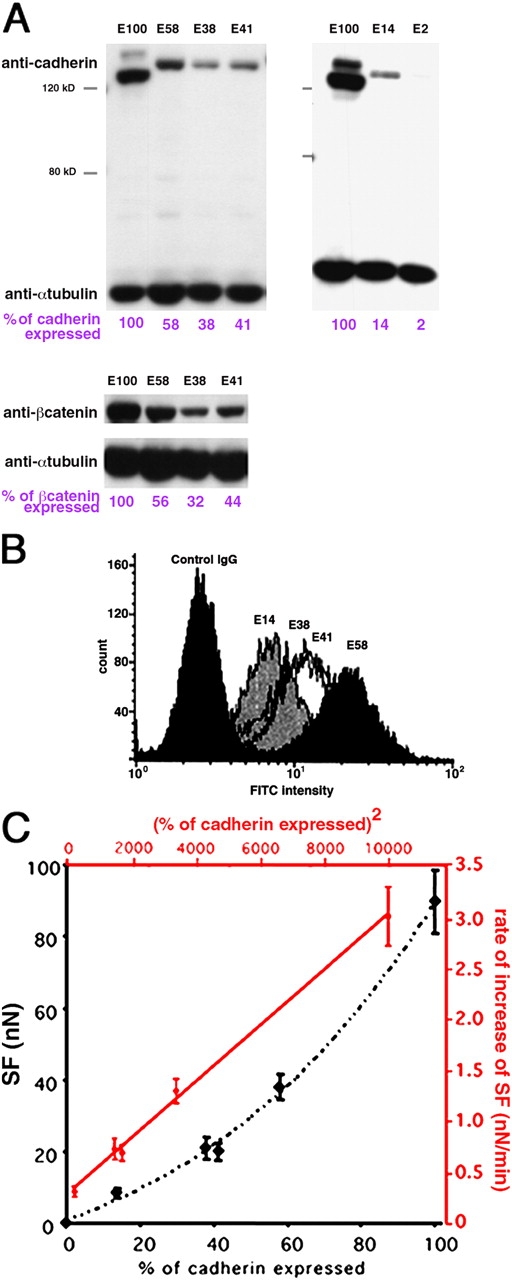
SF depends on cadherin expression at the surface. (A and B) Characterization of clones differing in E-cadherin expression level. (A) Western blot analysis of cell extracts with anti–E-cadherin or β-cat, paired with an anti–α-tubulin. Quantification of cadherin and β-cat in cell extracts is indicated in violet. (B) FACS analysis of E-cadherin expression on the cell surface of four different clones. (C) SF (y axis, nN) measured for 30-min doublets of various clones (x axis, relative cadherin content in %). In red, the rate of increase of SF (y axis, nN/min) varies linearly with the square of the % cadherin expression (x axis). The equation for the best fitting red line is Y = 3 × 10−4 X + 0.2661.
We compared the adhesiveness of these clones (Fig. 4 C, black curve) by measuring the SF for 30-min doublets prepared from each of them. E2 cells, which had the lowest levels of cadherin expression, displayed no significant adhesion at 30 min. Mean SF for the other clones were 8.5 nN for E14, 21 nN for E38, 19.8 nN for E41, 38.1 nN for E58, and 89.5 nN for E100 (Fig. 4 C). Furthermore, the rate of increase of SF, calculated by its augmentation in the first 30 min of contact for the E14–E100 clones, varied linearly with the square of cadherin expression level (Fig. 4 C, red curve). Thus, the SF for 30-min doublets is primarily determined by the amount of E-cadherin expressed at the cell surface.
Role of the cytoplasmic partners of cadherins in the modulation of SF
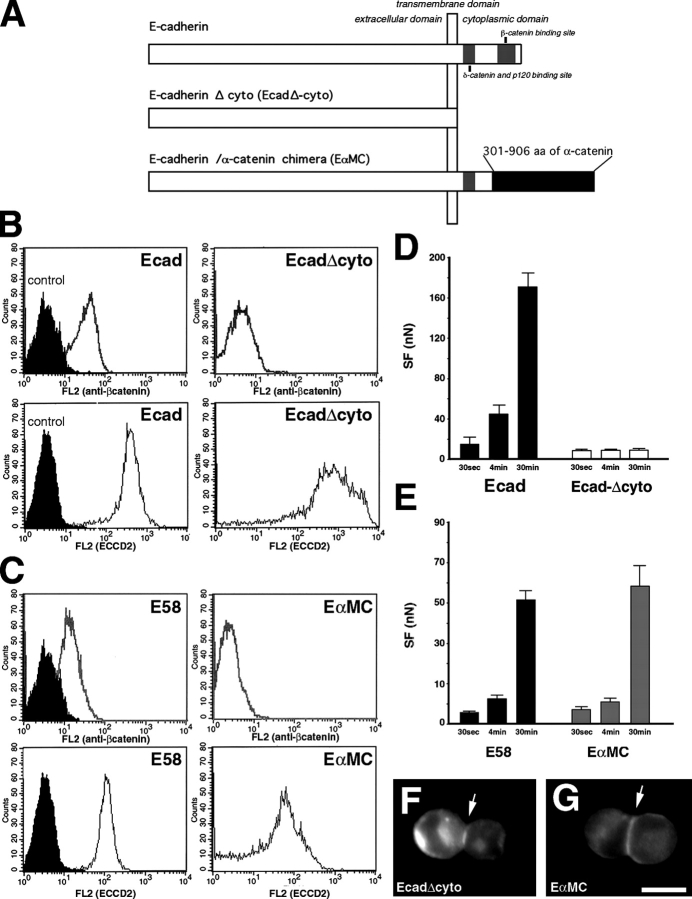
We determined the role of the E-cadherin cytoplasmic domain and its partners in the establishment of cell adhesion by comparing SF in cells expressing wild-type and cytoplasmically modified E-cadherins. Parental S180 cells were transiently cotransfected with pEGFPC1 and a plasmid encoding E-cadherin, E-cadherin lacking the cytoplasmic domain (Ecad-Δcyto) or E-cadherin–α-cat chimera (EαMC; Ozawa, 2002; Fig. 5 A). FACS analysis revealed that GFP-producing cells expressed higher levels of E-cadherin or Ecad-Δcyto (Fig. 5 B, bottom) than EαMC (Fig. 5 C, bottom), whereas the GFP-positive EαMC transfectants expressed an amount of mutant E-cadherin similar to that of the E-cadherin in the expressor clone E58 (Fig. 5 C, bottom). Only cells expressing E-cadherin were shown to coexpress β-cat (Fig. 5, B and C, top), indicating that the lack of a β-cat binding site prevented the mutated cadherins from recruiting this cytoplasmic partner.
Figure 5.
The time-dependent increase in SF depends on the connection of cadherin to the actin cytoskeleton. (A) Schematic representation of the structure of wild-type cadherin, E-cadherin lacking the cytoplasmic domain (Ecad-Δcyto), and E-cadherin–α-cat chimera (EαMC) expressed by transiently transfected S180 cells. FACS analysis of transiently cotransfected cells expressing Ecad (B), Ecad-Δcyto (B), EαMC (C), or E58 cells (C) with anti–β-cat (B and C, top, white peaks), anti–E-cadherin ECCD2 antibody (B and C, bottom, white peaks), or control antibodies (black peaks). (D and E) Mean SF for 30-s, 4- and 30-min doublets of GFP-positive cells expressing E-cadherin (D, black bars), Ecad-Δcyto (D, white bars), EαMC chimera (E, gray bars), and doublets of E58 cells (E, black bars). Immunodetection of Ecad-Δcyto (F) and EαMC (G) proteins in representative doublets formed after 30-min aggregation in suspension. Bar, 10 μm.
GFP-positive cells were held in contact for 30 s, 4 min, and 30 min. Cells expressing the Ecad-Δcyto did not exhibit a time-dependent increase in SF in contrast to the cells expressing E-cadherin or EαMC (Fig. 5, D and E). The Ecad-Δcyto (Fig. 5 F) and EαMC (Fig. 5 G) proteins accumulated at the contact zone (Fig. 5, F and G, arrows) in doublets. Moreover, E58 cells and EαMC transfectants expressing the same range of E-cadherin and EαMC chimera (Fig. 5 C, bottom), respectively, also displayed a similar time-dependent increase in SF. Together, these results indicate that the E-cadherin cytoplasmic domain and its connection to the actin cytoskeleton play a crucial role in the strengthening of cell–cell adhesion.
Role of the cytoskeleton in E-cadherin–mediated intercellular adhesion
The recruitment of actin microfilaments to cell–cell contacts has been shown to promote strong cadherin-mediated adhesion (Imamura et al., 1999). We assessed the impact of the actin cytoskeleton on the establishment of cell adhesion by measuring SF for paired Ecad cells in the presence of either Latrunculin B (LatB) or cytochalasin D, both of which inhibit actin polymerization (Flanagan and Lin, 1980; Spector et al., 1983), or Jasplakinolide (Jasp), a drug inhibiting actin disassembly or promoting actin filament aggregation in a dose-dependent manner (Bubb et al., 1994; Cramer, 1999).
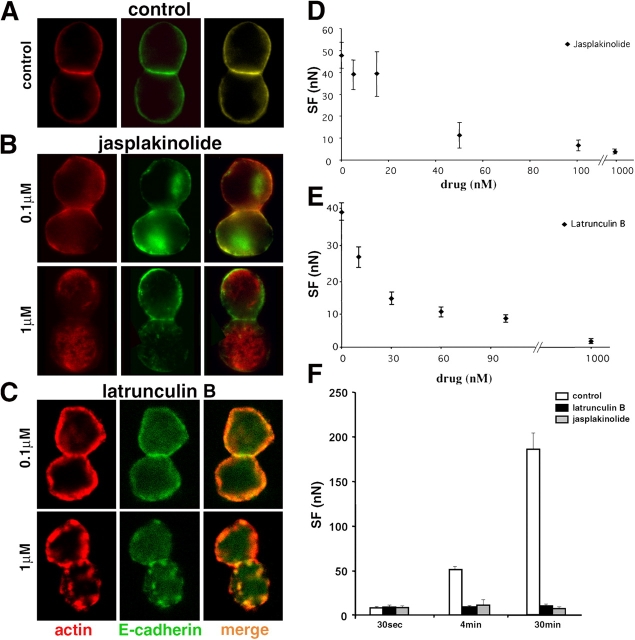
We determined the effects of Jasp and LatB on actin by labeling Ecad doublets with an anti-actin mAb (not depicted) or phalloidin-TRITC (Fig. 6, B and C, respectively). Under control conditions, paired cells displayed a uniform distribution of surface E-cadherin and cortical actin over most of the cell with higher density colocalization of both molecules at the cell–cell interface (Fig. 6 A). Treatment with Jasp at 0.1 μM caused cortical actin and E-cadherin to redistribute in a nonuniform manner everywhere. However, at the contact zone both molecules were still noticeably colocalized. Jasp at 1 μM dramatically reduced the thickness of cortical actin, produced actin aggregates throughout the cytoplasm and eliminated the characteristic E-cadherin/actin colocalization at the cell–cell interface. Immunostaining of actin with mAb or phalloidin gave similar results and showed that, for doublets in suspension, Jasp at both 0.1 and 1 μM mainly induces a disorganization of the actin network reflecting the aggregation/polymerization activity of this drug described by Cramer (1999). LatB at 0.1 μM had no marked effect on the localization of E-cadherin and actin in paired cells in suspension (Fig. 6 C) but 1 μM LatB treatment induced the formation of large actin aggregates in the cytoplasm, E-cadherin clusters at the cell surface and higher levels of E-cadherin staining in the cytoplasm. FACS analysis demonstrated that LatB or Jasp, at concentrations up to 0.3 μM, does not affect E-cadherin expression at the cell surface (unpublished data).
Figure 6.
Drugs affecting actin polymerization perturb actin cytoskeleton organization and decrease SF. Confocal analysis of Ecad doublets formed in suspension under control conditions (A), in the presence of Jasp (B) or LatB (C), and labeled for actin and E-cadherin. Merged images are shown in right panels. The images correspond to a medial transverse plane of the doublet. Dose-response curve of SF for 4-min Ecad doublets in medium containing Jasp (D) or LatB (E). (F) Mean SF for 30-s, 4- and 30-min doublets in the presence of 0.1 μM LatB (black bars), 0.1 μM Jasp (gray bars), or in control medium (white bars).
Jasp (Fig. 6 D), LatB (Fig. 6 E), and cytochalasin D (not depicted) all reduced the SF for Ecad 4-min doublets in a dose-dependent manner. The IC50 for LatB was 21.2 nM, although at this concentration the drug had no visible effect on the distribution of E-cadherin and actin in cells in suspension (Fig. 6 C). Ecad cells treated with 0.1 μM LatB or Jasp formed doublets that displayed initial SF (30 s of contact) identical to that of untreated cells but the treatment abolished the time-dependent increase in SF characteristic of control doublets (Fig. 6 F). Maximal inhibition of adhesion for LatB was achieved at 0.5 μM and was fully reversible upon removal of the drug.
To test whether LatB's effect on SF might be due to changes in cell viscoelasticity and deformability, we used a depletion force-induced adhesion test (Evans and Needham, 1988) on S180 cells with and without LatB. SFs measured in the presence of LatB at up to 0.1 μM were similar to those of the control condition. This result demonstrates that treatment of cells with LatB at concentrations as high as 0.1 μM does not interfere with force measurements in the dual pipette assay (unpublished data).
Thus, the time-dependent increase in the SF for Ecad cells depends principally on actin polymerization and actin cytoskeleton dynamics.
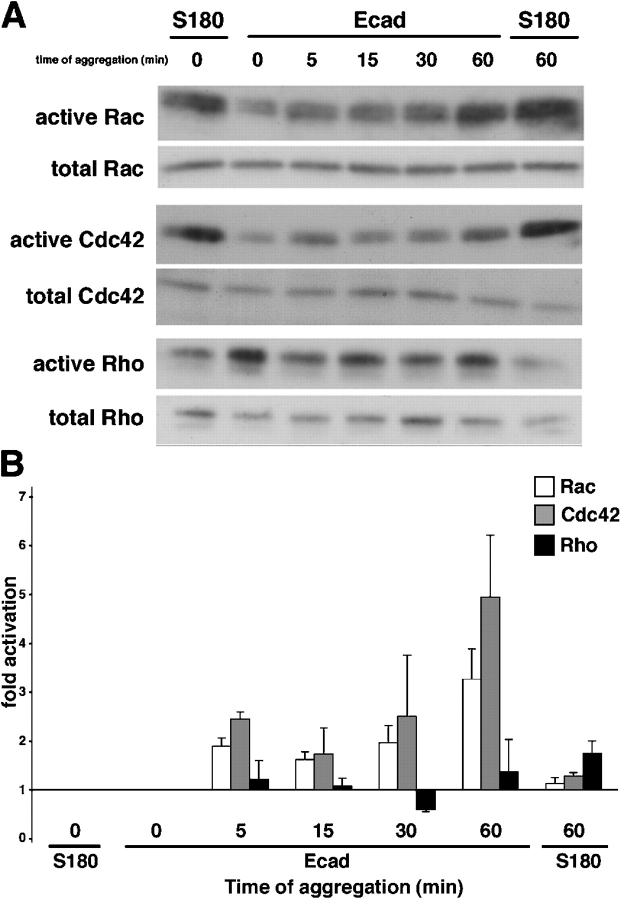
Activation of Rac and Cdc42, but not of Rho, during formation of aggregates of Ecad cells in suspension
We used GTPase pull down assays to test the effect of E-cadherin–mediated intercellular adhesion on endogenous activity of Rho-like GTPases in Ecad cells in suspension. The levels of endogenous active and total Rho-like GTPases were monitored in S180 cells and Ecad cells at different times during a 60-min aggregation assay (Fig. 7A). In S180 cells no change was observed in the activation levels of Rac, Cdc42, and Rho during the assay. In clear contrast with this result, activation of Rac was observed in Ecad cells as soon as 5 min after the start of aggregation and reached a maximum by the end of the assay (Fig. 7 B). The kinetics of activation for Cdc42 were comparable to those described for Rac, but activation of Rho followed a very different pattern (gray, white, and black bars, respectively; Fig. 7 B). The levels of activated Rho in Ecad cells did not significantly change throughout the aggregation assay (Fig. 7 B). Results from total lysates indicated that the differences observed in band densities after precipitation were not due to variations in the total amount of protein. Each GST pull-down assay was repeated three times.
Figure 7.
Activation of small GTPases during Ecad cell aggregation. Representative Western blot analysis of GTP-bound (active) and total Rac, Cdc42, and Rho on S180 and Ecad cells taken at different times of the aggregation assay in suspension (A). (B) Fold activation of the Rac (white bars), Cdc42 (gray bars) and Rho (black bars) GTPases; the activation level at time 0 serves as the reference level. Activation fold represents the mean ± SEM from three independent experiments.
Requirement of Rac and Cdc42, but not of Rho, for E-cadherin mediated adhesion, as evaluated by SF measurements
We transiently transfected Ecad cells with pEGFPC1 alone (transfection control), or with vectors encoding the GFP-tagged constitutively inactive constructs, Cdc42DN, RacDN, and RhoDN or the GFP-tagged constitutively active constructs, Cdc42DA, RacDA, and RhoDA. In suspended isolated cells, GFP (Fig. 8 B) was distributed uniformly throughout the cytoplasm. For Cdc42DN (Fig. 8 F), RacDN (Fig. 8 J), RhoDN (Fig. 8 N), and RacDA (Fig. 9, G and H), we observed homogeneous fluorescence in the cytoplasm and more intense fluorescence close to the cell membrane. This distribution was also observed for Cdc42DA and RhoDA, but to a lesser extent (unpublished data).
Figure 8.
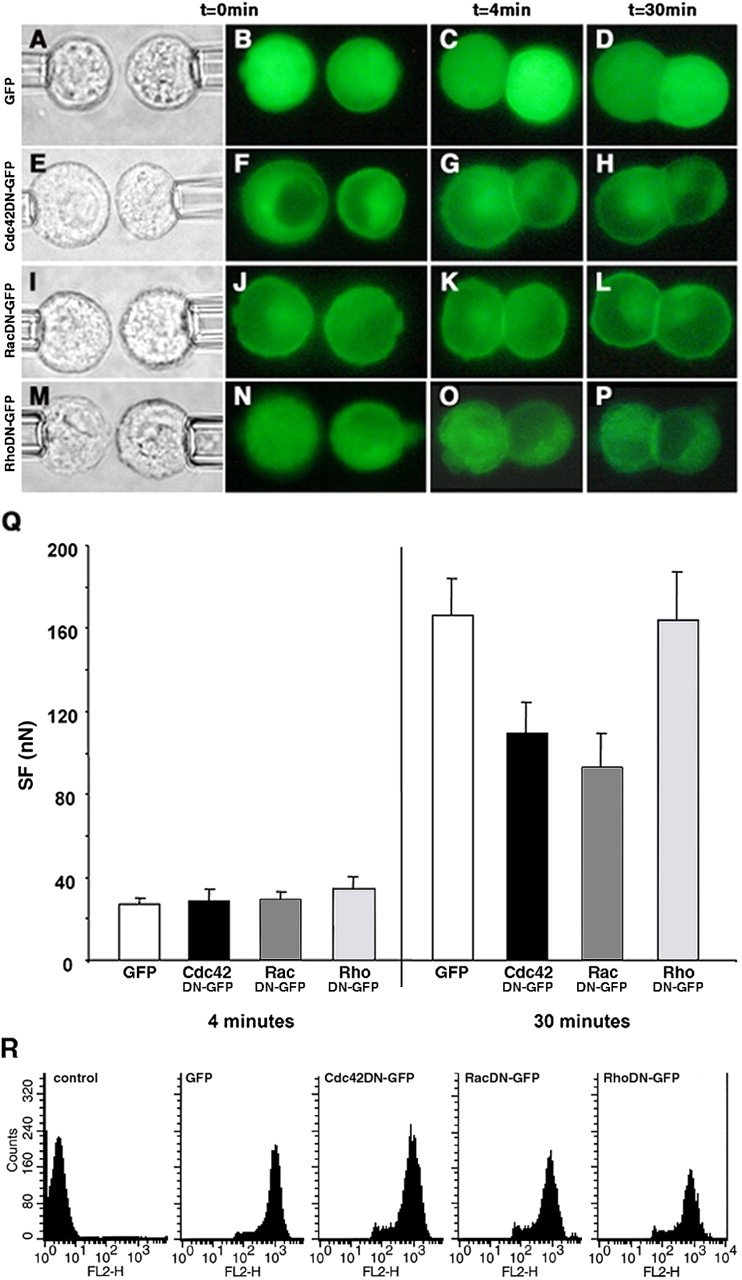
The effect of dominant negative GTPase protein expression on SF. Distribution of GFP-tagged proteins in transfected Ecad cells producing GFP (B–D), and the Cdc42DN (F–H), RacDN (J–L), and RhoDN (N–P) before contact (B, F, J, and N), in 4-min doublets (C, G, K, and O) and in 30-min doublets (D, H, L, and P). Each row represents a series of real-time images of a doublet monitored by light transmission or epifluorescence microscopy before and at 4 and 30 min of contact. Q, SF measured for 4- and 30-min Ecad doublets producing either GFP (white bars), Cdc42DN (black bars), RacDN (dark gray bars), or RhoDN (light gray bars). (R) FACS analysis of transiently transfected Ecad cells, positive for GFP, Cdc42DN, RacDN, or RhoDN, and immunostained with an antibody directed against the extracellular domain of E-cadherin (FL2 channel).
Figure 9.
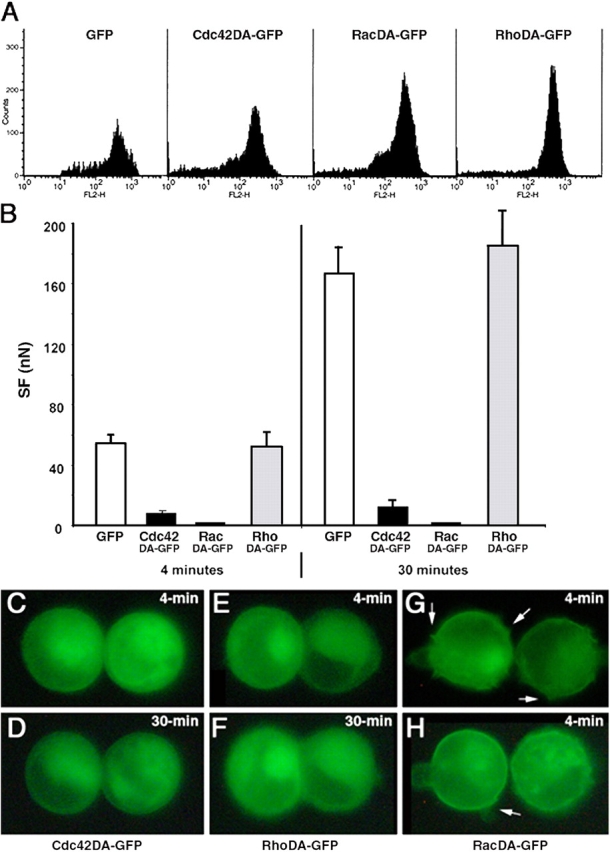
The effect of dominant active GTPase protein expression on SF. (A) FACS analysis of transiently transfected Ecad cells, positive for GFP, Cdc42DA, RacDA or RhoDA, and immunostained with an antibody directed against the extracellular domain of E-cadherin (FL2 channel). (B) SF measured for 4- and 30-min Ecad doublets producing GFP (white bars), Cdc42DA (black bars), RacDA (dark gray bars) and RhoDA (light gray bars). Real-time images showing the distribution of GFP-tagged proteins in 4-min (C, E, G, and H) and 30-min (D and F) doublets of Ecad cells producing Cdc42DA (C and D), RhoDA (E and F), or RacDA (G and H). Arrows in G and H indicate the membrane protrusions specifically observed in RacDA transfectants.
GFP-positive cells were held in contact for 4 or 30 min. In the transfection control cells, GFP remained uniformly distributed in the cytoplasm as cell adhesion developed (Fig. 8, C and D) whereas DN-forms of Rho GTPases were recruited at the cell–cell contact zone within 4 min (Fig. 8, G, K, and O) and were markedly accumulated at 30 min (PFig. 8, H, L, and P). By contrast, the Cdc42DA and RhoDA were not recruited (Fig. 9, C and D, and E and F, respectively).
Force measurements showed that all 4-min doublets (GFP controls and all DN-GTPases) developed similar SFs (Fig. 8 Q). Even at 30 min, variations in Rho levels had no impact; neither RhoDN nor RhoDA affected the adhesive phenotype of paired cells or the SF measured between them (Fig. 8, O–Q; Fig. 9, B, E, and F).
In striking contrast to these results are those for the other two GTPases. Production of Cdc42DN or RacDN significantly reduced adhesion (by 35% and 44%, respectively) relative to that of the control group in 30-min doublets (Fig. 8 Q). Dominant active forms had an even stronger effect. Although cells producing the Cdc42DA did adhere when pushed into contact, the contact interface less developed (Fig. 9, C and D) than for control cells or for cells producing any of the inactive constructs (PFig. 8, C and P), and a much lower SF was required to separate 4- and 30-min Cdc42DA doublets than for even the dominant negative forms of Cdc42 or Rac (Fig. 9 B vs. Fig. 8 Q). This indicates that no time-dependent strengthening of adhesion occurred in the presence of Cdc42DA. RacDA had the most dramatic effect of all. Cells transfected with RacDA were irregular in shape, with membrane protrusions easily visible on fluorescence microscopy and they did not form adhesive doublets at all (Fig. 9, G and H). These results suggest major changes in the membrane dynamics of RacDA transfectants.
FACS analysis showed that the transfected cells producing GFP, DN-forms (Fig. 8 R) or DA-forms (Fig. 9 A) of Rho GTPases expressed E-cadherin at their surface. Thus, the observed decrease in the SF of cells producing mutant forms of GTPases could not be accounted for by a loss of E-cadherin from the cell surface.
Discussion
In the dual pipette assay described here, we initiated adhesion between two cells by pushing them together and then measured the SF required to separate them. We manipulated cells in suspension using micropipettes, an approach that eliminates matrix-mediated signaling, minimizes the contribution of generalized membrane events, and bypasses the initiation of intercellular adhesion through lamellipodial and filopodial activities, as typically occurs during cell–cell adhesion on a substratum. The assay was not designed to quantify the strength of molecular interactions between individual adhesive receptors present at the cell surface. Instead, for the first time, it provides an overall quantification in terms of mechanical force of the adhesive properties conferred on the cell by adhesion receptors during the development of adhesion. In this cellular context, the assay provides insight into the functioning of specific cell surface receptors, their cytoplasmic partners and their connections to the cytoskeleton.
We used S180 cells stably transfected with the E-cadherin cDNA to investigate E-cadherin–dependent adhesive mechanisms and to minimize interference by other adhesion receptors; no classical intercellular adhesion molecules were detectable in the S180 cells, and they do not form doublets (however, they do form very weak cadherin-independent adhesions after very extended contact times; Fig. 3 B).
By contrast, S180 cells expressing E-cadherin display significant adhesion after a few seconds of contact, and a SF of a few to several hundred nanoNewtons can be measured, depending on the duration of contact and cadherin levels expressed at the cell surface. SF increased linearly over time for the first 30 min and then reached a plateau. Forces then continued to develop slowly between 30 and 60 min. Overall, our results clearly demonstrate that cadherins are required to initiate and to sustain the rapid intercellular adhesion that develops during the first hour of contact.
Here, we report a requirement for homophilic interactions between functional cadherin molecules (E or N) for the rapid development of strong adhesion (Table 1), although heterotypic cadherin interactions have been reported in other studies. N-cadherin expressing lens cells and E-cadherin expressing liver cells were found to form heterotypic junctions in primary cultures (Volk et al., 1987), and cadherins have been observed to interact in a heterophilic fashion in flow chamber and cell sorting assays (Niessen and Gumbiner, 2002; Duguay et al., 2003). In our assay, however, heterotypic contacts between E- and N-cadherin expressing cells were unable to induce the formation of doublets. This cannot be interpreted to completely exclude the possibility of the heterophilic interaction among different types of cadherins because heterotypic interactions may be constrained by the geometry of our assay or may simply produce adhesion too weak to be detected by our technique.
In this assay, the SF measured between two adherent cells considered to be identical in terms of E-cadherin expression because they are derived from the same clone. The comparison of SF in clones expressing different levels of E-cadherin shows that SF is a function of cadherin density at the cell surface, with greater SF resulting from higher levels of expression (Fig. 4 C). The curve best describing the relationship between force and cadherin expression level for 30-min doublets is a second-order polynomial function. In addition, the rate of increase of SF, an index representing how fast a pair of cells can interact each other to form a doublet within 30 min, associates linearly with the square of the percentage of cadherin expression (for clones with 14–100% expression). These two relationships clearly indicate a direct link between two parameters, E-cadherin density on two “identical” interacting cells and SF, in our dual pipette assay. Thus, we demonstrate that E-cadherin expression is the main parameter regulating initiation and development of E-cadherin–mediated adhesion.
We identified three phases in the kinetics of adhesion in paired cells: (1) initial contact (up to 30 s); (2) rapid increase of SF with duration of contact (30 s–30 min of contact); and (3) a phase in which the increase of SF after 30 min continues much more slowly over time. Table II chronologically summarizes the mechanisms required during the development of E-cadherin adhesion in paired cells.
Table II. Chronological order of mechanisms required during the development of adhesion in paired Ecad cells.
| Time (min)
|
0–0.5
|
0.5–4
|
4–30
|
30+
|
||
|---|---|---|---|---|---|---|
| Molecular manipulation or drug treatment |
Effects | First phase of adhesion |
Second phase of adhesion |
Third phase of adhesion |
||
| Deletion of E-cadherin cytoplasmic domain | No interaction with cytoplasmic partners | − | + | + | + | |
| Without calcium anti-cadherin antibody | Nonfunctional cadherins | + | + | + | + | |
| E-cadherin–α-catenin chimera | Direct interaction with the actin cytoskeleton |
− | − | − | − | |
| Latrunculin | Inhibition of actin polymerization | − | + | + | + | |
| Jasplakinolide | Aggregation/ polymerization of actin | |||||
| Brefeldin A | Inhibition of vesicular transport | − | − | − | nd | |
| Actin network organizationand membrane dynamics | ||||||
| Cdc42DN/RacDN | Inhibition of filopodia/ lamellipodia and membrane ruffles | − | − | + | nd | |
| Cdc42DA/RacDA | Stimulation of filopodia/ lamellipodia and membrane ruffles | + | + | + | + | |
| RhoDN/RhoDA | Inhibition/induction of stress fibers; cell contractility | − | − | − | − | |
| E-cadherin | E-cadherin | E-cadherin | E-cadherin | |||
| Dependency | Actin polymerization |
Actin polymerization |
||||
| Actin remodelling through Rac/Cdc42 activation |
Actin dynamics | |||||
| Membrane dynamics |
Membrane dynamics |
Membrane dynamics |
||||
+, sensitive.
−, insensitive.
nd, not done.
The deletion of the cytoplasmic domain of E-cadherin does not affect the initiation of adhesion (Fig. 5 D). Similarly, treatment of cells with LatB or Jasp has no effect on the SF measured for 30-s doublets (Fig. 6 F). These results indicate that the actin cytoskeleton is not required during the first phase of adhesion and we deduce, therefore, that SFs recorded during this phase reflect the trans-interactions of E-cadherin extracellular domains at the contact zone. In contrast, deleting the E-cadherin cytoplasmic domain or perturbing actin polymerization by drugs does affect the second and third phases of adhesion, abolishing the time-dependent increase in the measured SF. Thus, a controlled actin polymerization activity is essential for the development and stabilization of cadherin-mediated adhesion over time. Furthermore, the accumulation of the Ecad-Δcyto at the contact zone suggests that the rapid increase in SF with time is most likely due to an “inside-out” signaling rather than solely the increase in the number of binding sites. Together, our results indicate that the connection of the cadherin–catenin complex with actin cytoskeleton and its reinforcement is the prominent process controlling the second phase of adhesion whereas the third stage probably corresponds to contact stabilization through higher order cytoskeletal rearrangements, as previously described (Yonemura et al., 1995; Adams et al., 1996; Yap et al., 1997; Yap and Kovacs, 2003).
We also found that the inhibition of intracellular protein transport by BFA did not affect SF measured for Ecad cells during the initial and second phases of adhesion. This argues strongly that the early stages of adhesion in paired cells depend on interactions between cadherins already present at the contact zone or recruited from membrane regions proximal to it.
GTPases of the Rho family (Rho, Cdc42, and Rac) are known to participate in the signaling cascades activated by cadherin-mediated intercellular adhesion (Braga et al., 1997, Fukata and Kaibuchi, 2001; Nakagawa et al., 2001; Braga, 2002; Charasse et al., 2002; Kovacs et al., 2002; Yap and Kovacs, 2003), and their dominant negative forms have been shown to affect the organization of actin filaments and the recruitment and stabilization of cadherin and β-cat at cell–cell contact sites in cells attached to a substratum (Braga et al., 1997; Takaishi et al., 1997; Jou and Nelson, 1998). Our results support and extend these findings. We observed that the formation of Ecad cell aggregates in suspension was accompanied with the activation of Rac and Cdc42, but not Rho (Fig. 7). Because neither Rac nor Cdc42 was activated during aggregation of S180 cells (lacking cadherins), we conclude that this activation in Ecad cells was specifically mediated by E-cadherin interactions.
In paired cells in suspension, overproduction of Cdc42DN and RacDN attenuated the normal time-dependent increase in SF between 4 and 30 min of contact (Fig. 8). This attenuation was not caused by a decrease of the E-cadherin expression levels in the transfectants. Therefore, although Rac and Cdc42 GTPases are not involved in the first phase of adhesion, they clearly are involved late in the second phase of adhesion. Other reports (Braga et al., 1997; Kovacs et al., 2002; Lambert et al., 2002) have shown these GTPases are involved in nascent contacts between epithelial cells. The pushing together of isolated cells in our assay circumvents the need for filopodial or lamellipodial activity and may abolish the potential primary effect of these GTPases during the early stages of cell–cell contact. Our results are consistent with the observations that cadherin ligation can activate Rac and Cdc42 (Kim et al., 2000; Kovacs et al., 2002) and that inhibitory forms of Cdc42 and Rac disturb E-cadherin–mediated adhesion in gyratory cell aggregation assays (Fukata et al., 1999). They also confirm that Rac/Cdc42 are involved in the development of adhesion after 4 min and in strengthening the mechanical link between cadherin and actin filaments, reinforcing adhesion, as previously suggested (Ehrlich et al., 2002).
Dominant active forms of Cdc42 and Rac had more pronounced effect. Overproduction of Cdc42DA protein in paired Ecad cells in suspension resulted in much weaker than normal adhesion, with a dramatically lower SF (Fig. 9). RacDA protein drastically altered E-cadherin–based adhesion, preventing both its initiation and subsequent development. Transfectants producing RacDA had normal levels of E-cadherin at the cell surface, but the molecules were completely unable to mediate adhesion. Because even cells treated with LatB or Jasp generated some SF (Fig. 6 F), we suspect that Rac may affect cadherin activity directly, independently of actin remodeling, by an unknown mechanism. We do not exclude the possibility that activated Rac also modified the state of the actin cytoskeleton and otherwise affected general membrane dynamics. Such a possibility is consistent with our observation that RacDA-producing cells, unlike the other transfectants, displayed notable membrane protrusions (Fig. 9, G and H).
The results with the dominant active constructs evoke an interesting speculation. The Cdc42DA and RacDA proteins overproduced in isolated cells probably compete with endogenous Cdc42 and Rac activation, primed at sites of cadherin–cadherin interaction, when cells are pushed into contact. In a sense, they would “swamp out” the adhesion-based signal with an overbearing background noise. Thus, if Rac/Cdc42 activation occurs independently of and before cadherin ligation, it prevents E-cadherin–mediated adhesion. This interpretation would be consistent with the frequent overproduction of Rho family GTPases (Sahai and Marshall, 2002) and the associated loss of intercellular adhesiveness observed in tumor cells during metastasis. Moreover, sustained activation of Rac also disrupts cadherin junctions in keratinocytes (Braga et al., 2000).
Finally, Rho is not activated during Ecad cell aggregation in suspension, nor does overproduction of either RhoDA or RhoDN in any way affect the intercellular adhesion or the measured SF in our paired cell assay. Several reports (Braga et al., 1997; Takaishi et al., 1997; Noren et al., 2001) suggest a role for Rho in the establishment of adherent junctions regulated by E-cadherin in epithelial cell lines or keratinocytes adhering to ECM and indeed, under physiological conditions, most cells are in contact with ECM (circulating cells excepted). The paired, suspended cells used here differ from cells attached to ECM in two major aspects: (1) they are round and nonpolarized, so their capacity to establish intercellular junctions in three dimensions is not subject to shape constraints associated with 2D-substrata; and (2) they are exempt from matrix-dependent signaling and matrix-dependent actin cytoskeleton remodelling.
The assay described here provides a new tool for investigating the cytomechanics of intercellular adhesions. It will allow us to compare the adhesiveness of cells expressing different types of cadherin, visualize the contributing molecules, and elucidate the mechanisms involved.
Materials and methods
Reagents
LatB, cytochalasin D, and Jasp were purchased from Calbiochem. BFA, phalloidin-TRITC, anti-actin and DECMA-1 mAb were obtained from Sigma-Aldrich. The pAbs to P, E, N-cadherins, and the ECCD-2 mAb were obtained from Takara Biomedicals. The rabbit inhibitory antibody directed against the extracellular domain of chicken E-cadherin has been described elsewhere (Thiery et al., 1984). The mAbs directed against β-cat, α-cat, p120, and phosphotyrosine were obtained from Becton Dickinson Biosciences. The mAbs anti–α-tubulin and secondary antibodies conjugated to HRP were purchased from Amersham Biosciences. The secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. The anti-Rac and anti-RhoA mAbs were purchased from Upstate Biotechnology and Santa Cruz Biotechnology, Inc., respectively. Rabbit anti-Cdc42 antibody was provided by P. Chavrier (UMR144 CNRS-Institut Curie). Expression vectors encoding mutant forms of Rho, Rac, and Cdc42 GTPases fused to GFP (Roux et al., 1997; Gauthier-Rouviere et al., 1998; Ory et al., 2000) were obtained from M. Lambert (U440 INSERM/UPMC-Institut du Fer à Moulin). The GST-CRIB PAK1 and GST-RBD Rhotekin were described elsewhere (Ren and Schwartz, 2000; Patel et al., 2002) and were provided by F. Niedergang (UMR144 CNRS-Institut Curie) and I. Ader (U528 INSERM-Institut Curie), respectively. pEαMC encoding an E-cadherin–α-cat chimera and pEcad-GFP were a gift of M. Ozawa (Kagoshima University, Kagoshima, Japan; Ozawa, 2002 and 1998, respectively). pEGFPC1 was obtained from CLONTECH Laboratories, Inc.
Cell lines, constructs and transfections
We produced the pCE-EcadΔcyto expression vector encoding an E-cadherin with a deletion of its cytoplasmic domain, as follows: a DNA fragment was amplified from pCE-Ecad using 5′-AAAGACCAGGTGACCACG and 5′-ATCTGTACGTACCTACGGACCGACCACCGTTCTCC-TCCG primers to introduce sites for RsrII and SnaBI (underlined sequences) and a stop codon at the end of the transmembrane domain of E-cadherin. The 195-bp PCR fragment was digested by BstE2 and SnaB1 and ligated into theBstE2 and SnaB1 sites of pCE-Ecad.
The Ecad and Ncad clones are S180 cells stably transfected to express chicken E- and N-cadherin and are described elsewhere (Friendlander et al., 1989 and Dufour et al., 1999, respectively). Clones with different levels of mouse E-cadherin expression were obtained by stable transfection of S180 cells with the pCE-Ecad eukaryotic expression vector and pAG60 as described previously (Boyer et al., 1992). Alternatively, cells at 80% confluence were transiently transfected with 5 μg DNA: 12 μg lipofectamine 2000 (Invitrogen) or a mixture of 0.8 μg of pEGFPC1 and 8 μg DNA (pEcad, pEcadΔcyto, or pEαMC): 15 μg lipofectamine 2000 for 5 h and incubated in culture medium. After 15–48 h of transfection, we selected the GFP-positive cells for force measurements.
Tissue culture, cell dissociation, and drug treatments
Cells were maintained in DME with 10% FCS, and confluent cultures were routinely treated with TE buffer (0.05% trypsin + 0.02% EDTA). For force measurements and cell aggregation assay, cell dissociation was performed in TC buffer (0.01% trypsin + 10 mM calcium) as described previously (Nakagawa and Takeichi, 1995). Before SF measurement, cells were resuspended in working medium—a CO2-independent medium (Invitrogen) supplemented with 1% FCS—and used immediately. When necessary, drugs or similar amounts of solvents (as a control) were added to isolated cells in working medium 30 min to 2 h (Invitrogen) before force measurements. Cell aggregation assays are performed as described elsewhere (Dufour et al., 1999).
Western blotting
Extraction of cell monolayers and Western blot analysis were performed as described previously (Dufour et al., 1999) with a mixture of antibodies directed against α-tubulin and β-cat or E-cadherin and revealed by ECL detection (Amersham Biosciences). Quantitative analysis was done using the ImageQuant program on a representative Western blot of three independent experiments. The α-tubulin content was used to normalize for protein level. The levels of β-cat and E-cadherin of E100 cells were set at 100% in comparisons of transfected clones.
Pull down assay and the determination of the activity of Rho-like GTPases
Cell aggregation assays were performed as described above. 106 cells were used for each point of the kinetics. Cells were lysed on ice in lysis buffer and the Rac, Cdc42, and RhoA activity pull down assays were performed as described previously (Patel et al., 2002; Arthur and Burridge, 2001). Precipitation was performed in the presence of 0.5% BSA with GST-CRIB (30 μg) and GST-RBD (30 μg) and revealed by Western blotting.
Microscopy
Immunodetection of cadherins on cultured cells was performed as described previously (Dufour et al., 1999). Preparations were viewed by epifluorescence microscopy, using a DMRBE microscope (Leica) equipped with an objective of 63× (PL APO, NA/1.32-0.6 oil) and of 100× (NA/1.4) and with a cooled CCD camera (Hamamatsu C5985). Acquisitions were controlled by a Power Macintosh workstation operating IP-Lab software. Alternatively, preparations were analyzed by TCS4D confocal microscopy based on a DM microscope interfaced with an argon/krypton laser. Cadherin expression on the cell surface of TC- or TE-treated cells was analyzed by flow cytometry as described previously (Beauvais et al., 1995) with specific antibody directed against the extracellular domain of cadherins. Samples from three independent experiments were analyzed.
Measurement of SF between cells
We used a micromanipulation technique described previously in Daoudi et al. (2004). In brief, forces were measured on the stage of an inverted epifluorescence microscope (Leica) equipped with objectives of 63× (PL FLUOTAR; NA/0.7; C PLAN NA/0.75) and with a cooled CCD C5985 (Hamamatsu) or Coolpix 5000 camera (Nikon). Image acquisition was described in the previous paragraph. Cells were manipulated at 37°C with two micropipettes, each held by one micromanipulator connected to a combined hydraulic/pneumatic system and a pressure sensor making it possible to control and measure the aspiration applied to the cells. Micropipettes were pulled (model P-2000; Sutter Instrument), cut, and fire polished with a homemade microforge, such that their i.d. was 4.0–5.5 μm. The aspiration applied to the left pipette was measured by a pressure sensor (model DP103-38; Validyne). Aspiration was monitored continuously during the separation process (Fig. 3), and the values recorded for each of the last two cycles in the series (P n-1 and P n) were used to calculate the SF for each doublet using the equation: SF = π (d/2)2 (P n-1+P n)/2 where d is the i.d. of left pipette. Results for 30–50 measurements were used to obtain the mean force of separation for a specific contact time in at least three independent experiments.
Online supplemental material
Real-time (Videos 1 and 2) and time-lapse (Video 3) films are included as online videos. Video 1 shows two Ecad cells held by micropipettes and put in contact. Video 2 shows the separation process for a 4-min Ecad doublet and a SF of 47.5 nN was calculated. Videos 1 and 2 were taken under a 63× objective. Video 3 shows the separation process of a S180 cell doublet transiently expressing E-cadherin tagged with GFP. Images were taken under a 100× objective. For the details, refer to video legends. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200403043/DC1.
Acknowledgments
We thank F. Niedergang, I. Ader, and M. Ozawa for reagents; and C. Burger, J.-B. Sibarita, V. Fraisier, D. Meur, and D. Morineau for help with imaging and computerized video microscopy. We thank K.M. Yamada, R.J. Thomas-Mudge, M.J. Morgan, P. Nassoy, S.Y. Lee, and B. Janssens for helpful discussions.
This work was supported by the CNRS, the Institut Curie (Physicochimie des structures biologiques complexes), the Association pour la Recherche sur le Cancer (grant 5653), and EEC contract QLGI-CT-2001-00869. Y.-S. Chu benefited from a France-Taiwan Ministry of Foreign Affairs Ph.D. fellowship.
J.P. Thiery and S. Dufour were co-principal investigators.
Abbreviations used in this paper: α-cat, α-catenin; β-cat, β-catenin; BFA, brefeldin A; Ecad-Δcyto, E-cadherin lacking the cytoplasmic domain; Jasp, Jasplakinolide; LatB, Latrunculin B; SF, separation force; TC, trypsin-calcium.
References
- Adams, C.L., W.J. Nelson, and S.J. Smith. 1996. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell–cell adhesion. J. Cell Biol. 135:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angres, B., A. Barth, and W.J. Nelson. 1996. Mechanism for transition from initial to stable cell–cell adhesion: kinetic analysis of E-cadherin–mediated adhesion using a quantitative adhesion assay. J. Cell Biol. 134:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W.T., and K. Burridge. 2001. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 12:2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner, W., P. Hinterdorfer, W. Ness, A. Raab, D. Vestweber, H. Schindler, and D. Drenckhahn. 2000. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA. 97:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais, A., C.A. Erickson, T. Goins, S.E. Craig, M.J. Humphries, J.P. Thiery, and S. Dufour. 1995. Changes in the fibronectin-specific integrin expression pattern modify the migratory behaviour in the embryonic environment. J. Cell Biol. 128:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, B., S. Dufour, and J.-P. Thiery. 1992. E-cadherin expression during the acidic FGF-induced dispersion of a rat bladder carcinoma cell line. Exp. Cell Res. 201:347–357. [DOI] [PubMed] [Google Scholar]
- Braga, V.M. 2002. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14:546–556. [DOI] [PubMed] [Google Scholar]
- Braga, V.M., L.M. Machesky, A. Hall, and N.A. Hotchin. 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 137:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V.M., M. Betson, X. Li, and N. Lamarche-Vane. 2000. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell-cell adhesion in normal human keratinocytes. Mol. Biol. Cell. 11:3703–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb, M.R., A.M. Senderowicz, E.A. Sausville, K.L. Duncan, and E.D. Korn. 1994. Jasplakinolide a cytotoxic natural product induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269:14869–14871. [PubMed] [Google Scholar]
- Charasse, S., M. Meriane, F. Comunale, A. Blangy, and C. Gauthier-Rouvière. 2002. N-cadherin–dependent cell–cell contact regulates Rho GTPases and β-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, L.P. 1999. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr. Biol. 9:1095–1105. [DOI] [PubMed] [Google Scholar]
- Daoudi, M., E. Lavergne, A. Garin, N. Tarantino, P. Debre, F. Pincet, C. Combadiere, and P. Deterre. 2004. Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J. Biol. Chem. 279:19649–19657. [DOI] [PubMed] [Google Scholar]
- Dufour, S., A. Beauvais-Jouneau, A. Delouvée, and J.P. Thiery. 1999. Differential function of N-cadherin and cadherin-7 in the control of embryonic cell motility. J. Cell Biol. 146:501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguay, D., R.A. Foty, and M. Steinberg. 2003. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev. Biol. 253:309–323. [DOI] [PubMed] [Google Scholar]
- Ehrlich, J.S., M.D. Hansen, and W.J. Nelson. 2002. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell. 3:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E., and D. Needham. 1988. Attraction between lipid bilayer membranes in concentrated solutions of nonabsorbing polymers: comparison of mean-field theory with measurements of adhesion energy. Macromolecules. 21:1822–1831. [Google Scholar]
- Flanagan, M.D., and S. Lin. 1980. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J. Biol. Chem. 255:835–838. [PubMed] [Google Scholar]
- Friendlander, D.R., R.-M. Mege, B.A. Cunningham, and G.M. Edelman. 1989. Cell sorting-out is modulated by both the specificity and amount of different cell-cell adhesion molecules (CAMs) expressed on cell surfaces. Proc. Natl. Acad. Sci. USA. 86:7043–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata, M., and K. Kaibuchi. 2001. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2:887–897. [DOI] [PubMed] [Google Scholar]
- Fukata, M., S. Kuroda, M. Nakagawa, A. Kawajiri, N. Itoh, I. Shoji, Y. Matsuura, S. Yonehara, H. Fujisawa, A. Kikuchi, and K. Kaibuchi. 1999. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J. Biol. Chem. 274:26044–26050. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouviere, C., E. Vignal, M. Meriane, P. Roux, P. Montcourier, and P. Fort. 1998. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol. Biol. Cell. 9:1379–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimond, C., A. van Der Flier, S. van Delft, C. Brakebusch, I. Kuikman, J.G. Collard, R. Fassler, and A. Sonnenberg. 1999. Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function. J. Cell Biol. 147:1325–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2000. Regulation of cadherin adhesive activity. J. Cell Biol. 148:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, Y., M. Itoh, Y. Maeno, S. Tsukita, and A. Nagafuchi. 1999. Functional domains of α-catenin required for the strong state of cadherin-based adhesion. J. Cell Biol. 144:1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora, C., and E. Fuchs. 2002. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 4:E101–E108. [DOI] [PubMed] [Google Scholar]
- Jou, T.S., and W.J. Nelson. 1998. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler, R. 1993. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 9:317–321. [DOI] [PubMed] [Google Scholar]
- Kim, S.H., S. Li, and D.B. Sacks. 2000. E-cadherin-mediated cell-cell attachment activated Cdc42. J. Biol. Chem. 275:36999–37005. [DOI] [PubMed] [Google Scholar]
- Kovacs, E.M., R.G. Ali, A.J. McCormack, and A.S. Yap. 2002. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277:6708–6718. [DOI] [PubMed] [Google Scholar]
- Lambert, M., D. Choquet, and R.-M. Mege. 2002. Dynamics of ligand-induced Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J. Cell Biol. 157:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier-Gavelle, F., and J. Duband. 1997. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by β1 and β3 integrins in migrating neural crest cells. J. Cell Biol. 137:1663–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi, Y., K. Miki, A. Takatsuki, G. Tamura, and Y. Ikehara. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 261:11398–11403. [PubMed] [Google Scholar]
- Nakagawa, M., M. Fukata, M. Yamaga, N. Itoh, and K. Kaibuchi. 2001. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114:1829–1838. [DOI] [PubMed] [Google Scholar]
- Nakagawa, S., and M. Takeichi. 1995. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 121:1321–1332. [DOI] [PubMed] [Google Scholar]
- Niessen, C.M., and B.M. Gumbiner. 2002. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 156:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., C.M. Niessen, B.M. Gumbiner, and K. Burridge. 2001. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276:33305–33308. [DOI] [PubMed] [Google Scholar]
- Nose, A., A. Nagafuchi, and M. Takeichi. 1988. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 54:993–1001. [DOI] [PubMed] [Google Scholar]
- Ory, S., Y. Munari-Silem, P. Fort, and P. Jurdic. 2000. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J. Cell Sci. 113:1177–1188. [DOI] [PubMed] [Google Scholar]
- Ozawa, M. 1998. Identification of the region of alpha-catenin that plays an essential role in cadherin-mediated cell adhesion. J. Biol. Chem. 273:29524–29529. [DOI] [PubMed] [Google Scholar]
- Ozawa, M. 2002. Lateral dimerization of the E-cadherin extracellular domain is necessary but not sufficient for adhesive activity. J. Biol. Chem. 277:19600–19608. [DOI] [PubMed] [Google Scholar]
- Patel, J.C., A. Hall, and E. Caron. 2002. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol. Biol. Cell. 13:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret, E., A.M. Benoliel, P. Nassoy, A. Pierres, V. Delmas, J.-P. Thiery, P. Bongrand, and H. Feracci. 2002. Fast dissociation kinetics between individual E-cadherin fragments revealed by flow chamber analysis. EMBO J. 21:2537–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X.D., and M.A. Schwartz. 2000. Determination of GTP loading on Rho. Methods Enzymol. 325:264–272. [DOI] [PubMed] [Google Scholar]
- Roux, P., C. Gauthier-Rouviere, S. Doucet-Brutin, and P. Fort. 1997. The small GTPases Cdc42Hs Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr. Biol. 7:629–637. [DOI] [PubMed] [Google Scholar]
- Sahai, E., and C.J. Marshall. 2002. RHO-GTPases and cancer. Nat. Rev. Cancer. 2:133–142. [DOI] [PubMed] [Google Scholar]
- Sivasankar, S., B. Gumbiner, and D. Leckband. 2001. Direct measurements of multiple adhesive alignments and unbinding trajectories between cadherin extracellular domains. Biophys. J. 80:1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, I., N.R. Shochet, Y. Kashman, and A. Groweiss. 1983. Latrunculins: novel marine toxins that disrupt microfilaments organization in cultured cells. Science. 219:493–495. [DOI] [PubMed] [Google Scholar]
- Steinberg, M.S., and M. Takeichi. 1994. Experimental specification of cell sorting tissue spreading and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA. 91:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi, K., T. Sasaki, H. Kotani, H. Nishioka, and Y. Takai. 1997. Regulation of cell–cell adhesion by Rac and Rho small G proteins in MDCK cells. J. Cell Biol. 139:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery, J.-P., A. Delouvée, W.J. Gallin, B.A. Cunningham, and G.M. Edelman. 1984. Ontogenic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev. Biol. 102:61–78. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., C. Bauer, M. Yin, and E. Fuchs. 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 100:209–219. [DOI] [PubMed] [Google Scholar]
- Vestweber, D., and R. Kemler. 1985. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 4:3393–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk, T., O. Cohen, and B. Geiger. 1987. Formation of heterotypic adherens-type junctions between L-CAM-containing liver cells and A-CAM-containing lens cells. Cell. 50:987–994. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., and E.M. Kovacs. 2003. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 160:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A.S., W.M. Brieher, M. Pruschy, and B.M. Gumbiner. 1997. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr. Biol. 7:308–315. [DOI] [PubMed] [Google Scholar]
- Yonemura, S., M. Itoh, A. Nagafuchi, and S. Tsukita. 1995. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 108:127–142. [DOI] [PubMed] [Google Scholar]