Abstract
Alarge body of work indicates that chromosomes play a key role in the assembly of both acentrosomal and centrosome-containing spindles. In animal systems, the absence of chromosomes either prevents spindle formation or allows the assembly of a metaphase-like spindle that fails to evolve into an ana-telophase spindle. Here, we show that Drosophila secondary spermatocytes can assemble morphologically normal spindles in the absence of chromosomes. The Drosophila mutants fusolo and solofuso are severely defective in chromosome segregation and produce secondary spermatocytes that are devoid of chromosomes. The centrosomes of these anucleated cells form robust asters that give rise to bipolar spindles that undergo the same ana-telophase morphological transformations that characterize normal spindles. The cells containing chromosome-free spindles are also able to assemble regular cytokinetic structures and cleave normally. In addition, chromosome-free spindles normally accumulate the Aurora B kinase at their midzones. This suggests that the association of Aurora B with chromosomes is not a prerequisite for its accumulation at the central spindle, or for its function during cytokinesis.
Keywords: chromosome segregation; chromosome passengers; Aura B; centrosome; microtubule
Introduction
Although the basic structure of the spindle is similar in all cell types of higher eukaryotes, spindle assembly can occur through different pathways. In most animal somatic cells, spindle formation is mediated by a pair of microtubule (MT)* organizing centers, called the centrosomes. During prophase, the separating centrosomes nucleate astral arrays of MTs that are captured and stabilized by the chromosomes, allowing the formation of a bipolar spindle (for review see Compton, 2000). In contrast, meiotic cells of females of several animal species and mitotic cells of higher plants assemble their spindles via an acentrosomal pathway. In these cells, which do not possess centrosomes, MTs grow from multiple sites around the chromosomes and progressively self-organize into a bipolar spindle through the action of both plus-end– and minus-end–directed motor proteins (Compton, 2000). Growing evidence indicates that chromosomes play a key role in the formation of these acentrosomal spindles (for review see Karsenti and Vernos, 2001). Recent studies have suggested that this role reflects the ability of chromosomes to generate Ran-GTP, a Ras-like GTPase that promotes MT growth and stability. The chromatin-bound Ran-GEF, RCC1, is thought to catalyze the Ran-GDP/Ran-GTP transition, generating a high local concentration of Ran-GTP that stimulates MT nucleation (Carazo-Salas et al., 1999, 2001).
A large body of work indicates that chromosomes also play an essential role in the formation of centrosome-containing spindles. When the nucleus of grasshopper spermatocytes is removed by micromanipulation before nuclear envelope breakdown, astral MTs disassemble and the spindle fails to form (Zhang and Nicklas, 1995). Studies performed in echinoderm, Drosophila, and Xenopus early embryos have shown that centrosomes can duplicate and form robust asters in the absence of chromosomes, but these asters fail to evolve into real spindles and do not undergo the ana-telophase morphological transformations that characterize chromosome-containing spindles (Sluder et al., 1986; Picard et al., 1988; Raff and Glover, 1989; Sawin and Mitchison, 1991). Similar results have been recently obtained using PtK homokaryons, where centrosomes lacking associated chromosomes give rise to metaphase-like spindles that fail to turn into normal ana-telophase spindles (Faruki et al., 2002). Interestingly, also in acentrosomal systems, such as mouse meiosis, chromatin-free bipolar spindles do not have the ability to evolve into ana-telophase–like configurations (Brunet et al., 1998). Together, these results have led to the view that chromosomes play an essential role in spindle formation and dynamics both in acentrosomal and centrosome-containing systems (Waters and Salmon, 1997; Karsenti and Vernos, 2001).
Here, we show that Drosophila secondary spermatocytes devoid of chromosomes assemble metaphase-like spindles that evolve into telophase spindles. These chromosome-free cells also assemble regular cytokinetic structures and cleave normally. These results indicate that in Drosophila spermatocytes, spindle formation and dynamics are controlled by chromosome-independent factors.
Results and discussion
In the course of an extensive screen for mutations affecting Drosophila male meiosis (see Materials and methods), we isolated four mutants with severe defects in chromosome segregation. Two of these mutants map to the second and two to the third chromosome; complementation tests revealed that they identify two genes we call fusolo (fsl) and solofuso (suo). Deficiency mapping experiments showed that fsl and suo are uncovered by Df(3L)BK10 (71C3; 71E5) and Df(2L)VA17 (37C; 37F5), respectively. fsl 1/fsl 1, fsl 2/fsl 2, fsl 1/Df, and fsl 2/Df flies are viable but sterile in both sexes; suo 1/suo 1, suo 2/suo 2 homozygotes, and suo 2/Df hemizygotes are viable and also sterile in both sexes, whereas suo 1/Df hemizygotes are late lethals.
To characterize the meiotic phenotype of fsl and suo, we made larval and adult testis preparations that were simultaneously stained for tubulin, centrin, and DNA. The anti–human centrin (HsCen1p) antibody (Paoletti et al., 1996) decorates Drosophila centrioles (Riparbelli et al., 2002), facilitating distinction between first and second meiotic divisions, which display two and one centriole at each pole, respectively. The analysis of fsl 1/Df, fsl 1 /fsl 1, fsl 2/Df, and fsl 2/fsl 2 testes showed that these mutant combinations do not substantially differ in terms of severity of the phenotype, displaying a common defect in chromosome segregation. Thus, we focused on fsl 1/fsl 1 and fsl 1/Df for detailed characterization of the meiotic phenotype. In fsl 1/fsl 1 and fsl 1/Df, meiotic prometaphase and metaphase I figures are normal (Fig. 1 c). However, in most ana-telophases, chromosome segregation is disrupted (Fig. 1, d and e; Table I). In approximately half of mutant ana-telophase I cells, all chromosomes segregate to one pole only (Fig. 1 e and Table I), leading to the formation of secondary spermatocytes that are completely devoid of chromosomes (Fig. 2). Chromosome-containing fsl secondary spermatocytes form a regular spindle and exhibit the same aberrant chromosome behavior seen in the first meiotic division (unpublished data; see Fig. 5 a). In fsl secondary spermatocytes without chromosomes, centrosomes nucleate robust astral arrays of MTs that move to the opposite cell poles (Fig. 2 a′). These asters give rise to metaphase-like spindles devoid of chromosomes that differ from their wild-type counterparts only for the absence of kinetochore fibers (Fig. 2, a and a′). It should be noted that in these chromosome-free spindles, there is limited overlapping between the antiparallel MTs emanating from the opposite poles (Fig. 2 a′). However, little or no overlapping of these MTs is also seen in wild-type metaphase spindles (Fig. 2 a; Cenci et al., 1994). Chromosome-free spindles evolve into an anaphase A-like configuration, which again displays little or no MT overlapping at the center of the cell, as occurs in wild-type anaphases (Fig. 2, b and b′; Cenci et al., 1994). These anaphase A-like spindles undergo anaphase B (Fig. 2, c and c′), assemble a morphologically normal central spindle, and elongate to form telophase figures that are indistinguishable from their wild-type counterparts (Fig. 2, d–e′). It should be noted that in fsl mutants, the frequency of chromosome-free metaphase/early anaphase II figures and the frequency of chromosome-free telophase II figures are comparable (Table I). This indicates that most (if not all) metaphase-like spindles without chromosomes have the ability to form a central spindle and to proceed to telophase.
Figure 1.
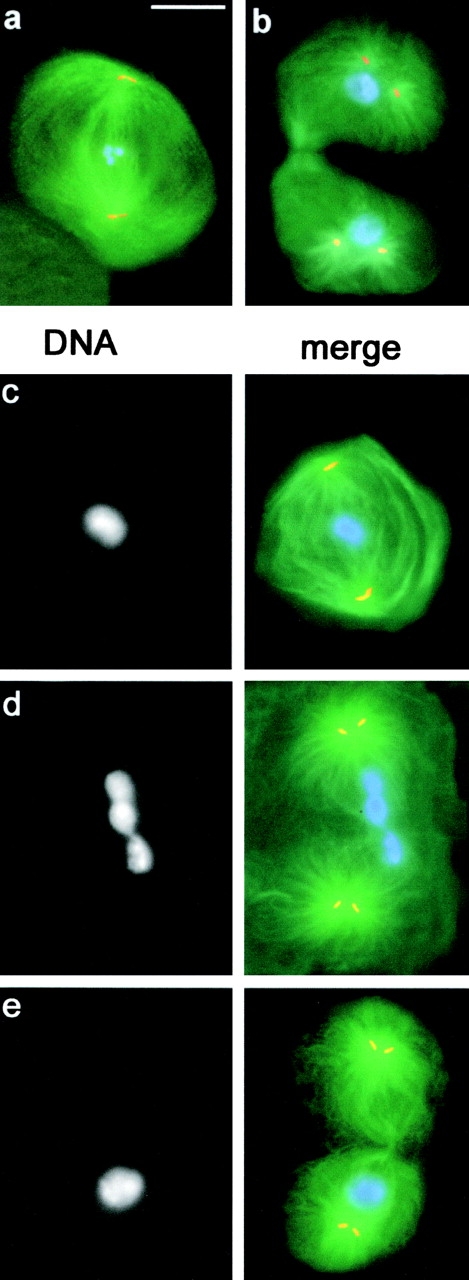
First meiotic division in fsl mutant males. Cells were stained for tubulin (green), centrin (orange), and DNA (blue). (a and b) Meiotic division in wild-type males. (a) Metaphase I; (b) Late telophase I; (c–e) Meiotic division in fsl males. (c) Metaphase I; (d) Late telophase I with nonsegregating chromosomes at the center of the cell; (e) Late telophase I with all chromosomes segregating to only one of the two presumptive daughter cells. Bar, 10 μm.
Table I.
Meiotic defects observed in fsl mutant males
| Mutant, meiotic division | Metaphases and early anaphases1
|
Telophases
|
||||
|---|---|---|---|---|---|---|
| A | B | A | B | C | D | |
| fsl1/fsl1, I | 190 | 0.0 | 95 | 0.0 | 49.5 | 50.5 |
| fsl1/fsl1, II | 304 | 49.3 | 254 | 49.2 | 20.1 | 30.7 |
| fsl1/Df, I | 90 | 0.0 | 62 | 0.0 | 45.2 | 54.8 |
| fsl1/Df, II | 99 | 54.5 | 78 | 53.9 | 20.5 | 25.6 |
| Control, I | 102 | 0.0 | 90 | 0.0 | 0.0 | 0.0 |
| Control, II | 140 | 0.0 | 108 | 0.0 | 0.0 | 0.0 |
A, number of cells scored; B, percentage of cells devoid of chromosomes; C, percentage of telophases with nonsegregating chromosomes at the center of the cell (see Fig. 1 d); D, percentage of telophases with chromosomes segregating to one daughter cell only (see Fig. 1 e).
This class also includes prometaphases.
Figure 2.
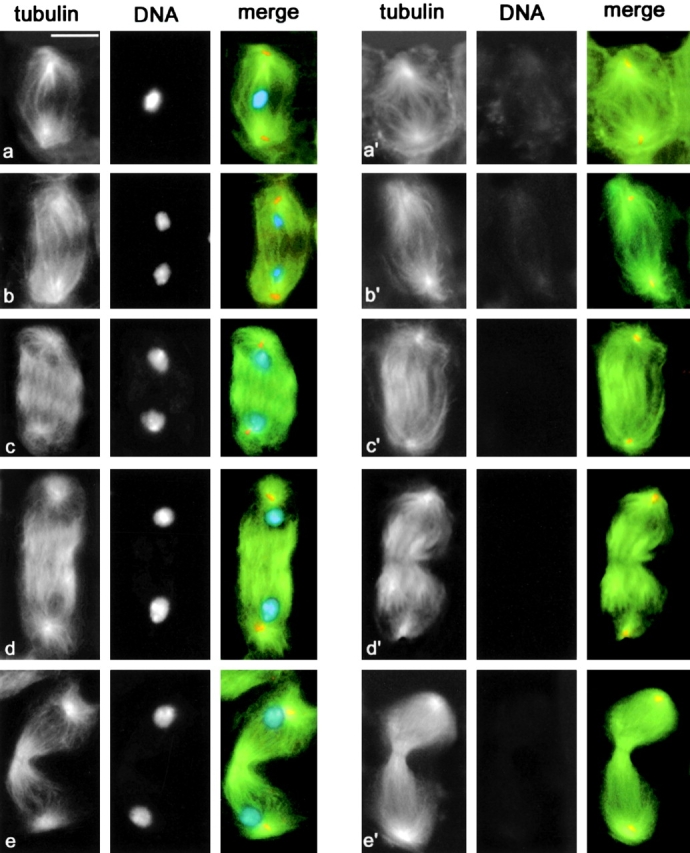
Spindle formation and dynamics in chromosome-free fsl secondary spermatocytes. Cells were stained for tubulin (green), centrin (orange), and DNA (blue). (a–e) Second meiotic division in wild type males. (a) Metaphase; (b) Early anaphase, (c) Late anaphase; (d) Early telophase; (e) Late telophase. (a′–e′) Spindles of chromosome-free fsl secondary spermatocytes. (a′) Metaphase-like; (b′) Early anaphase-like; (c′) Late anaphase-like; (d′) Early telophase-like; (e′) Late telophase-like. Bar, 10 μm.
Figure 5.
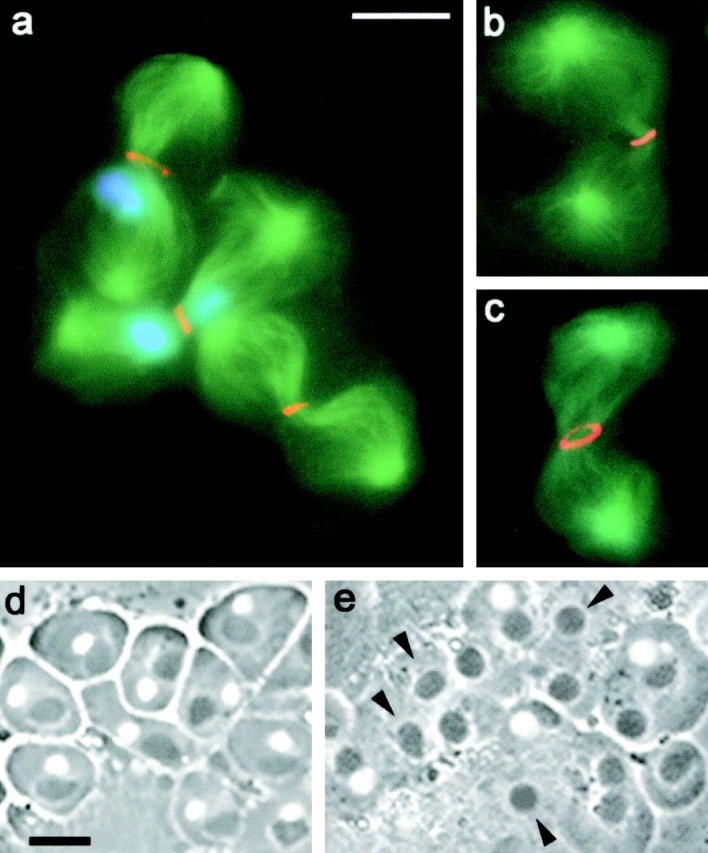
Cytokinesis in the absence of chromosomes in fsl mutants. (a–c) Telophase II figures stained for tubulin (green), DNA (blue), and either actin (a, orange), myosin II (b, orange), or anillin (c, orange). Note that the cytokinetic structures of chromosome-free cells are comparable to those of chromosome-containing cells. (d and e) Live spermatids from wild-type (d) and fsl (e) males. Note that in fsl mutants, some nebenkern (arrowheads) are not associated with nuclei. Bars, 10 μm.
The cytological characterization of suo mutants showed that they exhibit common alterations in chromosome segregation, which are more pronounced in suo 1 than in suo 2 mutant combinations. However, suo 1/Df mutants are severely defective in germline cell proliferation. The chromosome segregation defect in suo spermatocytes is different from that observed in fsl mutants. suo prometaphase and metaphase I figures are normal, as observed in fsl mutants, but anaphases and telophases are characterized by the presence of chromatin bridges that are usually not seen in fsl mutants. As a result of these bridges, in a fraction of suo ana-telophase I cells, all the chromosomes segregate to one pole only, giving rise to chromosome-free secondary spermatocytes [25% in suo 1/suo 1 (n = 97) and 29% in suo 2/Df (n = 61)]. The suo secondary spermatocytes devoid of chromosomes behave like those of fsl mutants; they form a bipolar spindle that undergoes the same dynamic transformations seen in chromosome-containing spindles (unpublished data). Together, these results indicate that the ability to form a spindle in the absence of chromosomes does not depend on a specific mutant background, but is an intrinsic characteristic of Drosophila spermatocytes.
In contrast to Drosophila spermatocytes, chromosome-free metaphase-like spindles of PtK homokaryons are unable to evolve into a typical telophase structure. In these peculiar spindles, the antiparallel MTs emanating from the centrosomes give rise to a compact MT bundle that fails to bind the MKLP (CHO1) kinesin, which accumulates at the central spindle midzone in chromosome-containing cells (Faruki et al., 2002). Thus, we asked whether the central spindles of chromosome-free telophases have the ability to bind Pavarotti (Pav), the Drosophila orthologue of MKLP (Adams et al., 1998). This analysis revealed that these telophases normally accumulate Pav in their midzones (Fig. 3), indicating a correct organization of central spindle MTs.
Figure 3.
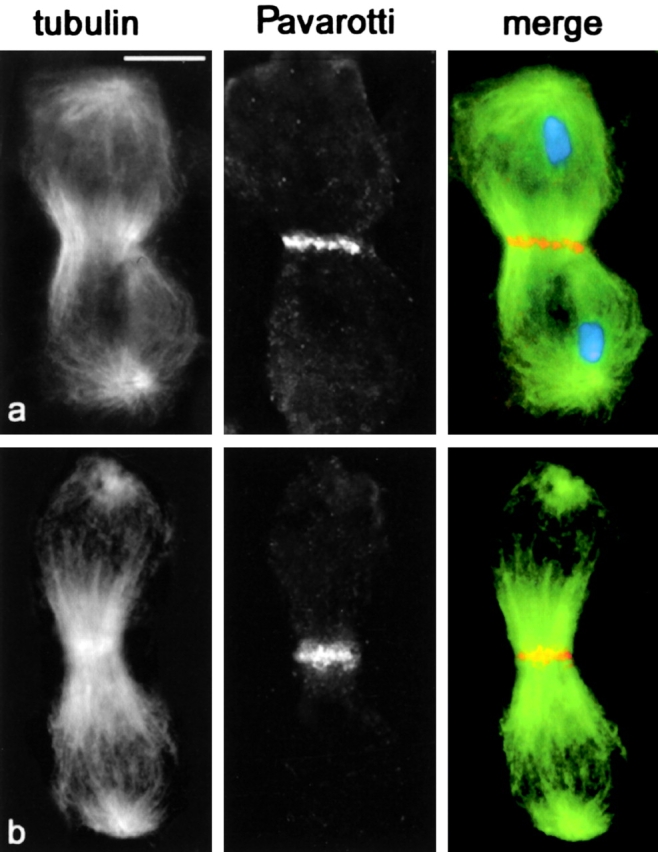
Pav accumulates at the central spindle midzone of chromosome-free telophases. Cells were stained for tubulin (green), Pav (orange), and DNA (blue). (a) Telophase II from wild-type males. (b) Chromosome-free telophase II from fsl mutants. Bar, 10 μm.
To further characterize the central spindles of chromosome-free spermatocytes, we asked whether they have the ability to bind Aurora B. Aurora B is in an evolutionary conserved macromolecular complex that contains the inner centromere protein and survivin (for review see Adams et al., 2001). The proteins of this complex are called chromosome passengers (Earnshaw and Bernat, 1991) because they accumulate at centromeres in metaphase, but move to the central spindle midzone in telophase. Given that both Aurora B and the inner centromere protein are essential for cell cleavage, it has been suggested that these proteins may help to integrate chromosomal events with cytokinesis (Adams et al., 2001). Immunostaining of wild-type spermatocytes for Aurora B showed that this protein is concentrated at metaphase kinetochores (Fig. 4 a). As spermatocytes progress through cell division, Aurora B accumulates in the central spindle midzone (Fig. 4 c). In chromosome-containing cells of fsl and suo mutants, Aurora B behavior is identical to wild type (Fig. 4 b and d; unpublished data). In chromosome-free metaphase-like figures from both fsl and suo mutants, Aurora B displays a diffuse staining (unpublished data). However, as these cells move toward telophase, Aurora B accumulates in the central spindle midzone, as occurs in wild type (Fig. 4, e and f). Together, these results clearly show that Aurora B concentration in the central spindle does not require its previous localization at kinetochores. In addition, they strongly suggest that the role played by Aurora B during cytokinesis is independent of that played in chromosome structure and segregation.
Figure 4.
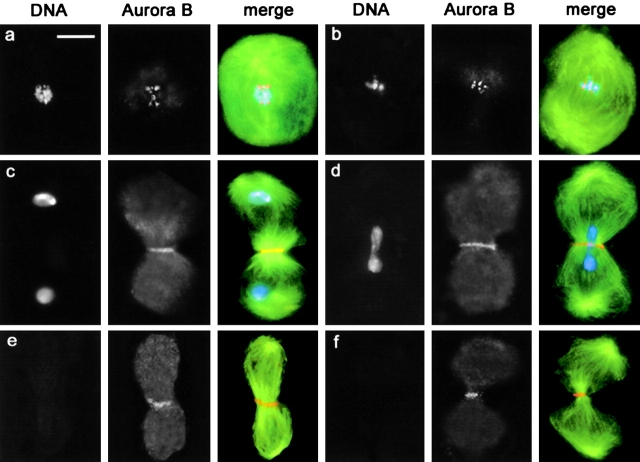
Aurora B distribution in wild-type and fsl spermatocytes. Cells were stained for tubulin (green), Aurora B (orange), and DNA (blue). (a) Wild-type metaphase I; (b) fsl metaphase I; (c) Wild-type telophase I; (d) fsl telophase I; (e) Chromosome-free fsl early telophase II; (f) Chromosome-free fsl late telophase II. Note that Aurora B concentrates in the central spindle midzone in the absence of chromosomes. Bar, 10 μm.
The findings that chromosome-free spermatocytes normally accumulate both Pav and Aurora B at their central spindle midzones suggest (but do not prove) that these cells have the ability to undergo cytokinesis. Thus, we stained both fsl and suo mutant testes for components of the cytokinetic apparatus such as F actin, myosin II, and anillin. F actin and myosin II are well-known components of the contractile ring that mediates cytokinesis in animal cells (for review see Glotzer, 2001). Anillin is a 190-kD protein that concentrates in the cleavage furrow of a variety of Drosophila cells, where it is thought to mediate membrane–ring interactions during cytokinesis (Field and Alberts, 1995; Giansanti et al., 1999; Somma et al., 2002). The analysis of fsl (Fig. 5) and suo preparations (unpublished data) revealed that secondary spermatocytes without chromosomes form morphologically regular cytokinetic structures across the central spindle midzone. In addition, we observed that these structures and those of their wild-type counterparts constrict to the same extent (Fig. 5).
To confirm that fsl and suo secondary spermatocytes can undergo cytokinesis in the absence of chromosomes we analyzed spermatid morphology in larval testes of both mutants. In wild type spermatocytes, mitochondria are equally partitioned between the two daughter cells at each meiotic division. At the end of meiosis II the mitochondria received by each spermatid fuse to form a spherical structure called the nebenkern. As a result, each wild type spermatid comprises two spherical structures of similar size: a phase-light nucleus and a phase-dark nebenkern (Fig. 5 d). If cytokinesis fails, abnormal spermatids are formed, containing a large nebenkern associated with either two or four normal-sized nuclei (Fuller, 1993). An examination of fsl and suo live spermatids revealed that in both mutants there are no large nebenkern resulting from failure in cytokinesis. Instead, both mutants display many regular-sized nebenkern that are not associated with nuclei (Fig. 5 e); these nebenkern are likely to originate from secondary spermatocytes without chromosomes that have successfully undergone the cytokinetic process.
We have shown that chromosome-free spermatocytes assemble regular cytokinetic structures and cleave normally, indicating that chromosomes are not the source of signals that stimulate cytokinesis. These findings are consistent with the micromanipulation experiments on grasshopper spermatocytes, showing that elimination of chromosomes from metaphase cells does not prevent them to proceed through anaphase and telophase and undergo cytokinesis (Zhang and Nicklas, 1996). They also agree with the classic Rappaport's experiments on echinoderm eggs (Rappaport, 1986), and with more recent experiments on vertebrate cells, showing that ectopic cytokinesis can occur between adjacent asters of different chromosome-containing spindles placed in the same cytoplasm (Eckley et al., 1997; Rieder et al., 1997; Savoian et al., 1999). Previously, we have shown that in Drosophila spermatocytes the signals for cytokinesis are not generated by the asters. asterless spermatocytes, which are devoid of asters due to a primary defect in centrosome assembly, form poorly focused anastral spindles. However, these spindles eventually organize morphologically normal central spindles that are fully able to support cytokinesis (Bonaccorsi et al., 1998). Thus, the results on asterless, fsl, and suo indicate that neither the asters nor the chromosomes are required for signaling cytokinesis in Drosophila spermatocytes. This suggests that in this system, the central spindle is both necessary and sufficient to stimulate cytokinesis.
Our results indicate that Drosophila secondary spermatocytes can form a morphologically normal spindle in the absence of chromosomes, and thus, in the absence of a high concentration of Ran-GTP in the center of the cell. The assembly of a metaphase-like bipolar spindle in the absence of chromosomes has been observed in several systems (see Introduction), including mouse oocytes (Brunet et al., 1998) and PtK homokaryons (Faruki et al., 2002). However, all these metaphase-like MT arrays are unstable and fail to proceed through ana-telophase. Thus, it has been suggested that in most centrosome-containing animal cells, chromosomes are not required for initial spindle morphogenesis, but for the stabilization of the structure and its evolution toward an ana-telophase configuration (Faruki et al., 2002).
In contrast with these systems, the metaphase-like chromosome-free spindles of Drosophila spermatocytes are sufficiently stable to undergo anaphase and telophase. We would like to point out that Drosophila spermatocytes behave differently from those of grasshopper, where enucleation of late prophase spermatocytes inhibits spindle assembly (Zhang and Nicklas, 1995). Yet, elimination of chromosomes from grasshopper metaphase spermatocytes does not affect the ability of the spindle to proceed through ana-telophase (Zhang and Nicklas, 1996). However, the latter finding may reflect incomplete elimination of a critical chromosome-associated factor (e.g., Ran-GTP) from the micromanipulated cell. Regardless the interpretation of these grasshopper experiments, it is clear that in Drosophila spermatocytes, chromosome-independent factors control spindle formation and dynamics. However, both the nature of these factors and the mechanisms underlying progression of chromosome-free spindles from a metaphase-like to a telophase-like structure remain to be determined.
Materials and methods
Drosophila stocks and mapping procedures
fsl 1, fsl 2, suo 1, and suo 2 have been isolated by a cytological screen of a collection of male sterile mutants. These mutants were selected by B. Wakimoto (Washington University, Seattle, WA) and D.L. Lindsley (University of California, San Diego, San Diego, CA) from 12,000 viable lines, generated by E. Koundakjian and C. Zuker (University of California, San Diego, San Diego, CA), each homozygous for either a second or a third EMS-mutagenized chromosome. We named our mutants fusolo and solofuso, two Italian terms that mean “only spindle,” after the cytological phenotype described here. The Oregon R laboratory strain was used as a wild-type control. All the stocks were grown at 25°C in Drosophila standard medium.
To map suo and fsl, we used the second and third chromosome deficiency kits (provided by the Bloomington Stock Center, Indiana University, Bloomington, IN; http://flystocks.bio.indiana.edu/), respectively. Each of these kits includes a set of selected deficiencies that uncover about two thirds of the chromosome. fsl/TM6C and suo/CyO females were crossed to males from each pertinent deficiency stock, and the mutant/Df males from each cross were tested for fertility. Sterile mutant/Df males were then examined cytologically to determine their meiotic phenotype.
Cytological procedures
The double-staining techniques for actin/tubulin, myosin II/tubulin, and anillin/tubulin (the anti-myosin and -anillin antibodies were provided by C. Field, Harvard Medical School, Boston, MA) were described previously (Giansanti et al., 1999). For double immunostaining of centrin/tubulin and Aurora B/tubulin, testes were fixed according to protocol 3 of Giansanti et al. (1999). Testis preparations were incubated for 1 h in 1% BSA in PBS. They were then incubated overnight with both a monoclonal anti-tubulin antibody (Amersham Biosciences) diluted 1:100 in PBS and either a rabbit anti-HsCen1p antibody (provided by M. Bornens, Institut Curie, Paris, France; Paoletti et al., 1996) or a rabbit anti-Aurora B antibody (provided by D. Glover, University of Cambridge, Cambridge, UK; Giet and Glover, 2001) diluted 1:500 and 1:200 in PBS, respectively. Primary antibodies were detected using a FITC-conjugated anti–mouse (diluted 1:20; Jackson ImmunoResearch Laboratories) and a CY3-conjugated anti–rabbit (diluted 1:100; Jackson ImmunoResearch Laboratories) secondary antibodies. After two washes (5 min each) in PBS, slides were mounted in Vectashield® plus DAPI (Vector Laboratories) to stain DNA. Live spermatid preparations were obtained as described by Cenci et al. (1994), and were analyzed by phase contrast optics.
Immunostained and live preparations were examined using a microscope (Axioplan; Carl Zeiss MicroImaging, Inc.) equipped with an HBO 50-W mercury lamp for epifluorescence and with a cooled charge-coupled device (CCD; Photometrics), as described by Bonaccorsi et al. (1998). Grayscale images were collected separately using the IPLab Spectrum software (Scanalytics). They were then converted into Photoshop® 5.5 and used as such, or merged in pseudocolors.
Acknowledgments
We thank B. Wakimoto, D.L. Lindsley, E. Koundakjian, and C. Zuker for the collection of male sterile mutants screened to isolate fsl and suo, the Bloomington stock center for the deficiency kits, C. Field for anti-myosin II and anti-anillin antibodies, and M. Bornens and D. Glover for anti-centrin and anti-Aurora B antibodies, respectively. We also thank G. Siriaco for comments on the manuscript.
E. Bucciarelli and M.G. Giansanti contributed equally to this paper.
Footnotes
Abbreviations used in this paper: fsl, fusolo; MT, microtubule; Pav, Pavarotti; suo, solofuso.
References
- Adams, R.R., A.A. Tavares, A. Salzberg, H.J. Bellen, and D.M. Glover. 1998. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12:1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R.R., M. Carmena, and W.C. Earnshaw. 2001. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11:49–54. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi, S., M.G. Giansanti, and M. Gatti. 1998. Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J. Cell Biol. 142:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet, S., Z. Polanski, M.H. Verlhac, J.Z. Kubiak, and B. Maro. 1998. Bipolar meiotic spindle formation without chromatin. Curr. Biol. 8:1231–1234. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., G. Guarguaglini, O.J. Gruss, A. Segref, E. Karsenti, and I.W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 400:178–181. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., O.J. Gruss, I.W. Mattaj, and E. Karsenti. 2001. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 3:228–233. [DOI] [PubMed] [Google Scholar]
- Cenci, G., S. Bonaccorsi, C. Pisano, F. Verni, and M. Gatti. 1994. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 107:3521–3534. [DOI] [PubMed] [Google Scholar]
- Compton, D.A. 2000. Spindle assembly in animal cells. Annu. Rev. Biochem. 69:95–114. [DOI] [PubMed] [Google Scholar]
- Earnshaw, W.C., and R.L. Bernat. 1991. Chromosomal passengers: towards an integrated view of mitosis. Chromosoma. 100:139–146. [DOI] [PubMed] [Google Scholar]
- Eckley, D.M., A.M. Ainsztein, A.M. MacKay, I.G. Goldberg, and W.C. Earnshaw. 1997. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J. Cell Biol. 136:1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruki, S., R.W. Cole, and C.L. Rieder. 2002. Separating centrosomes interact in the absence of associated chromosomes during mitosis in cultured vertebrate cells. Cell Motil. Cytoskeleton. 52:107–121. [DOI] [PubMed] [Google Scholar]
- Field, C., and B.M. Alberts. 1995. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 131:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, M.T. 1993. Spermatogenesis. The Development of Drosophila melanogaster. Vol. I. M. Bate and A.M. Arias, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 71–147.
- Giansanti, M.G., S. Bonaccorsi, and M. Gatti. 1999. The role of anillin in meiotic cytokinesis of Drosophila males. J. Cell Sci. 112:2323–2334. [DOI] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M. 2001. Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 17:351–386. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and I. Vernos. 2001. The mitotic spindle: a self-made machine. Science. 294:543–547. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., M. Moudjou, M. Paintrand, L. Salisbury, and M.J. Bornens. 1996. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109:3089–3102. [DOI] [PubMed] [Google Scholar]
- Picard, A., M.C. Harricane, J.C. Labbe, and M. Doree. 1988. Germinal vesicle components are not required for the cell-cycle oscillator of the early starfish embryo. Dev. Biol. 128:121–128. [DOI] [PubMed] [Google Scholar]
- Raff, J.W., and D.M. Glover. 1989. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell. 57:611–619. [DOI] [PubMed] [Google Scholar]
- Rappaport, R. 1986. Establishment of the mechanism of cytokinesis in animal cells. Int. Rev. Cytol. 105:245–281. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., A. Khodjakov, L.V. Paliulis, T.M. Fortier, R.W. Cole, and G. Sluder. 1997. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc. Natl. Acad. Sci. USA. 94:5107–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli, M.G., G. Callaini, D.M. Glover, and M. do C. Avides. 2002. A requirement of the abnormal spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J. Cell Sci. 115:913–922. [DOI] [PubMed] [Google Scholar]
- Savoian, M.S., W.C. Earnshaw, A. Khodjakov, and C.L. Rieder. 1999. Cleavage furrows formed between centrosomes lacking an intervening spindle and chromosomes contain microtubule bundles, INCENP, and CHO1 but not CENP-E. Mol. Biol. Cell. 10:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., and T.J. Mitchison. 1991. Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol. 112:925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder, G., F.J. Miller, and C.L. Rieder. 1986. The reproduction of centrosomes: nuclear versus cytoplasmic controls. J. Cell Biol. 103:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma, M.P., B. Fasulo, G. Cenci, E. Cundari, and M. Gatti. 2002. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol. Biol. Cell. 13:2448–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, J.C., and E.D. Salmon. 1997. Pathways of spindle assembly. Curr. Opin. Cell Biol. 9:37–43. [DOI] [PubMed] [Google Scholar]
- Zhang, D., and R.B. Nicklas. 1995. Chromosome initiate spindle assembly upon experimental dissolution of the nuclear envelope in grasshopper spermatocytes. J. Cell Biol. 131:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., and R.B. Nicklas. 1996. ‘Anaphase’ and cytokinesis in the absence of chromosomes. Nature. 382:466–468. [DOI] [PubMed] [Google Scholar]