Abstract
To efficiently bud off from infected cells, HIV and other enveloped viruses hijack the host cellular machinery that is normally involved in vacuolar protein sorting and multivesicular body (MVB) biogenesis. The HIV Gag protein mimics hepatocyte growth factor–regulated tyrosine kinase substrate (Hrs), a modular adaptor protein that links membrane cargo recognition to its degradation after delivery to MVBs. In contrast to T cells, where HIV budding occurs at the plasma membrane, virus buds into vacuoles of macrophages, a process that may facilitate its spread within the infected host.
For enveloped viruses to be released from cells, they must undergo budding from either the plasma membrane or intracellular membranes, followed by pinching off or fission. This results in release of infectious particles with a lipid bilayer coat acquired from the host cell. It has been appreciated for more than a decade that release of HIV particles from cells requires the p6 COOH-terminal product of processed Gag protein (Gottlinger et al., 1991; Huang et al., 1995; Freed, 2002). Deletion of this sequence results in arrested budding, with viral particles tethered to the plasma membrane by a thin stalk that has failed to undergo fission (Huang et al., 1995; Freed, 2002). Similar budding defects were also observed with other retroviruses harboring mutations in various regions, named late (L) assembly domains, within their Gag polyproteins (Freed, 2002).
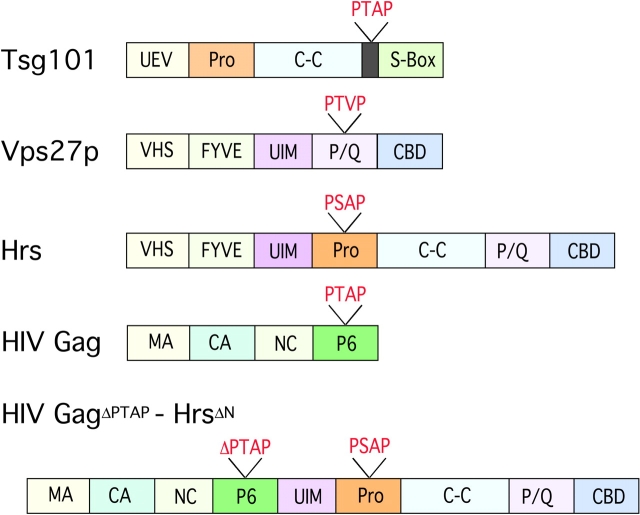
The Gag protein of HIV is synthesized as a 55-kD precursor, whose NH2 terminus is myristylated, thus targeting the protein to membranes, where it forms homotypic aggregates that induce membrane invagination and budding (Freed, 1998; Gottlinger, 2001). As the budding viral particles mature, the Gag precursor is cleaved into the myristylated NH2-terminal p17MA (matrix), p24CA (capsid), p7NC (nucleocapsid), and p6 (late domain) (Freed, 1998). Studies from several laboratories in the last few years have demonstrated that a highly conserved sequence in p6, PTAP, is required for completion of viral budding (Fig. 1) (Huang et al., 1995; Freed, 1998). This motif was found to serve as a docking site for the host cellular factor tumor susceptibility gene 101 (Tsg101), a protein that, in turn, is required for release of viral particles from HIV-producing cells (Garrus et al., 2001). A ubiquitin binding domain (designated ubiquitin E2 variant sequence [UEV]) at the NH2 terminus of Tsg101 binds to the PTAP motif, and the binding is enhanced if the Gag p6 polypeptide is ubiquitinated (Garrus et al., 2001; VerPlank et al., 2001; Pornillos et al., 2002a).
Figure 1.
Schematic representation of Tsg101, Vps27p, Hrs, HIV Gag, and HIV Gag Δ PTAP -Hrs ΔN . Pro, proline rich domain; S-Box, steadiness box; VHS, Vps27-Hrs-STAM domain; FYVE, PI(3)P interaction domain; C-C, coiled-coil domain; P/Q, proline- and glutamine-rich sequence; MA, matrix; CA, capsid; NC, nucleocapsid.
The yeast homologue of Tsg101, vacuolar protein sorting 23p (Vps23p), is a component, along with Vps28p and Vps37p, of a 350-kD complex required for sorting of endosomal membrane cargo proteins into MVBs (Katzmann et al., 2001). This complex, endosomal sorting complex required for transport (ESCRT)-I, is transiently recruited to the limiting membrane of endosomes, where it recruits two other complexes, ESCRT-II and ESCRT-III, to initiate MVB formation by invagination of membrane patches into the lumen (Babst et al., 2002a,b; Conibear, 2002; Katzmann et al., 2002). This process is topologically identical to HIV budding and viral particle formation (Pornillos et al., 2002b). During HIV assembly, Tsg101 and the associated ESCRT complexes thus appear to be hijacked from their physiological role to participate in the budding of virions (Pornillos et al., 2002b; Martin-Serrano et al., 2003).
Three articles in this issue (Bache et al., 2003; Katzmann et al., 2003; Pornillos et al., 2003) and another article elsewhere (Lu et al., 2003) show that the rightful cellular partner for Tsg101/Vps23p is the adaptor protein Hrs/Vps27p, which has proline-rich motifs similar to those found in viral L domains. Hrs serves the key function of delivering cargo from early endosomal membranes to the limiting membrane of late endosomes for formation of MVBs. These findings will be discussed along with the observation that in HIV-infected macrophages virions bud off into MVBs rather than at the plasma membrane, as occurs in T helper cells.
Role of class E Vps proteins in sorting of cargo to MVBs
Regulated degradation of cell surface molecules, particularly receptors involved in myriad signaling pathways, is essential for the control of many biological processes, including cell growth, tissue morphogenesis, and host defense (Seto et al., 2002). Endocytic membrane cargo can be partitioned either into recycling vesicles, for delivery back to the cell surface, or into late endosomes, for delivery to degradative compartments (Gruenberg, 2001). Recognition of cargo at the limiting membrane of the late endosome results in invagination of the bilayer, budding of cargo into intraluminal vesicles, and formation of MVBs. The vesicles and their contents are then degraded by proteases and hydrolases after fusion of the MVBs to lysosomes (Katzmann et al., 2002; Stahl and Barbieri, 2002).
Genetic and biochemical studies during the last several years have begun to elucidate the mechanism by which degradation of membrane cargo is regulated (Katzmann et al., 2002). Screens in Saccharomyces cerevisiae have identified multiple genes involved in trafficking of proteins from early endosomes to the degradative vacuole, the lysosome equivalent in yeast. Studies of mutants with defects in sorting of transmembrane proteins, such as the G protein–coupled Ste2p pheromone receptor and carboxypeptidase S, from the plasma membrane and Golgi, respectively, to the vacuolar lumen have led to the identification of class E Vps proteins required at different stages of this process (Katzmann et al., 2002). These include components of the three biochemically characterized high molecular weight ESCRT complexes and additional proteins, notably Vps27p and Vps4p, an ATPase required for the release of the ESCRT machinery at late stages of budding (Garrus et al., 2001; Conibear, 2002; Katzmann et al., 2002).
Despite the identification of many of the components required for cargo delivery to the MVBs, there remains a gap in understanding how cargo is recognized and targeted for invagination in late endosomes. Monoubiquitination of cargo has been proposed to be at least one of the signals involved in recognition by the vacuolar sorting machinery (Hicke, 2001; Clague, 2002), Thus, mutant carboxypeptidase S that fails to be ubiquitinated is delivered to the limiting membrane of endosomes but does not invaginate into MVBs (Katzmann et al., 2001). In mammalian cells, appending ubiquitin to the cytoplasmic tail of transferrin receptor, which normally recycles to the cell surface, results in its diversion to late endosomes (Raiborg et al., 2002). Several class E Vps proteins have ubiquitin interaction motifs (UIMs) or UEV domains that interact with ubiquitin in vitro (Katzmann et al., 2002). These include Vps23p/Tsg101 and Vps27p/Hrs (Pornillos et al., 2002a; Shih et al., 2002). The interactions of these proteins, resulting in recruitment of ESCRT-I to the limiting membrane of the late endosome, are described in the current articles.
Hrs/Vps27p links early endosomal cargo to MVB formation
The recent characterization of the interaction of viral L domains with Tsg101 has spurred efforts to identify cellular components that normally recruit the ESCRT-I complex in the process of MVB formation. The current works demonstrate that the yeast adaptor protein Vps27p and its mammalian ortholog, Hrs, serve as key links to deliver early endosomal cargo to the MVB and vacuole (Bache et al., 2003; Katzmann et al., 2003; Lu et al., 2003; Pornillos et al., 2003). Clues were forthcoming from previous studies on Hrs protein function in several species. Hrs had been shown to associate with several proteins involved in endocytosis, including clathrin and Eps15 (Raiborg and Stenmark, 2002). It was also found to colocalize primarily with early endosomes and to contain a FYVE domain, which interacts with phosphatidylinositol 3′-phosphate (PI[3]P), a marker of early endosomes (Fig. 1) (Komada et al., 1997; Raiborg et al., 2001; Stahelin et al., 2002). Hrs also has a UIM, which is involved both in binding to monoubiquitinated proteins and in directing ubiquitination of Hrs itself (Raiborg and Stenmark, 2002; Shih et al., 2002). In yeast, the UIM is required for delivery of cargo to the vacuole (Bilodeau et al., 2002). Mouse embryos homozygous for a targeted inactivation of hrs display abnormally large early endosomes, and Hrs-deficient Drosophila have defective endosomal invagination and MVB formation (Komada and Soriano, 1999; Lloyd et al., 2002). In addition, Hrs and Vps27p have several proline-rich motifs that resemble those in viral L domains.
The new studies have approached the problem of how ESCRT-I is recruited to the site of MVB formation from different angles. Emr and colleagues have used fluorescently tagged proteins to examine their relationship in yeast (Katzmann et al., 2003). They show that GFP-tagged Vps27p colocalizes with DS-Red–tagged FYVE domain of EEA1, an early endosomal marker, and that this requires the FYVE domain of Vps27p (Fig. 1). Moreover, the protein was diffusely distributed in the cytoplasm in PI 3-kinase–deficient vps34 mutant cells, consistent with a requirement for PI(3)P in the recruitment of Vps27p to endosomes (Katzmann et al., 2003). Vps23p was coprecipitated with Vps27p from membrane fractions, but not soluble fractions, of cells transfected with epitope-tagged versions of both proteins, suggesting a direct interaction between Vps27p and ESCRT-I only on endosomal membranes. This interaction did not require either the UIM or the conserved VHS domain of unknown function in the NH2-terminal part of Vps27p. GFP-Vps27p was localized to endosomal structures in a Vps23p-deficient strain, but Vps23p-GFP was diffusely distributed in the cytoplasm in the absence of either Vps27p or Vps34p (Katzmann et al., 2003). These results suggest that Vps27 acts upstream of Vps23, recruiting it to the endosomal limiting membrane once it is activated due to interaction with cargo protein (Fig. 2). Targeting of Vps23p-GFP to endosomal membranes required expression of Vps27p with an intact P/Q-rich COOH-terminal domain, including PSDP and PTVP sequences resembling those in retroviral L domains.
Figure 2.
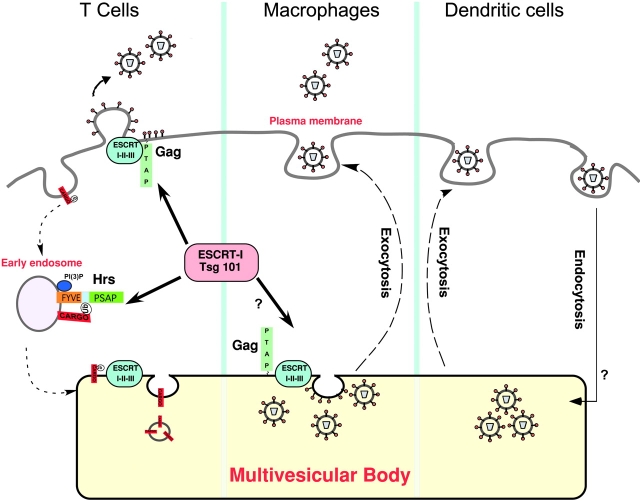
Mimicry of Hrs function by HIV Gag. The normal function of Hrs, shown in the left panel, is to recruit Tsg101 and the ESCRT-I complex for sorting ubiquitinated (Ub) cargo into the MVB. During HIV infection, HIV Gag mimics Hrs and redirects the ESCRT machinery to the site of viral budding. In T cells (left panel), viral assembly and release occur at the plasma membrane, whereas in macrophages (center) HIV particles accumulate in late endosomes and MVBs. In DCs (right panel), HIV undergoes receptor-mediated endocytosis after binding to DC-SIGN and is recycled to the cell surface. Although the vesicular compartments involved have not been identified, macrophages and DCs may share a mechanism for releasing HIV particles upon contact with T lymphocytes.
Consistent results were obtained by Stenmark's group, which used RNA interference to reduce levels of Hrs in HeLa cells and showed that Tsg101 association with membranes is dependent on Hrs expression (Bache et al., 2003). Moreover, reduction in Hrs levels resulted in reduced numbers of MVBs. Conversely, when Hrs was overexpressed Tsg101 was relocalized from LAMP1+ late endosomes to the EEA1+ early endosomal compartment. These results are consistent with the ability of Hrs to interact with early endosomal cargo and subsequently recruit Tsg101/ESCRT-I to the late endosome. When Hrs levels are abnormally high, more of the protein may be expected to associate with PI(3)P in early endosomes, resulting in aberrant binding of Tsg101 at an early stage of endosomal maturation (Bache et al., 2003).
Sundquist and colleagues approached the general problem of targeting of cargo to MVBs from the perspective of the Tsg101-recruiting sequence in HIV Gag (Pornillos et al., 2003). They surveyed Vps proteins for the PTAP or PSAP sequences present in p6 and found such a motif in Hrs. Using Biacore and yeast two-hybrid analyses, they found that Hrs interacts with Tsg101, although the PSAP-containing peptide from Hrs bound with lower affinity than that from HIV p6 to the UEV domain of Tsg101 (Pornillos et al., 2003). Yeast two-hybrid analyses of Stenmark, Sundquist, and Cohen together show that the NH2-terminal VHS and FYVE domains and the UIM of Hrs are dispensable for binding to Tsg101 but that the PSAP motif and a second region encompassing the coiled-coil domain and part of the P/Q-rich region are required for full binding activity (Fig. 1) (Bache et al., 2003; Lu et al., 2003; Pornillos et al., 2003). When this segment of Hrs was fused to the COOH terminus of a budding-defective HIV Gag mutated in the p6 PTAP motif, there was restoration of Gag particle release. Release of this fusion protein was abrogated by RNAi depletion of Tsg101 and was dependent on the PTAP-binding sequence of the Tsg101 UEV (Pornillos et al., 2003).
These studies are consistent with the view that Hrs/Vps27p has key roles in cargo recognition early in the process of endocytosis and in subsequent invagination of the endosomal limiting membrane. This protein is recruited to the early endosome by virtue of its FYVE domain interaction with PI(3)P. The interaction of the Hrs UIM domain with ubiquitinated cargo and with other monoubiquitinated proteins involved in endocytosis, such as Eps15 and epsins, may also contribute to its localization at the membrane of early endosomes. The UIM of Hrs directs its own monoubiquitination, and this may contribute to its regulated interaction with Tsg101, which results in recruitment of the ESCRT machinery and subsequent budding of the limiting membrane into the lumen to form MVBs.
Budding of HIV into late endosomes in macrophages
The principal cellular targets for infection with HIV are CD4+ T helper cells and macrophages. Although HIV budding is observed to occur at the plasma membrane in T cells, it has long been known that HIV particles accumulate in vacuolar compartments of infected macrophages (Orenstein et al., 1988). Marsh and colleagues now demonstrate that in primary macrophages HIV accumulates in vacuoles containing late endosome/MVB markers (Pelchen-Matthews et al., 2003). This observation is consistent with another recent report from Raposo et al. (2002) indicating that macrophages accumulate HIV particles in the MIIC compartment, the site where MHC class II complexes assemble with peptides derived from late endosomes.
Using immunogold EM, Pelchen-Matthews et al. (2003) found that p24/p55+ immature viral particles are often near the limiting membrane of vesicles. By labeling with gold particles of different sizes, they found p24 in the same compartment as CD63, LAMP-1, and other endosomal markers (Pelchen-Matthews et al., 2003). Infectious virus released from the macrophages was absorbed very efficiently with antibody specific for CD63, a marker for vesicles within MVBs, suggesting that the viral envelope had incorporated this protein in the course of budding into this compartment.
What advantage would HIV have by being sequestered in such vesicles? Pelchen-Matthews et al. (2003) and Raposo et al. (2002) propose that HIV particles are assembled within secretory vesicles in macrophages and are then released by exocytosis when these cells receive specific signals. MVBs of antigen-presenting cells are also sites for accumulation of exosomes, vesicles that have been proposed to efficiently prime T lymphocytes (Thery et al., 2002). HIV, like exosomes, may undergo regulated release in an environment enriched for T helper cells, thus facilitating viral spread. Unlike macrophages, dendritic cells (DCs) are not readily infected with HIV. However, interaction of HIV with DCs results in substantial enhancement of infectivity of adjacent T cells, and this requires the endocytosis of HIV into a yet undefined vesicular compartment within the DCs (Kwon et al., 2002). It is possible that regulated exocytosis in macrophages, which are productively infected with HIV, and in DCs, which may simply transcytose virions, has a similar outcome in both cell types, resulting in more efficient infection of T helper cells when these cells come into contact with the antigen-presenting cells (Kwon et al., 2002; McDonald et al., 2003) (Fig. 2).
It remains to be determined whether release of virus from macrophages is constitutive or regulated. It is also unclear if Tsg101 and the ESCRT machinery have similar roles in HIV budding in T cells and macrophages. The role of these host cell molecules has been established in T cell lines, but similar studies have not yet been reported in macrophages or monocytes (Garrus et al., 2001). It is possible that interaction of HIV Gag with a macrophage-specific protein overrides its targeting to the plasma membrane and allows it to assemble at the MVB, the normal site of action of the ESCRT complexes. Although ubiquitination of p6 increases its affinity for the UEV of Tsg101, abrogation of ubiquitination by mutating the relevant lysines in p6 had no effect on viral release from transfected HeLa cells (Freed, 2002). The role of Gag ubiquitination in release of HIV in primary T cells versus macrophages has not been reported and is a plausible explanation for how the virus employs different pathways to bud in different cell types. It will also be informative to learn whether depletion of Hrs in T cells versus macrophages influences budding of HIV in these cells.
The progress in understanding how Hrs functions in endosomal sorting will very likely provide us with a deeper appreciation of the mechanisms employed for budding by diverse enveloped viruses. HIV and Ebola virus, a filovirus, have PTAP motifs that interact with Tsg101, but other viruses, such as Rous sarcoma and Moloney murine leukemia viruses, have L domains with PPPY motifs that do not interact with Tsg101, but that, nonetheless, utilize the class E Vps pathway (Freed, 2002). Further studies on how these viruses and HIV entrain the Vps machinery during their assembly in different cell types will result in a better understanding of protein sorting and viral pathogenesis.
Acknowledgments
A. Amara is supported by a Dr. Bernard Levine postdoctoral fellowship, and D.R. Littman is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper: DC, dendritic cell; ESCRT, endosomal sorting complex required for transport; Hrs, hepatocyte growth factor–regulated tyrosine kinase substrate; MVB, multivesicular body; PI(3)P, phosphatidylinositol 3′-phosphate; UEV, ubiquitin E2 variant sequence; UIM, ubiquitin interaction motif; Vps, vacuolar protein sorting.
References
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Meerloo, and S.D. Emr. 2002. a. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 3:271–282. [DOI] [PubMed] [Google Scholar]
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. b. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 3:283–289. [DOI] [PubMed] [Google Scholar]
- Bache, K.G., A. Brech, A. Mehlum, and H. Stenmark. 2003. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 162:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau, P.S., J.L. Urbanowski, S.C. Winistorfer, and R.C. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539. [DOI] [PubMed] [Google Scholar]
- Clague, M.J. 2002. Membrane transport: a coat for ubiquitin. Curr. Biol. 12:R529–R531. [DOI] [PubMed] [Google Scholar]
- Conibear, E. 2002. An ESCRT into the endosome. Mol. Cell. 10:215–216. [DOI] [PubMed] [Google Scholar]
- Freed, E.O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 251:1–15. [DOI] [PubMed] [Google Scholar]
- Freed, E.O. 2002. Viral late domains. J. Virol. 76:4679–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus, J.E., U.K. von Schwedler, O.W. Pornillos, S.G. Morham, K.H. Zavitz, H.E. Wang, D.A. Wettstein, K.M. Stray, M. Cote, R.L. Rich, et al. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 107:55–65. [DOI] [PubMed] [Google Scholar]
- Gottlinger, H.G. 2001. The HIV-1 assembly machine. AIDS. 15:S13–S20. [DOI] [PubMed] [Google Scholar]
- Gottlinger, H.G., T. Dorfman, J.G. Sodroski, and W.A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA. 88:3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg, J. 2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2:721–730. [DOI] [PubMed] [Google Scholar]
- Hicke, L. 2001. A new ticket for entry into budding vesicles-ubiquitin. Cell. 106:527–530. [DOI] [PubMed] [Google Scholar]
- Huang, M., J.M. Orenstein, M.A. Martin, and E.O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, D.J., M. Babst, and S.D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 106:145–155. [DOI] [PubMed] [Google Scholar]
- Katzmann, D.J., G. Odorizzi, and S.D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3:893–905. [DOI] [PubMed] [Google Scholar]
- Katzmann, D.J., C.J. Stefan, M. Babst, and S.D. Emr. 2003. Vps27 recruits ESCRT machinery to endosomes during MBV sorting. J. Cell Biol. 162:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada, M., and P. Soriano. 1999. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 13:1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada, M., R. Masaki, A. Yamamoto, and N. Kitamura. 1997. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J. Biol. Chem. 272:20538–20544. [DOI] [PubMed] [Google Scholar]
- Kwon, D.S., G. Gregorio, N. Bitton, W.A. Hendrickson, and D.R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 16:135–144. [DOI] [PubMed] [Google Scholar]
- Lloyd, T.E., R. Atkinson, M.N. Wu, Y. Zhou, G. Pennetta, and H.J. Bellen. 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 108:261–269. [DOI] [PubMed] [Google Scholar]
- Lu, Q., L.W. Hope, M. Brasch, C. Reinhard, and S.N. Cohen. 2003. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. USA. 100:7626–7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano, J., T. Zang, and P.D. Bieniasz. 2003. Role of ESCRT-I in retroviral budding. J. Virol. 77:4794–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, D., L. Wu, S.M. Bohks, V.N. KewalRamani, D. Unutmaz, and T.J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 300:1295–1297. [DOI] [PubMed] [Google Scholar]
- Orenstein, J.M., M.S. Meltzer, T. Phipps, and H.E. Gendelman. 1988. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62:2578–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews, A., B. Kramer, and M. Marsh. 2003. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 162:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos, O., S.L. Alam, R.L. Rich, D.G. Myszka, D.R. Davis, and W.I. Sundquist. 2002. a. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 21:2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos, O., J.E. Garrus, and W.I. Sundquist. 2002. b. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12:569–579. [DOI] [PubMed] [Google Scholar]
- Pornillos, O., D.S. Higginson, K.M. Stray, R.D. Fisher, J.E. Garrus, M. Payne, G. He, H. Wang, S. Morhan, and W.I. Sundquist. 2003. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J. Cell Biol. 162:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., and H. Stenmark. 2002. Hrs and endocytic sorting of ubiquitinated membrane proteins. Cell Struct. Funct. 27:403–408. [DOI] [PubMed] [Google Scholar]
- Raiborg, C., B. Bremnes, A. Mehlum, D.J. Gillooly, A. D'Arrigo, E. Stang, and H. Stenmark. 2001. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci. 114:2255–2263. [DOI] [PubMed] [Google Scholar]
- Raiborg, C., K.G. Bache, D.J. Gillooly, I.H. Madshus, E. Stang, and H. Stenmark. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4:394–398. [DOI] [PubMed] [Google Scholar]
- Raposo, G., M. Moore, D. Innes, R. Leijendekker, A. Leigh-Brown, P. Benaroch, and H. Geuze. 2002. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic. 3:718–729. [DOI] [PubMed] [Google Scholar]
- Seto, E.S., H.J. Bellen, and T.E. Lloyd. 2002. When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev. 16:1314–1336. [DOI] [PubMed] [Google Scholar]
- Shih, S.C., D.J. Katzmann, J.D. Schnell, M. Sutanto, S.D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389–393. [DOI] [PubMed] [Google Scholar]
- Stahelin, R.V., F. Long, K. Diraviyam, K.S. Bruzik, D. Murray, and W. Cho. 2002. Phosphatidylinositol 3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J. Biol. Chem. 277:26379–26388. [DOI] [PubMed] [Google Scholar]
- Stahl, P.D., and M.A. Barbieri. 2002. Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci STKE. 2002:PE32. [DOI] [PubMed]
- Thery, C., L. Zitvogel, and S. Amigorena. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2:569–579. [DOI] [PubMed] [Google Scholar]
- VerPlank, L., F. Bouamr, T.J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C.A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. USA. 98:7724–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]