Abstract
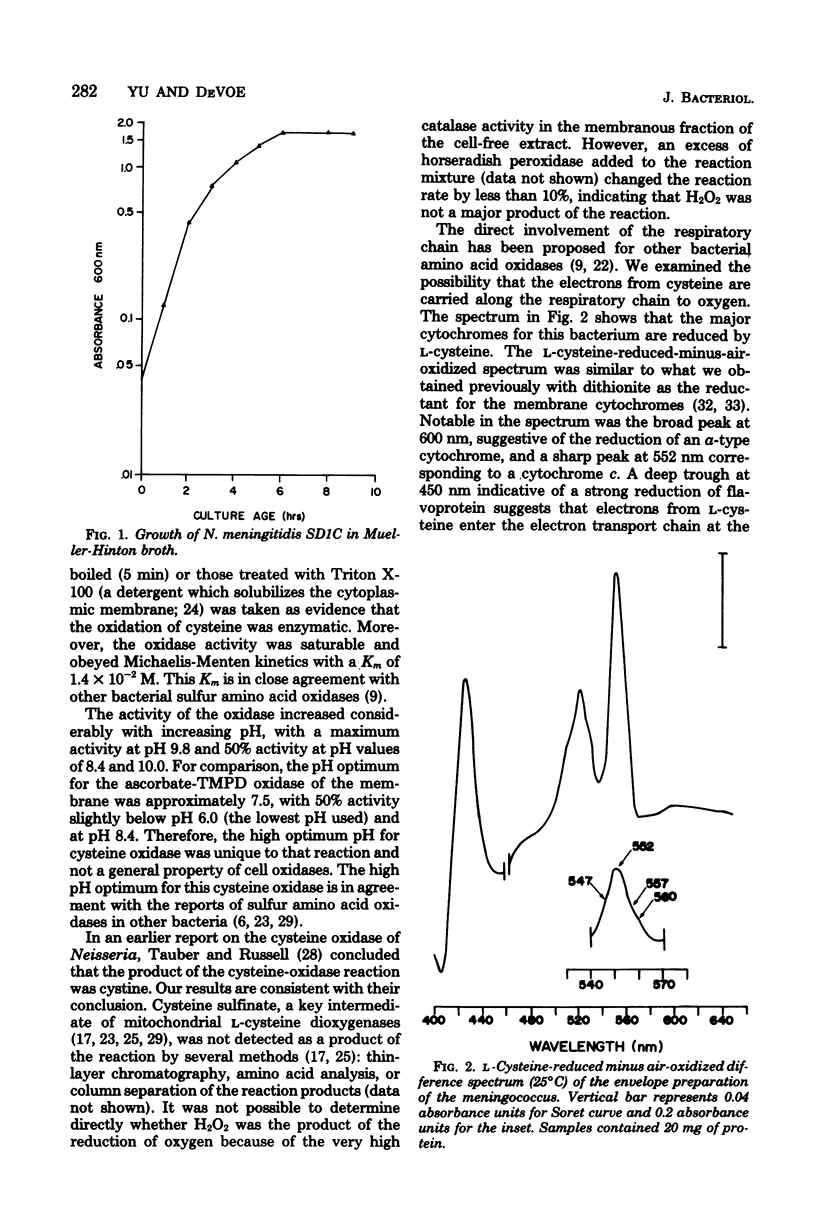
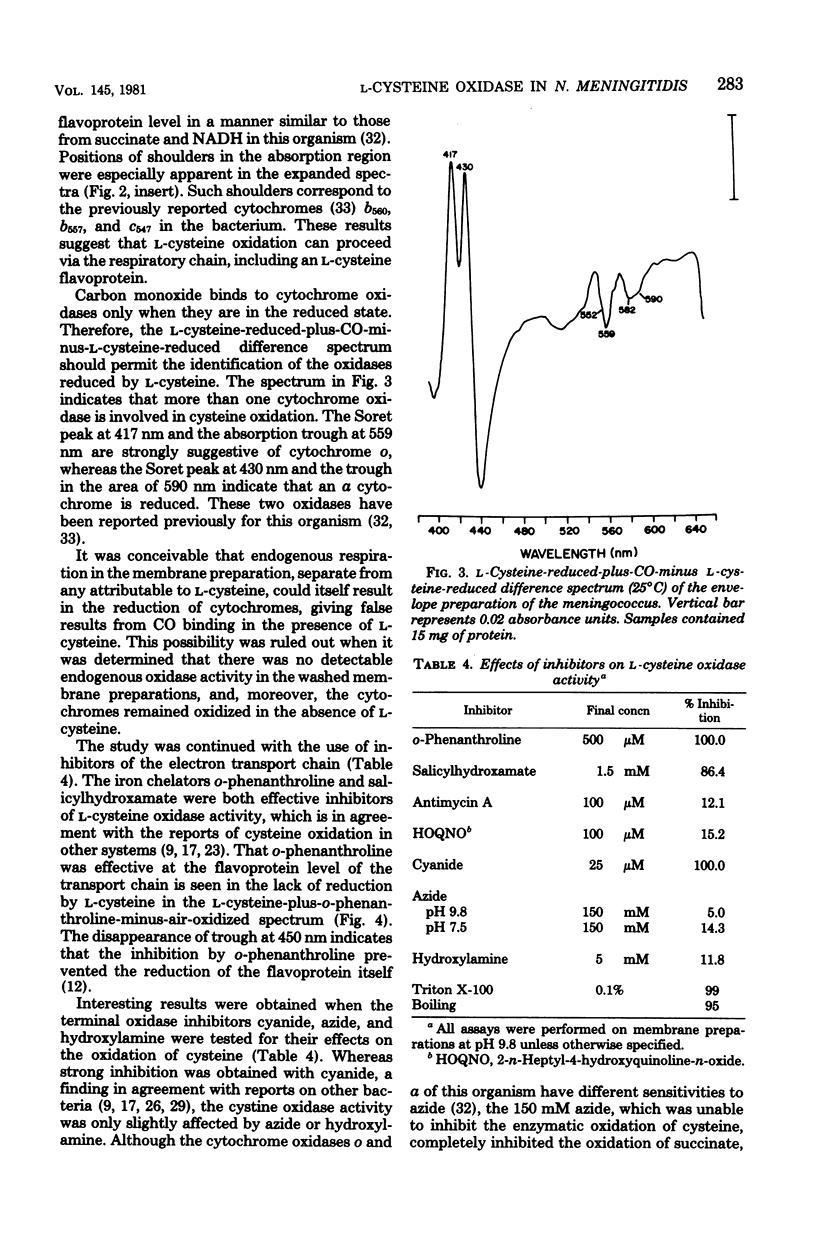
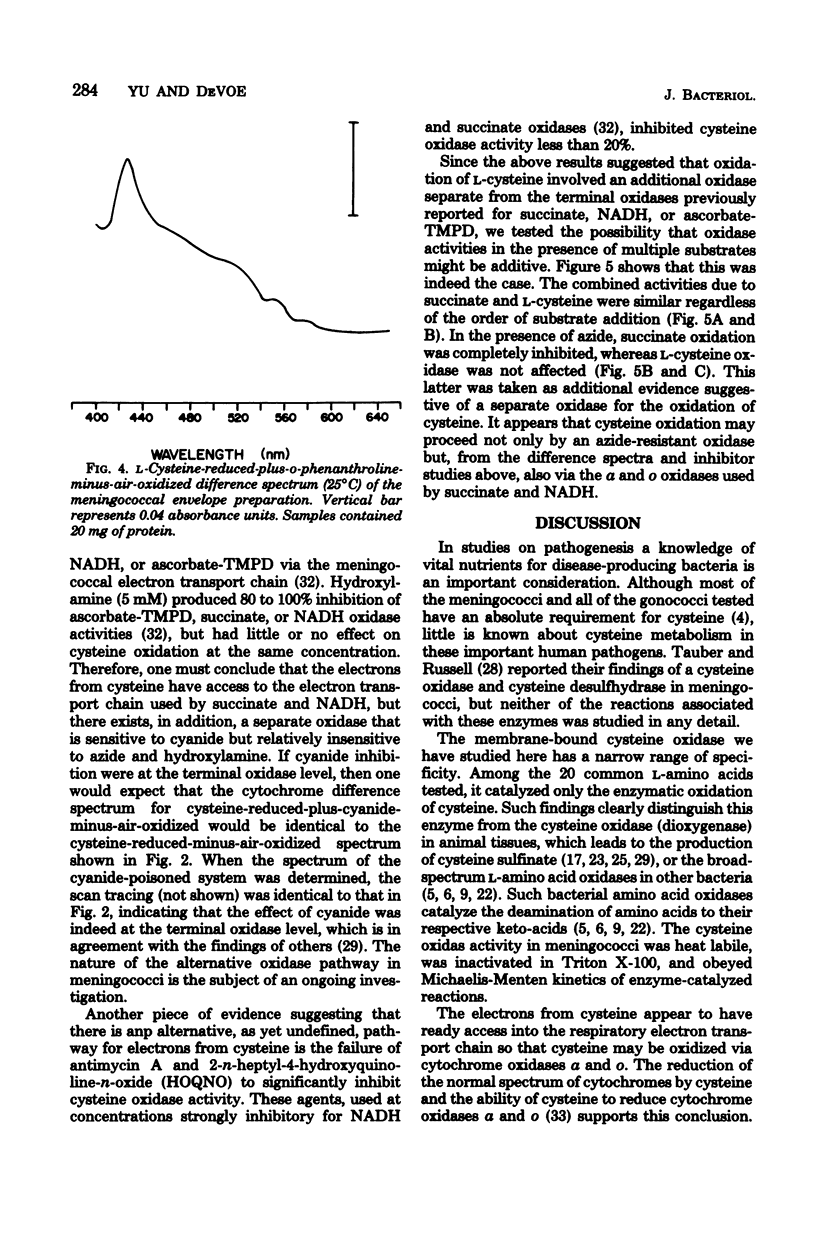
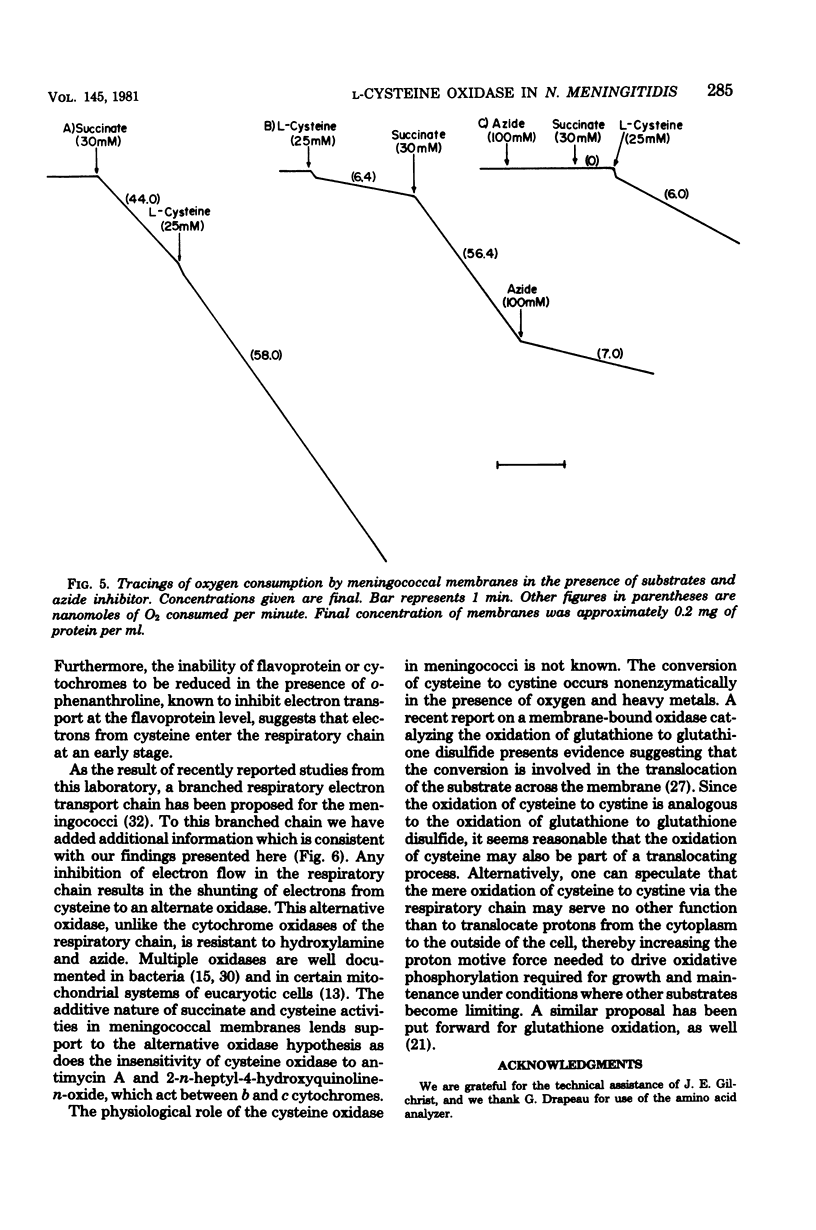
Among the L-amino acids, only L-cysteine was oxidized by isolated washed membranes of group B Neisseria meningitidis SD1C. The cysteine oxidase in the membrane obeyed Michaelis-Menten kinetics and was heat labile. The pH optimum for the maximum velocity of the reaction was 9.8. Specific activity of the enzyme increased as cell growth progressed through the exponential phase toward the stationary phase of growth. The enzyme activity was markedly sensitive to inhibition by metal chelators, but was resistant to inhibitors of terminal oxidases with the exception of cyanide. All known cytochromes in the membrane, except b563, were reduced with L-cysteine. The additive nature of L-cysteine oxidase and succinate oxidase activities suggests that an unidentified oxidase is involved in the oxidation of cysteine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Walgate J. H., Duerre J. A. Oxidative deamination of sulfur amino acids by bacterial and snake venom L-amino acid oxidase. Arch Biochem Biophys. 1971 Sep;146(1):54–63. doi: 10.1016/s0003-9861(71)80040-1. [DOI] [PubMed] [Google Scholar]
- Coudert M. Charcterization and physiological function of a soluble L-amino acid oxidase in Corynebacterium. Arch Microbiol. 1975;102(2):151–153. doi: 10.1007/BF00428360. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W. Egestion of degraded meningococci by polymorphonuclear leukocytes. J Bacteriol. 1976 Jan;125(1):258–266. doi: 10.1128/jb.125.1.258-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerre J. A., Chakrabarty S. l-amino acid oxidases of Proteus rettgeri. J Bacteriol. 1975 Feb;121(2):656–663. doi: 10.1128/jb.121.2.656-663.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Preliminary characterization studies on the Neisseria catarrhalis respiratory electron transport chain. J Bacteriol. 1974 Oct;120(1):552–555. doi: 10.1128/jb.120.1.552-555.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenimer E. A., Lapp D. F. Effects of selected inhibitors on electron transport in Neisseria gonorrhoeae. J Bacteriol. 1978 May;134(2):537–545. doi: 10.1128/jb.134.2.537-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morse S. A. The biology of the gonococcus. CRC Crit Rev Microbiol. 1978;7(2):93–189. doi: 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- Painter A. A., Hunter F. E., Jr Phosphorylation coupled to the transfer of electrons from glutathione to cytochrome c. Science. 1970 Oct 30;170(3957):552–553. doi: 10.1126/science.170.3957.552. [DOI] [PubMed] [Google Scholar]
- Pelmont J., Arlaud G., Rossat A. M. L-aminoacide oxydases des enveloppes de Proteus mirabilis: propriétés générales. Biochimie. 1972;54(10):1359–1374. doi: 10.1016/s0300-9084(72)80076-2. [DOI] [PubMed] [Google Scholar]
- SOERBO B., EWETZ L. THE ENZYMATIC OXIDATION OF CYSTEINE TO CYSTEINESULFINATE IN RAT LIVER. Biochem Biophys Res Commun. 1965 Feb 3;18:359–363. doi: 10.1016/0006-291x(65)90714-x. [DOI] [PubMed] [Google Scholar]
- Sakakibara S., Yamaguchi K., Hosokawa Y., Kohashi N., Ueda I. Purification and some properties of rat liver cysteine oxidase (cysteine dioxygenase). Biochim Biophys Acta. 1976 Feb 13;422(2):273–279. doi: 10.1016/0005-2744(76)90138-8. [DOI] [PubMed] [Google Scholar]
- TAUBER H., RUSSELL H. Enzymes of Neisseria gonorrhoeae and other Neisseria. Proc Soc Exp Biol Med. 1962 Jul;110:440–443. doi: 10.3181/00379727-110-27543. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Grau E. M., Meister A. Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2715–2719. doi: 10.1073/pnas.76.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer A. The production of cysteinesulfinic acid from cysteine in vitro. Biochim Biophys Acta. 1965 Jul 8;104(2):405–412. doi: 10.1016/0304-4165(65)90346-6. [DOI] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E. K., DeVoe I. W. Terminal branching of the respiratory electron transport chain in Neisseria meningitidis. J Bacteriol. 1980 Jun;142(3):879–887. doi: 10.1128/jb.142.3.879-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


