Abstract
A mitosis-specific Aurora-A kinase has been implicated in microtubule organization and spindle assembly in diverse organisms. However, exactly how Aurora-A controls the microtubule nucleation onto centrosomes is unknown. Here, we show that Aurora-A specifically binds to the COOH-terminal domain of a Drosophila centrosomal protein, centrosomin (CNN), which has been shown to be important for assembly of mitotic spindles and spindle poles. Aurora-A and CNN are mutually dependent for localization at spindle poles, which is required for proper targeting of γ-tubulin and other centrosomal components to the centrosome. The NH2-terminal half of CNN interacts with γ-tubulin, and induces cytoplasmic foci that can initiate microtubule nucleation in vivo and in vitro in both Drosophila and mammalian cells. These results suggest that Aurora-A regulates centrosome assembly by controlling the CNN's ability to targeting and/or anchoring γ-tubulin to the centrosome and organizing microtubule-nucleating sites via its interaction with the COOH-terminal sequence of CNN.
Keywords: centrosomes; γ-tubulin; microtubule nucleation; mitotic spindle; MTOC
Introduction
In animal cells, microtubules are organized from the centrosome/microtubule-organizing center (MTOC), composed of a pair of centrioles and the surrounding pericentriolar material. Individual microtubules are nucleated from an ∼25-nm γ-tubulin–containing ring complex (γ-TuRC; Zheng et al., 1995). At the onset of M phase, the centrosome becomes “mature” and organizes more microtubules (Kuriyama and Borisy, 1981), which is accompanied with an increased level of γ-tubulin accumulation at each spindle pole (Khodjakov and Rieder, 1999). One of the molecules that has been implicated in the mechanism of centrosome maturation is Aurora-A (Hannak et al., 2001; Berdnik and Knoblich, 2002), a mitosis-specific Ser/Thr kinase located at mitotic poles and spindle microtubules (Roghi et al., 1998). The kinase, originally identified as a gene product important in spindle assembly and function in Drosophila (Glover et al., 1995), has recently been shown to be in the Ran-signaling pathway and to play an important role in efficient transmission of Ran-GTP gradient established by the condensed chromosomes for the control of spindle assembly and dynamics (Tsai et al., 2003). Aurora-A binds to spindle components, such as TACC/XMAP215 (Giet et al., 2002) and TPX2 (Kufer et al., 2002; Eyers et al., 2003). Although possible functions of those molecules and their interaction with Aurora-A in bipolar spindle formation have been elucidated, mechanisms of how Aurora-A stimulates the recruitment of γ-tubulin to the centrosome at spindle poles have not yet been evaluated. To address this question, we sought centrosomal proteins that interact with Aurora-A and regulate the process of microtubule nucleation onto the centrosome.
Results and discussion
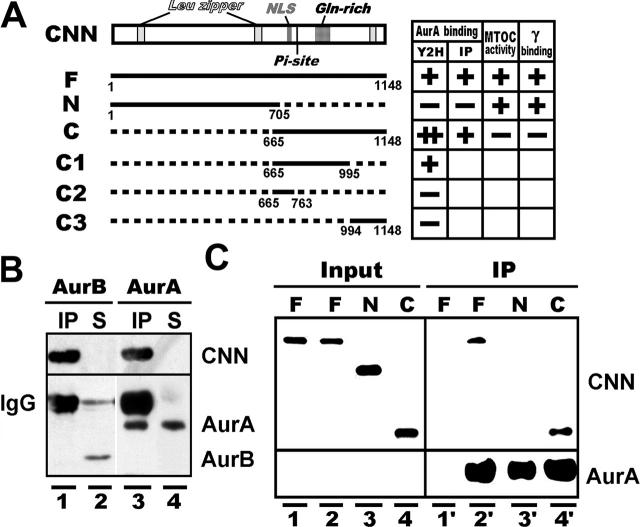
By screening of a Drosophila two-hybrid library, we isolated two clones encoding a molecule capable of interaction with Aurora-A. The sequence corresponds to the COOH-terminal domain of centrosomin (CNN; Fig. 1 A, clone C), a core component of the centrosome important for assembly of mitotic centrosomes in Drosophila (Heuer et al., 1995; Megraw et al., 1999). Although the truncated polypeptide covered by clone CNN-C1 appears to be sufficient for interaction with Aurora-A, the binding intensity was weaker than CNN-C. Fig. 1 B demonstrates that endogenous Aurora-A (Fig. 1 B, lanes 3 and 4), but not Aurora-B (Fig. 1 B, lanes 1 and 2), was immunoprecipitated with HA-tagged CNN expressed in S2 cells. Specificity of the COOH-terminal domain of CNN for interaction with Aurora-A was further confirmed by in vitro binding assays as summarized in Fig. 1 C.
Figure 1.
Interaction of Aurora-A with CNN and γ-tubulin. (A) A map of CNN and its deletion constructs capable of association with Aurora-A assayed by yeast two-hybrid screening (Y2H) and immunoprecipitation (IP). Microtubule-nucleating activity (MTOC activity) and γ-tubulin interaction (γ binding) are also summarized on the right. CNN contains characteristic regions, including leucine zipper motifs (Leu zipper), a potential nuclear localization signal (NLS), a putative Aurora-A phosphorylation site (Pi-site), and a glutamine-enriched region (Gln-rich). Numbers indicate the positions of amino acids. (B) HA-tagged CNN binds to Aurora-A (lanes 3 and 4), but not Aurora-B (lanes 1 and 2), in S2 cells. Proteins in immunoprecipitated (IP) and nonprecipitated supernatant (S) fractions, prepared from HA-CNN–expressing (lanes 1 and 3) and –nonexpresssing (lanes 2 and 4) cells, were identified by blotting with HA, Aurora-A, and Aurora-B antibodies. (C) Aurora-A binds to the COOH-terminal domain of CNN. In vitro synthesized full (F), NH2-terminal (N), and COOH-terminal (C) domains of CNN (Input, lanes 1 to 4) were mixed with (IP, lanes 2′ to 4′) and without (IP, lane 1′) His-tagged Aurora-A purified from bacteria.
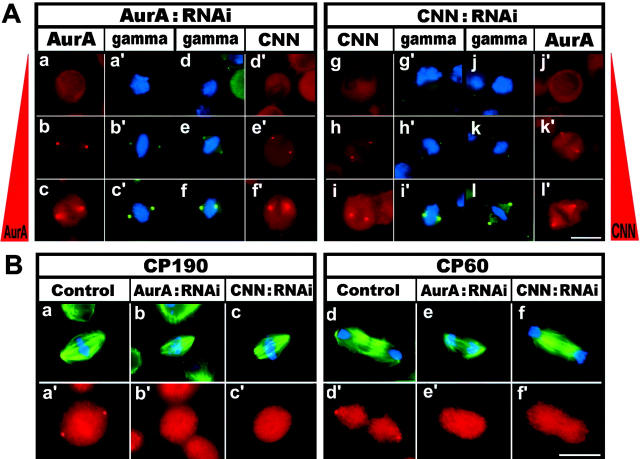
To investigate the role of protein interaction in the centrosome, we prepared S2 cells from which Aurora-A or CNN was depleted by RNA interference (RNAi; Fig. 2 A). In cells lacking Aurora-A (a), not only CNN (d′), but also γ-tubulin (a′ and d), were absent at each spindle pole, which agrees with a previous report (Berdnik and Knoblich, 2002). When CNN was depleted (g), neither γ-tubulin (g′ and j; Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999) nor Aurora-A (j′) was seen at the spindle pole. In cells with partially depleted Aurora-A (b) or CNN (h), we detected comparable amounts of γ-tubulin (b′, e, h′, and k) and CNN (e′) or Aurora-A (k′) at each pole. Fig. 2 B demonstrates that, besides γ-tubulin, other centrosome proteins, CP190 (a–c) and CP60 (d–f), became dislocated from the spindle poles in RNAi cells. Therefore, we concluded that CNN and Aurora-A are mutually dependent for localization at spindle poles, which is required for proper targeting of other centrosomal proteins to the centrosome. This is consistent with previous observations that the centrosomal association of CNN is not dependent on the presence of γ-tubulin/γ-TuRC (Barbosa et al., 2000).
Figure 2.
Immunolocalization of centrosome proteins at the spindle pole. (A) Double staining of mitotic S2 cells with Aurora-A/γ-tubulin and CNN/γ-tubulin after depletion of either Aurora-A (a–f) or CNN (g–l) by RNAi. DAPI staining is also shown in cells labeled with γ-tubulin (gamma). Different amounts of Aurora-A and CNN remained at the pole in depleted cells. Aurora-A and CNN are dependent on one another to localize at the spindle pole, and two proteins are required for recruiting γ-tubulin to the centrosome. (B) Localization of CP190 (a′–c′) and CP60 (d′–f′) in control (a and d), Aurora-A–depleted (b and e), and CNN-depleted (c and f) cells. The cells were also stained with α-tubulin and DAPI (a–f). Although depletion of Aurora-A and CNN predominantly induced abnormal spindles in monopolar/multipolar organization, spindles in bipolar orientation were selected to demonstrate the absence of centrosomal proteins at each pole. Bars, 10 μm.
To confirm the role of CNN in recruiting γ-tubulin, we analyzed protein interaction in vitro. In Fig. 3 A (lanes 1–4), nickel beads conjugated with His-tagged CNN were mixed with cell extracts prepared from colcemid-treated S2 cells. γ-Tubulin was specifically sedimented by the full (Fig. 3 A, lane 2) and NH2-terminal (Fig. 3 A, lane 3) sequence, but not the COOH-terminal sequence (Fig. 3 A, lane 4) of CNN. Because neither in vitro binding assays nor two-hybrid screens demonstrated direct binding between two molecules (unpublished data), CNN may interact with a γ-tubulin complex, rather than γ-tubulin directly (Barbosa et al., 2000). Further, we expressed HA-tagged CNN in S2 cells. As shown in Fig. 3 (B–B′′), exogenous proteins caused formation of γ-tubulin–containing cytoplasmic aggregates capable of microtubule formation and association with microtubule asters. These results clearly indicate that the NH2-terminal domain of CNN interacts with γ-tubulin/γ-TuRC and plays an important role in assembly of MTOCs.
Figure 3.
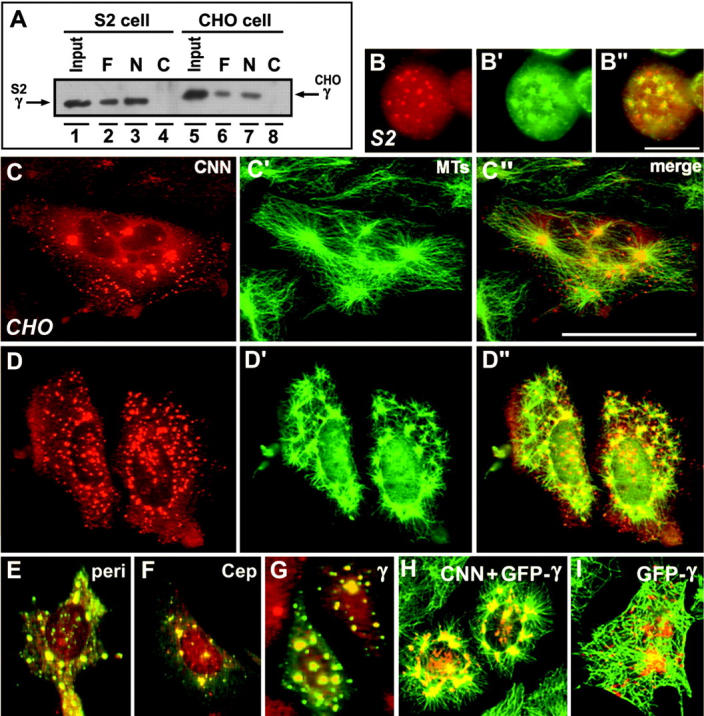
CNN interacts with γ-tubulin and induces microtubule-nucleating sites. (A) The NH2-terminal domain of CNN binds to γ-tubulin. Nickel beads conjugated with His-tagged full (F), NH2-terminal (N), and COOH-terminal (C) domains of CNN were incubated with cell extracts prepared from mitotic S2 (lanes 2–4) and CHO cells (lanes 6–8). Endogenous γ-tubulin is shown in lanes 1 and 5. (B–D) Immunostaining of HA-CNN–expressing S2 (B) and CHO cells (C–D) with HA (B–D) and α-tubulin antibodies (B′–D′). Merged images were shown in B′′–D′′. CNN expression caused the formation of cytoplasmic aggregates associated with microtubule asters. In D–D′′, cells were briefly recovered from nocodazole treatment before fixation. (E–G) Colocalization of pericentrin (E), Cep135 (F), and γ-tubulin (G) at the cytoplasmic foci induced by CNN expression. Merged images of CNN (green) and other centrosomal proteins (red) were shown. (H–I) Induction of GFP-tagged γ-tubulin in CHO cells coexpressing (H) or not coexpressing HA-CNN (I). To merge images, GFP was converted to red in double-stained cells with microtubules (green) and HA (green). Although expression of γ-tubulin alone induced cytoplasmic dots, microtubule-nucleating activity of the aggregates was detected only when γ-tubulin was coexpressed with CNN. Bars, 10 μm (B) and 50 μm (C–I).
γ-Tubulin/γ-TuRC–mediated microtubule assembly is believed to be common among species. Thus, it is highly likely that an Aurora-A–binding molecule(s) equivalent to CNN is functioning in a variety of organisms. Although Drosophila CNN was unable to associate with mammalian Aurora-A in transfected mammalian cells (Fig. S1 and Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200305048/DC1) as well as by two-hybrid screens (unpublished data), the NH2-terminal domain of CNN could still interact with γ-tubulin/γ-TuRC in mammalian cells as in S2 cells (Fig. 3 A, lanes 5–8). To analyze a possible role of CNN–γ-tubulin interaction in initiation of microtubule assembly, we thus overexpressed Drosophila CNN in mammalian cells. HA-tagged CNN induced cytoplasmic foci in various sizes and numbers (Fig. 3 C). Significantly, the pattern of microtubule distribution was profoundly affected as a result of microtubule association with virtually every dot containing CNN (Fig. 3, C′–C′′). These sites can initiate microtubule formation as evidently shown in cells where short microtubules were assembled during brief recovery from nocodazole treatment (Fig. 3, D–D′′). All cells overexpressing CNN induced microtubule-organizing sites, which were associated with centrosome proteins, such as pericentrin (Fig. 3 E) and Cep135 (Fig. 3 F; Ohta et al., 2002). Particularly prominent was γ-tubulin, which was probably recruited from a large cytoplasmic pool (Fig. 3 G). In support of this view, we found that GFP-tagged exogenous γ-tubulin became colocalized with HA-CNN to participate in the formation of microtubule-nucleating sites (Fig. 3 H). This was in striking contrast with cells expressing γ-tubulin alone, where cytoplasmic aggregates induced by γ-tubulin expression could not contribute to microtubule formation (Fig. 3 I; Kofron et al., 1998). These results suggest that microtubules are directly nucleated from the CNN aggregates through the mechanism mediated by γ-tubulin/γ-TuRC.
To confirm the microtubule-nucleating activity of the CNN aggregates, we polymerized microtubules in vitro by incubating isolated GFP-tagged CNN dots with X-rhodamine–conjugated brain tubulin. Fig. 4 (A–D) shows microtubule asters detected by phase-contrast and fluorescence microscopy. There is always a dot positive in GFP fluorescence at the center of the microtubule asters. Although variable numbers of microtubules emanated from the center, more microtubules tended to polymerize onto the GFP dots in larger sizes (Fig. 4, B–D). In Fig. 4 E, the process of aster formation was monitored by time-lapse microscopy. A fluorescence image taken 10 min after mounting the sample on a microscopic stage revealed several microtubules growing from a GFP-positive site. As time progressed, more microtubules appeared to emanate from the center, indicating that microtubules were formed by direct polymerization onto the CNN-containing foci, rather than that preformed microtubules were gathered around the center.
Figure 4.
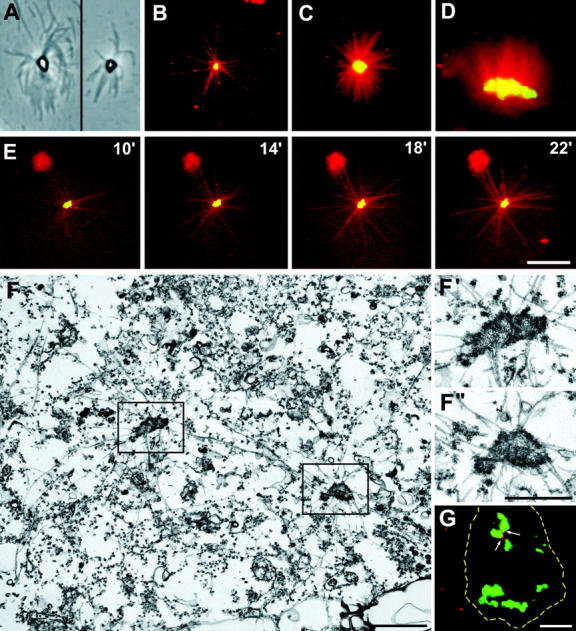
Cytoplasmic aggregates induced by CNN overexpression in mammalian cells. (A–D) In vitro microtubule nucleation onto CNN-containing sites detected by phase-contrast (A) and fluorescence (B–D) microscopy. GFP-tagged CNN aggregates were fractionated from CHO cells and incubated with X-rhodamine–conjugated brain tubulin. CNN aggregates in different sizes and shapes nucleated various numbers of microtubules. (E) Time-lapse images of GFP-tagged CNN-containing aggregates with assembled microtubules. CNN aggregates mixed with X-rhodamine tubulin were placed on a microscopic stage at time zero, and fluorescence images were taken at indicated times after the temperature was shifted to 37°C. (F) Thin-section EM of CHO cells expressing GFP-tagged CNN. The cells were briefly extracted with a detergent containing microtubule-stabilizing buffer before fixation. Two microtubule asters are seen in the field, and there is an electron-dense particle of different shape at each center. F′ and F′′ are close-ups of the areas outlined in F. (G) Immunofluorescence staining of 293 cells with anti-human centrin-2 antibodies (red). Dotted lines indicate the outline of a cell expressing CNN aggregates (green). There are two centrioles (arrows) that were not included in all sites induced by CNN overexpression. Bars, 10 μm (A–E, and G) and 1 μm (F–F′′).
Microtubules are nucleated from the pericentriolar material that surrounds the centrioles of the centrosome. To compare ultrastructure of microtubule-initiating sites induced by CNN with that of the pericentriolar material/centrosome, we examined CHO cells expressing GFP-tagged CNN by EM. Fig. 4 F shows the presence of two microtubule asters formed in a cell that were briefly extracted before fixation for better visualization of microtubules and microtubule asters. Located at each focal point of microtubule asters is an electron-dense particle in various sizes and shapes (Fig. 4, F′–F′′). Unlike the pericentriolar material, which has been described as an ill-defined amorphous cloud, the entire structure induced by CNN was well delineated by electron-dense materials to which microtubules were attached. In favorable sections, we could see microtubules penetrating to the interior region of the aggregates. Neither centrioles nor centrosomal substructures, such as satellites, appendages, and CHO cell–specific virus particles (Gould and Borisy, 1977), were generally seen at the site induced by CNN expression. Because CNN is a coiled-coil structural protein (Heuer et al., 1995), the dense particles likely represent the aggregated form of overexpressed CNN proteins.
Multiple centrosomes/MTOCs have been detected in cells in which the mechanism of centrosome duplication coupled with the cell cycle control becomes deregulated (Hinchcliffe and Sluder, 2001). In the case of CNN-containing MTOCs, their number and size formed during relatively short periods (8–12 h) varied greatly according to the level of protein expression. Moreover, no centrioles were found at ectopic MTOCs by EM (Fig. 4 F) and immunostaining with centriole-specific centrin-2 antibodies (Fig. 4 G, arrows). Therefore, it is plausible that CNN expression causes the formation of protein aggregates that acquire the microtubule-nucleating capacity by recruiting γ-tubulin/γ-TuRC. This unique property of CNN to generate microtubule-nucleating sites by interacting with γ-tubulin/γ-TuRC allowed us to speculate that CNN may function as an adaptor for connecting γ-tubulin to the centrosome.
By expressing truncated polypeptides, it was concluded that CNN's ability to interact with γ-tubulin/γ-TuRC and induce ectopic microtubule-nucleating sites resides in the NH2-terminal sequence of CNN from which the Aurora-A–binding domain is omitted. In contrast, cytoplasmic aggregates formed in cells expressing the COOH-terminal domain failed to initiate microtubule formation in both S2 and mammalian cells (unpublished data). These results lead us to conclude that CNN consists of two functionally distinct subdomains: the Aurora-A–binding site is at the COOH terminus capable of formation of the protein complex to be recruited to the spindle pole, and the NH2-terminal sequence is involved in assembling centrosomes/MTOCs by recruiting γ-tubulin/γ-TuRC. Although no CNN homologues have yet been identified outside Drosophila, Aurora-A would likely be involved in the control of microtubule nucleation through its association with the COOH terminus of a CNN-related molecule(s) in mammalian cells.
Control of mitotic spindle assembly onto the centrosome could be achieved by several mechanisms, including nucleation of individual microtubules onto γ-tubulin–containing protein complexes (Zheng et al., 1995), stimulation of microtubule nucleation and stabilization of polymerized microtubules by MAPs (Popov et al., 2002), and recruitment of minus ends of preexisting microtubules by the action of motor activity to the centrosome (Heald et al., 1997). Aurora-A binds not only CNN but also the D-TACC/MSPS/XMAP215 complex (Giet et al., 2002) and a spindle component of TPX2 (Kufer et al., 2002). These components appear to be required for microtubule assembly on mitotic centrosomes/poles controlled through the distinct mechanisms from that of γ-tubulin recruitment. Therefore, it is reasonable that Aurora-A plays a role in regulating the overall process of centrosome maturation by orchestrating multiple pathways of microtubule assembly during mitosis. It is worth mentioning that individual mechanisms of microtubule assembly may show a distinct requirement for protein phosphorylation and the Aurora-A kinase activity; although both Aurora-A and CNN were still able to locate at the centrosome, D-TACC/MSPS complex failed to be recruited to spindle poles in the absence of enzymatic activity of Aurora-A kinase (Giet et al., 2002).
Aurora kinases are highly expressed in cells derived from many human tumor cell types (Bischoff et al., 1998; Tatsuka et al., 1998; Zhou et al., 1998), which frequently contain multiple centrosomes. Because defects in the number, structures, and function of centrosomes are closely associated with the genetic instability in transformed cells (Nigg, 2002), Aurora-A might be involved in tumorigenesis by inducing abnormal numbers of MTOCs as a result of inappropriate distribution of CNN-like molecule(s).
Materials and methods
Yeast two-hybrid screen
Full-length Drosophila Aurora-A was fused in-frame to the GAL4 DNA-binding domain using the pGBT vector (CLONTECH Laboratories, Inc.). The resulting “bait” plasmid was used to screen a pACT2-Drosophila embryo library (CLONTECH Laboratories, Inc.) by the yeast two-hybrid method in Y190 cells.
RNAi in S2 cells
cDNAs of Aurora-A and CNN were amplified by PCR using primers as follows: Aurora-A sense, 5′-TAATACGACTCACTATAGGATGTCCCATCCGTCTGACCA-3′ and antisense 5′-TAATACGACTCACTATAGGAGTTGTTGAGCTCCCAGGTC-3′; CNN sense, 5′-TAATACGACTCACTATAGGGAAACAGCTGTTCCAGCGCGCTTCATTTTC-3′ and antisense 5′-TAATACG-ACTCACTATAGGCACCAGTTCCTCCTCCAATCTCTGCACCTC-3′. dsRNAs were synthesized using the MEGAscript™ T7 transcription kit (Ambion), denatured for 20 min at 94°C, and were then annealed at RT overnight. dsRNA samples were run on 1% agarose gels to ensure that they migrated as a single band.
For RNAi, S2-R cells (a gift from Dr. F. Kafatos, Harvard University, Cambridge, MA) were transferred into 6-well plates 1 d before transfection. This particular cell line appears to yield high efficiency of protein depletion by RNAi. 20 μg dsRNA was incubated with 40 μl of 4 mg/ml dimethyl dioctadecyl ammonium bromide and 80 μl M3 insect medium (Sigma-Aldrich). After transfection, cells were incubated for 3 or 4 d before fixation.
Transfection and immunofluorescence staining
The entire coding sequences of Aurora-A and CNN were cloned by PCR from a Drosophila embryo cDNA library (a gift from Dr. F. Kafatos) and ligated into expression vectors of pcDNA3 (Invitrogen) and pFBDA (a gift from Dr. A. Straight, Harvard Medical School, Boston, MA), respectively. 1.5 μg purified plasmid DNA was mixed with FuGENE™ 6 (Roche Diagnostics), and added to S2 cells in a 3.5-cm dish cultured in 10% FCS containing Schneider's Drosophila medium. Cells were further cultured up to 3 d before fixing with cold methanol. CHO and 293 cells were grown as monolayers and transfected with CNN/γ-tubulin constructs as described before (Kofron et al., 1998; Ohta et al., 2002).
For immunostaining, fixed cells were rehydrated with 0.05% Tween 20 containing PBS, and were incubated at 37°C for 30 min with primary antibodies as follows: polyclonal anti-centrosomin (a gift from Dr. W. Theurkauf, University of Massachusetts Medical School, Worcester, MA), polyclonal anti-Drosophila Aurora-A (a gift from Dr. J. Knoblich, Research Institute of Molecular Pathology, Vienna, Austria), polyclonal anti–human Aurora-A (a gift from Dr. M. Kimura, Gifu University, Gifu, Japan), monoclonal anti-α-tubulin (Sigma-Aldrich), monoclonal anti-γ-tubulin (Sigma-Aldrich), monoclonal rat anti-HA (Roche Diagnostics), and polyclonal anti-HA (Santa Cruz Biotechnology, Inc.). Antibodies specific to CP60, CP190 (gifts from Dr. M. Moritz, University of California, San Francisco, San Francisco, CA), pericentrin (Covalence), human centrin-2 (a gift from Dr. J. Salisbury, Mayo Clinic Foundation, Rochester, MN), and Cep135 (Ohta et al., 2002) were used to probe additional centrosomal components.
Protein-binding assays
S2 cells expressing HA-tagged CNN were lysed in TBSN (20 mM Tris-HCl at pH 8.0, 150 mM NaCl, 1.5 mM EDTA, 5 mM EGTA, and 0.5% Nonidet P-40) containing protease inhibitors. The cytosolic fraction recovered after centrifugation at 10,000 g for 20 min was incubated with polyclonal anti-HA antibody for 1 h at 4°C followed by incubation with Protein A–conjugated Sepharose beads for 3 h. Beads were subsequently washed three times with TBSN, and boiled in an SDS-containing sample buffer. Endogenous kinases were detected by immunoblot analysis using monospecific anti-Aurora-A and anti-Aurora-B antibodies (Aurora-B antibody was a gift from Dr. M. Carmena, Wellcome Center for Cell Biology, Edinburgh, UK).
For identification of the Aurora-A–binding domain of CNN, 35S-labeled full (F), NH2-terminal (N), and COOH-terminal (C) sequences of CNN were synthesized in vitro using a TNT T7/T3-coupled reticulocyte lysates system kit (Promega). The full-length Drosophila Aurora-A was subcloned into pREST B (CLONTECH Laboratories, Inc.), and T7/His6-tagged protein was expressed in the Escherichia coli strain BL21DE3 (Novagen) at 30°C for 1 h. After purification through nickel Sepharose beads according to the manufacturer's instructions, Aurora-A fusion proteins were mixed with HA-tagged CNN polypeptides in a buffer (50 mM Hepes at pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.1% Tween 20, and protease inhibitors), and were further incubated overnight at 4°C in the presence of anti-T7 antibodies (Novagen).
To assay the γ-tubulin–binding activity of CNN, His-tagged CNN fusion proteins corresponding to F, N, and C in Fig. 1 A were first prepared in bacteria. After conjugation with nickel agarose, beads were incubated with extracts of mitotic S2 and CHO cells prepared in TBSN as above. γ-Tubulin sedimented with nickel beads was detected by immunoblot analysis.
In vitro microtubule nucleation
CHO cells expressing full-length GFP-CNN were lysed in a medium containing 2 mM Pipes, pH 6.8, and 0.25% Triton X-100, and centrifuged at 1,500 rpm for 2 min. CNN-containing aggregates recovered in a post-nuclear fraction were sedimented on a sucrose cushion at 10,000 g for 20 min and resuspended in PEMG (100 mM Pipes, pH 6.8, 1 mM EGTA, 1 mM MgCl2, and 1 mM GTP). Next, the sample was mixed with purified bovine brain tubulin supplemented with X-rhodamine–conjugated tubulin (Sammak and Borisy, 1988), and microtubule polymerization was monitored by fluorescence microscopy as described previously (Tournebize et al., 1997). Microtubule formation was also assayed by phase-contrast microscopy using the procedure described previously (Sellitto and Kuriyama, 1988).
EM
Transfected CHO cells were cultured for 8–12 h to express GFP-tagged full-length CNN. After incubation with 2 μg/ml nocodazole for 2 h, cells were briefly recovered from drug treatment, and were extracted in PEMG containing 0.1% Triton X-100 and 10% glycerol for 30 s at 37°C before fixation with 2% glutaraldehyde. Positions of GFP-positive cells were identified by fluorescence microscopy, and the sample was postfixed with 1% OsO4, embedded in Epon-Araldite according to the standard procedures.
Online supplemental material
Fig. S1 shows that mammalian Aurora-A (Aik; arrows) does not colocalize with the full-coding (CNN-F), COOH-terminal (CNN-C), and NH2-terminal (CNN-N) sequence of Drosophila CNN expressed in mammalian U-2OS cells. In Fig. S2, CNN-F was expressed in human cells from which Aik was depleted by RNAi. After monitoring immunostaining pattern of Aik and CNN, the same cells were further stained with anti-α-tubulin antibody. The CNN aggregates retained their capacity to nucleate microtubules in vivo in the absence of mammalian Aurora-A. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200305048/DC1.
Supplemental Material
Acknowledgments
We would like to thank Drs. O. Shimmi and M. O'Connor (University of Minnesota, Minneapolis, MN) for providing us with valuable Drosophila reagents. Thanks are also due to Drs. M. Camena, F. Kafatos, M. Kimura, J. Knoblich, M. Moritz, J. Salisbury, A. Straight, and W. Theurkauf for the experimental reagents.
This work was supported by grants from the National Institutes of Health (GM55735) and the Minnesota Medical Foundation to R. Kuriyama, and from the Uehara Memorial Foundation to Y. Terada.
The online version of this article includes supplemental material.
Abbreviations used in this paper: CNN, centrosomin; γ-TuRC, γ-tubulin–containing ring complex; MTOC, microtubule-organizing center; RNAi, RNA interference.
References
- Barbosa, V., R.R. Yamamoto, D.S. Henderson, and D.M. Glover. 2000. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14:3126–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik, D., and J.A. Knoblich. 2002. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 12:640–647. [DOI] [PubMed] [Google Scholar]
- Bischoff, J.R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginthe, et al. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers, P.A., E. Erikson, L.G. Chen, and J.L. Maller. 2003. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13:691–697. [DOI] [PubMed] [Google Scholar]
- Giet, R., D. McLean, S. Descamps, M.J. Lee, J.W. Raff, C. Prigent, and D.M. Glover. 2002. Drosophila Aurora A kinase is required to localize D-TACC to centrosome and to regulate astral microtubules. J. Cell Biol. 156:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, D.M., M.H. Leibowitz, D.A. McLean, and H. Parry. 1995. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 81:95–105. [DOI] [PubMed] [Google Scholar]
- Gould, R.R., and G.G. Borisy. 1977. The pericentriolar material in Chinese hamster ovary cells nucleated microtubule formation. J. Cell Biol. 73:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., M. Kirkham, A.A. Hyman, and K. Oegema. 2001. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155:1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald, R., R. Tournebize, A. Habermann, E. Karsenti, and A. Hyman. 1997. A. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, J.G., K. Li, and T.C. Kaufman. 1995. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development. 121:3861–3876. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E.H., and G. Sluder. 2001. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 15:1167–1181. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and C.L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron, M., E. Nadezdina, A. Vassilev, J. Matuliene, R. Essner, J. Kato, and R. Kuriyama. 1998. Interaction of an overexpressed γ-tubulin with microtubules in vivo and in vitro. Zool. Sci. 15:477–487. [DOI] [PubMed] [Google Scholar]
- Kufer, T.A., H.H. Sillje, R. Korner, O.J. Gruss, P. Meraldi, and E.A. Nigg. 2002. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama, R., and G.G. Borisy. 1981. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 91:822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw, T.L., K. Li, L.-R. Kao, and T.C. Kaufman. 1999. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 126:2829–2839. [DOI] [PubMed] [Google Scholar]
- Nigg, E.A. 2002. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2:815–825. [DOI] [PubMed] [Google Scholar]
- Ohta, T., R. Essner, J.-H. Ryu, R.E. Palazzo, Y. Uetake, and R. Kuriyama. 2002. Characterization of Cep135, a novel centrosomal protein involved in microtubule organization in mammalian cells. J. Cell Biol. 156:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, A.V., F. Severin, and E. Karsenti. 2002. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 12:1326–1330. [DOI] [PubMed] [Google Scholar]
- Roghi, C., R. Giet, R. Uzbekov, N. Morin, I. Chartrain, R. Le Guellec, A. Couturier, M. Doree, M. Philippe, and C. Prigent. 1998. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111:557–572. [DOI] [PubMed] [Google Scholar]
- Sammak, P.J., and G.G. Borisy. 1988. Detection of single fluorescent microtubules and methods for determining their dynamics in living cells. Cell Motil. Cytoskeleton. 10:237–245. [DOI] [PubMed] [Google Scholar]
- Sellitto, C., and R. Kuriyama. 1988. Distribution of pericentriolar material in multiple spindles induced by colcemid treatment in Chinese hamster ovary cells. J. Cell Sci. 89:57–65. [DOI] [PubMed] [Google Scholar]
- Tatsuka, M., H. Katayama, T. Ota, T. Tanaka, S. Odashima, F. Suzuki, and Y. Terada. 1998. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 58:4811–4816. [PubMed] [Google Scholar]
- Tournebize, R., S.S. Andersen, F. Verde, M. Doree, E. Karsenti, and A.A. Hyman. 1997. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 16:5537–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M.Y., C. Wiese, K. Cao, O. Martin, P. Donovan, J. Ruderman, C. Prigent, and Y. Zheng. 2003. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5:242–248. [DOI] [PubMed] [Google Scholar]
- Vaizel-Ohayon, D., and E.D. Schejter. 1999. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 9:889–898. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., M.L. Wong, B. Alberts, and T. Mitchison. 1995. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 378:578–583. [DOI] [PubMed] [Google Scholar]
- Zhou, H., J. Kuang, L. Zhong, W.L. Kuo, J.W. Gray, A. Sahin, B.R. Brinkley, and S. Sen. 1998. Tumour amplified kinase ATK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20:189–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.