Abstract
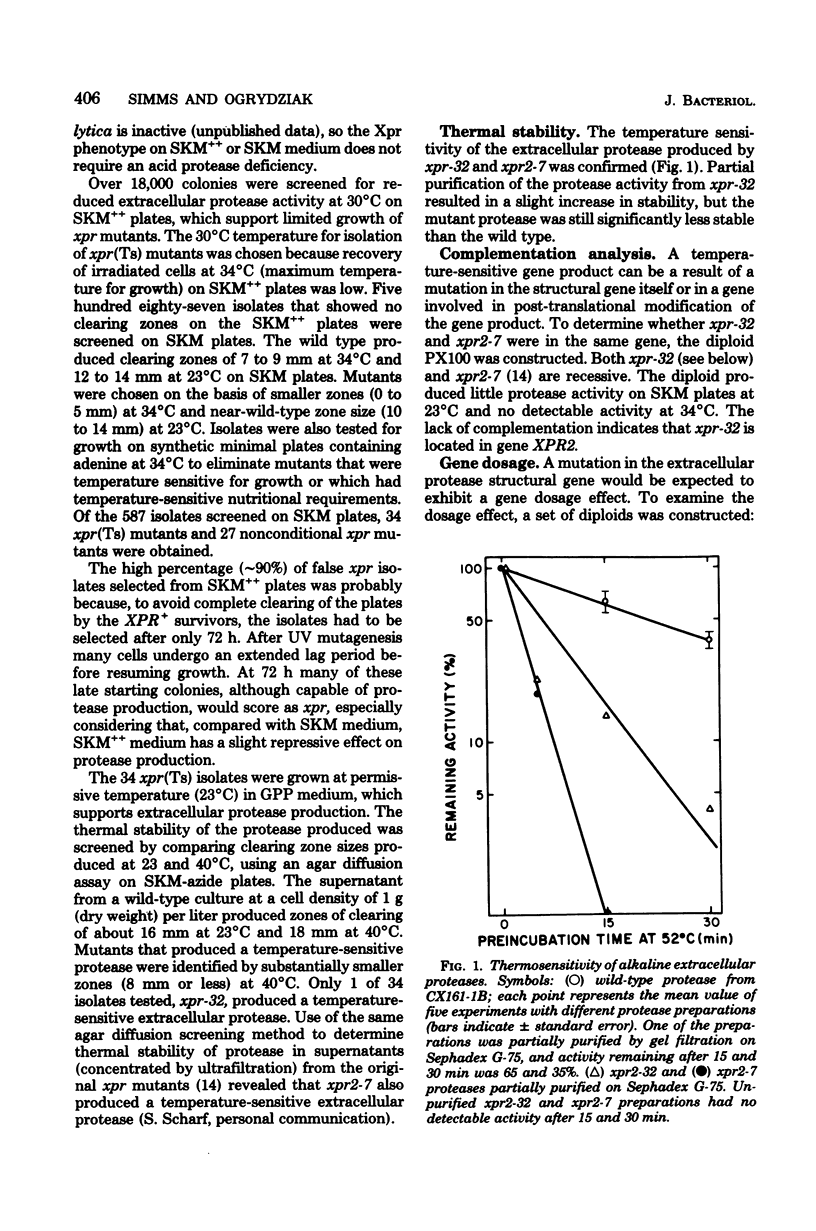
Mutants for Saccharomycopsis lipolytica temperature sensitive for alkaline extracellular protease production, but not for growth, were isolated. Thirty-three isolates were temperature sensitive for protease production, and one (xpr-32) produced a temperature-sensitive protease. Genetic analysis indicated that xpr-32 was located in gene XPR2, and allele xpr2-7 was found to also produce a temperature-sensitive protease. None of five independently isolated xpr2 mutations affects the production of extracellular ribonucleases and acid protease(s). Diploids with zero, one, or two active alleles of the XPR2 locus were constructed, and the XPR2 locus was shown to exhibit a gene dosage effect on alkaline extracellular protease synthesis (enzyme activity/cell protein). These results suggest that the XPR2 gene is the structural gene for the alkaline extracellular protease of S. lipolytica.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty V., Johnson G. Post-Translational Modification as a Potential Explanation of High Levels of Enzyme Polymorphism: Xanthine Dehydrogenase and Aldehyde Oxidase in DROSOPHILA MELANOGASTER. Genetics. 1979 Apr;91(4):695–722. doi: 10.1093/genetics/91.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free S. J., Schimke R. T. Effects of a post-translational modification mutation on different developmentally regulated glycosidases in Dictyostelium discoideum. J Biol Chem. 1978 Jun 25;253(12):4107–4111. [PubMed] [Google Scholar]
- Free S. J., Schimke R. T., Freeze H., Loomis W. F. Characterization and genetic mapping of modA. A mutation in the post-translational modification of the glycosidases of Dictyostelium discoideum. J Biol Chem. 1978 Jun 25;253(12):4102–4106. [PubMed] [Google Scholar]
- Free S. J., Schimke R. T. The structural gene for alpha-mannosidase-1 in Dictyostellium discoideum. Genetics. 1976 Oct;84(2):159–174. doi: 10.1093/genetics/84.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen A. T., Verheijen F. W., d'Azzo A., Galjaard H. Genetic heterogeneity in human neuraminidase deficiency. Nature. 1980 Jun 12;285(5765):500–502. doi: 10.1038/285500a0. [DOI] [PubMed] [Google Scholar]
- Loppes R. A mutation altering some properties of the neutral phosphatase in Chlamydomonas reinhardi: possible post-translational modification of phosphatase structure. J Bacteriol. 1978 Aug;135(2):551–558. doi: 10.1128/jb.135.2.551-558.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrydziak D. M., Demain A. L., Tannenbaum S. R. Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta. 1977 Apr 27;497(2):525–538. doi: 10.1016/0304-4165(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Mortimer R. K. Genetics of Extracellular Protease Production in SACCHAROMYCOPSIS LIPOLYTICA. Genetics. 1977 Dec;87(4):621–632. doi: 10.1093/genetics/87.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H., Yamane K., Maruo B. Thermosensitive, extracellular neutral proteases in Bacillus subtilis: isolation, characterization, and genetics. J Bacteriol. 1979 Aug;139(2):583–590. doi: 10.1128/jb.139.2.583-590.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Septum formation, cell division, and sporulation in mutants of yeast deficient in proteinase B. Proc Natl Acad Sci U S A. 1979 May;76(5):2395–2399. doi: 10.1073/pnas.76.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]



