Abstract
Kinesin II is a heterotrimeric plus end–directed microtubule motor responsible for the anterograde movement of organelles in various cell types. Despite substantial literature concerning the types of organelles that kinesin II transports, the question of how this motor associates with cargo organelles remains unanswered. To address this question, we have used Xenopus laevis melanophores as a model system. Through analysis of kinesin II–mediated melanosome motility, we have determined that the dynactin complex, known as an anchor for cytoplasmic dynein, also links kinesin II to organelles. Biochemical data demonstrates that the putative cargo-binding subunit of Xenopus kinesin II, Xenopus kinesin II–associated protein (XKAP), binds directly to the p150Glued subunit of dynactin. This interaction occurs through aa 530–793 of XKAP and aa 600–811 of p150Glued. These results reveal that dynactin is required for transport activity of microtubule motors of opposite polarity, cytoplasmic dynein and kinesin II, and may provide a new mechanism to coordinate their activities.
Keywords: kinesin II; dynactin; dynein; melanophores; organelle transport
Introduction
Kinesin II (KIF3) is a member of the kinesin superfamily of motor proteins, which are found in species ranging from Chlamydomonas to humans. It is involved in a number of different processes, including interflagellar transport, axonal transport, cell division, protein sorting and secretion, and vesicular transport (Marszalek and Goldstein, 2000). The best studied role for kinesin II is in the transport of protein rafts in the cilia and flagella of eukaryotes (Cole et al., 1998). However, kinesin II is also important for the transport of membrane-bound organelles such as axonal vesicles, Golgi-derived vesicles, and pigment organelles (Kondo et al., 1994; Le Bot et al., 1998; Tuma et al., 1998). Kinesin II consists of three subunits: two homologous motor subunits and an accessory subunit known as kinesin-associated protein (KAP)* (Cole et al., 1992), which is thought to mediate cargo binding. Although much work has been done to study this motor, the mechanism by which kinesin II interacts with its cargo has yet to be identified.
Whereas very little is known about how kinesin II associates with cargo organelles, this issue has been extensively characterized for cytoplasmic dynein. It has been demonstrated that dynein associates with many of its cargoes through the dynactin complex (Karki and Holzbaur, 1999). Dynactin is a complex of at least 10 polypeptides that range in size from 24 to 150 kD (Schroer, 1996). The best characterized subunits of dynactin are p150Glued and p50 (dynamitin).
p150Glued contains both microtubule-binding and dynein-binding domains. p150Glued binds microtubules through the NH2-terminal CAP-Gly motif (Vaughan and Vallee, 1995; Waterman-Storer et al., 1995), and phosphorylation near this motif has been shown to regulate microtubule binding (Vaughan et al., 2002). p150Glued interacts directly with the intermediate chain of cytoplasmic dynein (DIC) (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995), and this interaction is thought to be essential for dynein-mediated organelle transport (Gill et al., 1991; Holzbaur et al., 1991; Holleran et al., 1998; King and Schroer, 2000). Regulation of this interaction by DIC phosphorylation suggests an important function (Pfister et al., 1996; Vaughan et al., 2001).
Dynamitin is localized in the shoulder region of the complex and is thought to hold p150 and the rest of the complex together (Schroer, 1996; Eckley et al., 1999). Exogenous levels of dynamitin disrupt the dynactin complex and provide a tool to study dynactin function (Echeverri et al., 1996).
Mechanisms of kinesin II cargo recognition can be investigated using Xenopus melanophores, which contain hundreds of pigment organelles (melanosomes). These cells can be stimulated to aggregate melanosomes to the cell center by melatonin, which reduces the concentration of intracellular cAMP or disperse them by melanocyte stimulating hormone (MSH), which increases intracellular cAMP (Daniolos et al., 1990). Aggregation is accomplished by cytoplasmic dynein (Nilsson and Wallin, 1997), whereas dispersion requires the combined action of myosin V and kinesin II (Rogers and Gelfand, 1998; Tuma et al., 1998; Gross et al., 2002a). The identification of kinesin II as the microtubule motor responsible for melanosome dispersion and the ability to activate pigment dispersion facilitates functional studies of kinesin II in melanophores, making them an ideal system to study this motor.
In this report, we have investigated the role of dynactin in bidirectional melanosome transport. This work demonstrates that p150Glued and the KAP subunit of Xenopus kinesin II interact and that this interaction is required for melanosome dispersion. In addition, our results show that p150Glued does not bind XKAP and the DICs at the same time, suggesting a novel regulatory mechanism to control the direction of motility.
Results and discussion
Previous work in cultured cells and Drosophila has suggested that dynactin could function in both anterograde and retrograde microtubule transport (Waterman-Storer et al., 1997; Martin et al., 1999; Valetti et al., 1999; Gross et al., 2002b). A powerful tool used to disrupt the dynactin complex in vivo is overexpression of dynamitin (Echeverri et al., 1996; Burkhardt et al., 1997). To determine the effect of disrupting the dynactin complex on melanosome transport, we expressed an EGFP–dynamitin fusion construct in melanophores. Because melanosomes can move in an outward direction on both microtubules and actin filaments, the contribution of acto-myosin transport was eliminated by depolymerizing actin filaments with latrunculin B. Anterograde transport was then stimulated by MSH.
Visual observations of these cells indicated that melanosomes ceased to move either toward or away from the cell center. We then determined the percentage of melanosomes undergoing directed movements, which were defined as any linear melanosome movement with a distance of at least 2 μm, at a rate of at least 0.2 μm/s. This analysis (Table I) demonstrates that overexpression of EGFP–dynamitin strongly suppressed both anterograde and retrograde components of organelle movement, suggesting that a disruption of the dynactin complex inhibits both dynein- and kinesin II–based transport. At the same time, staining with a tubulin antibody showed that overexpression of dynamitin did not result in depolymerization of microtubules, and only a small fraction of transfected cells (<5%) showed microtubule arrays that were not tightly focused at the centrosome (unpublished data).
Table I. Directed melanosome movement in cells transfected with dynamatin and XKAP constructs.
| Transfection
|
Anterograde movement
|
Retrograde movement
|
n
|
|---|---|---|---|
| GFP | 17.4 ± 8.2 | 8.7 ± 5.3 | 720 |
| GFP–dynamitin | 2.6 ± 4.6a | 2.1 ± 3.8a | 400 |
| GFP–XKAP | 2.0 ± 2.3a | 1.7 ± 2.4a | 300 |
| GFP–N-XKAP | 9 ± 5.7a | 8 ± 7.5 | 200 |
| GFP–C-XKAP | 0.9 ± 3.0a | 1.4 ± 2.3a | 200 |
Melanosome movement was classified as any directional movement ≥2 μm with a rate ≥0.2 μm/s.
Significant from GFP cells at 99.5% confidence level. Statistics were analyzed using Student's t test.
Kinesin II–dynactin interaction
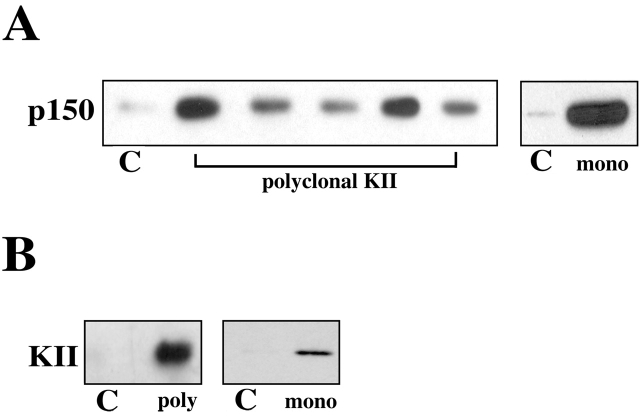
One explanation for these results is that both kinesin II and dynein share the same membrane receptor. To test this hypothesis, we performed immunoprecipitations of melanophore extracts using a monoclonal antibody raised against the 85-kD subunit of kinesin II (K2.4). Coimmunoprecipitation of dynactin with kinesin II was observed with this antibody but not control antibodies (Fig. 1 A). To exclude cross-reaction with dynactin as an explanation, we repeated this experiment using five different affinity-purified polyclonal antibodies against kinesin II. Each polyclonal antibody, but not control IgG, was able to pull down dynactin from melanophore extracts (Fig. 1 A). In these experiments, monoclonal and polyclonal antibodies against kinesin II pulled out 2–6% of the total dynactin pool. In the reverse experiment, both polyclonal and monoclonal antibodies to the p150Glued subunit of the dynactin complex pulled out ∼1% of the total kinesin II pool (Fig. 1 B). These results provided an indication that kinesin II and dynactin interact.
Figure 1.
Kinesin II and the dynactin complex interact in melanophore extracts. (A) Kinesin II was precipitated from melanophore extracts with five different polyclonal (left) and one monoclonal (right) antibodies against the 95- and 85-kD subunits of kinesin II, respectively. Blots were probed with monoclonal anti-p150. Quantification of these blots shows that different kinesin II antibodies pull down 2–6% of the total p150 in the extract. (B) Dynactin was precipitated with polyclonal (left) or monoclonal (right) antibodies against p150. Blots were probed with monoclonal anti–kinesin II. Quantification of these blots shows that p150 antibodies pull down ∼1% of the total kinesin II in the extract. C indicates control samples precipitated either by normal rabbit IgG (left) or an unrelated monoclonal antibody (right).
Molecular basis of kinesin II–dynactin interactions
The fact that dynactin interacts with cytoplasmic dynein via p150Glued raised the possibility of a similar interaction with kinesin II. Based on previous work (Vaughan and Vallee, 1995), we performed blot overlays on purified melanosomes using recombinant p150, residues 1–811. These assays revealed binding to a ∼100-kD protein in purified melanosomes, consistent with the molecular weight of XKAP (unpublished data). To confirm this result, we prepared Escherichia coli expression constructs encoding GST fusions with the NH2-terminal 350 aa of XKAP (N-XKAP) or the COOH-terminal 263 aa (C-XKAP) and purified these proteins for use in blot overlays. In these assays, p150Glued 1–811 bound to GST–C-XKAP but not to GST–N-XKAP (Fig. 2 A). To more accurately map the region of the p150Glued –XKAP interaction, a more limited p150Glued construct encoding residues 600–811 was used in blot overlays. p150Glued 600–811 bound to purified DIC's and GST–C-XKAP, whereas no binding was observed to GST–N-XKAP (Fig. 2 B).
Figure 2.
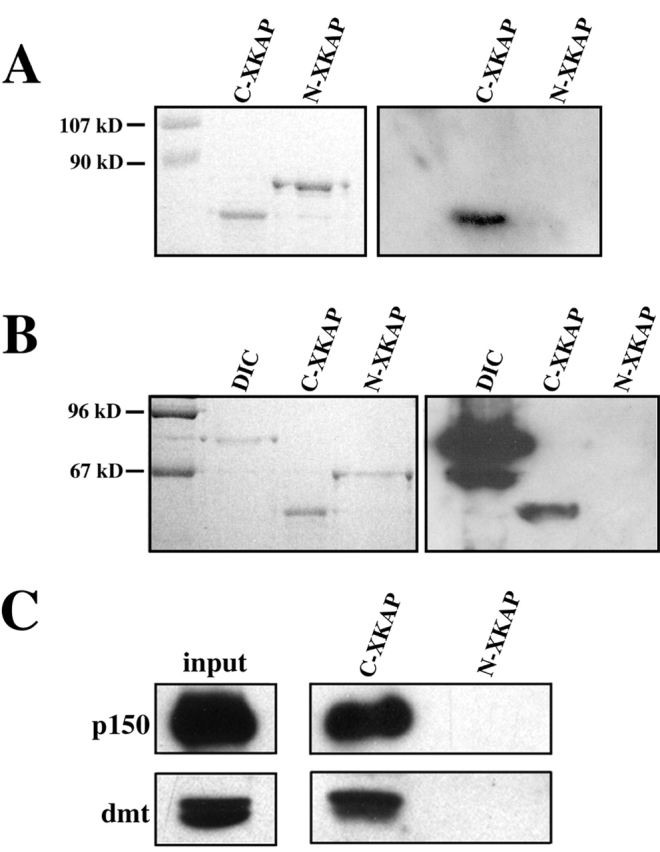
Direct interaction of p150 and XKAP. (A) 2.5 μg each of GST–C-XKAP and GST–N-XKAP were separated on a polyacrylamide gel and overlaid with 2 μg/ml p150Glued 1–811. Purified p150Glued 1–811 binds to GST–C-XKAP but not GST–N-XKAP. (B) Blot overlay assay using 2 μg/ml purified p150Glued 600–811. In this assay, p150Glued 600–811 showed robust binding to 1 μg each of purified DIC and GST–C-XKAP but not GST–N-XKAP. In A and B, the left panel is a Coomassie stain of purified proteins, and the right panel is blot overlay. (C) C-XKAP binds endogenous dynactin from melanophore extracts. GST–C-XKAP bound to glutathione agarose beads pulls p150 and dynamitin from cell extracts, whereas GST–N-XKAP does not.
Independent confirmation of binding was achieved through pull-down assays in which GST–XKAP fragments were purified by glutathione bead affinity and incubated with melanophore extracts. In these experiments, C-XKAP but not N-XKAP bound endogenous dynactin as shown by the fact that C-XKAP pulled down both p150 and p50-dynamitin (Fig. 2 C). These results demonstrate a direct interaction between the XKAP subunit of kinesin II and the p150Glued subunit of dynactin.
Competition between kinesin II and dynein for binding to p150Glued
The region of p150Glued implicated in kinesin II binding was also sufficient to bind the cytoplasmic dynein intermediate chain. This raised the question of whether dynactin could bind kinesin II and dynein simultaneously. To assess this possibility, immunoprecipitations were performed using polyclonal antibodies against the 95-kD subunit of kinesin II and DIC (Fig. 3 A). Both kinesin II and DIC were able to pull out p150Glued. However, no kinesin II was detected in antidynein immunoprecipitates and vice versa, suggesting that the two motors cannot bind to p150Glued at the same time.
Figure 3.
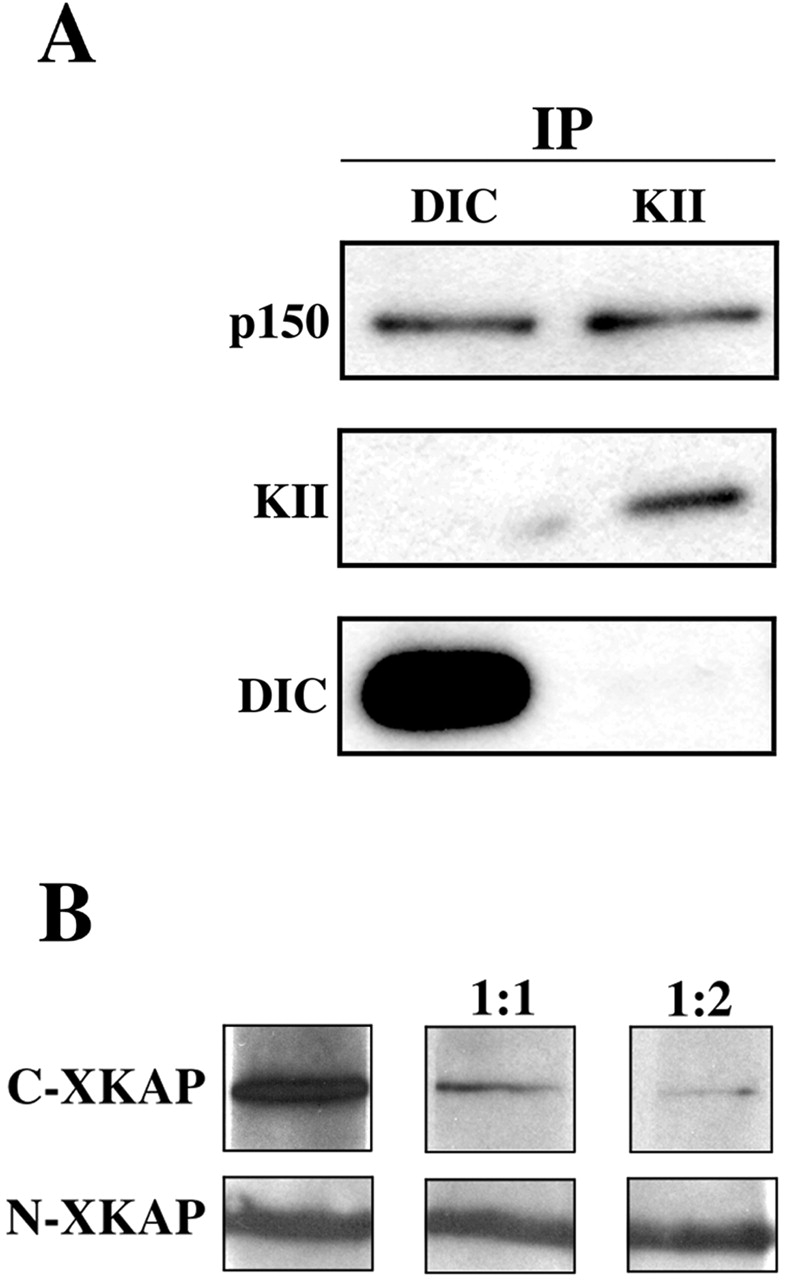
Kinesin II and dynein compete for binding to dynactin. (A) Polyclonal antibodies against DIC and the 95-kD subunit of kinesin II are each able to pull down p150 from melanophore extracts, but they do not pull down each other. Antibodies used for immunoprep are shown at the top. Antibodies used to probe the blot are indicated on the sides. (B) The ability of p150 to bind to purified DIC via blot overlay diminishes in the presence of increasing amounts of C-XKAP but not N-XKAP. Purified DIC was overlayed with myc- p150Glued 600–811 alone or in the presence of a 1:1 or 1:2 molar ratio of C-XKAP or N-XKAP. Blots were probed with an anti-myc antibody.
To further test this question, we designed a competition assay in which the binding of p150Glued 600–811 to DIC via blot overlay was assessed in the presence of soluble XKAP fragments (Fig. 3 B). As observed previously, p150Glued bound DICs. However, when p150Glued was preincubated with C-XKAP binding to DIC decreased as the molar ratio of the fragment increased. N-XKAP, which does not bind p150Glued, had no impact on the p150Glued–DIC interaction.
Functional analysis of the kinesin II–dynactin interaction
The data presented above demonstrates that disruption of the dynactin complex inhibits both retrograde and anterograde melanosome transport and that kinesin II and dynactin physically interact. If dynactin is truly required for bidirectional melanosome transport, then blocking the DIC–XKAP binding site on p150Glued should inhibit both anterograde and retrograde transport.
To test this hypothesis, we measured organelle movements in cells overexpressing XKAP or its COOH- and NH2-terminal fragments. Overexpression of XKAP greatly reduced both anterograde and retrograde melanosome transport (Table I) with no detectable change in microtubule distribution (unpublished data). Furthermore, this inhibition was observed in cells transfected with C-XKAP, confirming that the binding of XKAP to p150Glued is mediated by the COOH-terminal portion of XKAP (Table I). Cells transfected N-XKAP showed some inhibition of anterograde but not retrograde melanosome transport, possibly by binding one or both of the motor subunits of kinesin II. These results provide functional confirmation to the suggestion that kinesin II utilizes p150Glued as a binding platform on the melanosome surface.
The results presented here demonstrate that p150Glued interacts with both kinesin II and dynein on melanosomes. The identification of the KAP subunit as being responsible for the interaction of kinesin II with other proteins agrees with the findings of several different studies. Yeast two-hybrid analysis demonstrated that KAP interacts with a member of the Wnt signaling pathway, adenomatous polyposis coli, and is required for adenomatous polyposis coli localization to the tips of growing microtubules (Jimbo et al., 2002). Other KAP binding partners have been identified in this way, including Smg-GDS (Shimizu et al., 1996), XCAP (Xenopus chromosome–associated polypeptide) (Shimizu et al., 1998), and nonerythroid spectrin (Takeda et al., 2000). Conversely, it was shown that p150Glued interacts with the mitotic kinesin, HsEg5, suggesting that dynactin may interact with members of the kinesin superfamily other than kinesin II (Blangy et al., 1997).
Our results for the first time provide physical evidence to the theory that anterograde and retrograde motility of organelles on microtubules is coordinated. This idea was first put forth by Schroer et al. (1988), who showed that immunodepletion of conventional kinesin inhibited both plus and minus end– directed vesicle transport. A similar result was obtained when antibodies against conventional kinesin inhibited bidirectional vesicle movement in axoplasm (Brady et al., 1990). Waterman-Storer et al. (1997) demonstrated that inhibitory antibodies against p150Glued blocked the motility of organelles obtained from extruded squid axoplasm along microtubules in both directions. Indications of motor coordination were also observed in genetic studies with Drosophila. Welte et al. (1998) demonstrated that a mutation in the klarsicht gene inhibited microtubule transport of lipid droplets in both directions. In addition it was shown that mutations in the gene encoding Drosophila Glued inhibited plus end–directed vesicle transport in addition to dynein based transport (Martin et al., 1999; Gross et al., 2002b). The inhibition of anterograde and retrograde vesicle movement was also observed in fibroblasts transfected with dynamitin (Valetti et al., 1999). Exactly how the coordination of anterograde and retrograde microtubule motors is accomplished remains to be seen, but this report demonstrates that for cytoplasmic dynein and kinesin II the inhibition of one motor can lead to inhibition of the opposite polarity motor as a result of both motors sharing the same binding component on the cargo surface.
Materials and methods
Cell culture
Immortalized Xenopus melanophores were cultured as described (Rogers et al., 1997). Aggregation and dispersion of pigment was induced by the addition of 10 nM melatonin or 100 nM MSH, respectively. Actin filaments were depolymerized by the addition of 5 μM latrunculin B for 60 min.
Molecular biology and transfection
pEGFP–dynamitin was a gift from R. Vallee (Columbia University, New York, NY). Partial XKAP cDNA overlapping clones were isolated from a Xenopus oocyte cDNA library using as a probe a cDNA encoding SMAP (a gift from Pr Tokai, Osaka University Medical School, Osaka, Japan). Full-length XKAP cDNA was obtained by PCR on A6 cells cDNA and cloned into the pCR2.1 vector using the TA cloning kit (Invitrogen). Several constructs were prepared using internal restriction sites to express fragments of XKAP fused to either GST or GFP. For expression of GFP, dynamitin, and XKAP constructs, melanophores were plated on poly-lysine–coated coverslips and transfected with the FuGENE6 transfection reagent (Roche Diagnostics).
Melanosome tracking
To study the direction and length of melanosome movements, melanosomes were selected at random and observed for 60 s. Melanosomes exhibiting a linear movement of at least 2 μm with a velocity of at least 0.2 μm/s were counted. In addition, the direction of melanosome movement was determined, with movements away from the nucleus scored as anterograde and movements toward the nucleus scored as retrograde.
Biochemical techniques
Cells used in biochemical experiments were rinsed in PBS and detached from plates with a rubber policeman. Cells were lysed in IMB50 (50 mM imidizole, pH 7.4, 1 mM EGTA, 0.5 mM EDTA, 5 mM magnesium acetate, 175 mM sucrose, and 1 mM DTT) with protease inhibitors (PMSF, chymostatin, leupeptin, and pepstatin).
Immunoprecipitations were performed in IMB50. Cells were lysed and centrifuged at 16,000 g for 10 min. Extracts were precleared for 60 min with 10 μl normal rabbit IgG or myc antibody prebound to 100 μl of a 50% solution of protein A beads (Bio-Rad laboratories). Extracts were incubated for 60 min at RT with antibodies prebound to 25 μl of a 50% solution of protein A beads. Beads were washed in IMB50, and IMB50 with 250 mM NaCl. Antibodies used were as follows: normal rabbit IgG, anti-myc (Evan et al., 1985), K2.4 (monoclonal anti–kinesin II) (Cole et al., 1993), Kif3B (polyclonal anti–kinesin II) (a gift from Dr. Virgil Muresan, Case Western Reserve University, Cleveland, OH), 74.1 (monoclonal anti-DIC) (Steffen et al., 1997), polyclonal anti-DIC (Vaughan and Vallee, 1995), monoclonal anti-p150Glued (BD Transduction Laboratories), and polyclonal anti-p150Glued (Vaughan et al., 1999).
For peptide pull-down assays, extracts were incubated for 60 min at RT with 150 μl glutathione agarose beads (Sigma-Aldrich) to which protein was prebound. Beads were washed in IMB50 and IMB50 plus 200 mM NaCl. Beads were resuspended in laemmli buffer supplemented with 5 mM glutathione. These samples were probed with monoclonal anti-p150Glued and a polyclonal antibody against dynamitin (a gift from Dr. Erika Holzbaur, University of Pennsylvania, Philadelphia, PA).
Blot overlay assays were performed as described (Vaughan and Vallee, 1995) using recombinant p150Glued fragments 1–811 and 600–811. Overlays were probed with anti-p150Glued antibodies or anti-myc. For analysis of competition between kinesin II and dynein, recombinant DIC was probed by overlay using p150Glued 1–811 alone or after preincubation with recombinant XKAP (1:1 or 1:2 molar ratios).
Acknowledgments
This work was supported by National Institutes of Health grants (GM52111) to V.I. Gelfand, (GM60560) K.T. Vaughan, (T32 GM07283), and S.W. Deacon, and an American Heart Association grant (30099N) to K.T. Vaughan.
Footnotes
Abbreviation used in this paper: C-XKAP, COOH-terminal 263 aa X-KAP; DIC, dynein intermediate chain; KAP, kinesin II–associated protein; MSH, melanocyte stimulating hormone; N-XKAP, NH2-terminal 350 aa XKAP; XKAP, Xenopus kinesin II–associated protein.
References
- Blangy, A., L. Arnaud, and E.A. Nigg. 1997. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J. Biol. Chem. 272:19418–19424. [DOI] [PubMed] [Google Scholar]
- Brady, S.T., K.K. Pfister, and G.S. Bloom. 1990. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA. 87:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt, J.K., C.J. Echeverri, T. Nilsson, and R.B. Vallee. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, D.G., W.Z. Cande, R.J. Baskin, D.A. Skoufias, C.J. Hogan, and J.M. Scholey. 1992. Isolation of a sea urchin egg kinesin-related protein using peptide antibodies. J. Cell Sci. 101:291–301. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., S.W. Chinn, K.P. Wedaman, K. Hall, T. Vuong, and J.M. Scholey. 1993. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 366:268–270. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., D.R. Diener, A.L. Himelblau, P.L. Beech, J.C. Fuster, and J.L. Rosenbaum. 1998. Chlamydomonas kinesin-II–dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141:993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniolos, A., A.B. Lerner, and M.R. Lerner. 1990. Action of light on frog pigment cells in culture. Pigment Cell Res. 3:38–43. [DOI] [PubMed] [Google Scholar]
- Echeverri, C.J., B.M. Paschal, K.T. Vaughan, and R.B. Vallee. 1996. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132:617–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley, D.M., S.R. Gill, K.A. Melkonian, J.B. Bingham, H.V. Goodson, J.E. Heuser, and T.A. Schroer. 1999. Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the arp1 minifilament pointed end. J. Cell Biol. 147:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan, G.I., G.K. Lewis, G. Ramsay, and J.M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S.R., T.A. Schroer, I. Szilak, E.R. Steuer, M.P. Sheetz, and D.W. Cleveland. 1991. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115:1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S.P., M.C. Tuma, S.W. Deacon, A.S. Serpinskaya, A.R. Reilein, and V.I. Gelfand. 2002. a. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S.P., M.A. Welte, S.M. Block, and E.F. Wieschaus. 2002. b. Coordination of opposite-polarity microtubule motors. J. Cell Biol. 156:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran, E.A., S. Karki, and E.L. Holzbaur. 1998. The role of the dynactin complex in intracellular motility. Int. Rev. Cytol. 182:69–109. [DOI] [PubMed] [Google Scholar]
- Holzbaur, E.L., J.A. Hammarback, B.M. Paschal, N.G. Kravit, K.K. Pfister, and R.B. Vallee. 1991. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 351:579–583. [DOI] [PubMed] [Google Scholar]
- Jimbo, T., Y. Kawasaki, R. Koyama, R. Sato, S. Takada, K. Haraguchi, and T. Akiyama. 2002. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat. Cell Biol. 4:323–327. [DOI] [PubMed] [Google Scholar]
- Karki, S., and E.L. Holzbaur. 1995. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 270:28806–28811. [DOI] [PubMed] [Google Scholar]
- Karki, S., and E.L Holzbaur. 1999. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11:45–53. [DOI] [PubMed] [Google Scholar]
- King, S.J., and T.A. Schroer. 2000. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2:20–24. [DOI] [PubMed] [Google Scholar]
- Kondo, S., R. Sato-Yoshitake, Y. Noda, H. Aizawa, T. Nakata, Y. Matsuura, and N. Hirokawa. 1994. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J. Cell Biol. 125:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bot, N., C. Antony, J. White, E. Karsenti, and I. Vernos. 1998. Role of xklp3, a subunit of the Xenopus kinesin II heterotrimeric complex, in membrane transport between the endoplasmic reticulum and the Golgi apparatus. J. Cell Biol. 143:1559–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek, J.R., and L.S. Goldstein. 2000. Understanding the functions of kinesin-II. Biochim. Biophys. Acta. 1496:142–150. [DOI] [PubMed] [Google Scholar]
- Martin, M., S.J. Iyadurai, A. Gassman, J.G. Gindhart, Jr., T.S. Hays, and W.M. Saxton. 1999. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 10:3717–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, H., and M. Wallin. 1997. Evidence for several roles of dynein in pigment transport in melanophores. Cell Motil. Cytoskeleton. 38:397–409. [DOI] [PubMed] [Google Scholar]
- Pfister, K.K., M.W. Salata, J.F. Dillman III, E. Torre, and R.J. Lye. 1996. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol. Biol. Cell. 7:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, S.L., and V.I. Gelfand. 1998. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 8:161–164. [DOI] [PubMed] [Google Scholar]
- Rogers, S.L., I.S. Tint, P.C. Fanapour, and V.I. Gelfand. 1997. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc. Natl. Acad. Sci. USA. 94:3720–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer, T.A. 1996. Structure and function of dynactin. Cell Dev. Biol. 7:321–328. [Google Scholar]
- Schroer, T.A., B.J. Schnapp, T.S. Reese, and M.P. Sheetz. 1988. The role of kinesin and other soluble factors in organelle movement along microtubules. J. Cell Biol. 107:1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, K., H. Kawabe, S. Minami, T. Honda, K. Takaishi, H. Shirataki, and Y. Takai. 1996. SMAP, an Smg GDS-associating protein having arm repeats and phosphorylated by Src tyrosine kinase. J. Biol. Chem. 271:27013–27017. [DOI] [PubMed] [Google Scholar]
- Shimizu, K., H. Shirataki, T. Honda, S. Minami, and Y. Takai. 1998. Complex formation of SMAP/KAP3, a KIF3A/B ATPase motor-associated protein, with a human chromosome-associated polypeptide. J. Biol. Chem. 273:6591–6594. [DOI] [PubMed] [Google Scholar]
- Steffen, W., S. Karki, K.T. Vaughan, R.B. Vallee, E.L. Holzbaur, D.G. Weiss, and S.A. Kuznetsov. 1997. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell. 8:2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, S., H. Yamazaki, D.H. Seog, Y. Kanai, S. Terada, and N. Hirokawa. 2000. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J. Cell Biol. 148:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma, M.C., A. Zill, N. Le Bot, I. Vernos, and V. Gelfand. 1998. Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores. J. Cell Biol. 143:1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti, C., D.M. Wetzel, M. Schrader, M.J. Hasbani, S.R. Gill, T.E. Kreis, and T.A. Schroer. 1999. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 10:4107–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, K.T., and R.B. Vallee. 1995. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, K.T., S.H. Tynan, N.E. Faulkner, C.J. Echeverri, and R.B. Vallee. 1999. Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J. Cell Sci. 112:1437–1447. [DOI] [PubMed] [Google Scholar]
- Vaughan, P.S., J.D. Leszyk, and K.T. Vaughan. 2001. Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J. Biol. Chem. 276:26171–26179. [DOI] [PubMed] [Google Scholar]
- Vaughan, P.S., P. Miura, M. Henderson, B. Byrne, and K.T. Vaughan. 2002. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J. Cell Biol. 158:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., S. Karki, and E.L. Holzbaur. 1995. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc. Natl. Acad. Sci. USA. 92:1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., S.B. Karki, S.A. Kuznetsov, J.S. Tabb, D.G. Weiss, G.M. Langford, and E.L. Holzbaur. 1997. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. USA. 94:12180–12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte, M.A., S.P. Gross, M. Postner, S.M. Block, and E.F. Wieschaus. 1998. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 92:547–557. [DOI] [PubMed] [Google Scholar]