Abstract
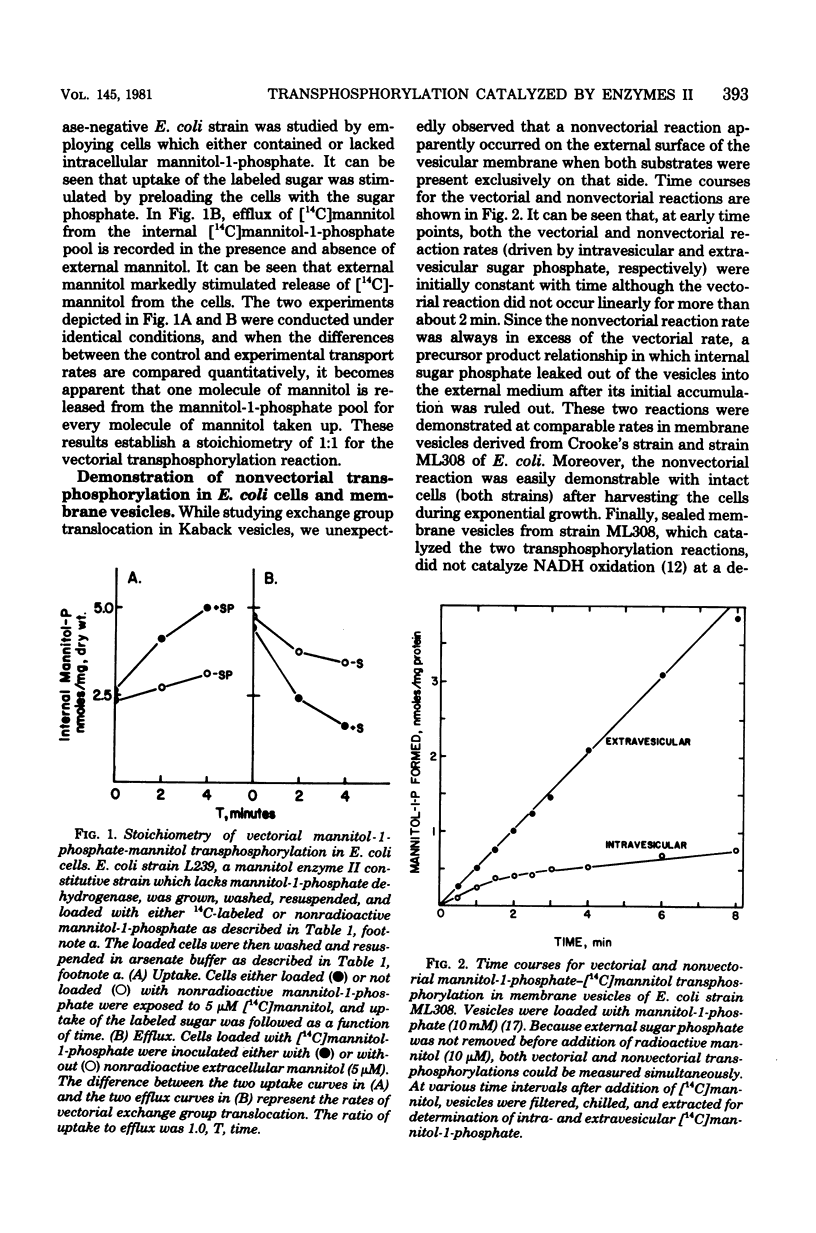
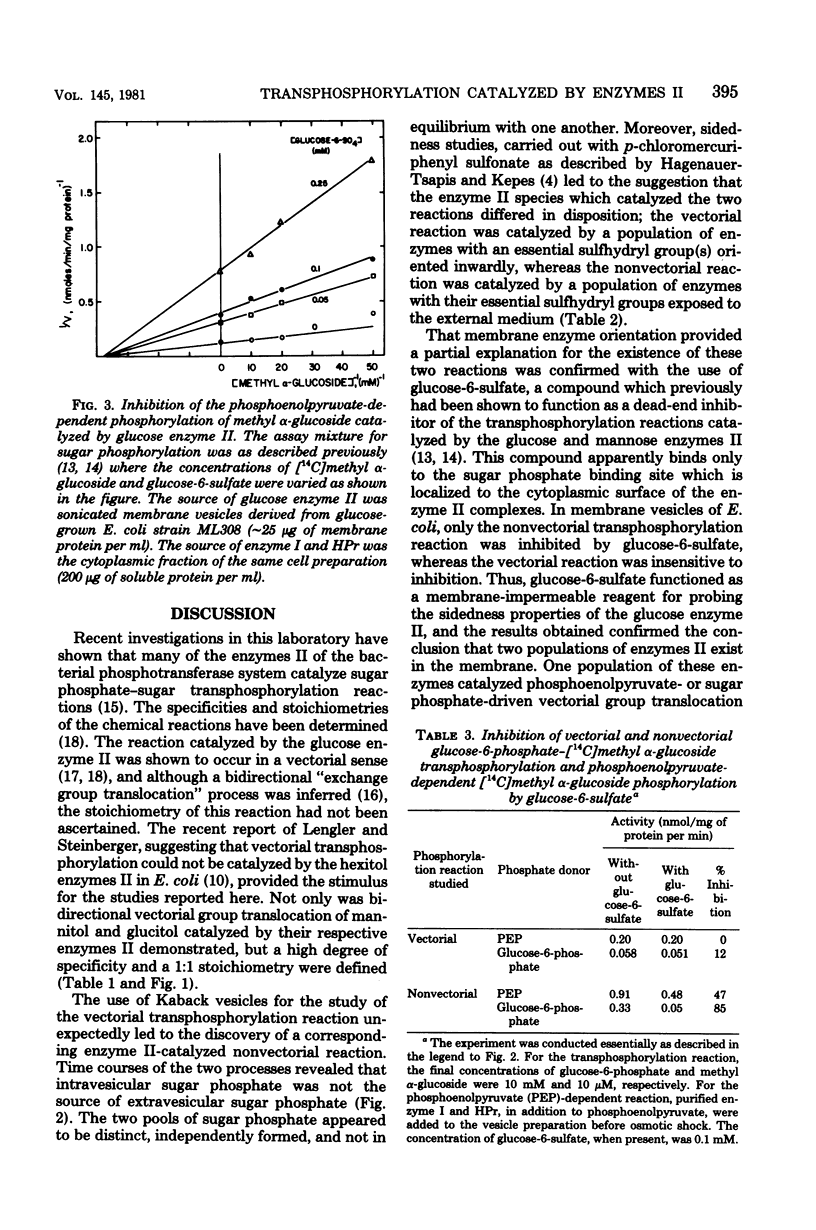
Vectorial transphosphorylation of hexitols, catalyzed by enzymes II of the bacterial phosphotransferase system, was studied in intact cells and membrane vesicles of Escherichia coli. In strains depleted of phosphoenolpyruvate and unable to metabolize the internal hexitol phosphate, internal mannitol-1-phosphate stimulated uptake of extracellular [14C]mannitol, whereas external mannitol stimulated release of [14C]mannitol from the intracellular [14C]mannitol-1-phosphate pool. The stoichiometry of mannitol uptake to mannitol release was 1:1. Glucitol did not promote release of [14C]mannitol from the mannitol phosphate pool but stimulated release of [14C]glucitol from internal glucitol phosphate pools when the glucitol enzyme II was induced to high levels. In E coli cells and membrane vesicles, both vectorial and nonvectorial transphosphorylation reactions of hexitols and hexoses were demonstrated. The nonvectorial reactions, but not the vectorial reactions, catalyzed by the mannitol and glucose enzymes II, were inhibited by p-chloromercuriphenyl sulfonate, a membrane-impermeable sulfhydryl reagent which inactivates enzymes II. Similarly, glucose-6-sulfate, an inhibitor of the glucose enzyme II-catalyzed transphosphorylation reaction, specifically inhibited the nonvectorial reaction. This compound was shown to be a noncompetitive inhibitor of methyl alpha-glucoside phosphorylation employing phospho-HPr as the phosphate donor. It apparently exerts its inhibitory effect by exclusive binding to the sugar phosphate binding site on the enzyme II complex. The results are consistent with the conclusion that enzymes II can exist in two distinct dispositions in the membrane, one of which catalyzes vectorial transphosphorylation, and the other catalyzes nonvectorial transphosphorylation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L. W., Rosen B. P. Functional mosaicism of membrane proteins in vesicles of Escherichia coli. J Bacteriol. 1977 Feb;129(2):959–966. doi: 10.1128/jb.129.2.959-966.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Different sidedness of functionally homologous essential thiols in two membrane-bound phosphotransferase enzymes of Escherichia coli detected by permeant and nonpermeant thiol reagents. J Biol Chem. 1980 Jun 10;255(11):5075–5081. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Saier M. H., Jr Purification of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1979 Jan 25;254(2):249–252. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Lengeler J., Steinberger H. Analysis of regulatory mechanisms controlling the activity of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978 Nov 16;167(1):75–82. doi: 10.1007/BF00270323. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Kinetic analyses of the sugar phosphate:sugar transphosphorylation reaction catalyzed by the glucose enzyme II complex of the bacterial phosphotransferase system. J Biol Chem. 1978 Nov 10;253(21):7595–7597. [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Substrate specificity and kinetic characterization of sugar uptake and phosphorylation, catalyzed by the mannose enzyme II of the phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1980 Sep 25;255(18):8585–8591. [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Moczydlowski E. G. Sugar phosphate:sugar transphosphorylation coupled to exchange group translocation catalyzed by the enzyme II complexes of the phosphoenolpyruvate:sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):8908–8916. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kaczorowski G., Fisher J., Walsh C. T., Silverstein S. C. Determination of the absolute number of Escherichia coli membrane vesicles that catalyze active transport. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5032–5036. doi: 10.1073/pnas.71.12.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Meadow N. D., Roseman S. Modified assay procedures for the phosphotransferase system in enteric bacteria. Anal Biochem. 1979 May;95(1):293–304. doi: 10.1016/0003-2697(79)90219-7. [DOI] [PubMed] [Google Scholar]



