Abstract
Interaction of macrophages with apoptotic cells involves multiple steps including recognition, tethering, phagocytosis, and anti-inflammatory macrophage responses. Defective apoptotic cell clearance is associated with pathogenesis of autoimmune disease. CD14 is a surface receptor that functions in vitro in the removal of apoptotic cells by human and murine macrophages, but its mechanism of action has not been defined. Here, we demonstrate that CD14 functions as a macrophage tethering receptor for apoptotic cells. Significantly, CD14−/− macrophages in vivo are defective in clearing apoptotic cells in multiple tissues, suggesting a broad role for CD14 in the clearance process. However, the resultant persistence of apoptotic cells does not lead to inflammation or increased autoantibody production, most likely because, as we show, CD14−/− macrophages retain the ability to generate anti-inflammatory signals in response to apoptotic cells. We conclude that CD14 plays a broad tethering role in apoptotic cell clearance in vivo and that apoptotic cells can persist in the absence of proinflammatory consequences.
Introduction
Apoptosis culminates in the engulfment of apoptotic cells and bodies where the phagocyte provides a safe haven for apoptotic cell degradation, preventing environmental damage that is typical of necrotic cell death (for reviews see Henson et al., 2001; Savill et al., 2002; Grimsley and Ravichandran, 2003; Gregory and Devitt, 2004). In addition, the phagocytosis of apoptotic cells normally results either in neutral responses from phagocytes or active suppression of inflammation through production of anti-inflammatory mediators such as TGF-β1, PGE2, and PAF (Voll et al., 1997; Fadok et al., 1998a, 2001; Kurosaka et al., 2003) and triggering of tolerogenic responses in the adaptive immune system (for reviews see Savill et al., 2002; Albert, 2004). However, under certain circumstances, apoptotic cell engulfment may be immunostimulatory, and the persistence of apoptotic cells is associated in specific mouse strains with the pathogenesis of autoimmune disease (Botto et al., 1998; Scott et al., 2001; Cohen et al., 2002; Szondy et al., 2003; Hanayama et al., 2004).
The removal of apoptotic cells by macrophages involves a complex array of macrophage receptors, ill-defined apoptotic cell–associated molecules, and soluble factors that link apoptotic cells and macrophage surfaces (for reviews see Savill et al., 2002; Gregory and Devitt, 2004). An attractive model has been proposed where the molecules of apoptotic cell clearance function in one or both of two major components of the clearance process, (1) tethering and (2) “tickling” (Hoffmann et al., 2001), the former events signifying binding of apoptotic cells to phagocytes leading to the latter events that mediate signal transduction to effect phagocytosis and anti-inflammatory responses. Taken from the perspective of the macrophage, available evidence now points to multiple phases in the process of apoptotic cell clearance, including (a) discriminatory recognition of apoptotic cells, (b) tethering of phagocyte receptors to apoptotic cell–associated ligands, (c) phagocytic signaling, and (d) anti-inflammatory signaling (Hoffmann et al., 2001; Savill et al., 2002; Brown et al., 2002; Grimsley and Ravichandran, 2003; Gregory and Devitt, 2004).
CD14 is a pattern recognition receptor (PRR) that is renowned for its ability to generate proinflammatory responses following interaction with bacterial endotoxin, LPS (Goyert et al., 1988; Wright et al., 1990; Ulevitch and Tobias, 1995). In vitro studies have shown that CD14 also plays a significant role in the clearance of apoptotic cells by both human and murine macrophages (Flora and Gregory, 1994; Devitt et al., 1998; Fadok et al., 1998b; Schlegel et al., 1999). However, CD14-dependent engulfment of apoptotic cells is not accompanied by proinflammatory macrophage responses (Devitt et al., 1998). Here, we investigate the mechanism of action of CD14 in apoptotic cell clearance by macrophages. In both human and mouse macrophage models, we demonstrate activity of CD14 as a tethering receptor for apoptotic cells. We show in binding studies that soluble CD14 receptors can discriminate between viable and apoptotic cells. Quantitative histological studies indicate that CD14−/− mice have defective clearance capacity leading to persistence of apoptotic cells in multiple tissues. However, such persistent apoptotic cells do not engender inflammatory responses, and CD14−/− animals fail to develop increased titers of autoantibodies or autoimmune disease. Indeed, macrophages from CD14−/− mice retain the ability to mount anti-inflammatory responses to apoptotic cells. These findings identify CD14 as a macrophage receptor that recognizes and tethers apoptotic cells in preparation for engulfment. The mechanism of action of CD14 can be functionally uncoupled from the anti-inflammatory signaling events that accompany apoptosis. These results demonstrate a mechanism by which apoptotic cells can persist in vivo in the absence of inflammatory consequences.
Results
Macrophage CD14 functions in apoptotic cell recognition and tethering in vitro
We first demonstrated a role for CD14 in the clearance of apoptotic cells by human macrophages using the inhibitory mAb 61D3. The capacity of this CD14 mAb (but not the CD14 mAb 63D3) to inhibit interaction of apoptotic cells with macrophages is confirmed in Fig. 1 A. To determine whether macrophages from CD14-deficient mice were similarly inhibited in their capacity to interact with apoptotic cells, peritoneal or bone marrow–derived macrophages (BMDMs) from CD14+/+ versus CD14−/− mice were compared. As shown in Fig. 1 (B and C), BMDMs from CD14−/− mice were substantially less efficient than their CD14+/+ counterparts in interacting with apoptotic cells, including both binding and phagocytic phases (Fig. 1 C). In contrast, phagocytosis of IgG-opsonized cells was equivalent for both CD14−/− and CD14+/+ BMDMs (Fig. 1 D). Macrophages isolated from the peritoneal cavities of CD14−/− mice were also less effective in interacting with apoptotic cells than their CD14+/+ counterparts, although the difference was less marked than with BMDMs (Fig. 1 E). These results confirm and extend previous observations demonstrating a role for macrophage CD14 as a receptor for apoptotic cells.
Figure 1.
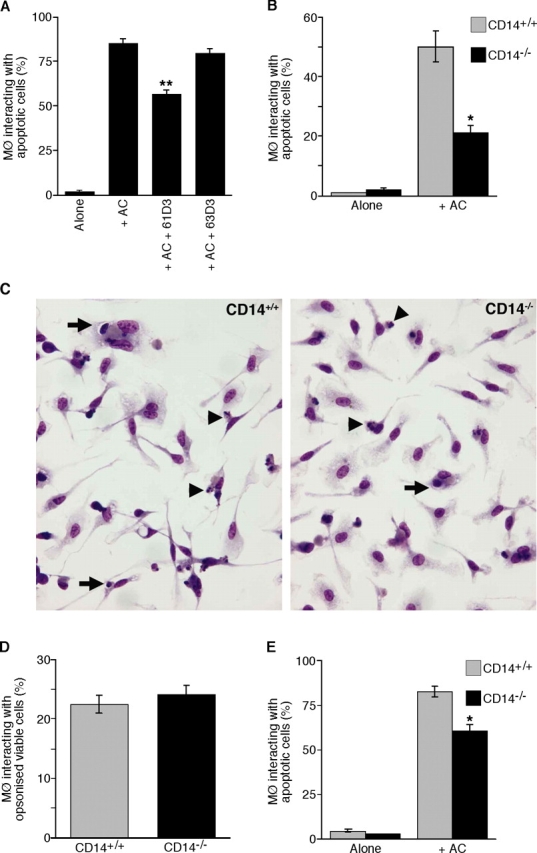
CD14 dependence of interaction of apoptotic cells with human and murine macrophages. (A) Interaction (binding and phagocytosis) of 7-d HMDMs with apoptotic BL cells. Interaction between HMDMs and apoptotic BL cells (AC) was assessed after 60-min coculture at 37°C in the presence or absence of CD14 mAbs 61D3 or 63D3. Background interaction of apoptotic cells in the macrophage cultures without added BL cells is also shown (Alone). Results represent the percentage of macrophages interacting with apoptotic cells. Data shown are means ± SEM (n = 2). ANOVA: **, P < 0.01. (B) Interaction of 10-d BMDMs from CD14+/+ (gray bar) and CD14−/− (black bar) mice with apoptotic BL cells assessed after 30-min coculture at 37°C. Data shown are means ± SEM (n = 3). ANOVA: *, P < 0.05. Similar results were observed when syngeneic apoptotic thymocytes were tested in place of BL cells. (C) Photomicrographs showing 10-d BMDMs' interaction with apoptotic BL cells, comprising binding (arrowheads) and phagocytic (arrows) events. (D) Interaction of IgG-opsonized BL cells with the same macrophages as in B. Data are means ± SEM (n = 3). (E) Interaction of peritoneal macrophages from CD14+/+ (gray bar) and CD14−/− (black bar) mice with apoptotic BL cells assessed after 30-min coculture at 37°C. Data shown are means ± SEM (n = 3). ANOVA: *, P < 0.05.
To investigate tethering events independently of phagocytosis, macrophage–apoptotic cell interactions were conducted at 16–20°C, which permitted binding, but not phagocytic, events to occur (Fig. 2 and not depicted). Tethering of apoptotic cells to human monocyte-derived macrophages (HMDMs) was reduced significantly by mAb 61D3 but not by mAb 63D3 (Fig. 2 A). Enhanced tethering of apoptotic cells induced by transient expression of CD14 in COS transfectants (Fig. 2 C) was returned to background levels by 61D3 (Fig. 2 B). BMDMs (Fig. 2 D) and peritoneal macrophages (Fig. 2 E) from CD14−/− mice also bound apoptotic cells less effectively than their CD14+/+ counterparts. Finally, to determine whether or not CD14 is involved directly in the discrimination of viable and apoptotic cells, purified recombinant forms of CD14 were assessed for cell-binding ability. As shown in Fig. 2 F, recombinant CD14 bound apoptotic, but not viable, cells. The latter result raises the possibility that soluble CD14, which is present in high amounts in plasma and secretions might also be involved in apoptotic cell clearance. Together, these results demonstrate in both human and murine systems that CD14 can function independently of phagocytic events by tethering apoptotic cells to macrophages. Because of its relative specificity for apoptotic over viable cells, we conclude that CD14 also contributes to apoptotic cell recognition by macrophages.
Figure 2.
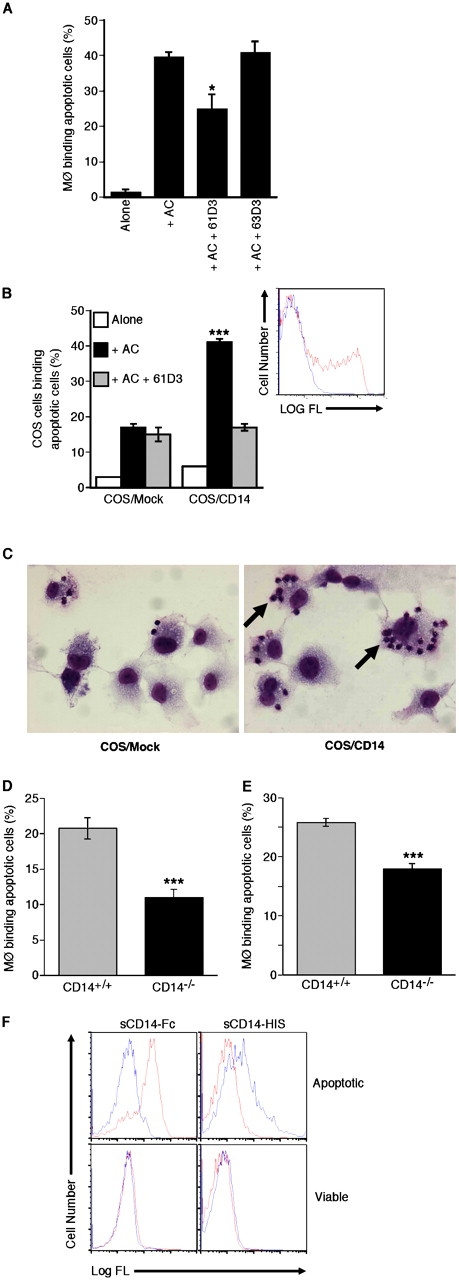
Macrophage tethering of apoptotic cells by CD14. (A) Binding (in the absence of phagocytosis) of apoptotic BL cells (AC) by HMDMs assessed after coculture at 16–20°C for 60 min in the presence or absence of CD14 mAbs 61D3 or 63D3. Data shown are means ± SEM (n = 2). ANOVA: *, P < 0.05. (B) Binding of apoptotic BL cells (AC) by either mock-transfected or CD14-transfected COS-1 cells after coculture at 16–20°C for 60 min in the presence (gray bar) or absence (black bar) of CD14 mAb 61D3. (inset) Expression of CD14 by COS-1 cells as assessed by flow cytometry following immunofluorescence staining using mAb 63D3 and goat anti–mouse-FITC (shown in red) versus staining of mock transfectants (shown in blue). ANOVA: ***, P < 0.001. (C) Photomicrographs showing binding (but not phagocytosis) of apoptotic BL cells by COS cells. Binding is promoted by the expression of CD14 (arrow). (D) Binding of 10-d BMDMs from CD14+/+ (gray bar) and CD14−/− (black bar) mice with apoptotic BL cells assessed after 30-min coculture at 16–20°C. Data shown are means ± SEM (n = 3). ANOVA: ***, P < 0.001. (E) Binding of peritoneal macrophages from CD14+/+ (gray bar) and CD14−/− (black bar) mice with apoptotic BL cells assessed after 30-min coculture at 16–20°C. Data shown are means ± SEM (n = 3). ANOVA: ***, P < 0.001. (F) Binding of soluble recombinant human CD14 (sCD14) to apoptotic cells. BL cells undergoing spontaneous apoptosis were identified as “apoptotic” or “viable” according to light-scatter properties and confirmed by morphological analyses (Dive et al., 1992). Spontaneous apoptosis, a characteristic feature of group I BL cell lines (Gregory et al., 1991) was confirmed by morphological analysis as described previously (Devitt et al., 2003). Histograms of recombinant forms of CD14, sCD14-Fc, and sCD14-HIS binding to cells in these zones are shown. Control staining is shown in blue against staining with SCD14-Fc and SCD14-HIS in red.
Increased incidence of apoptotic cells in tissues of CD14−/− mice
If CD14's role in the clearance process were redundant in vivo, the frequency of apoptotic cells in histological sections should be equivalent in tissues of CD14−/− and CD14+/+ mice. We initially analyzed the normal thymus of young adult animals because apoptosis normally occurs at high rate in this tissue and apoptotic thymocytes can be readily observed in situ (Surh and Sprent, 1994). Macroscopically, no differences were noted between CD14−/− and CD14+/+ thymi, and no overt microscopic differences in cellularity or tissue architecture were observed. However, quantitative analyses of apoptotic thymocytes identified by in situ end labeling (ISEL; Fig. 3 A) revealed increased frequencies of apoptotic events in both the cortex and medulla of the CD14−/− thymus compared with its CD14+/+ counterpart. Detailed morphological analyses of thymic cortex in toluidine blue–stained, resin-embedded sections allowed apoptotic cells to be defined as either “free” or “macrophage-associated” (Fig. 3 B). Quantitative analyses indicated that, although there were increased numbers both of free and of macrophage-associated apoptotic cells in the cortices of CD14−/− compared with CD14+/+ thymi, the ratio of free/macrophage-associated events was substantially higher in the CD14−/− animals (Fig. 3 B) despite equivalent distribution of macrophages throughout the thymus as assessed by F4/80 staining (Fig. 3 C). Similar results showing the presence of free apoptotic cells in thymus of CD14−/− and CD14+/+ mice were obtained after annexin V (AxV) and propidium iodide (PI) staining of dissociated thymi (see Fig. 5 A). Earlier studies have reported marginally lower levels of free apoptotic cells in the murine thymus than those reported here (Surh and Sprent, 1994; McIlroy et al., 2000), although it is important to note that different mouse strains and methodologies were used.
Figure 3.
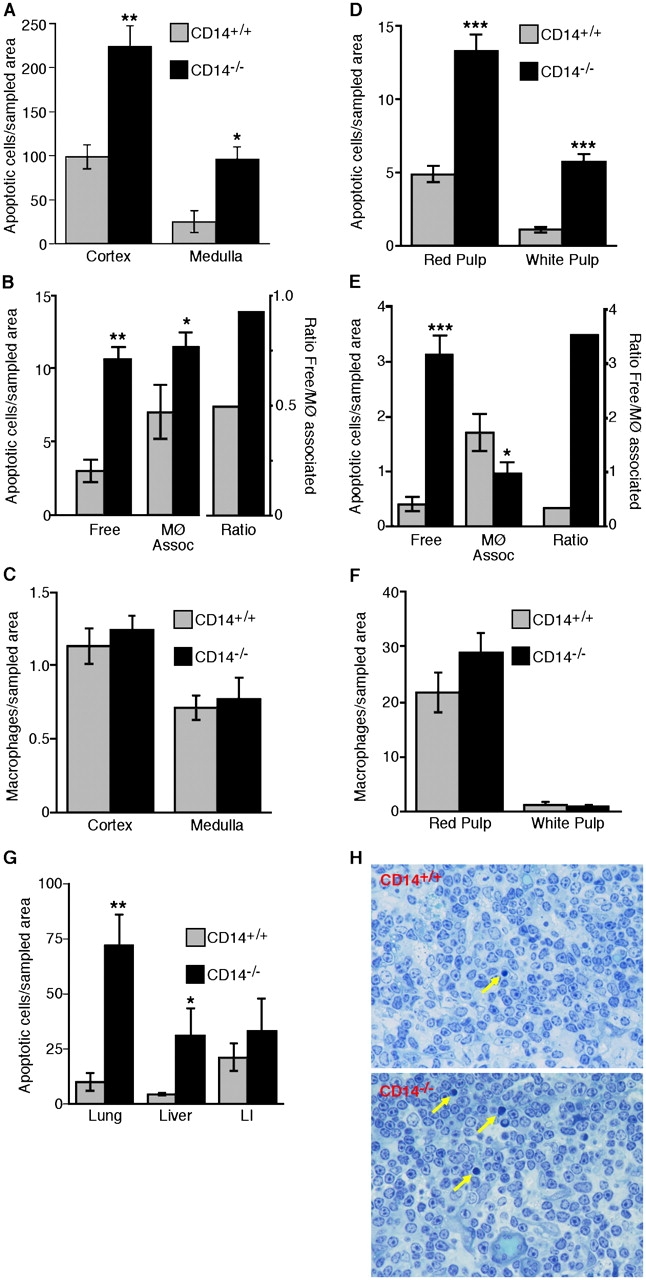
Persistence of apoptotic cells in tissues of normal CD14 − / − mice. (A) Morphometric analysis of the frequency of ISEL+ nuclei present in the thymic cortex and medulla of CD14+/+ and CD14−/− mice. (B) Quantitative analyses of thymic sections showing the numbers of free and macrophage-associated apoptotic cells together with the ratios of free/macrophage-associated apoptotic cells for CD14+/+ and CD14−/− cortices. (C) Morphometric analyses of the distribution of macrophages (F4/80-reactive material) in the cortex and medulla of CD14+/+ and CD14−/− thymus. All thymus data shown are means ± SEM (n = 3 animals in each case). ANOVA: *, P < 0.05; **, P < 0.01. (D) Morphometric analysis of the frequency of ISEL+ nuclei present in the splenic red and white pulp of CD14+/+ and CD14−/− mice. ANOVA: ***, P < 0.001. (E) Quantitative analysis of the numbers of free and apoptotic cells together with the ratios of free/macrophage-associated apoptotic cells for CD14+/+ and CD14−/− splenic red pulp. (F) Morphometric analyses of the distribution of F4/80+ macrophages in the cortex and medulla of CD14+/+ and CD14−/− spleen. All spleen data shown are means ± SEM (n = 3 animals in each case). ANOVA: *, P < 0.05; ***, P < 0.001. (G) Persistence of apoptotic cells in non-lymphoid tissues of CD14−/− mice. Morphometric analyses of the frequency of ISEL+ nuclei present in the lung, liver, and large intestine (LI) of CD14+/+ and CD14−/− mice. Data are means ± SEM (n = 3 animals in each case). ANOVA: *, P < 0.1; **, P < 0.01. (H) Photomicrographs showing histological detail of CD14+/+ versus CD14−/− thymus. Note preponderance of free apoptotic cells in CD14−/− thymus (arrows) and absence of inflammatory cell infiltrate.
Figure 5.
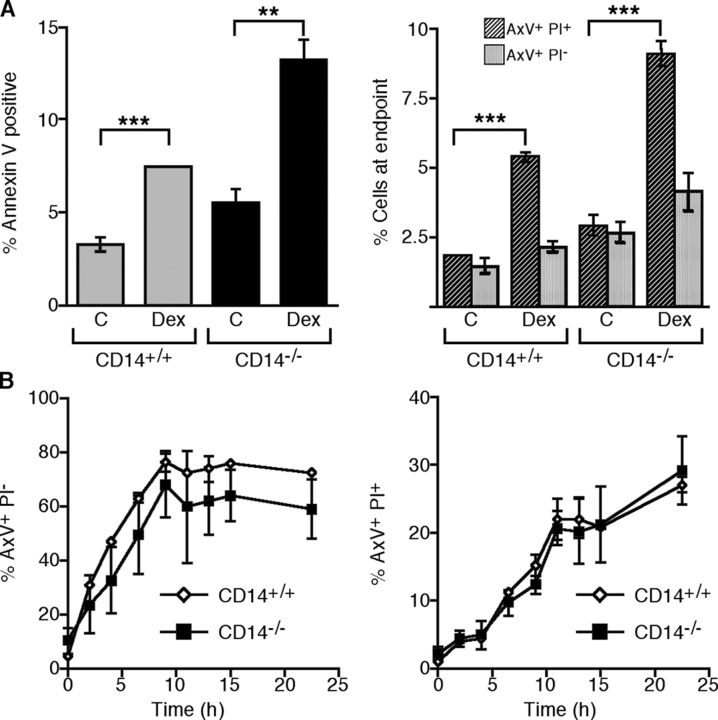
Persistence of apoptotic cells in thymus of CD14 mice after dexamethasone treatment. (A) Flow cytometric analyses of thymocytes harvested from mock (C) or dexamethasone (DEX)-treated CD14+/+ or CD14−/− mice. Left, percentage of total AxV+ cells; right, percentage of apoptotic (AxV+PI−) or secondarily necrotic (AxV+PI+) cells. ANOVA: **, P < 0.01; ***, P < 0.001. (B) Induction of apoptosis by dexamethasone treatment of thymocytes from CD14+/+ (open diamonds) or CD14−/− (closed squares) mice in vitro. Left, generation of apoptotic (AxV+PI−) cells with time; right, generation of necrotic (AxV+PI+) cells with time. Data shown are means ± SEM (n = 3 animals per group).
To extend these observations of basal apoptosis to other tissues, we compared the spleens of CD14−/− and CD14+/+ animals using identical approaches. As with the thymus, no overt macroscopic differences between CD14−/− and CD14+/+ spleens were noted. However, the numbers of positive apoptotic events by ISEL were substantially elevated in the red and white pulp of the spleens of CD14−/− animals (Fig. 3 D). This finding was confirmed in detailed morphological analysis of the red pulp where it was found that the majority of apoptotic cells were free, rather than macrophage-associated, in the CD14−/− animals (Fig. 3 E). Conversely, in the CD14+/+ mice, the ratio of free/macrophage-associated apoptotic cells was reversed with most apoptotic events being macrophage-associated (Fig. 3 E). Splenic macrophage distribution was similar throughout the CD14−/− and CD14+/+ spleen (Fig. 3 F). Quantitative ISEL analyses of lung, liver, and large intestine confirmed the trends observed in thymus and spleen, with increased frequencies of apoptotic events in the CD14−/− tissues (Fig. 3 G) although the differences between the large intestines of CD14−/− and CD14+/+ animals were not statistically significant. No signs of inflammatory cell infiltration were observed in any of the tissues studied as illustrated in sections of thymus shown in Fig. 3 H.
These data suggest either that apoptosis was increased at multiple sites as a result of CD14 deficiency or that the absence of CD14 led to persistence of apoptotic cells as a result of their inefficient clearance. Given the mode of action of CD14 indicated by the in vitro studies, together with the relative preponderance of free, as opposed to macrophage-associated, apoptotic cells in the spleen and thymus of CD14−/− animals, these results strongly argue that the increased frequencies of apoptotic events encountered in the tissues of CD14−/− mice results from reduced clearance rather than from increased apoptosis.
Defective apoptotic cell clearance by CD14−/− macrophages in vivo
To define more precisely if CD14−/− mice display defects in their ability to clear apoptotic cells in vivo, we determined the extent to which apoptotic cells administered i.p. could be cleared by resident macrophages. To this end, CD14+/+ or CD14−/− mice were injected i.p. with fluorescent, apoptotic thymocytes and after 15 min coincubation in situ the proportion of peritoneal macrophages interacting with apoptotic cells was enumerated by flow cytometry following F4/80 immunofluorescence staining (Fig. 4). As shown, effective discrimination of apoptotic thymocytes, F4/80+ macrophages, and macrophages interacting with apoptotic thymocytes was possible (Fig. 4 A). Microscopic analyses of sorted populations indicated that those events displaying dual fluorescence (i.e., F4/80+, apoptotic cell+) represented macrophages with bound and/or engulfed apoptotic thymocytes (unpublished data). Quantitative analysis of multiple experiments (Fig. 4 B) showed that peritoneal macrophages from CD14−/− animals were markedly reduced in their ability to interact with apoptotic cells to a level that was approximately half the capacity of CD14+/+ macrophages. These results provide further evidence that apoptotic cells persist in CD14−/− mice as a result of defective clearance by macrophages.
Figure 4.
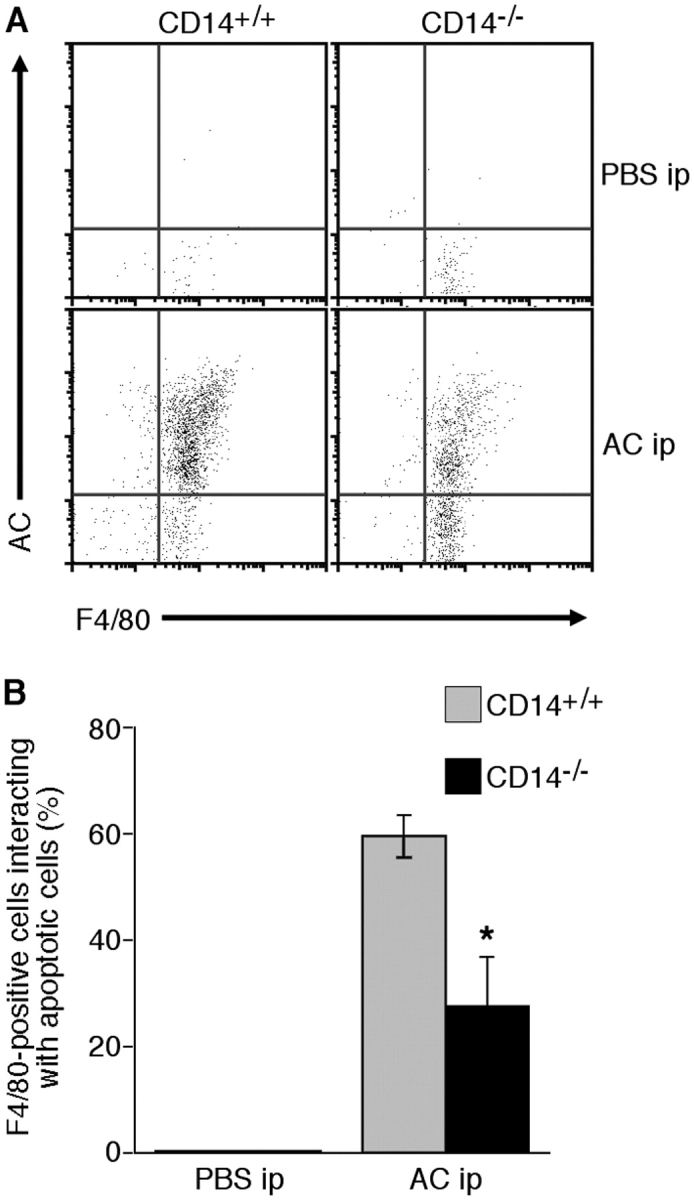
Inefficient interaction of apoptotic thymocytes with resident peritoneal macrophages in CD14 −/− mice. Fluorescent (green) autologous apoptotic thymocytes in PBS were introduced into the peritoneal cavity of CD14+/+ or CD14−/− mice by i.p. injection. Control animals received i.p. PBS alone. After 15 min, peritoneal cells were harvested, stained with F4/80, and analyzed by flow cytometry. Similar numbers of resident F4/80+ peritoneal macrophages were obtained from CD14+/+ and CD14−/− animals. (A) Representative flow cytometric dot plots of apoptotic (green) cells (AC) versus (red) F4/80+ events (macrophages). (B) Collated data from CD14+/+ and CD14−/− mice tested (means ± SEM; n = 3 animals in each case). ANOVA: *, P < 0.05.
In situ apoptotic cell clearance is inefficient in CD14−/− thymi after triggering synchronous thymocyte apoptosis
The i.p. investigations outlined in the previous section suggested that CD14−/− macrophages were less able than their CD14+/+ counterparts to clear a burden of apoptotic cells in situ. We wished to determine whether or not macrophages elsewhere were similarly deficient. Therefore, we used dexamethasone treatment to elicit massive, synchronous thymocyte apoptosis in situ in CD14−/− and CD14+/+ mice and assessed the persistence of these cells quantitatively by flow cytometry. Thymi from dexamethasone-treated animals were markedly smaller than those of mock (PBS)-treated animals. ISEL of thymic sections demonstrated substantial apoptosis induced by dexamethasone notably in the cortices of both CD14−/− and CD14+/+ thymi (unpublished data). In contrast, control, mock-treated animals displayed much less ISEL product, with CD14−/− animals showing obviously greater numbers of apoptotic events than their CD14+/+ counterparts confirming our earlier results (illustrated in Fig. 3 A). Disruption of thymi and flow cytometric assessment of apoptosis in the resulting cell suspensions showed that higher levels of dying and dead thymocytes could be isolated from CD14−/−, as compared with CD14+/+, mice. These levels were significantly increased by dexamethasone treatment (Fig. 5 A). When the AxV-positive cells were assessed for loss of membrane integrity (secondary necrosis) using PI, significantly higher levels of PI+ cells following dexamethasone treatment were observed both in CD14−/− and CD14+/+ populations (Fig. 5 A). Because these AxV+ PI+ cells were apoptotic cells (as judged by their morphology; unpublished data) that had lost membrane integrity, these results suggested that the massive, synchronous apoptosis induced by dexamethasone overloaded the phagocytic capacity of the thymi of both CD14+/+ and CD14−/− animals. These observations are consistent with the conclusion that thymocytes that were not cleared rapidly were permitted to undergo post-apoptotic changes akin to those occurring in vitro in the absence of phagocytes. However, the overload was more marked in the samples from CD14−/− thymi because significantly higher levels of total AxV+ and of AxV+ PI+ cells were isolated in comparison to CD14+/+ thymi (Fig. 5 A).
To address the possibility that thymocytes from CD14−/− animals were more sensitive to dexamethasone-induced apoptosis, we isolated thymocytes from wild-type and CD14−/− animals, treated them with dexamethasone in vitro, and followed the kinetics of apoptosis. No significant differences in the rates of apoptosis (AxV+ PI−) or secondary necrosis (AxV+ PI+) were demonstrable between CD14+/+ and CD14−/− thymocytes, indicating that CD14+/+ and CD14−/− thymocytes were not differentially sensitive to dexamethasone (Fig. 5 B). These results provided further evidence that apoptotic cell clearance was defective in CD14−/− mice.
CD14 deficiency does not promote inflammation or autoantibody production, and CD14−/− macrophages retain the capacity to generate anti-inflammatory responses to apoptotic cells
Persistence of apoptotic cells in vivo was expected to be associated with inflammation and increased autoantibody production. However, CD14−/− mice showed no overt inflammatory lesions (Fig. 3 H and not depicted) or increased serum concentration of TNF-α (not depicted) despite the chronic presence of apoptotic cells within tissues. We also monitored autoantibody titers and autoimmune disease pathology in aged animals and found titers of anti-nuclear antibodies (ANA) were comparable in sera of CD14+/+ and CD14−/− animals (Fig. 6 A). Extensive histopathological analyses of tissues from these animals (unpublished data) indicated no end organ effects. Thus, in these animals, persistence of apoptotic cells resulting from the absence of CD14 does not augment autoantibody production or lead to increased susceptibility to autoimmune disease.
Figure 6.
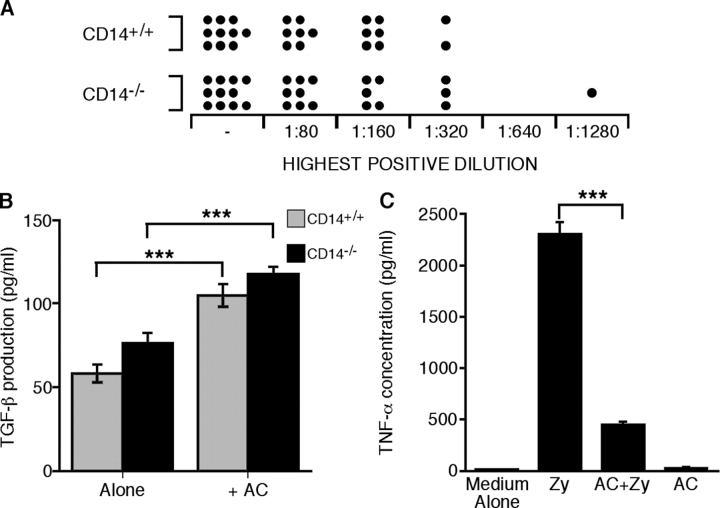
Persistence of apoptotic cells in CD14 − / − mice does not lead to increased autoantibody production or inflammation. (A) Autoantibody production is not increased in CD14−/− mice. Titers of ANA antibodies in sera from aged CD14−/− and CD14+/+ mice were assessed by indirect immunofluorescence. See text for details. “−” denotes negative samples. (B) CD14−/− macrophages produce TGF-β effectively in response to apoptotic cells. TGF-β concentration in the supernatants of peritoneal macrophages following 18-h stimulation alone or with apoptotic thymocytes (AC). Data shown are means ± SEM, n = 5. ANOVA: ***, P < 0.001. (C) Apoptotic cells can inhibit proinflammatory responses of CD14-deficient macrophages. (left) TNF-α concentration in the supernatants of 10-d BMDMs following 18-h stimulation with both or either opsonized zymosan (Zy) and apoptotic thymocytes (AC). Data shown are means ± SEM, n = 3. ANOVA: ***, P < 0.001.
In light of these data, we wished to assess whether or not CD14−/− macrophages were sensitive to the induction of anti-inflammatory pathways in response to interactions with apoptotic cells as defined in previous studies (Voll et al., 1997; Fadok et al., 1998a). As shown in Fig. 6 B, CD14−/− macrophages were found to be as effective as their CD14+/+ counterparts in producing the anti-inflammatory cytokine TGF-β in response to apoptotic cells. Furthermore, CD14−/− macrophages elicit significant TNF-α production in response to opsonized zymosan (a CD14-independent proinflammatory stimulus) that is inhibited following macrophage interaction with apoptotic cells (Fig. 6 C). These results indicate that the immuno-modulatory effects of apoptotic cells on macrophages are functional in the absence of CD14, providing a rationale for the observed absence of inflammatory consequences of CD14 deficiency in vivo.
Discussion
The present work was undertaken as a direct extension of our previous work demonstrating that CD14 on human macrophages plays a key role in the clearance of apoptotic cells in vitro (Devitt et al., 1998, 2003). Here, we show (a) that CD14 acts as a tethering receptor of apoptotic cells, (b) that, in animals deficient in CD14, apoptotic cells are cleared sub-optimally, resulting in increased frequencies of apoptotic cells and bodies at multiple sites in situ, and (c) that such persistent apoptotic cells are not proinflammatory.
Our conclusion that the observed phenotype of CD14−/− mice is due to the persistence of apoptotic cells as a result of impaired clearance, rather than to increased apoptosis, is based on several observations. First, our quantitative histological analyses suggested that, in tissues of CD14−/− animals, fewer apoptotic cells were associated with macrophages as compared with those of wild-type animals. Second, we found that macrophages from CD14−/− animals were less effective than their normal counterparts in clearing apoptotic cells in standard in vitro assays despite equivalent clearance of antibody-opsonized cells regardless of CD14 expression, indicating that CD14−/− macrophages are not fundamentally flawed in their capacity to phagocytose. CD14-deficient macrophages are similarly unimpaired in their ability to phagocytose whole bacteria (Moore et al., 2000). Third, when apoptotic cells were introduced into the peritoneal cavity and permitted to interact with peritoneal macrophages in a well-established short-term in vivo assay (Taylor et al., 2000), CD14−/− macrophages were found to be less effective than their CD14+/+ counterparts in binding and phagocytosing apoptotic cells. Finally, we used dexamethasone to induce massive, synchronous thymocyte apoptosis in situ. Again, we found that the phagocytosis of the apoptotic cells so induced in the CD14−/− animals was less effective than in CD14+/+ animals, leading to persistence of greater numbers of free apoptotic cells. Significantly, thymocytes from CD14−/− and CD14+/+ animals were similarly sensitive to apoptosis induction by dexamethasone.
As well as playing a role in apoptotic cell clearance, CD14 is functional in innate immune responses against microbial products, particularly LPS (for reviews see Ulevitch and Tobias, 1995; Kitchens, 2000). However, subtle infections resulting from the absence of CD14 are unlikely to account for the increased numbers of apoptotic cells observed in the tissues of CD14−/− mice. Although infectious agents can induce apoptosis in tissues, including the thymus (Ayala et al., 1996; Hotchkiss et al., 2001), the animals used for these studies were healthy and fertile and it should be noted that the normal phagocytic capacity of mice is such that increased numbers of free apoptotic cells are observed only when massive levels of synchronous apoptosis are induced causing overload of the clearance mechanisms (for review see Ogasawara et al., 1993) or when the clearance mechanisms themselves are compromised (Botto et al., 1998; Hamon et al., 2000; Scott et al., 2001; Li et al., 2003; Hanayama et al., 2004). Here, we have used a range of in vitro and in vivo assays of apoptotic cell clearance that accord with each other and are consistent with the conclusion that CD14−/− animals are inefficient in clearing apoptotic cells rather than being more susceptible to the induction of apoptosis.
CD14 is one of several macrophage receptors implicated, mainly through in vitro studies, in the clearance of apoptotic cells. The results presented here indicate that CD14 plays a non-redundant or only partially redundant role in apoptotic cell clearance in normal animals. We observed persistence of apoptotic cells in all tissues studied—thymus, spleen, lung, liver, and gut. This finding contrasts with animals that are functionally deficient in the Mer tyrosine kinase, which also display an apoptotic cell clearance defect in vivo. In unchallenged animals, clearance of apoptotic cells in the thymus, the only tissue thus far reported, was found to be normal in Mer-deficient mice; treatment with dexamethasone was required to reveal the clearance defect in situ (Scott et al., 2001). SR-A–deficient thymic macrophages, which are defective in their capacity to engulf apoptotic thymocytes in vitro, are uncompromised in SR-A–deficient thymi in situ even when thymocyte apoptosis is accelerated by irradiation (Platt et al., 2000). Clearly, therefore, the functions of CD14, Mer, and SR-A in enabling or supporting apoptotic cell clearance in vivo are separable. Besides CD14, the only other molecules whose absence has thus far been reported to generate apoptotic cell clearance defects in unchallenged adult tissue in situ are the bridging molecules C1q and MFG-E8 (Botto et al., 1998; Hanayama et al., 2004). C1q deficiency leads to the persistence of apoptotic cells specifically in glomeruli of genetically susceptible mice (Botto et al., 1998). In MFG-E8–deficient mice, apoptotic cell engulfment is impaired in the germinal centers of secondary lymphoid follicles (Hanayama et al., 2004). Apoptotic cells appear not to persist at other sites in either C1q- or MFG-E8–deficient mice. Significantly, consequences of the absence of CD14 are manifest widely with all adult tissues examined thus far displaying persistence of apoptotic cells in situ. We conclude that CD14 has a broad role, being required for efficient apoptotic cell clearance at multiple tissue sites.
Although the detailed molecular mechanisms underlying CD14's involvement in apoptotic cell clearance have yet to be defined, they differ from those involving C1q, Mer, and MFG-E8. C1q appears to bridge apoptotic cells to CD91/calreticulin on phagocytes (Ogden et al., 2001), Mer probably associates with apoptotic cell surface phosphatidylserine (PS) via the bridging protein Gas6 (Ishimoto et al., 2000), and MFG-E8 bridges PS to phagocyte vitronectin receptor integrins (Hanayama et al., 2002). Notably, tethering of apoptotic cells appears to be normal in both Mer- and MFG-E8–deficient animals (Scott et al., 2001; Hanayama et al., 2004). Previous work has suggested that CD14 functions to tether apoptotic cells to the phagocyte surface (Devitt et al., 1998; Hoffmann et al., 2001). We now provide strong evidence in support of this notion, demonstrating a role for CD14 in tethering apoptotic cells to macrophages and showing that purified CD14 can bind effectively to apoptotic, but not viable, cells. Therefore, CD14's major, and perhaps sole, function in clearing apoptotic cells may be in the initial recognition and binding phase of the process. In this model, additional receptor–ligand interactions (e.g., PS exposed on apoptotic cells binding via Gas6 to Mer [Ishimoto et al., 2000] or via MFG-E8 to vitronectin receptors [Hanayama et al., 2002]) would be required to induce phagocytosis/anti-inflammatory responses in the phagocyte.
In the present scenario, even though CD14 deficiency leads to persistence of apoptotic cells in many locations, such persistence is not accompanied by inflammatory reactions at these sites. This contrasts with the effects of massive apoptosis, which can generate inflammatory effects (Uchimura et al., 2000; Lorimore et al., 2001). Evidence has been provided that defective apoptotic cell clearance, given appropriate genetic background (C57BL/6,129 or mixed), is associated with increased productivity of autoantibodies and increased incidence of autoimmune disease (Botto et al., 1998; Scott et al., 2001; Mitchell et al., 2002; Cohen et al., 2002; Szondy et al., 2003; Hanayama et al., 2004). However, a causative link between defective apoptotic cell clearance and autoimmune disease pathogenesis is far from clear because of the strains of mice used (Bygrave et al., 2004). Significantly, autoantibody production is not enhanced in the CD14−/− animals investigated here. Balb/c animals, the background strain used here, are not inherently resistant to autoimmune disease (Horai et al., 2000; Rudner et al., 2003). Our results are consistent with the idea that CD14 provides a non-redundant tethering mechanism that facilitates the interaction of apoptotic cells with macrophages. Although this mechanism is absent in CD14−/− mice, the signaling mechanisms that prevent inflammatory and possibly autoimmune responses to persistent apoptotic cells appear to remain intact. Therefore, the tethering activity of CD14 in clearing apoptotic cells can be functionally uncoupled from the mechanisms that prevent such cells generating inflammatory or immunostimulatory responses. This supports the view that different receptor–ligand interactions are required for different phases of the clearance process as we and others have previously proposed. The functional separation of anti-inflammatory and engulfment signaling events has been described recently (Cvetanovic and Ucker, 2004).
Because apoptotic cell phagocytosis appears to be reduced rather than blocked in CD14−/− mice, it would appear that additional clearance mechanisms are also operable. Such mechanisms may include lower affinity tethering mechanisms and mechanisms that relate specifically to apoptotic cells at later stages than those normally detected by CD14. The relative inefficiency of these mechanisms that causes apoptotic cell persistence is indicative of a non-redundant or only partially redundant role for CD14 in the clearance of apoptotic cells.
The binding of purified CD14, a prototypical PRR, to apoptotic but not viable cells indicates that activation of the apoptosis program leads to the display of cellular CD14-ligands. In line with the conserved microbial PRR ligands (such as LPS) being termed pathogen-associated molecular patterns (PAMPs), such host PRR ligands have been collectively termed apoptotic cell–associated molecular patterns (ACAMPs; Franc et al., 1999; Gregory, 2000). A key difference between ligation of PRRs by PAMPs versus ACAMPs is the response of the host cell, with PAMPs generating proinflammatory and ACAMPs anti-inflammatory responses. Of critical importance to understanding CD14's role in apoptotic cell clearance is defining the mechanistic differences underlying responses to PAMPs versus ACAMPs. We have previously proposed several scenarios including a “minimalist” view of CD14 acting solely as a tethering receptor (Gregory, 2000; Gregory and Devitt, 2004). The present results support this view but do not preclude CD14 playing additional roles in the clearance process, for example cooperating with molecular partners to generate or facilitate intracellular signals for engulfment. In this context, cooperation with toll-like receptors, as in the generation of proinflammatory responses to PAMPs, appears unlikely (Li et al., 2001; Blander and Medzhitov, 2004; Cvetanovic and Ucker, 2004; Shiratsuchi et al., 2004).
The results presented here first demonstrate that absence of the mechanism(s) served by CD14 in the phagocytic clearance of apoptotic cells reveals apoptosis in multiple tissues. The lack of the CD14-dependent mechanism(s) of rapid apoptotic cell disposal appears not to be detrimental to the host. Notably, CD14−/− animals are healthy, inflammatory lesions associated with persistent apoptotic cells are absent, autoantibody production is similar to that of wild-type animals, and no autoimmune disease is detectable despite detailed histopathological analyses of aged animals. Therefore, the results presented here clearly demonstrate that apoptotic cells can persist without consequent inflammation or autoimmune disease development despite the susceptibility of the Balb/c strain to autoimmune disease (Horai et al., 2000; Rudner et al., 2003). Indeed, our findings raise the possibility that persistence under these circumstances may prolong the anti-inflammatory effects of apoptotic cells.
Materials and methods
Animals
Balb/c CD14−/− mice were produced as described previously (Haziot et al., 1996). For most studies, animals were used at 8–12 wk; for investigations of autoantibody production and autoimmune disease pathogenesis, ages ranged from 10 to 20 mo. For some experiments, animals were injected i.p. with 200 μg dexamethasone (Organon) 24 h before sacrifice. All animals were killed by cervical dislocation. All animal work was performed under licence from the UK Home Office and with approval of the Review Boards of the Universities of Nottingham and Edinburgh.
Qualitative and quantitative histology
Tissues were prepared either (1) by fixation in 4% PFA in 0.1 M of phosphate buffer, pH 7.3, followed by wax embedding or (2) by fixation in 1% PFA, 3% glutaraldehyde in 0.1 M of cacodylate buffer, pH 7.2, followed by embedding in araldite resin. 6-μm sections were cut from wax blocks for standard hematoxylin and eosin staining, ISEL, or immunohistochemistry. 1-μm sections were cut from resin blocks for toluidine blue staining and detailed light microscopic analyses. ISEL was performed using a FragEl kit (Calbiochem) as per the manufacturer's instructions followed by counterstaining with methyl green. Separate de-waxed sections were immunostained with mAb F4/80 and visualized using a Vectastain kit (Vector Laboratories). Quantitative light microscopic analyses were performed according to standard morphometric procedures (Weibel, 1979) using random systematic sampling of blocks, sections, and microscopic fields from three CD14−/− animals and matched CD14+/+ controls. In the case of ISEL-labeled sections, numbers of labeled nuclei per unit area of tissue were measured. For resin-embedded tissue (thymus and spleen), the frequency of apoptotic cells/large apoptotic bodies per unit area was recorded. In addition, these events were subdivided according to whether they were “free” or macrophage-associated (i.e., juxtaposed with a macrophage or appearing within the cytoplasm of a macrophage). Given the reticular nature of the macrophages identified by F4/80 staining, point-counting (Weibel, 1979) was used to provide a measurement of the proportional area of macrophage cytoplasm distributed throughout the tissues analyzed (thymus and spleen). Hematoxylin- and eosin-stained sections of kidney and secondary lymphoid tissues from aged animals were analyzed for histopathological evidence of autoimmune disease.
Cell lines, cell isolation, and culture
The Burkitt lymphoma (BL) line Mutu I (Gregory et al., 1990) was cultured in RPMI 1640 medium containing 2 mM l-glutamine supplemented with 10% Serum Supreme (BioWhittaker). COS-1 cells were cultured and transfected with human CD14 cDNA as described previously (Devitt et al., 1998). Monocytes were isolated from defibrinated blood (Flora and Gregory, 1994) and cultured for 7 d in IMDM (Invitrogen) containing 10% autologous serum on multi-well glass slides as described previously (Devitt et al., 1998). Thymocyte suspensions were prepared by mechanical disruption of thymi between frosted glass slides. Macrophages from bone marrow were isolated and cultured for 10 d on multi-well glass slides (Hendley-Essex) in DME supplemented with 10% FCS, 2 mM l-glutamine, and 10% L cell–conditioned medium as described previously (Fadok et al., 1992). Non-elicited peritoneal macrophages were lavaged from the peritoneal cavities of mice and adherent cells cultured on multi-well glass slides in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, and 10% L cell–conditioned medium for 1–2 d before use.
Apoptosis-induction and measurement in vitro
Apoptosis was induced by treatment (16–18 h) at 37°C of Mutu I cells with 1 μg ml−1 ionomycin (Sigma-Aldrich) or thymocytes with 1 μM dexamethasone (Sigma-Aldrich). Apoptosis was assessed routinely by fluorescence microscopy of DAPI (Sigma-Aldrich)-stained cells (Devitt et al., 2003). Flow cytometric analysis of apoptosis in thymocytes was undertaken using AxV binding in combination with PI staining. Cells were washed into binding buffer (10 mM Hepes, pH 7.4, 2.5 mM CaCl2, and 150 mM NaCl) and stained with AxV-FITC (BioWhittaker). PI (20 μg ml−1; Sigma-Aldrich) was added subsequently and samples were run directly on the Coulter XL flow cytometer (Beckman Coulter). Percentages of cells that were (1) AxV−PI− (viable), (2) AxV+PI− (apoptotic), or (3) AxV+PI+ (necrotic) were enumerated.
Quantitative analyses of macrophage–apoptotic cell interactions
Human monocyte–derived, murine bone marrow–derived, or peritoneal macrophages cultured on multi-well glass slides were coincubated with apoptotic cells (106 per well in RPMI containing 0.2% [wt/vol] BSA; Sigma-Aldrich). In some experiments, the CD14 mAbs 61D3 or 63D3 (Devitt et al., 1998) were included. After 30–60 min at 16–20°C (for tethering assays) or at 37°C (to include phagocytosis), unbound cells were removed by extensive washing, and slides were fixed in methanol, stained with Jenner/Giemsa (BDH), and mounted in DePeX (BDH) before examination by light microscopy (Devitt et al., 2003). In certain tethering experiments, COS-1 cells or COS-1/CD14 transfectants were used as “surrogate” macrophages. For the i.p. interaction assay, a method modified from Taylor et al. (2000) was used. In brief, freshly isolated autologous thymocytes were fluorescently labeled with 1 μM CMFDA (Molecular Probes) in PBS as per the manufacturer's instructions, and cultured at a density of 5 × 106/ml for 16–18 h in RPMI containing10% FCS, 2 mM l-glutamine, and 1 μM dexamethasone (Sigma-Aldrich). Apoptotic thymocytes (>90% apoptotic as judged by DAPI-stained nuclear morphology) were washed thoroughly, and 5 × 106 cells in 1 ml of sterile PBS were injected i.p. into CD14+/+ or CD14−/− mice. After 15-min interaction, i.p. cells were harvested and immunostained with F4-80-tri-color (Caltag Laboratories). Flow cytometry was performed immediately to enumerate the proportion of F4/80-positive cells (macrophages) that were associated with green (apoptotic) cells.
Phagocytosis of antibody-opsonized cells
BL cells were opsonized with excess CD19 mAb (mouse IgG1) at 4°C for 15 min, washed, and coincubated with BMDMs as described in the previous section. After 30 min at 37°C, unbound cells were removed and phagocytosis was assessed by light microscopy of Jenner/Giemsa-stained preparations. Duplicate wells of each sample were scored, with >200 macrophages per well being analyzed.
Production and binding of recombinant human CD14
Recombinant human CD14 was produced either as an Fc fusion protein or his-tagged protein in 293 cells and purified using either protein G or nickel affinity purification techniques. For staining, 200,000 cells were incubated with 1 μg of tagged CD14 for 30 min at 4°C. CD14-Fc was detected by sequential staining steps with biotinylated goat anti–human IgG (Sigma-Aldrich) and streptavidin-quantum red (Sigma-Aldrich). CD14-HIS was similarly detected using murine anti-HIS mAb (Roche) and goat anti–mouse-PE (Sigma-Aldrich).
Cytokine titers
Levels of TNF-α present in mouse sera collected postmortem were assessed using an anti-mouse TNF-α ELISA kit (R&D Systems). The anti-inflammatory effect of apoptotic cells was assessed as previously described (Fadok et al., 1998a). In brief, murine macrophages were treated with apoptotic thymocytes or apoptotic BL cells for 18 h in X-vivo 10 medium (BioWhittaker). For some wells, zymosan, opsonized with human serum for 15 min at RT before washing, was added to a final concentration of 100 μg/ml. After the period of stimulation, culture supernatants were assayed for TNF-α or TGF-β using anti-mouse TNF-α or anti-mouse TGF-β ELISA kits (R&D Systems).
Autoantibody titers
ANA titers were assessed using previously described methods (Botto et al., 1998; Cohen et al., 2002). In brief, sera collected immediately postmortem were diluted in PBS. Indirect immunofluorescence was performed on acetone-fixed Hep-2 cell monolayers. The visualization reagent was goat anti–mouse FITC (Sigma-Aldrich) slides being analyzed by standard epifluorescence microscopy (model Zeiss Axioskop 2; Carl Zeiss MicroImaging, Inc.). Dilutions of sera were tested from 1:80 to 1:2,560, and the last dilution in the series to show reactivity was recorded for each animal. A “negative” titer was given to those samples that showed no reactivity at a 1:80 dilution.
Acknowledgments
We are very grateful to Dr. Marina Botto for advice on the ANA methodology.
This work was supported by the Medical Research Council (UK), the European Union, and the Leukaemia Research Fund (UK).
S.C. Gangloff's present address is University of Reims Champagne Ardenne, EA2070 -IFR53, 51 100 Reims, France.
Abbreviations used in this paper: ACAMP, apoptotic cell–associated molecular pattern; ANA, anti-nuclear antibodies; AxV, annexin V; BL, Burkitt lymphoma; BMDM, bone marrow–derived macrophage; HMDM, human monocyte-derived macrophage; ISEL, in situ end labelling; PAMP, pathogen-associated molecular pattern; PI, propidium iodide; PRR, pattern recognition receptor; PS, phosphatidylserine.
References
- Albert, M.L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4:223–231. [DOI] [PubMed] [Google Scholar]
- Ayala, A., C.D. Herdon, D.L. Lehman, C.A. Ayala, and I.H. Chaudry. 1996. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 87:4261–4275. [PubMed] [Google Scholar]
- Blander, J.M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from toll-like receptors. Science. 304:1014–1018. [DOI] [PubMed] [Google Scholar]
- Botto, M., C. DellAgnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. [DOI] [PubMed] [Google Scholar]
- Brown, S., I. Heinisch, E. Ross, K. Shaw, C.D. Buckley, and J. Savill. 2002. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 418:200–203. [DOI] [PubMed] [Google Scholar]
- Bygrave, A.E., K.L. Rose, J. Cortes-Hernandez, J. Warren, R.J. Rigby, H.T. Cook, M.J. Walport, T.J. Vyse, and M. Botto. 2004. Spontaneous autoimmunity in 129 and C57BL/6 mice-implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2:E243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, P.L., R. Caricchio, V. Abraham, T.D. Camenisch, J.C. Jennette, R.A.S. Roubey, H.S. Earp, G. Matsushima, and E.A. Reap. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetanovic, M., and D.S. Ucker. 2004. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J. Immunol. 172:880–889. [DOI] [PubMed] [Google Scholar]
- Devitt, A., O.D. Moffatt, C. Raykundalia, J.D. Capra, D.L. Simmons, and C.D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 392:505–509. [DOI] [PubMed] [Google Scholar]
- Devitt, A., S. Pierce, C. Oldreive, W.H. Shingler, and C.D. Gregory. 2003. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death Differ. 10:371–382. [DOI] [PubMed] [Google Scholar]
- Dive, C., C.D. Gregory, D.J. Phipps, D.L. Evans, A.E. Milner, and A.H. Wyllie. 1992. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim. Biophys. Acta. 1133:275–285. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., J.S. Savill, C. Haslett, D.L. Bratton, D.E. Doherty, P.A. Campbell, and P.M. Henson. 1992. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149:4029–4035. [PubMed] [Google Scholar]
- Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. a. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., M.L. Warner, D.L. Bratton, and P.M. Henson. 1998. b. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha(v)beta(3)). J. Immunol. 161:6250–6257. [PubMed] [Google Scholar]
- Fadok, V.A., D.L. Bratton, L. Guthrie, and P.M. Henson. 2001. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J. Immunol. 166:6847–6854. [DOI] [PubMed] [Google Scholar]
- Flora, P.K., and C.D. Gregory. 1994. Recognition of apoptotic cells by human macrophages: inhibition by a monocyte/macrophage-specific monoclonal antibody. Eur. J. Immunol. 24:2625–2632. [DOI] [PubMed] [Google Scholar]
- Franc, N.C., K. White, and R.A.B. Ezekowitz. 1999. Phagocytosis and development: back to the future. Curr. Opin. Immunol. 11:47–52. [DOI] [PubMed] [Google Scholar]
- Goyert, S.M., E. Ferrero, W.J. Rettig, A.K. Yenamandra, F. Obata, and M.M. Lebeau. 1988. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 239:497–500. [DOI] [PubMed] [Google Scholar]
- Gregory, C.D. 2000. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr. Opin. Immunol. 12:27–34. [DOI] [PubMed] [Google Scholar]
- Gregory, C.D., and A. Devitt. 2004. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 113:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, C.D., M. Rowe, and A.B. Rickinson. 1990. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J. Gen. Virol. 71:1481–1495. [DOI] [PubMed] [Google Scholar]
- Gregory, C.D., C. Dive, S. Henderson, C.A. Smith, G.T. Williams, J. Gordon, and A.B. Rickinson. 1991. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 349:612–614. [DOI] [PubMed] [Google Scholar]
- Grimsley, C., and K.S. Ravichandran. 2003. Cues for apoptotic cell engulfment: eat-me, don't eat-me and come-get-me signals. Trends Cell Biol. 13:648–656. [DOI] [PubMed] [Google Scholar]
- Hamon, Y., C. Broccardo, O. Chambenoit, M.F. Luciani, F. Toti, S. Chaslin, J.M. Freyssinet, P.F. Devaux, J. McNeish, D. Marguet, and G. Chimini. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2:399–406. [DOI] [PubMed] [Google Scholar]
- Hanayama, R., M. Tanaka, K. Miwa, A. Shinohara, A. Iwamatsu, and S. Nagata. 2002. Identification of a factor that links apoptotic cells to phagocytes. Nature. 417:182–187. [DOI] [PubMed] [Google Scholar]
- Hanayama, R., M. Tanaka, K. Miyasaka, K. Aozasa, M. Koike, Y. Uchiyama, and S. Nagata. 2004. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 304:1147–1150. [DOI] [PubMed] [Google Scholar]
- Haziot, A., E. Ferrero, F. Kontgen, N. Hijiya, S. Yamamoto, J. Silver, C.L. Stewart, and S.M. Goyert. 1996. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 4:407–414. [DOI] [PubMed] [Google Scholar]
- Henson, P.M., D.L. Bratton, and V.A. Fadok. 2001. Apoptotic cell removal. Curr. Biol. 11:R795–R805. [DOI] [PubMed] [Google Scholar]
- Hoffmann, P.R., A.M. deCathelineau, C.A. Ogden, Y. Leverrier, D.L. Bratton, D.L. Daleke, A.J. Ridley, V.A. Fadok, and P.M. Henson. 2001. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai, R., S. Saijo, M. Tanioka, S. Nakae, K. Sudo, A. Okahara, T. Ikuse, M. Asano, and Y. Iwakura. 2000. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 191:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss, R.S., W.M. Dunne, P.E. Swanson, C.G. Davis, K.T. Tinsley, K.C. Chang, T.G. Buchman, and I.E. Karl. 2001. Role of apoptosis in Pseudomonas aeruginosa pneumonia. Science. 294:1783. [DOI] [PubMed] [Google Scholar]
- Ishimoto, Y., K. Ohashi, K. Mizuno, and T. Nakano. 2000. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. (Tokyo). 127:411–417. [DOI] [PubMed] [Google Scholar]
- Kitchens, R.L. 2000. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem. Immunol. 74:61–82. [DOI] [PubMed] [Google Scholar]
- Kurosaka, K., M. Takahashi, N. Watanabe, and Y. Kobayashi. 2003. Silent cleanup of very early apoptotic cells by macrophages. J. Immunol. 171:4672–4679. [DOI] [PubMed] [Google Scholar]
- Li, M., D.F. Carpio, Y. Zheng, P. Bruzzo, V. Singh, F. Ouaaz, R.M. Medzhitov, and A.A. Beg. 2001. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 166:7128–7135. [DOI] [PubMed] [Google Scholar]
- Li, M.O., M.R. Sarkisian, W.Z. Mehal, P. Rakic, and R.A. Flavell. 2003. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 302:1560–1563. [DOI] [PubMed] [Google Scholar]
- Lorimore, S.A., P.J. Coates, G.E. Scobie, G. Milne, and E.G. Wright. 2001. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 20:7085–7095. [DOI] [PubMed] [Google Scholar]
- McIlroy, D., M. Tanaka, H. Sakahira, H. Fukuyama, M. Suzuki, K. Yamamura, Y. Ohsawa, Y. Uchiyama, and S. Nagata. 2000. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14:549–558. [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D.A., M.C. Pickering, J. Warren, L. Fossati-Jimack, J. Cortes-Hernandez, H.T. Cook, M. Botto, and M.J. Walport. 2002. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J. Immunol. 168:2538–2543. [DOI] [PubMed] [Google Scholar]
- Moore, K.J., L.P. Andersson, R.R. Ingalls, B.G. Monks, R. Li, M.A. Arnaout, D.T. Golenbock, and M.W. Freeman. 2000. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J. Immunol. 165:4272–4280. [DOI] [PubMed] [Google Scholar]
- Ogasawara, J., R. Watanabefukunaga, M. Adachi, A. Matsuzawa, T. Kasugai, Y. Kitamura, N. Itoh, T. Suda, and S. Nagata. 1993. Lethal effect of the anti-fas antibody in mice. Nature. 364:806–809. [DOI] [PubMed] [Google Scholar]
- Ogden, C.A., A. deCathelineau, P.R. Hoffmann, D. Bratton, B. Ghebrehiwet, V.A. Fadok, and P.M. Henson. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, N., H. Suzuki, T. Kodama, and S. Gordon. 2000. Apoptotic thymocyte clearance in scavenger receptor class A-deficient mice is apparently normal. J. Immunol. 164:4861–4867. [DOI] [PubMed] [Google Scholar]
- Rudner, L.A., J.T. Lin, I.K. Park, J.M. Cates, D.A. Dyer, D.M. Franz, M.A. French, E.M. Duncan, H.D. White, and J.D. Gorham. 2003. Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J. Immunol. 170:4785–4792. [DOI] [PubMed] [Google Scholar]
- Savill, J., I. Dransfield, C.D. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975. [DOI] [PubMed] [Google Scholar]
- Schlegel, R.A., S. Krahling, M.K. Callahan, and P. Williamson. 1999. CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes. Cell Death Differ. 6:583–592. [DOI] [PubMed] [Google Scholar]
- Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi, A., I. Watanabe, O. Takeuchi, S. Akira, and Y. Nakanishi. 2004. Inhibitory effect of toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. J. Immunol. 172:2039–2047. [DOI] [PubMed] [Google Scholar]
- Surh, C.D., and J. Sprent. 1994. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 372:100–103. [DOI] [PubMed] [Google Scholar]
- Szondy, Z., Z. Sarang, P. Molnar, T. Nemeth, M. Piacentini, P.G. Mastroberardino, L. Falasca, D. Aeschlimann, J. Kovacs, I. Kiss, et al. 2003. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. USA. 100:7812–7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P.R., A. Carugati, V.A. Fadok, H.T. Cook, M. Andrews, M.C. Carroll, J.S. Savill, P.M. Henson, M. Botto, and M.J. Walport. 2000. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura, E., N. Watanabe, O. Niwa, M. Muto, and Y. Kobayashi. 2000. Transient infiltration of neutrophils into the thymus in association with apoptosis induced by whole-body X-irradiation. J. Leukoc. Biol. 67:780–784. [DOI] [PubMed] [Google Scholar]
- Ulevitch, R.J., and P.S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437–457. [DOI] [PubMed] [Google Scholar]
- Voll, R.E., M. Herrmann, E.A. Roth, C. Stach, J.R. Kalden, and I. Girkontaite. 1997. Immunosuppressive effects of apoptotic cells. Nature. 390:350–351. [DOI] [PubMed] [Google Scholar]
- Weibel, E.R. 1979. Stereological Methods Volume 1: Practical Methods for Biological Morphometry. Academic Press, London. 415 pp.
- Wright, S.D., R.A. Ramos, P.S. Tobias, R.J. Ulevitch, and J.C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 249:1431–1433. [DOI] [PubMed] [Google Scholar]