Abstract
We searched by a cDNA subtraction screen for differentially expressed transcripts in MCF-7 mammary carcinoma cells grown on tenascin-C versus fibronectin. On tenascin-C, cells had irregular shapes with many processes, whereas on fibronectin they were flat with a cobble stone–like appearance. We found elevated levels of 14-3-3 tau transcripts and protein in cells grown on tenascin-C. To investigate the consequences of an increased level of this phospho-serine/threonine–binding adaptor protein, we transfected MCF-7 cells with a construct encoding full-length 14-3-3 tau protein and selected clones with the highest expression levels. The morphology of these cells on tenascin-C was flat, resembling that of cells on fibronectin. This was reflected by a similar pattern of F-actin staining on either substratum. Furthermore, the growth rate on tenascin-C was increased compared with the parental cells. After transient transfection of HT1080 fibrosarcoma and T98G glioblastoma cells with 14-3-3 tau, only the 14-3-3 tau–expressing cells were able to adhere and survive on tenascin-C, whereas all cells adhered well on fibronectin. Therefore, we postulate that tenascin-C promotes the growth of tumor cells by causing an increase in the expression of 14-3-3 tau, which in turn has a positive effect on tumor cell adhesion and growth.
Keywords: extracellular matrix; cancer; growth; cell adhesion; adapter protein
Introduction
Tenascin-C is an ECM protein well known for its high expression in almost all solid tumors and for its antiadhesive properties for cells in culture (for reviews see Chiquet-Ehrismann, 1993; Crossin, 1996; Vollmer, 1997; Jones and Jones, 2000; Orend and Chiquet-Ehrismann, 2000). The level of tenascin-C expression and the nature of the isoform expressed seems in a variety of tumors to correlate with a higher incidence of metastases and poor prognosis (Goepel et al., 2000; Emoto et al., 2001; Salmenkivi et al., 2001; Adams et al., 2002; Herold-Mende et al., 2002).
Therefore, it is of great interest to find out how tumor cells react to the presence of tenascin-C. In most of the published studies to date, effects of tenascin-C on cell adhesion, spreading, migration, and growth have been reported (for review see Orend and Chiquet-Ehrismann, 2000). However, the molecular mechanisms mediating these responses to tenascin-C remain largely unknown. From previous studies we know that a tenascin-C substratum supports the growth of rat primary mammary carcinoma cells more than fibronectin (Chiquet-Ehrismann et al., 1986). Furthermore, we have shown that MCF-7 human mammary carcinoma cells induce tenascin-C expression in cocultured fibroblasts surrounding the tumor cell nests (Chiquet-Ehrismann et al., 1989).
We therefore decided to screen for transcripts that are differentially expressed in MCF-7 cells grown on a tenascin-C versus a fibronectin substratum. We now report on our discovery that a tenascin-C substratum induces the expression of the phospho-serine/threonine–binding adaptor protein 14-3-3 tau in MCF-7 mammary carcinoma cells and describe its influence on cell adhesion and cell growth.
Results and discussion
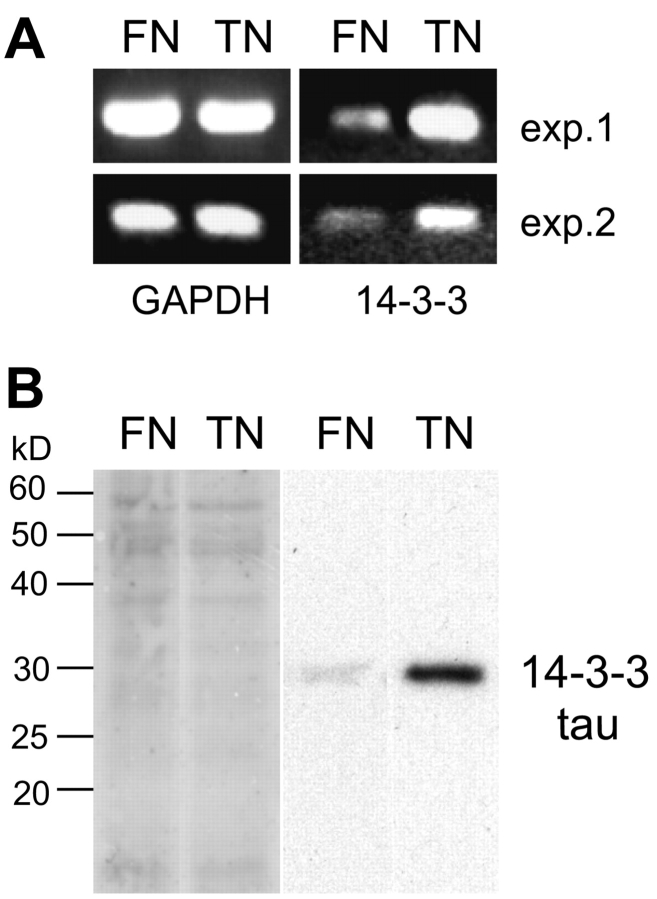
MCF-7 cells were grown in medium containing 10% FCS for 24 h on plates coated with fibronectin or tenascin-C, respectively. The cells show completely different morphologies on these two substrates. They grow in epithelial patches on fibronectin but lose their cell–cell contacts and adopt irregular shapes with long processes on a tenascin-C substratum (Fig. 1). This is reminiscent of the results obtained with MCF-7 cells grown on collagen gels in the presence and absence of tenascin-C where the cells in the presence of tenascin-C were found to loose their cell–cell contacts and detached from the substratum (Chiquet-Ehrismann et al., 1989). To identify molecular differences between the cells grown on fibronectin versus tenascin-C, we isolated mRNA from these cells and performed a screen for differentially expressed transcripts. This screen resulted in the identification of a cDNA clone encoding the adaptor protein 14-3-3 tau. 14-3-3 proteins are a family of phospho-serine/threonine–binding proteins with >70 known ligands as diverse as kinases, phosphatases, receptors, structural proteins, and transcription factors (for reviews see Fu et al., 2000; van Hemert et al., 2001; Tzivion and Avruch, 2002). We confirmed the higher level of 14-3-3 tau transcripts in two mRNA batches isolated from independently cultured MCF-7 cells on tenascin-C versus fibronectin substrates by semiquantitative PCR. As shown in Fig. 2 A, transcript levels of GAPDH were equal, whereas 14-3-3 tau was highly increased in the samples from the cells cultured on tenascin-C compared with fibronectin. We next tested whether the level of the 14-3-3 tau protein was also affected. We loaded equal amounts of cellular protein on an SDS-PAGE followed by detection of 14-3-3 tau by a specific antiserum on an immunoblot. As shown in Fig. 2 B, the level of the 14-3-3 protein was also much higher in cell extracts of cells grown on a tenascin-C substratum than on fibronectin.
Figure 1.
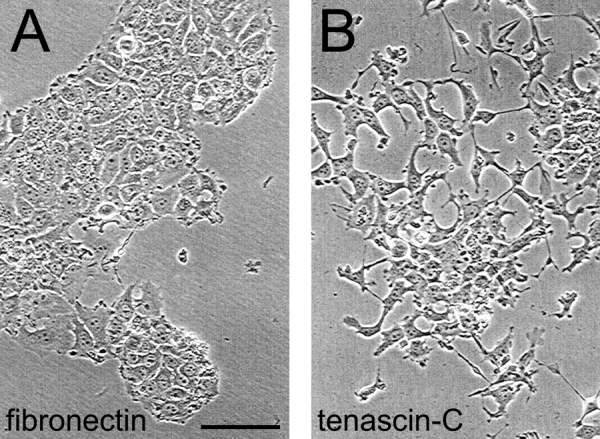
Morphological changes of MCF-7 cells grown on a tenascin-C substratum. MCF-7 cells were cultured for 24 h in complete medium on fibronectin-coated (A) and on tenascin-C–coated (B) dishes and photographed. On fibronectin, they adopt a cobble-stone–like epithelial morphology, whereas on tenascin-C they lose the cell–cell contacts and adopt irregular shapes. Bar, 200 μm.
Figure 2.
Induction of 14-3-3 tau in cells grown on tenascin-C. (A) Photographs of ethidium bromide-stained agarose gels shows RT-PCR reactions of mRNA isolated from MCF-7 cells grown for 24 h on a fibronectin (FN) or on a tenascin-C (TN) substratum. They reveal an increase in 14-3-3 transcripts in cells from the tenascin-C substratum, whereas GAPDH expression is equal in both situations. The same result was obtained in two independently performed experiments (exp. 1 and exp. 2). (B) Amidoblack staining of a 12% SDS gel blotted to a PVDF membrane showing equal protein content of cell extracts loaded from cells grown on fibronectin (FN) or tenascin-C (TN; left). The immunoblot of the same membrane obtained with anti–14-3-3 tau reveals a stronger signal in the extract from cells grown on tenascin-C than on fibronectin (right).
From the literature we know that 14-3-3 proteins are implicated in the regulation of cell growth and oncogenic transformation (Takihara et al., 2000) and that they exhibit antiapoptotic activity (Xing et al., 2000; Masters and Fu, 2001). Therefore, we decided to test for an effect of elevated 14-3-3 tau levels in MCF-7 cells on their adhesion and growth behavior when cultured on tenascin-C versus fibronectin substrates. We transfected MCF-7 cells with a construct encoding 14-3-3 tau containing an NH2-terminal Flag tag. Cell clones were isolated and tested on immunoblots for expression of the transfected 14-3-3 tau in comparison to the endogenous 14-3-3 tau protein as shown in Fig. 3 A. Clones 2 and 5 exhibiting the highest amount of the transfected 14-3-3 relative to the endogenous protein were selected for further experiments. When they were grown on tenascin-C–coated plates they exhibited a cobble stone–like morphology as on fibronectin, whereas the parental cells showed an irregular morphology on tenascin-C (Fig. 3 B). This was particularly well visible in phalloidin-stained cells (Fig. 4 A).
Figure 3.
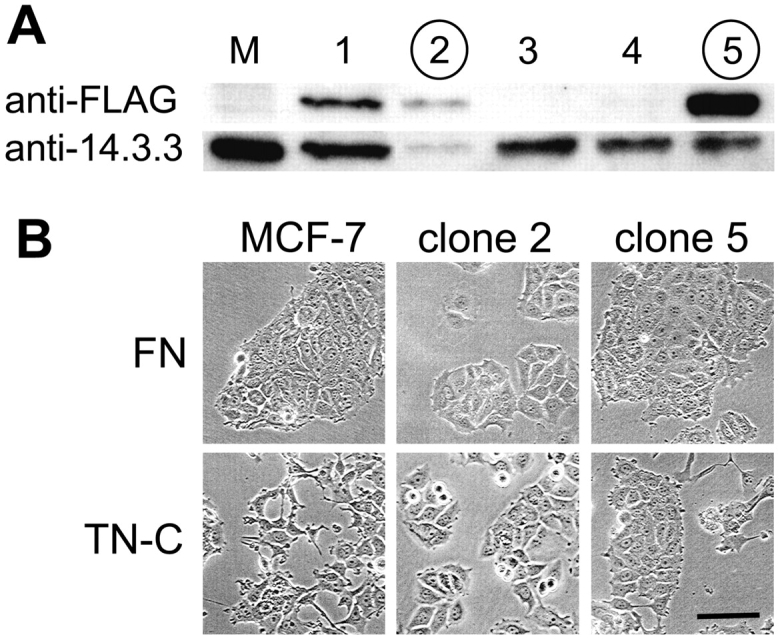
Isolation of MCF-7 clones overexpressing 14-3-3 tau. Cell extracts (not normalized to protein content) of the parental MCF-7 cells (M) and of clones 1–5 isolated after transfection with Flag-tagged 14-3-3 tau were separated by 12% SDS-PAGE. Immunoblots thereof were performed using anti-Flag to identify the product from the transfected construct and anti–14-3-3 tau to detect the endogenously expressed protein. The two bands could easily be distinguished, since the Flag-tagged protein migrated slightly slower than the endogenous protein. Clones 2 and 5 (circled numbers), exhibiting the strongest band with anti-Flag relative to the presence of the endogenous protein were selected for further studies. (B) Parental MCF-7 cells and clones 2 and 5 were cultured for 24 h in complete medium on a fibronectin versus a tenascin-C substratum and photographed. The parental cells showed an altered morphology induced by the tenascin-C substratum, whereas no difference was visible in the 14-3-3–overexpressing clones 2 and 5. Bar, 20 μm.
Figure 4.
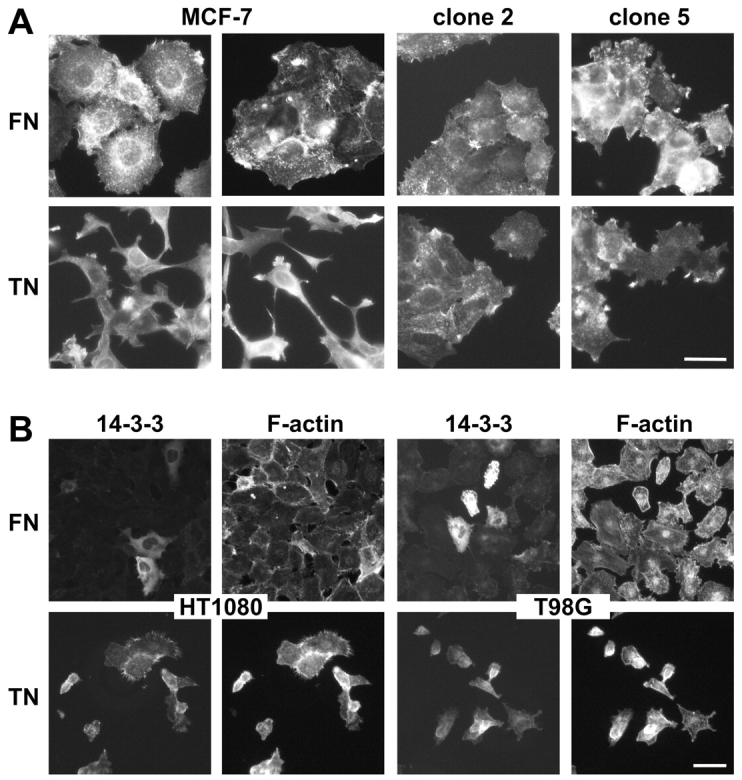
Effect of 14-3-3 tau on cell morphology and adhesion on fibronectin versus tenascin-C substrata. (A) TRITC-phalloidin staining of MCF-7 parental cells and clones 2 and 5 overexpressing 14-3-3 tau grown in fibronectin-coated (FN) or tenascin-C–coated (TN) wells. MCF-7 cells are well spread on fibronectin but elongated with many actin-rich processes on tenascin-C as opposed to clones 2 and 5, which how similar morphologies on either substratum with actin accumulations at the cell peripheries of the well spread cells. Bar, 50 μm. (B) Anti-Flag staining reveals cells overexpressing transfected 14-3-3–Flag (14-3-3), and RITC-phalloidin staining shows F-actin in all cells present in the same field of cultures of HT1080 cells and T98G cells grown on either fibronectin (FN) or tenascin-C (TN). Only the transfected cells were able to adhere and survive on the tenascin-C substratum, whereas on fibronectin most cells remained present. Bar, 50 μm.
Two more cell lines were analyzed for a similar effect of 14-3-3 tau on cell adhesion on tenascin-C. HT1080 fibrosarcoma cells and T98G glioblastoma cells were transiently transfected with 14-3-3 tau after plating them on either a fibronectin or a tenascin-C substratum. 1 d later, transfected cells were visualized by staining the Flag tag of the transfected 14-3-3 tau, and all cells were stained by phalloidin (Fig. 4 B). On fibronectin, a small proportion of the large number of adhering cells expressed 14-3-3 tau, whereas on tenascin-C only a few cells were present but all expressing 14-3-3 tau. This implies that in these cells overexpression of 14-3-3 tau also led to an increased cell adhesion on tenascin-C.
Possibly, this effect is related to the reported interaction of 14-3-3 tau with rap1/B-Raf (Berruti, 2000), since rap1 signaling has been implicated in the regulation of integrin-mediated cell adhesion (Bos et al., 2001). Another possibility could be an interaction of 14-3-3 with integrins themselves as has been reported for 14-3-3 beta and integrin β1 (Han et al., 2001) or for 14-3-3 zeta with the integrin-associated docking protein p130 (Cas). Finally, an interaction of 14-3-3 zeta with the actin depolymerizing factor cofilin and its regulatory kinase LIM kinase 2 has been observed (Birkenfeld et al., 2003), and 14-3-3 zeta seems to stabilize phosphocofilin levels (Gohla and Bokoch, 2002), pointing to a further pathway for 14-3-3 proteins by affecting cell morphology through direct action on the actin cytoskeleton.
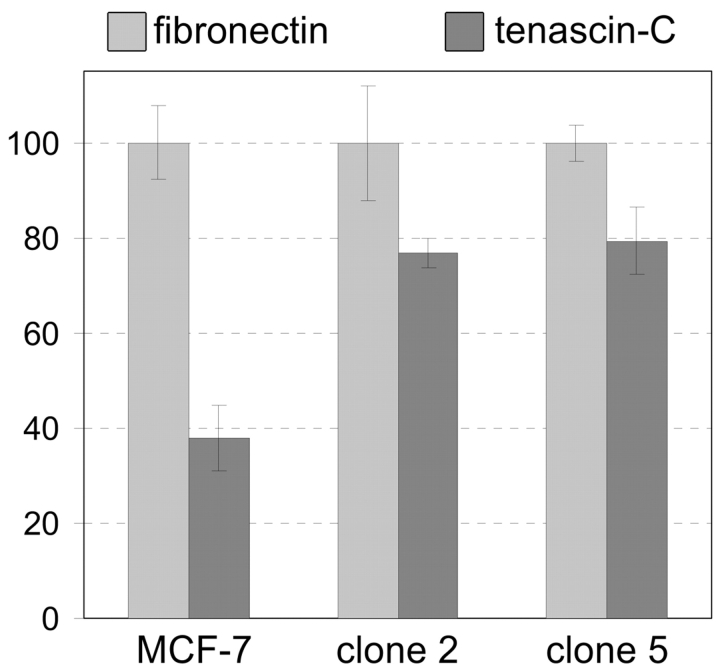
We next tested the effect of the overexpression of 14-3-3 tau on cell growth. We plated equal numbers of cells on fibronectin versus tenascin-C substrates in complete medium and cultured the parental versus the 14-3-3–transfected clones 2 and 5 for 3 d. After 3 d in culture, the MCF-7 cells reached higher densities on fibronectin than on tenascin-C, whereas no distinction could be made for clone 2 or 5, respectively (unpublished data). The lower cell number of the parental MCF-7 cells on a tenascin-C substratum coincided with a reduced level of DNA replication on a tenascin-C substrate in comparison to fibronectin as measured by 3H-thymidine incorporation (Fig. 5). The 3H-thymidine incorporation by the 14-3-3 tau–overexpressing clones 2 and 5 was recovered on tenascin-C and reached values almost as high as on fibronectin (Fig. 5). A possible mechanism is that cell cycle progression is stimulated by an interaction of 14-3-3 with phosphorylated p27Kip1 and by retaining this cell cycle inhibitor in the cytoplasm (Fujita et al., 2002). Alternatively, stimulation of cell growth by 14-3-3 could be indirect by its known inhibitory action on apoptosis (Xing et al., 2000; Masters and Fu, 2001). To test whether cells on tenascin-C were protected from apoptosis by overexpression of 14-3-3 tau, we plated the parental MCF-7 cells and clones 2 and 5 on tenascin-C– and fibronectin-coated wells, respectively, and analyzed the number of apoptotic cells under both conditions. However, not even the parental MCF-7 cells showed increased apoptosis on tenascin-C compared with the cells plated on fibronectin. Reduction of the serum level in the medium to 1% lead to a concomitant increase in apoptotic cells on both substrates. Therefore, the hypothesis that 14-3-3 tau could protect MCF-7 cells from apoptosis cannot be the reason for the observed increase in growth.
Figure 5.
DNA replication in cells cultured on fibronectin versus tenascin-C. 3H-thymidine incorporation was measured in MCF-7 cells and clones 2 and 5 cultured in medium with 0.1% FCS on fibronectin-coated versus tenascin-C–coated wells. In MCF-7 cells, DNA replication was reduced by >60% on tenascin-C compared with fibronectin, whereas the difference for clones 2 and 5 was greatly diminished.
Clearly 14-3-3 proteins are important adaptor proteins with many target proteins such as Raf-1 (Fantl et al., 1994; Fu et al., 1994; Li et al., 1995), PKC (Van Der Hoeven et al., 2000), phosphatidylinositol 3-kinase (Bonnefoy-Berard et al., 1995), Bad (Zha et al., 1996), and Cdc25 (Kumagai and Dunphy, 1999) and thus seem to have a central and integrating role in orchestrating signaling pathways regulating cell growth and death. Furthermore, their positive effect on cell adhesion presents an additional input in their capability of influencing cell behavior. For cells that have not completely lost their anchorage dependence, this again has a positive effect on cell growth. Therefore, it is interesting that tenascin-C, an extracellular matrix protein highly overexpressed in cancer tissues, causes an increase in the expression of 14-3-3 tau in cancer cells. Tenascin-C may, therefore, alter the physiological status of the tumor cells by induction of 14-3-3 tau, thereby promoting their growth.
Materials and methods
Cell cultures
MCF-7 mammary carcinoma cells (HTB-22; American Type Culture Collection), HT1080 fibrosarcoma cells (CCL-121; American Type Culture Collection), and T98G glioblastoma cells (CRL-1690; American Type Culture Collection) were cultured in Dulbecco's medium (Life Technologies) containing 10% FCS (Amimed) at 37°C and 5% CO2. Substrates of tenascin-C or fibronectin were prepared by coating these proteins at 25 μg/ml in PBS including 0.01% Tween for 1 h at RT. Tenascin-C and fibronectin were purified as described previously (Fischer et al., 1997).
Cell growth was analyzed by measuring the incorporation of 3H-thymidine. Equal numbers of cells were plated on tenascin-C versus fibronectin in complete medium. After 22 h, the medium was removed, the cell layers were washed with medium without FCS, and medium containing 0.1% FCS was added. 22 h later, the cells were labeled with 3H-thymidine for 6 h and harvested and analyzed as described previously (Huang et al., 2001). Apoptosis was analyzed by TUNEL staining using the In Situ Cell Death Detection kit (Roche Diagnostics AG) according to the procedure of the manufacturer.
For immunofluorescence and F-actin staining, cells were fixed with 4% PFA in PBS for 30 min and permeabilized with 0.1% Trition X-100 in PBS for 5 min. Then, they were either incubated for 1 h with 0.05 μM TRITC-phalloidin (Sigma-Aldrich) or with anti-Flag (M2; Stratagene) at a 1:500 dilution, or with both simultaneously, all in PBS containing 3% goat serum for 1 h. Secondary goat anti–mouse Alexa488-conjugated antibodies (Molecular Probes) diluted 1:1,000 in PBS/3% goat serum were applied for detection of the primary antibodies. Cells were washed in PBS after each incubation. Finally, the specimens were mounted in Mowiol (Calbiochem) and examined and photographed using a Axiophot microscope (Carl Zeiss MicroImaging, Inc.) connected to a 3CCD camera (Sony).
Subtractive cDNA cloning
An Oligotex Direct mRNA kit (QIAGEN) was used to isolate mRNA from two 10-cm plates of MCF-7 cells grown on either fibronectin or tenascin-C substrata for 24 h in complete medium. These mRNA samples were used for RT-PCR reactions and subtractive hybridization using the Advantage cDNA PCR kit (CLONETECH Laboratories, Inc.) and the PCR-Select cDNA Subtraction kit (CLONETECH Laboratories, Inc.) according to the manuals supplied. The PCR products were cloned into pKS and analyzed by sequencing. Primers were derived from the insert sequences to verify a differential expression of the respective transcripts in the two cDNA batches by semiquantitative PCR reactions using Taq polymerase (Roche Diagnostics AG). To amplify the 14-3-3 tau, we used the following primer pair: 5′-AGTTGCGTGTGGTGATGATCG-3′ and 5′-GTATGAGTCTTCATTCAGTG-3′. Samples were taken from each reaction after completion of 15, 20, 25, and 30 cycles and analyzed on agarose gels after ethidium bromide staining. This allowed us to verify the linear range of amplification that we used for comparison.
Transfection of 14-3-3–Flag and Western blots
A full-length 14-3-3 tau construct containing an NH2-terminal Flag tag was engineered by RT-PCR from mRNA of MCF-7 cells using the Advantage cDNA PCR kit (CLONETECH Laboratories, Inc.). A first set of 14-3-3-specific primers was used (5′-GCGGCCGCGGAGACGTGAAC-3′ and 5′-AAGGATGACACCCTGTATGG-3′) followed by a second round of PCR with (5′-ATGCGGCCGCACCATGGACTACAAGGATGACGATGACAAGATGGAGAAGACTGAGCTG-3′ and 5′-ATCTCGAGTTAGTTTTCAGCCCCTTCTGC-3′) to add the Flag tag and the appropriate restriction sites NotI and XhoI at respective ends needed for subsequent cloning into the expression vector pcDNA3 (Invitrogen). The resulting construct was verified by sequencing and was called 14-3-3–Flag. It contained the complete coding sequence of human 14-3-3 tau available from GenBank/EMBL/DDBJ under accession no. X56468 with an NH2-terminal extension of a Flag tag. MCF-7 cells were transfected with 14-3-3–Flag using the transfection reagent fugene (Roche Diagnostics AG), and clones were selected for by the addition of G418 (Life Technologies). The resistant cell clones were tested for the production of the 14-3-3–Flag protein on Western blots of cell extracts using anti-Flag (M2; Stratagene), a peroxidase-labeled anti–mouse IgG (Cappel/ICN), and the ECL reagent (Amersham Biosciences) for chemiluminescent detection of the reactive bands. Endogenous synthesis of 14-3-3 tau was detected on the same blot using anti–14-3-3 theta/tau (Research Diagnostics Inc.). The Flag-tagged 14-3-3 migrates slightly slower than the endogenous protein due to the presence of the tag.
HT1080 and T98G cells were each plated on fibronectin and tenascin-C–coated plates. 1 d later, they were transiently transfected with the 14-3-3–Flag construct using the transfection reagent fugene. On the next day, cells were fixed and stained with anti-Flag and phalloidin as described above for the MCF-7 cells.
Acknowledgments
We thank Wentao Huang for his expert help with the 3H-thymidine incorporation assays.
References
- Adams, M., J.L. Jones, R.A. Walker, J.H. Pringle, and S.C. Bell. 2002. Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Res. 62:3289–3297. [PubMed] [Google Scholar]
- Berruti, G. 2000. A novel rap1/B-Raf/14-3-3 theta protein complex is formed in vivo during the morphogenetic differentiation of postmeiotic male germ cells. Exp. Cell Res. 257:172–179. [DOI] [PubMed] [Google Scholar]
- Birkenfeld, J., H. Betz, and D. Roth. 2003. Identification of cofilin and LIM-kinase as novel interaction partners of 14-3-3zeta. Biochem. J. 369:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy-Berard, N., Y.C. Liu, M. von Willebrand, A. Sung, C. Elly, T. Mustelin, H. Yoshida, K. Ishizaka, and A. Altman. 1995. Inhibition of phosphatidylinositol 3-kinase activity by association with 14-3-3 proteins in T cells. Proc. Natl. Acad. Sci. USA. 92:10142–10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, J.L., J. de Rooij, and K.A. Reedquist. 2001. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2:369–377. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R. 1993. Tenascin and other adhesion-modulating proteins in cancer. Semin. Cancer Biol. 4:301–310. [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R., E.J. Mackie, C.A. Pearson, and T. Sakakura. 1986. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 47:131–139. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann, R., P. Kalla, and C.A. Pearson. 1989. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 49:4322–4325. [PubMed] [Google Scholar]
- Crossin, K.L. 1996. Tenascin: a multifunctional extracellular matrix protein with a restricted distribution in development and disease. J. Cell. Biochem. 61:592–598. [DOI] [PubMed] [Google Scholar]
- Emoto, K., Y. Yamada, H. Sawada, H. Fujimoto, M. Ueno, T. Takayama, K. Kamada, A. Naito, S. Hirao, and Y. Nakajima. 2001. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 92:1419–1426. [DOI] [PubMed] [Google Scholar]
- Fantl, W.J., A.J. Muslin, A. Kikuchi, J.A. Martin, A.M. MacNicol, R.W. Gross, and L.T. Williams. 1994. Activation of Raf-1 by 14-3-3 proteins. Nature. 371:612–614. [DOI] [PubMed] [Google Scholar]
- Fischer, D., M. Brown-Luedi, T. Schulthess, and R. Chiquet-Ehrismann. 1997. Concerted action of tenascin-C domains in cell adhesion, anti-adhesion and promotion of neurite outgrowth. J. Cell Sci. 110:1513–1522. [DOI] [PubMed] [Google Scholar]
- Fu, H., K. Xia, D.C. Pallas, C. Cui, K. Conroy, R.P. Narsimhan, H. Mamon, R.J. Collier, and T.M. Roberts. 1994. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science. 266:126–129. [DOI] [PubMed] [Google Scholar]
- Fu, H., R.R. Subramanian, and S.C. Masters. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40:617–647. [DOI] [PubMed] [Google Scholar]
- Fujita, N., S. Sato, K. Katayama, and T. Tsuruo. 2002. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277:28706–28713. [DOI] [PubMed] [Google Scholar]
- Goepel, C., J. Buchmann, R. Schultka, and H. Koelbl. 2000. Tenascin-A marker for the malignant potential of preinvasive breast cancers. Gynecol. Oncol. 79:372–378. [DOI] [PubMed] [Google Scholar]
- Gohla, A., and G. Bokoch. 2002. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 12:1704–1712 [DOI] [PubMed] [Google Scholar]
- Han, D.C., L.G. Rodriguez, and J.L. Guan. 2001. Identification of a novel interaction between integrin beta1 and 14-3-3beta. Oncogene. 20:346–357. [DOI] [PubMed] [Google Scholar]
- Herold-Mende, C., M.M. Mueller, M.M. Bonsanto, H.P. Schmitt, S. Kunze, and H.H. Steiner. 2002. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int. J. Cancer. 98:362–369. [DOI] [PubMed] [Google Scholar]
- Huang, W., R. Chiquet-Ehrismann, J.V. Moyano, A. Garcia-Pardo, and G. Orend. 2001. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 61:8586–8594. [PubMed] [Google Scholar]
- Jones, P.L., and F.S. Jones. 2000. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol. 19:581–596. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., and W.G. Dunphy. 1999. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 13:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., P. Janosch, M. Tanji, G.C. Rosenfeld, J.C. Waymire, H. Mischak, W. Kolch, and J.M. Sedivy. 1995. Regulation of Raf-1 kinase activity by the 14-3-3 family of proteins. EMBO J. 14:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, S.C., and H. Fu. 2001. 14-3-3 proteins mediate an essential anti-apoptotic signal. J. Biol. Chem. 276:45193–45200. [DOI] [PubMed] [Google Scholar]
- Orend, G., and R. Chiquet-Ehrismann. 2000. Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp. Cell Res. 261:104–110. [DOI] [PubMed] [Google Scholar]
- Salmenkivi, K., C. Haglund, J. Arola, and P. Heikkila. 2001. Increased expression of tenascin in pheochromocytomas correlates with malignancy. Am. J. Surg. Pathol. 25:1419–1423. [DOI] [PubMed] [Google Scholar]
- Takihara, Y., Y. Matsuda, and J. Hara. 2000. Role of the beta isoform of 14-3-3 proteins in cellular proliferation and oncogenic transformation. Carcinogenesis. 21:2073–2077. [DOI] [PubMed] [Google Scholar]
- Tzivion, G., and J. Avruch. 2002. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 277:3061–3064. [DOI] [PubMed] [Google Scholar]
- Van Der Hoeven, P.C., J.C. Van Der Wal, P. Ruurs, and W.J. Van Blitterswijk. 2000. Protein kinase C activation by acidic proteins including 14-3-3. Biochem. J. 347:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert, M.J., H.Y. Steensma, and G.P. van Heusden. 2001. 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 23:936–946. [DOI] [PubMed] [Google Scholar]
- Vollmer, G. 1997. Biologic and oncologic implications of tenascin-C/hexabrachion proteins. Crit. Rev. Oncol. Hematol. 25:187–210. [DOI] [PubMed] [Google Scholar]
- Xing, H., S. Zhang, C. Weinheimer, A. Kovacs, and A.J. Muslin. 2000. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO J. 19:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha, J., H. Harada, E. Yang, J. Jockel, and S.J. Korsmeyer. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell. 87:619–628. [DOI] [PubMed] [Google Scholar]