Abstract
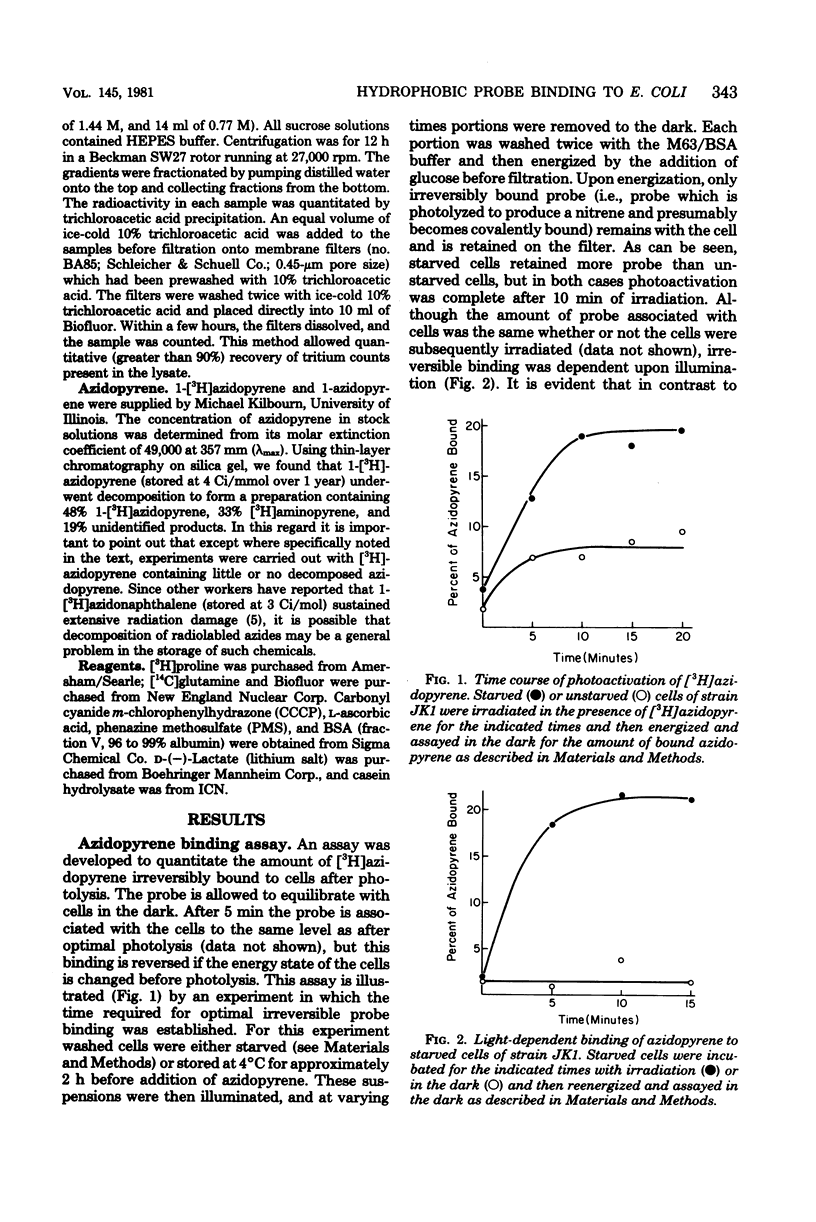
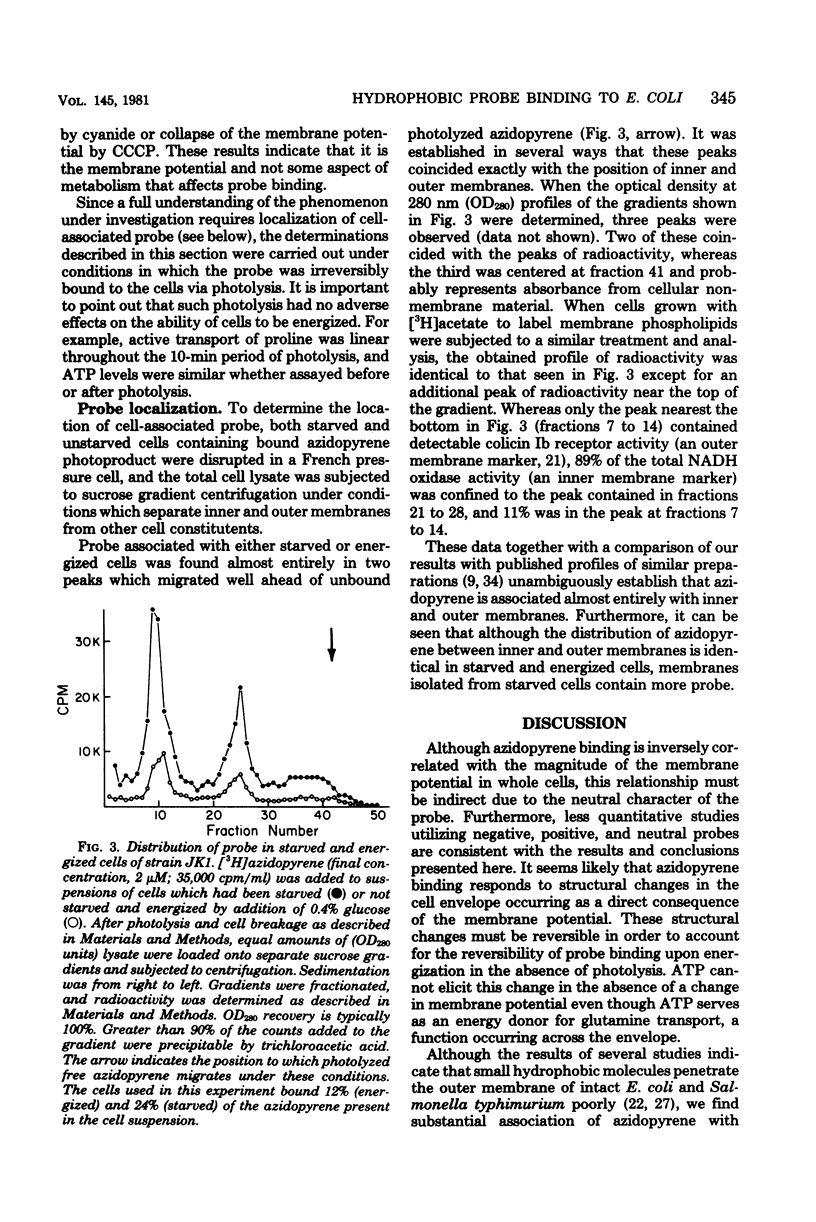
We describe conditions for a quantitative determination of azidopyrene binding to Escherichia coli cells. In addition, we define conditions whereby irradiation of azidopyrene in the presence of cells leads to irreversible association of probe with cells. This is presumably due to the light-dependent generation of reactive nitrenes and subsequent incorporation of nitrenopyrene moieties into cellular components. These methods allowed us to determine that the amount of azidopyrene bound to cells was inversely correlated with the magnitude of the cellular membrane potential, but was not correlated with high or low adenosine 5-triphosphate levels per se. Cells bound more azidopyrene if the delta psi was low. Cell-bound azidopyrene was found to be entirely associated with the inner and outer membrane. We suggest that the decreased association of hydrophobic probes upon energization of whole cells reflects a rapid transition in structural properties of the cell envelope.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. C. Probes of membrane structure. Annu Rev Biochem. 1978;47:359–383. doi: 10.1146/annurev.bi.47.070178.002043. [DOI] [PubMed] [Google Scholar]
- Azzi A. The application of fluorescent probes in membrane studies. Q Rev Biophys. 1975 May;8(2):237–316. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 1. Phenylnitrene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- Bercovici T., Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978 Apr 18;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- Conrad M. J., Singer S. J. Evidence for a large internal pressure in biological membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5202–5206. doi: 10.1073/pnas.76.10.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowlesmith I., Schindler M., Osborn M. J. Bacteriophage P22 is not a likely probe for zones of adhesion between the inner and outer membranes of Salmonella typhimurium. J Bacteriol. 1978 Jul;135(1):259–269. doi: 10.1128/jb.135.1.259-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A. M., Schaechter M. Translocation of phospholipids from the inner to the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1867–1871. doi: 10.1073/pnas.77.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galey W. R., Owen J. D., Solomon A. K. Temperature dependence of nonelectrolyte permeation across red cell membranes. J Gen Physiol. 1973 Jun;61(6):727–746. doi: 10.1085/jgp.61.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C. M., Radhakrishnan R., Gerber G. E., Olsen W. L., Quay S. C., Khorana H. G. Intermolecular crosslinking of fatty acyl chains in phospholipids: use of photoactivable carbene precursors. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2595–2599. doi: 10.1073/pnas.76.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in E. coli cell envelope structure caused by uncouplers of active transport and colicin E1. J Supramol Struct. 1976;5(3):291–308. doi: 10.1002/jss.400050304. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in Escherichia coli cell envelope structure and the sites of fluorescence probe binding caused by carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Biochemistry. 1977 Sep 6;16(18):4109–4117. doi: 10.1021/bi00637a026. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Harris J. M., Lytle F. E. Evidence for a microviscosity increase in the Escherichia coli cell envelope caused by colicin E1. Biochemistry. 1974 Jul 16;13(15):3057–3061. doi: 10.1021/bi00712a010. [DOI] [PubMed] [Google Scholar]
- Heller R. A., Klotzbücher R., Stoffel W. Interactions of a photosensitive analog of cholesterol with hydroxymethyglutaryl-CoA reductase (NADPH) and acyl-CoA:cholesterol acyltransferase. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1721–1725. doi: 10.1073/pnas.76.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu V. W., Wisnieski B. J. Photoreactive labeling of M13 coat protein in model membranes by use of a glycolipid probe. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5460–5464. doi: 10.1073/pnas.76.11.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S., Gilchrist M. J. Colicin Ia and Ib binding to Escherichia coli envelopes and partially purified cell walls. J Supramol Struct. 1973;1(3):208–219. doi: 10.1002/jss.400010306. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Acriflavine-binding capacity of Escherichia coli in relation to acriflavine sensitivity and metabolic activity. J Bacteriol. 1966 Nov;92(5):1447–1452. doi: 10.1128/jb.92.5.1447-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gomez D., Gennis R. B. Affinity of intact Escherichia coli for hydrophobic membrane probes is a function of the physiological state of the cells. Proc Natl Acad Sci U S A. 1977 May;74(5):1811–1815. doi: 10.1073/pnas.74.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gomez D., Konisky J. Membrane changes in Escherichia coli induced by colicin Ia and agents known to disrupt energy transduction. Biochemistry. 1976 Jun 29;15(13):2747–2753. doi: 10.1021/bi00658a006. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Purdy D. R., Koch A. L. Energy cost of galactoside transport to Escherichia coli. J Bacteriol. 1976 Sep;127(3):1188–1196. doi: 10.1128/jb.127.3.1188-1196.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- SILVER S. ACRIFLAVINE RESISTANCE: A BACTERIOPHAGE MUTATION AFFECTING THE UPTAKE OF DYE BY THE INFECTED BACTERIAL CELLS. Proc Natl Acad Sci U S A. 1965 Jan;53:24–30. doi: 10.1073/pnas.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Tecoma E. S., Wu D. Membrane deenergization by colicin K affects fluorescence of exogenously added but not biosynthetically esterified parinaric acid probes in Escherichia coli. J Bacteriol. 1980 Jun;142(3):931–938. doi: 10.1128/jb.142.3.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Mode of action of colicin Ia: effect of colicin on the Escherichia coli proton electrochemical gradient. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2579–2583. doi: 10.1073/pnas.75.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Helgerson S. L., Cramer W. A., Mitchell G. W. Changes in rotational motion of a cell-bound fluorophore caused by colicin E1: a study by fluorescence polarization and differential polarized phase fluorometry. Biochemistry. 1976 Oct 5;15(20):4429–4432. doi: 10.1021/bi00665a014. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor proteins of liver plasma membrane preparations. Biochemistry. 1980 Jan 8;19(1):70–76. doi: 10.1021/bi00542a011. [DOI] [PubMed] [Google Scholar]


