Abstract
Zona pellucida (ZP)–induced acrosome reaction in sperm is a required step for mammalian fertilization. However, the precise mechanism of the acrosome reaction remains unclear. We previously reported that PLCδ4 is involved in the ZP-induced acrosome reaction in mouse sperm. Here we have monitored Ca2+ responses in single sperm, and we report that the [Ca2+]i increase in response to ZP, which is essential for driving the acrosome reaction in vivo, is absent in PLCδ4−/− sperm. Progesterone, another physiological inducer of the acrosome reaction, failed to induce sustained [Ca2+]i increases in PLCδ4−/− sperm, and consequently the acrosome reaction was partially inhibited. In addition, we observed oscillatory [Ca2+]i increases in wild-type sperm in response to these acrosome inducers. Calcium imaging studies revealed that the [Ca2+]i increases induced by exposure to ZP and progesterone started at different sites within the sperm head, indicating that these agonists induce the acrosome reaction via different Ca2+ mechanisms. Furthermore, store-operated channel (SOC) activity was severely impaired in PLCδ4−/− sperm. These results indicate that PLCδ4 is an important enzyme for intracellular [Ca2+]i mobilization in the ZP-induced acrosome reaction and for sustained [Ca2+]i increases through SOC induced by ZP and progesterone in sperm.
Keywords: phospholipase C; acrosome reaction; zona pellucida; calcium; sperm
Introduction
Phosphoinositide metabolism is an important intracellular signaling system involved in a variety of cell functions, such as secretion of hormones, transduction of neurotransmitters and growth factor signaling, membrane traffic, ion channel activity, and regulation of the cytoskeleton (Fukami et al., 1992; Takenawa et al., 1999; Martin, 2001). PLC is one of the key enzymes in this system and acts by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PIP2) to generate two second messengers, inositol 1,4,5-trisphosphate (IP3)*and DAG. DAG mediates the activation of PKC, and IP3 releases Ca2+ from intracellular stores. Among PLC family members, PLCδ is considered to be the most basal isoform because its structure is the simplest, comprising a pleckstrin homology domain, an EF hand domain, X and Y domains, and a C2 domain, and it is evolutionarily conserved even in plants and yeast (Rebecchi and Pentyala, 2000; Rhee, 2001). The activation mechanism of PLCδ remains unclear, although it is the most sensitive to Ca2+. PLCδ4, one of the δ isozymes, was first identified from a cDNA library from regenerating liver (Liu et al., 1996), and therefore it is thought to be involved in cell growth. In addition, based on the fact that PLCδ4 is abundantly expressed in brain and testis (Lee and Rhee, 1996; Nagano et al., 1999), we proposed that PLCδ4 might have a specific role in reproductive functions. In fact, through the generation of PLCδ4-deficient mice, we have recently reported that PLCδ4 is involved in the acrosome reaction in sperm (Fukami et al., 2001).
The acrosome reaction, which entails exocytosis of the acrosomal vesicles, is an essential step for fertilization. In mammalian sperm, the acrosome reaction is thought to be initiated in vivo by binding to the zona pellucida (ZP), the extracellular matrix of the egg, and only sperm that have completed the acrosome reaction can penetrate the ZP and fuse with the egg plasma membrane (Wassarman, 1999; Wassarman et al., 2001; Primakoff and Myles, 2002). In general, it is thought that sperm binding to ZP3, one of the glycoprotein components of the ZP, induces a transient Ca2+ influx into the sperm through voltage-dependent nonselective cation channels, which in turn leads to activation of a pertussis toxin–sensitive trimeric Gi/o protein–coupled PLC (Patrat et al., 2000; Darszon et al., 2001). A tyrosine kinase–regulated PLCγ may also be activated during ZP3 binding (Patrat et al., 2000). Activation of PLCs generates IP3, thereby mobilizing [Ca2+]i from the sperm's intracellular Ca2+ store, the acrosome, although the Ca2+ storing capacity of this organelle seems very limited (Rossato et al., 2001). Nonetheless, these early responses appear to promote a subsequent sustained Ca2+ influx signal via store-operated channels (SOCs) that results in the acrosome reaction (Florman, 1994; O'Toole et al., 2000; Breitbart, 2002). Recent studies have provided evidence for the expression in sperm of transient receptor potential protein channels 1, 3, and 6 (Trp1, Trp3, and Trp6), all putative Ca2+-permeant SOCs (Trevino et al., 2001), and Trp2 has been described to play a role in the ZP3-induced acrosome reaction in mouse sperm (Jungnickel et al., 2001). However, the precise molecular mechanism by which the acrosome reaction occurs has remained unclear.
In addition to ZP, thapsigargin, a specific blocker of the sarco/endoplasmic reticulum Ca2+-ATPase, which causes Ca2+ depletion from internal stores and leads to capacitative Ca2+ entry (Sabala et al., 1993), is also able to induce the acrosome reaction (Llanos, 1998). Furthermore, progesterone released from the cumulus cells, and thus one of the major components of follicular fluid, has also been shown to induce the acrosome reaction in a presumed physiological manner (Roldan et al., 1994; Kobori et al., 2000). Although the mechanism of action of progesterone on sperm is not yet fully understood, it is thought to induce Ca2+ influx by activating a GABAA-like progesterone receptor/Cl− channel (Meizel et al., 1997).
On the basis of the consensus that the acrosome reaction requires Ca2+ influx, global patterns of [Ca2+]i changes in single sperm have been observed. However, only a few studies have reported detailed spatio-temporal analysis of [Ca2+]i rises at the single-sperm level because of difficulties of the measurement in these cells. Here we report, using Ca2+ imaging of single sperm, that sperm show Ca2+ waves in response to ZP, progesterone, and thapsigargin, although the site of initiation of the rises appears to differ with the agonist, and that PLCδ4 is an important protein in the regulation of the Ca2+ responses that drive the acrosome reaction.
Results
Expression of PLCδ4/ATL-II in sperm
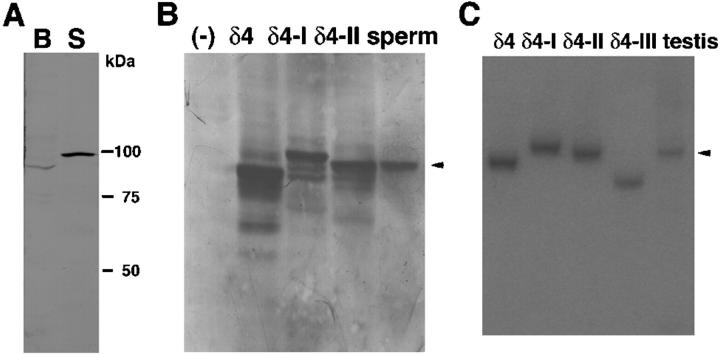
Western blot analysis using a specific antibody against PLCδ4 (Liu et al., 1996) revealed that an 85-kD PLCδ4 protein is expressed in brain, whereas a 90-kD protein is expressed in sperm (Fig. 1 A). There are three splicing variants, termed PLCδ4/ATL-I, PLCδ4/ATL-II, and PLCδ4/ATL-III in addition to PLCδ4 in testis (Nagano et al., 1999). PLCδ4/ATL-I and PLCδ4/ATL-II contain an additional 32 and 14 amino acids in their respective sequences in the linker region between the X and Y domains, and PLCδ4/ATL-III lacks PLC activity because the COOH-terminal region of the X domain and the linker region are substituted for 32 amino acids corresponding to the insert sequence of PLCδ4/ATL-I. To understand which splicing isoform exists in sperm and contributes to the acrosome reaction, we compared a band of molecular weight of ∼90 kD in sperm with PLCδ4 splicing variants overexpressed in COS-7 cells (Fig. 1 B). Western blot analysis showed that the 90-kD protein coincided with PLCδ4/ATL-II. There were no other PLCδ4 proteins in sperm. To confirm further the predominant role/expression of PLCδ4/ATL-II in sperm, we performed RT-PCR using total testis RNA and compared the molecular weight of the major PCR products using testis cDNA and plasmids coding for each of the splicing variants as templates. The major testis PCR product had a similar molecular weight to that produced from the PLCδ4.ATLII plasmid (Fig. 1 C, arrowhead), although a faint band corresponding to PLCδ4.ATLI was also detected under this condition. Therefore, we concluded that PLCδ4/ATL-II is the predominant PLCδ4 isoform expressed in sperm.
Figure 1.
PLCδ4/ATL-II is expressed in sperm. (A) The molecular weight of PLCδ4 in brain (B) and sperm (S) was compared by Western blot analysis. 30 μg of each homogenate was subjected to SDS-PAGE. Western blot analysis was performed using an antibody against PLCδ4 raised in the laboratory. (B) The molecular weight of sperm PLCδ4 was compared with PLCδ4 and alternative splicing isoforms of PLCδ4. PLCδ4 (δ4) and splicing isoforms of PLCδ4, PLCδ4/ALT-I (δ4-I) and PLCδ4/ALT-II (δ4-II), were overexpressed in COS-7 cells. 40 μg of lysates was subjected to SDS-PAGE, and then Western blotting was performed. The arrowhead denotes that the sperm protein closely resembles in size the product from the PLCδ4/ALT-II splice variant. (C) The products of RT-PCR using testis RNA were compared in size with authentic products using PLCδ4 (491 bp), PLCδ4/ALT-I (δ4-I; 587 bp), PLCδ4/ALT-II (δ4-II; 533 bp), and PLCδ4/ALT-III (δ4-III; 398 bp) plasmids. The arrowhead denotes that the major testis PCR product coincides in size with that of the PLCδ4/ALT-II splice variant.
ZP- and progesterone-induced acrosome reaction is impaired in PLCδ4−/− sperm
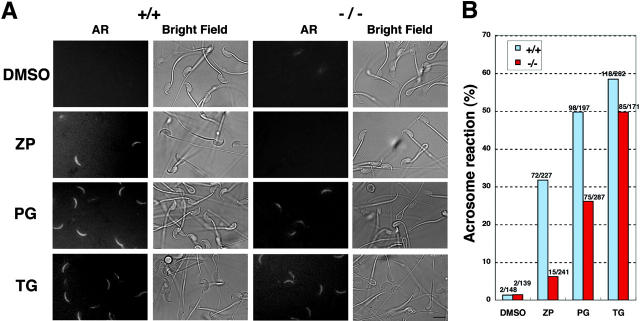
We have reported that PLCδ4 is an essential protein for ZP-induced acrosome reaction. To understand how PLCδ4 is involved in this process, we first examined whether PLCδ4 is required for the acrosome reaction induced by progesterone and thapsigargin. A soybean trypsin inhibitor (SBTI) was used to monitor the acrosome reaction. SBTI is known to tightly bind to acrosin (Tollner et al., 2000), which is located on the inner acrosomal membrane and only becomes exposed extracellularly after the acrosome reaction; therefore, we could easily recognize acrosome-reacted live sperm using fluorescence-labeled SBTI (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200210057/DC1). Moreover, this method enabled us to avoid spontaneously acrosome-reacted sperm before the addition of the agonists. In this study we used Alexa Fluor®594–labeled SBTI to monitor the acrosome reaction induced by ZP, progesterone, and thapsigargin (Fig. 2 A). The fluorescence signal began to be detected at ∼2–5 min after the addition of these reagents, and 31.7% of wild-type sperm completed the acrosome reaction within 15 min of addition of 3 ZP/μl, whereas only 6.2% of PLCδ4−/− sperm underwent the acrosome reaction (Fig. 2 B). 100 μM progesterone induced higher rates of acrosome reaction with 49.7% of PLCδ4+/+ sperm undergoing the reaction, whereas only 26.1% of the PLCδ4−/− sperm completed the process. These results suggest that PLCδ4 has an important role in the progesterone- and ZP-induced acrosome reaction. Nonetheless, because some of the sperm from PLCδ4−/− males underwent the acrosome reaction, we cannot exclude the possibility that other PLC isozymes might be involved in these acrosome reactions in the absence of PLCδ4, especially when progesterone is used to induce the reactions. What is more, we also cannot discount the possibility that some of these sperm might have undergone spontaneous acrosome reactions during the monitoring. There was no apparent difference in the number of wild-type and mutant sperm that underwent the acrosome reaction in response to thapsigargin (58.4 and 49.7%, respectively) (Fig. 2 B).
Figure 2.
Requirement of PLCδ4 in ZP- and progesterone-induced acrosome reaction. (A) Fluorescence and phase images of sperm treated with several acrosome reaction–inducing reagents. PLCδ4+/+ (+/+) and PLCδ4−/− (−/−) sperm capacitated for 1 h were treated with 1.0% DMSO, 3 Zp/μl solubilized mouse ZP (ZP), 100 μM progesterone (PG), or 5 μM thapsigargin (TG) for 15 min at 32°C in the presence of 2 μM Alexa Fluor®594–conjugated SBTI to monitor the occurrence of the acrosome reaction. The same microscopic fields are shown for acrosome reaction (AR) and bright field images. Bar, 10 μm. (B) Percent of sperm that underwent the acrosome reaction. The numbers above each column represent the number of sperm examined. Data were obtained from total amount of sperm by four independent experiments for progesterone and ZP and three independent experiments for thapsigargin.
Events of capacitation are normal in PLCδ4−/− sperm
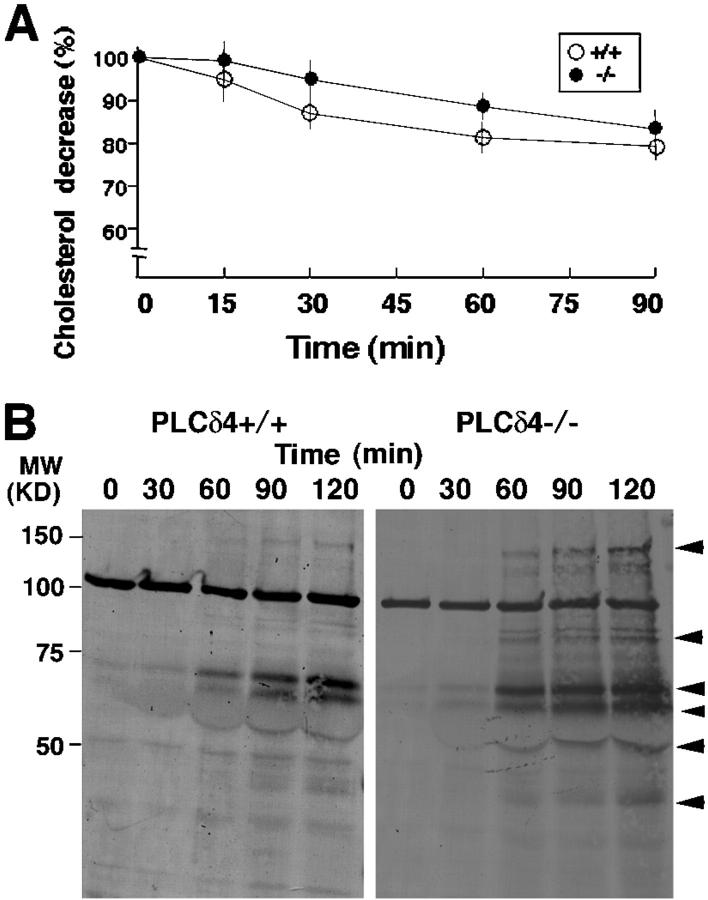
Before successful acrosome reaction can take place, the sperm have to undergo capacitation, a series of poorly defined molecular and biochemical changes including efflux of cholesterol, changes in the phospholipid content of the plasma membrane, hyperpolarization, and tyrosine phosphorylation (Arnoult et al., 1996; Nolan and Hammerstedt, 1997; Darszon et al., 2001). Capacitation occurs in vivo during sperm transit through the female tract and is mimicked in vitro by incubation of epididymal sperm in medium containing HCO3−, BSA, and Ca2+ (Breitbart, 2002). Several putative assays, such as assessment of cholesterol efflux, cAMP concentration, and protein tyrosine phosphorylation, can be used to know whether or not capacitation has occurred (Visconti et al., 1995, 1999; Iborra et al., 2000). To determine whether defects in capacitation may explain, at least in part, the abnormal acrosome reaction in PLCδ4−/− sperm, we measured the content of cholesterol during capacitation. The decrease in cholesterol levels began to be observed 15 min after capacitation in PLCδ4+/+ sperm and by 30 min in PLCδ4−/− sperm, although the overall decrease in cholesterol content by 90 min of capacitation was similar in both groups (Fig. 3 A). In addition, time-dependent specific protein tyrosine phosphorylation began to be detected in both PLCδ4+/+ and PLCδ4−/− after ∼60 min of incubation (Fig. 3 B, arrowheads). From these data, we conclude that PLCδ4 is likely not involved in the capacitation process.
Figure 3.
Changes in cholesterol content and tyrosine phosphorylation during capacitation. (A) Decrease in cholesterol content during capacitation is shown as percent decrease compared with the levels before capacitation. Approximately 2 × 106 sperm from PLCδ4+/+ (+/+) and PLCδ4−/− (−/−) mice were capacitated in HS buffer for the indicated times, and the content of cholesterol was measured as described in the Materials and methods. The data represent the average of four independent experiments. Error bars show standard errors. (B) Levels of tyrosine phosphorylation during capacitation. The sperm were capacitated for the indicated times and subjected to a 7.5% SDS-PAGE, and Western blot analysis was performed using the antibody 4G10. Arrowheads show the specific proteins that underwent tyrosine phosphorylation.
PLCδ4 is required for [Ca2+]i mobilization and sustained Ca2+ influx in sperm
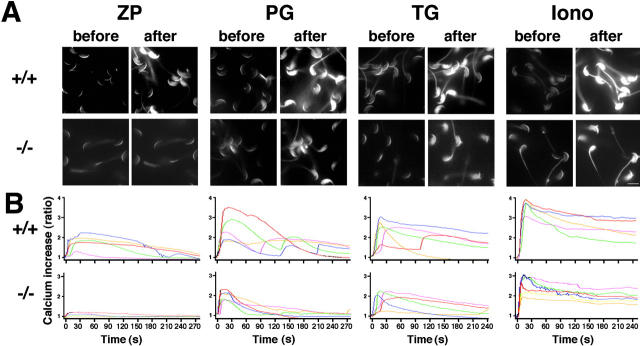
As it has been reported that Ca2+ has a primary role in the execution of the acrosome reaction (Patrat et al., 2000; Barratt and Publicover, 2001; Darszon et al., 2001; Breitbart, 2002), we next examined the Ca2+ responses in single sperm treated with ZP, progesterone, thapsigargin, or ionomycin (Fig. 4 A). We selected a single excitation calcium indicator fluo-4, because we could obtain calcium images of high resolution with this dye, and the images were temporally and spatially comparable to those obtained with the ratiometric dye fura-2 (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200210057/DC1). PLCδ4+/+ sperm preloaded with fluo-4 and treated with ZP exhibited a continuous [Ca2+]i increase (Fig. 4 B). This [Ca2+]i rise peaked at ∼30–60 s after the stimulation and lasted for more than 3–4 min before slowly returning to baseline. Approximately 50% of the sperm exhibited the described response (n = 120), and some sperm even showed repetitive [Ca2+]i increases after returning to baseline. In contrast, the addition of ZP induced a minor [Ca2+]i increase in PLCδ4−/− sperm, suggesting a role for PLCδ4 in the Ca2+ response during the ZP-induced acrosome reaction.
Figure 4.
[Ca 2+ ] i mobilization is impaired in PLCδ4 − / − sperm during acrosome reaction. Capacitated sperm were loaded with 4 μM fluo4-AM for 15 min, and Ca2+ responses were monitored every 2 s. (A) Ca2+ images of PLCδ4+/+ (+/+) and PLCδ4−/− (−/−) sperm before and after (10–30 s) treatment with 3 Zp/μl solubilized mouse ZP (ZP), 100 μM progesterone (PG), 5 μM thapsigargin (TG), or 5 μM ionomycin (Iono). The same microscopic fields are shown before and after the stimulus. The experiments were done at 32°C. Bar, 10 μm. (B) Ca2+ patterns of five sperm representative of the responses induced by the agonists used here. Levels of [Ca2+]i are expressed as F/F0 ratios. The data are representative from five (ZP), nine (PG), eight (TG), and three (Iono) independent experiments. Progesterone induced repetitive [Ca2+]i increases.
Treatment of PLCδ4+/+ sperm with 50–100 μM progesterone showed a faster, and on occasions oscillatory, Ca2+ response (n = 180; Fig. 4 B). The majority of PLCδ4+/+ sperm showed a large initial [Ca2+]i increase that declined slowly within 90–210 s, and that was followed by repetitive spikes. The wave-like Ca2+ influx induced by progesterone has been demonstrated in human sperm (Meizel et al., 1997), although repetitive Ca2+ responses were not reported in that work. It is worth noting that the responses to progesterone seem to be dose dependent. For instance, Kobori et al. (2000) reported that addition of 40 μM progesterone induced rises of longer duration than those induced by 20 μM. In agreement with those findings, we observed longer and repetitive [Ca2+]i increases with higher doses of progesterone (unpublished data). Although progesterone induced a Ca2+ response in PLCδ4−/− sperm, the amplitude of the rise was smaller and the duration was very transient, returning to basal levels within 60–120 s.
Treatment of PLCδ4+/+ sperm with thapsigargin or ionomycin induced continuous [Ca2+]i increases, and in most sperm, the [Ca2+]i rises were sustained for at least 240 s. Treatment of PLCδ4−/− sperm with these reagents produced a similar pattern, but with less amplitude (Fig. 4 B). These results raise the possibility that PLCδ4 may have an important role in the regulation of [Ca2+]i mobilization in sperm in response to numerous agonists.
Agonist-specific spatial distribution of [Ca2+]i rises in sperm
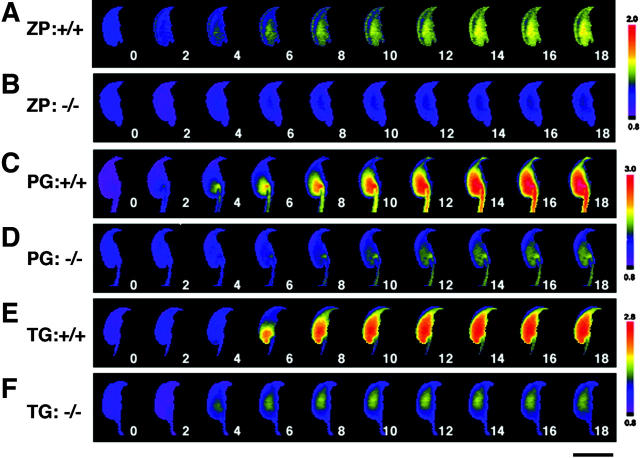
Typical [Ca2+]i increase patterns of sperm in response to ZP, progesterone, or thapsigargin over time are shown in Figs. 5 and 6 and Video 1 (available at http://www.jcb.org/cgi/content/full/jcb.200210057/DC1). When PLCδ4+/+ sperm preloaded with fluo-4 were treated with ZP, the [Ca2+]i increase was first detected in the acrosome area (2 s), then in the equatorial segment (4 s), and finally extended to the whole sperm head (around 14 s) (Fig. 6 A). Although there are a few reports that describe the global [Ca2+]i elevations in bovine or hamster sperm head during the ZP-induced acrosome reaction (Florman, 1994; Shirakawa and Miyazaki, 1999), this is the first observation that clearly notes the sequential [Ca2+]i mobilization induced by ZP. These data support the notion that the acrosome vesicle serves as the intracellular Ca2+ store and that ZP mobilizes [Ca2+]i before promoting Ca2+ influx during acrosome reaction. Interestingly, the acrosome reaction always occurred after peak [Ca2+]i values were attained, and the occurrence of the reaction was confirmed by simultaneous monitoring of fluo-4 and Alexa Fluor®594–labeled SBTI (unpublished data). As expected, only a minor [Ca2+]i increase was detected in PLCδ4−/− sperm after the addition of ZP (Fig. 5 B; Fig. 6 B).
Figure 5.
Spatio-temporal [Ca 2+ ] i dynamics in a single sperm treated with acrosome reaction inducers. Capacitated sperm were loaded with 4 μM fluo4-AM for 15 min, and [Ca2+]i mobilization at the single-sperm level was monitored. Sperm from PLCδ4+/+ (A, C, and E) or PLCδ4−/− (B, D, and F) were treated with 3 Zp/μl solubilized mouse ZP (A and B), 100 μM progesterone (C and D), or 5 μM thapsigargin (E and F). Images were collected every 2 s. The relative [Ca2+]i change is calculated as a ratio to F0 images and shown in pseudo-color. Bar, 10 μm. Figures show time (sec) after treatment of the reagents.
Figure 6.
Magnification of the initial images of sperm treated with acrosome reaction inducers. Sperm from PLCδ4+/+ (A, C, and E) or PLCδ4−/− (B, D, and F) were treated with ZP (A and B), progesterone (C and D), or thapsigargin (E and F). Bar, 10 μm. Figures show time (sec) after treatment of the reagents.
The Ca2+ responses induced by progesterone and thapsigargin in PLCδ4+/+ sperm appeared to have a different spatial distribution than those triggered by ZP. Addition of progesterone or thapsigargin intensively induced a Ca2+ response at the base of the sperm head (2–6 s) (Fig. 6, C and E; Videos 2 and 3, available at http://www.jcb.org/cgi/content/full/jcb.200210057/DC1). It is known that thapsigargin induces Ca2+ influx through SOC by inhibiting the Ca2+-ATPase on Ca2+ stores; therefore, we suspect that this post-acrosomal region near the neck region may be the site of the primary Ca2+ influx. Because progesterone promotes a [Ca2+]i rise with similar spatial distribution to that of thapsigargin, our results suggest that the progesterone-induced [Ca2+]i increase is primarily due to Ca2+ influx. This notion is further supported by the finding that [Ca2+]i increases in response to progesterone were not detected in PLCδ4+/+ sperm when progesterone was applied in Ca2+-free medium (unpublished data). Remarkably, the second Ca2+ wave also originated from the same post-acrosomal area (132 s) (Fig. 5 C). The spatial distribution of the [Ca2+]i increase induced by progesterone and thapsigargin in PLCδ4−/− sperm was also initiated in the post-acrosomal region (Fig. 5, D and F; Fig. 6, D and F), although the magnitude of the Ca2+ influx was very limited. It was also evident that the progesterone-induced [Ca2+]i increase was less sustained in PLCδ4−/− sperm. In spite of the lower Ca2+ responses induced by thapsigargin in PLCδ4−/− sperm, the timing of the acrosome reaction was not disturbed in mutant sperm and occurred between 176 and 178 s (Fig. 5 F). Collectively, these results demonstrate that ZP induces the acrosome reaction by increasing [Ca2+]i from intracellular Ca2+ stores followed by Ca2+ influx, whereas progesterone induces [Ca2+]i increase by promoting Ca2+ influx. Therefore, it appears that PLCδ4 has an important role in the regulation of [Ca2+]i mobilization in the ZP-induced acrosome reaction, and possibly in the Ca2+ influx promoted during the ZP-, progesterone-, and thapsigargin-induced acrosome reaction.
Monitoring of [Ca2+]i mobilization on sperm at the population level suggests a role of PLCδ4 on Ca2+ influx
To further understand the role of PLCδ4 in sperm [Ca2+]i mobilization and to confirm the validity of the Ca2+ responses obtained using single-sperm measurements, we examined at the population level the [Ca2+]i changes induced by the same agonists using a fluorescence microplate reader corresponding to a 96-well plate. We detected comparatively weak and slow increases in fluorescence in PLCδ4+/+ sperm after addition of ZP (Fig. 7 A), reflecting, perhaps, the fact that the response to ZP may start over a course of several minutes within a population of sperm, as shown by others. In contrast, progesterone triggered a very rapid and large [Ca2+]i increase and so did ionomycin and thapsigargin (Fig. 7 B). This pattern of [Ca2+]i increase was also observed in PLCδ4−/− sperm; however, the maximum intensity of [Ca2+]i uptake was about half of that observed in PLCδ4+/+ sperm, which agrees with the results reported in Figs. 4 and 5. Because thapsigargin appears to promote SOC channel activity but not generation of IP3 (Sabala et al., 1993), and ionomycin induces Ca2+ influx through the formation of synthetic Ca2+ channels, these results raise the possibility that PLCδ4 may play in sperm a novel functional role in the regulation of Ca2+ influx.
Figure 7.
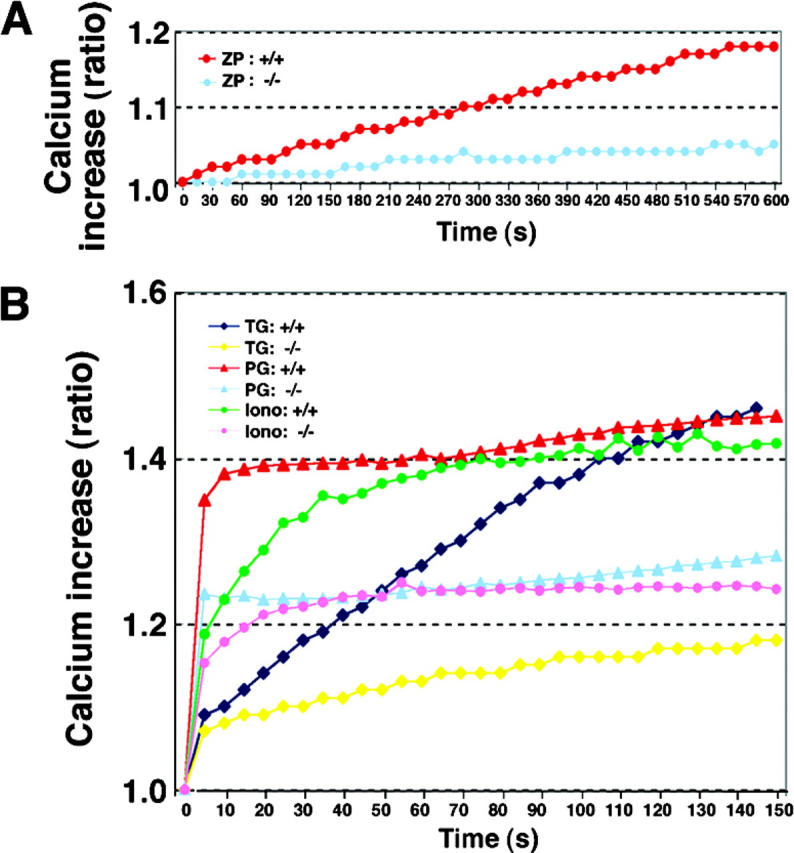
Measurement of [Ca 2+ ] i increase in sperm responded to various acrosome inducers by mass level. Capacitated cauda epidermal sperm were loaded with 4 μM fluo4-AM for 15 min at 37°C. Approximately 1 × 106 sperm in a 96-well plate were treated with ZP, progesterone (PG), thapsigargin (TG), or ionomycin (Iono). Increasing levels of intracellular Ca2+ are expressed as an F/F0 ratio. The data were collected from three independent experiments.
SOC activity is impaired in PLCδ4−/− sperm
Having noticed a difference in the amplitude of the [Ca2+]i increase between PLCδ4+/+ and PLCδ4−/− sperm in response to thapsigargin, we examined directly whether SOC activity was deficient in PLCδ4−/− sperm. To evaluate SOC activity, the sperm's Ca2+ stores were depleted by exposure to thapsigargin in Ca2+-free medium, followed by the addition of 1 mM extracellular Ca2+ (Fig. 8 A). The addition of thapsigargin induced a small [Ca2+]i increase in both wild-type and mutant sperm, suggesting that the content of the Ca2+ stores is comparable in both sperm populations. However, addition of extracellular Ca2+ induced a large Ca2+ influx and [Ca2+]i increase in PLCδ4+/+ sperm that decreased after driving the acrosome reaction (Fig. 8 A, top, arrowhead), whereas it produced a weak and transient [Ca2+]i increase in PLCδ4−/− sperm, demonstrating that the absence of PLCδ4 significantly affected the function of SOC channels.
Figure 8.
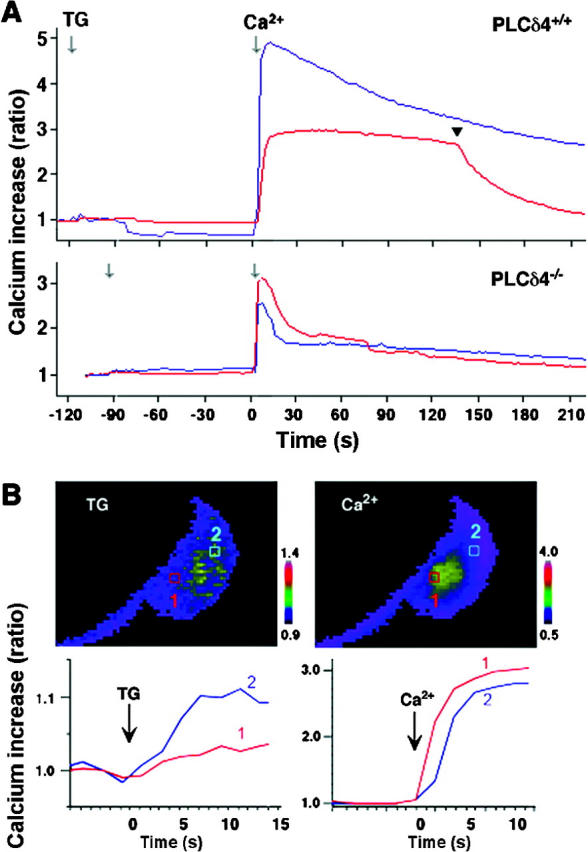
SOC activity is impaired in PLCδ4 − / − sperm. (A) PLCδ4+/+ and PLCδ4−/− sperm were incubated in Ca2+-free HS buffer containing 1 mM EGTA; the Ca2+ stores were depleted by treatment with 5 μM thapsigargin (TG). SOC activity was measured by addition of 1 mM extracellular Ca2+. Ca2+ patterns of two representative sperm responses are shown. The arrowhead denoted the time of acrosome reaction. (B) Changes in [Ca2+]i increases in different areas within a sperm are shown by pseudo-color images and time course plots. Two spots of post-acrosome (1) and equatorial segment (2) were selected for images and time course plots. The images shown were collected 6 s after addition of thapsigargin (TG) and immediately after (2 s) addition of 1 mM extracellular Ca2+ (Ca2+). The fluorescence intensity of images and plots is shown with different ratio scales.
To attempt to pinpoint the site of Ca2+ influx, and possibly to determine the position of SOC channels in sperm, we next examined the spatial distribution of the Ca2+ entry promoted by the addition of extracellular Ca2+ in sperm previously treated with thapsigargin in the absence of extracellular Ca2+. [Ca2+]i changes were compared in two different areas of the sperm head, one in the post-acrosomal region near the neck of the sperm head (Fig. 8 B, 1), which was anticipated to report Ca2+ influx and hence SOC activity, and the second in the equatorial segment (Fig. 8 B, 2), which was expected to indicate intracellular Ca2+ release. When PLCδ4+/+ sperm were treated with thapsigargin, a small [Ca2+]i increase was observed at position 2, in the equatorial region, and after addition of extracellular Ca2+, a large [Ca2+]i increase was detected primarily at position 1, the post-acrosomal area, which then propagated to position 2 (Fig. 8 B). These results indicate that release of intracellular [Ca2+]i in sperm occurs primarily in the acrosome and in the equatorial area, and that the post-acrosomal region is the SOC-rich area, which mediates Ca2+ influx.
Discussion
It is well known that Ca2+ release and Ca2+ influx play a significant role in the induction of the acrosome reaction (Patrat et al., 2000; Barratt and Publicover, 2001; Darszon et al., 2001; Breitbart, 2002). In this manuscript, we have characterized in sperm of wild-type and in PLCδ4-deficient mice the temporal and spatial distribution of the [Ca2+]i increases induced by treatment with ZP, progesterone, thapsigargin, or ionomycin (Figs. 4, 5, and 6). Our results show that absence of PLCδ4/ATLII causes major impairment in the release of intracellular Ca2+ induced by the addition of ZP, which is the mechanism responsible for inducing the acrosome reaction during natural fertilization. Moreover, PLCδ4 appears to affect Ca2+ influx, which may be necessary to complete the acrosome reaction, as the Ca2+ responses induced by the above-mentioned agonists were altered in PLCδ4 mutant sperm. We can therefore conclude that PLCδ4/ATLII may be involved in the regulation of both intracellular Ca2+ release and Ca2+ influx through SOC channels.
Using high resolution single-sperm Ca2+ imaging, we demonstrate that the agonists used in this study to induce the acrosome reaction trigger distinct Ca2+ responses, and that these responses are altered in intensity and duration in PLCδ4−/− sperm. First, we confirmed that in wild-type sperm, the addition of solubilized ZP induces the acrosome reaction by triggering Ca2+ release. We extended those observations by showing that the ZP-induced [Ca2+]i increase started in the acrosome region and spread to the whole sperm head (Fig. 6 A). As it has been reported that both the IP3 receptors (IP3Rs) and PLCδ4 localize to the acrosome (Walensky and Snyder, 1995; Fukami et al., 2001), our results suggest that the ZP induces [Ca2+]i mobilization initially from the acrosome and that PLCδ4 is responsible for the presumed generation of IP3. Evidence in support of this notion is provided by the findings that ZP almost failed to induce a [Ca2+]i increase in PLCδ4−/− sperm. Therefore, we conclude that the abnormal acrosome reaction induced by ZP in PLCδ4−/− sperm is most likely due to impaired intracellular [Ca2+]i mobilization in these sperm, and that this protein plays a crucial role in the acrosome reaction during natural fertilization.
Although the progesterone-induced [Ca2+]i increases in sperm exhibited different spatial and temporal patterns than those evoked by ZP, the intensity of these responses was still altered in PLCδ4−/− sperm. Our data show that most of the progesterone-induced Ca2+ response initiated in the post-acrosomal region, and that it was primarily due to Ca2+ influx, as it was not observed in Ca2+-free medium. Remarkably, in PLCδ4−/− sperm, the amplitude and duration of the progesterone-induced Ca2+ response were greatly decreased, paralleled by a reduction in the number of sperm that underwent the acrosome reaction (Fig. 2 B), supporting the notion that a sustained [Ca2+]i increase is required for the completion of the acrosome reaction and that PLCδ4 may regulate this aspect of the progesterone-induced Ca2+ signal.
The thapsigargin-induced Ca2+ responses were similar to those evoked by progesterone in that they depended on Ca2+ influx to elevate [Ca2+]i in sperm. In addition, direct assessment of SOC function using thapsigargin showed that capacitative Ca2+ entry through SOC channels is severely impaired in PLCδ4−/− sperm (Fig. 8 A), further supporting a role of PLCδ4 in the regulation of Ca2+ influx through these channels. Moreover, in this manuscript, we determined for the first time that the site of Ca2+ influx after the addition of thapsigargin (and progesterone) appears to be the post-acrosomal region, near the connective piece. The question then arises as to why this site might mediate Ca2+ influx. It is possible that SOC channels may be localized to this region. Toward this end, it is worth noting that recent reports localize IP3Rs to an additional region on the base of the head and neck region in human and bovine sperm (Ho and Suarez, 2001; Naaby-Hansen et al., 2001). Whether there is a direct link between IP3R and SOC channels in sperm and whether the site of Ca2+ influx is conserved across species remain to be investigated.
It also remains unclear how PLCδ4 might regulate SOC channels. It has recently been shown that some Trp channels operate as SOC channels (Montell et al., 2002), and TrpC3 has been reported to associate directly with the IP3R (Boulay et al., 1999; Kiselyov et al., 1999). In addition, recent evidence has shown that TrpC2 is implicated in the acrosome reaction (Jungnickel et al., 2001) and the phosphoinositide pathway may regulate the activity of Trp channels (Montell et al., 2002; Runnels et al., 2002). Therefore it is possible that defective stimulation of the phosphoinositide pathway may be responsible for the abnormal Ca2+ responses detected in the mutant PLCδ4 sperm. Lastly, we cannot discount the possibility that in PLCδ4 mutant sperm, the number and/or function of SOC channels may be altered in a manner unrelated to the possibilities considered here. Nonetheless, our finding that PLCδ4 is one of the important enzymes in the regulation of [Ca2+]i mobilization in sperm significantly contributes to the elucidation of the molecular pathways of mammalian fertilization. In addition, its possible role in the regulation of Ca2+ influx suggests novel areas of research. Regulation of Ca2+ influx is important for many vital cellular functions, such as cell growth, apoptosis, exocytosis, muscle contraction, and gene transcription (Berridge et al., 1999, 2000), and its abnormal function may lead to the development of disease (Peng and Hediger, 2002; Torbergsen, 2002). Further understanding of the relationship between phosphoinositide metabolism and Ca2+ channel regulation may contribute to elucidating the molecular basis of these diseases.
Materials and methods
Detection of acrosome reaction
Cauda epididymal sperm were obtained from 8–18-wk-old PLCδ4+/+ and PLCδ4−/− mice and capacitated for 1 h in HS medium (135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 30 mM Hepes, 10 mM glucose, 10 mM lactic acid, 1 mM purvic acid, 5 mg/ml BSA, 15 mM NaCO3, pH 7.4) at 37°C under CO2. Mouse ZP was isolated from the ovaries of 10–21-wk-old mice and solubilized as previously described (Ward et al., 1992). Progesterone, thapsigargin, and ionomycin were purchased from Fuji Pharmaceutical and Sigma-Aldrich, respectively. The acrosome reaction was induced by the addition of the above-mentioned agonist, and it was performed at 32°C for 15 min in the presence of 2 μM SBTI (Nacalai Tesque), which was labeled using an Alexa Fluor®594 protein labeling kit (Molecular Probes). After the acrosome reaction, the fluorescence-labeled sperm were counted under a fluorescence microscope with the same optical condition described below for the fluo-4 and Alexa Fluor®594–labeled SBTI double-labeling experiment. The ratio of the acrosome reaction was calculated by dividing the number of acrosome-reacted sperm by the number of total sperm.
Measurements of [Ca2+]i mobilization in single sperm
Capacitated cauda epididymal sperm were loaded with 4 μM fluo4-AM (Molecular Probes) for 15 min at 37°C and immobilized on a laminin-coated glass-bottom dish just before calcium measurement. We monitored simultaneously in single sperm the fluorescence emission of two dyes, fluo-4 and Alexa Fluor®594–labeled SBTI. Fluorescence Ca2+ images of fluo-4 and Alexa Fluor®594–labeled SBTI images were collected alternately for 200 msec every 2 s with altering excitation filters (470–490 nm for fluo-4 and 535–550 nm for SBTI), a dual-band dichroic mirror (51016bs; Chroma Technology Corp.), and a dual-band emission filter (51016em; Chroma Technology Corp.) on an inverted microscope (IX-70; Olympus) through a 60× objective (n.a. 1.4; Olympus) with a digital CCD camera (ORCA-ERG; Hamamatsu Photonics). On-line control of the system, acquisition, and off-line analysis of the collected data were done with TI Workbench software (written by T. Inoue) running on a Macintosh computer. Levels of [Ca2+]i are calculated as F/F0 ratios after background subtraction in both images and time course plots. Five image frames just before drug application were averaged and used as an F0 image. The experiments were performed at 32°C with a heating chamber covering the stage and objective lens of the microscope. For measurements of SOC activity, immobilized sperm were washed three times in Ca2+-free HS buffer (containing 1 mM EGTA) and treated with thapsigargin, followed by the addition of 1 mM extracellular Ca2+.
Measurements of [Ca2+]i mobilization at the population level
Capacitated cauda epididymal sperm were loaded with 4 μM fluo4-AM for 15 min at 37°C. Approximately 1 × 106 sperm were placed on a 96-well plate and treated with ZP, progesterone, thapsigargin, or ionomycin at 32°C. The fluorescence output changes were monitored at 5-s intervals using a Fusion-α fluorescence microplate analyzer (Packard BioScience) with a 495-nm excitation filter and a 515-nm emission filter. Increasing levels of [Ca2+]i were expressed as F/F0 ratios.
Expression of PLCδ4 isoforms, Western blot analysis, and RT-PCR analysis
COS-7 cells were maintained in DME supplemented with 10% FBS. PLCδ4, PLCδ4/ALT-I, and PLCδ4/ALT-II were subcloned into the expression vector pcDNA3 and transiently expressed in COS-7 cells by electroporation. At 48 h after transfection, cells were lysed with SDS sample buffer, and the proteins were separated by SDS-polyacrylamide electrophoresis, followed by transfer onto nitrocellulose membranes (Schleicher & Schuell). Western blot analysis was performed using a specific polyclonal antibody against PLCδ4.
Sperm capacitated for the indicated period were collected by centrifugation, solubilized with SDS sample buffer, and subjected to electrophoresis. Tyrosine phosphorylation was assessed by immunoblot analysis using a 4G10 antibody (Upstate Biotechnology) that recognizes tyrosine-phosphorylated proteins.
Total RNA from testis and sperm was prepared using the QIAGEN RNeasy kit. 10 μg RNA was used for reverse transcription. PCR was performed for 40 cycles of 30 s at 95°C, 30 s at 54°C, and 60 s at 72°C with 5 μCi [32P]dCTP and PLCδ4-specific primers (forward primer, TGGCACACCATCTGATTGCG; reverse primer, TACACGGCATAGCTGTCTGG) to produce 491-bp (PLCδ4), 587-bp (PLCδ4/ALT-I), 533-bp (PLCδ4/ALT-II), and 398-bp (PLCδ4/ALT-III) fragments. PCR products were separated on a 5.0% acrylamide gel, followed by autoradiography.
Cholesterol assay
Cauda epididymal sperm were capacitated for the indicated times in HS medium at 37°C under CO2. Sperm were collected by centrifugation, and lipids were extracted by chloroform/methanol/HCl as previously described (Schacht, 1978). An aliquot of the extracted lipids was used for measuring cholesterol content using an Amplex Red Cholesterol Assay kit (Molecular Probes). The cholesterol content was normalized by protein content and expressed relative to the concentration before capacitation.
Online supplemental material
The supplemental material (Figs. S1 and S2 and Videos 1–3, available at http://www.jcb.org/cgi/content/full/jcb.200210057/DC1) shows [Ca2+]i mobilization in wild sperm treated with acrosome reaction inducers. Capacitated wild sperm were loaded with 4 μM fluo4-AM for 15 min, and Ca2+ images were monitored every 2 s after treatment with 3 Zp/μl solubilized mouse ZP (ZP/WT), 100 μM progesterone (PG/WT), or 5 μM thapsigargin (TG/WT).
Acknowledgments
We thank G.N. Cherr and S. Oda for technical advice on the acrosome reaction assay and the immobilization of sperm for Ca2+ imaging, respectively. We also thank Jeremy Smyth for critical reading of the manuscript.
This work was supported by a special coordination fund for promoting science and technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a grant from the United States Department of Agriculture to R.A. Fissore.
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; SBTI, soybean trypsin inhibitor; SOC, store-operated channel; ZP, zona pellucida.
References
- Arnoult, C., Y. Zeng, and H.M. Florman. 1996. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J. Cell Biol. 134:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt, C.L., and S.J. Publicover. 2001. Interaction between sperm and zona pellucida in male fertility. Lancet. 358:1660–1662. [DOI] [PubMed] [Google Scholar]
- Berridge, M., P. Lipp, and M. Bootman. 1999. Calcium signalling. Curr. Biol. 9:R157–R159. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. [DOI] [PubMed] [Google Scholar]
- Boulay, G., D.M. Brown, N. Qin, M. Jiang, A. Dietrich, M.X. Zhu, Z. Chen, M. Birnbaumer, K. Mikoshiba, and L. Birnbaumer. 1999. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. USA. 96:14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart, H. 2002. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol. Cell. Endocrinol. 187:139–144. [DOI] [PubMed] [Google Scholar]
- Darszon, A., C. Beltran, R. Felix, T. Nishigaki, and C.L. Trevino. 2001. Ion transport in sperm signaling. Dev. Biol. 240:1–14. [DOI] [PubMed] [Google Scholar]
- Florman, H.M. 1994. Sequential focal and global elevations of sperm intracellular Ca2+ are initiated by the zona pellucida during acrosomal exocytosis. Dev. Biol. 165:152–164. [DOI] [PubMed] [Google Scholar]
- Fukami, K., K. Furuhashi, M. Inagaki, T. Endo, S. Hatano, and T. Takenawa. 1992. Requirement of phosphatidylinositol 4,5-bisphosphate for α-actinin function. Nature. 359:150–152. [DOI] [PubMed] [Google Scholar]
- Fukami, K., K. Nakao, T. Inoue, Y. Kataoka, M. Kurokawa, R.A. Fissore, K. Nakamura, M. Katsuki, K. Mikoshiba, N. Yoshida, and T. Takenawa. 2001. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science. 292:920–923. [DOI] [PubMed] [Google Scholar]
- Ho, H.C., and S.S. Suarez. 2001. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 65:1606–1615. [DOI] [PubMed] [Google Scholar]
- Iborra, A., M. Companyo, P. Martinez, and A. Morros. 2000. Cholesterol efflux promotes acrosome reaction in goat spermatozoa. Biol. Reprod. 62:378–383. [DOI] [PubMed] [Google Scholar]
- Jungnickel, M.K., H. Marrero, L. Birnbaumer, J.R. Lemos, and H.M. Florman. 2001. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat. Cell Biol. 3:499–502. [DOI] [PubMed] [Google Scholar]
- Kiselyov, K., G.A. Mignery, M.X. Zhu, and S. Muallem. 1999. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol. Cell. 4:423–429. [DOI] [PubMed] [Google Scholar]
- Kobori, H., S. Miyazaki, and Y. Kuwabara. 2000. Characterization of intracellular Ca2+ increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol. Reprod. 63:113–120. [DOI] [PubMed] [Google Scholar]
- Lee, S.B., and S.G. Rhee. 1996. Molecular cloning, splice variants, expression, and purification of phospholipase Cδ4. J. Biol. Chem. 271:25–31. [DOI] [PubMed] [Google Scholar]
- Liu, N., K. Fukami, H. Yu, and T. Takenawa. 1996. A new phospholipase Cδ4 is induced at S-phase of the cell cycle and appears in the nucleus. J. Biol. Chem. 271:355–360. [DOI] [PubMed] [Google Scholar]
- Llanos, M.N. 1998. Thapsigargin stimulates acrosomal exocytosis in hamster spermatozoa. Mol. Reprod. Dev. 51:84–91. [DOI] [PubMed] [Google Scholar]
- Martin, T.F. 2001. PI(4,5)P2 regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13:493–499. [DOI] [PubMed] [Google Scholar]
- Meizel, S., K.O. Turner, and R. Nuccitelli. 1997. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev. Biol. 182:67–75. [DOI] [PubMed] [Google Scholar]
- Montell, C., L. Birnbaumer, and V. Flockerzi. 2002. The TRP channels, a remarkably functional family. Cell. 108:595–598. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen, S., M.J. Wolkowicz, K. Klotz, L.A. Bush, V.A. Westbrook, H. Shibahara, J. Shetty, S.A. Coonrod, P.P. Reddi, J. Shannon, et al. 2001. Co-localization of the inositol 1,4,5-trisphosphate receptor and calreticulin in the equatorial segment and in membrane bounded vesicles in the cytoplasmic droplet of human spermatozoa. Mol. Hum. Reprod. 7:923–933. [DOI] [PubMed] [Google Scholar]
- Nagano, K., K. Fukami, T. Minagawa, Y. Watanabe, C. Ozaki, and T. Takenawa. 1999. A novel phospholipase Cδ4 (PLCδ4) splice variant as a negative regulator of PLC. J. Biol. Chem. 274:2872–2879. [DOI] [PubMed] [Google Scholar]
- Nolan, J.P., and R.H. Hammerstedt. 1997. Regulation of membrane stability and the acrosome reaction in mammalian sperm. FASEB J. 11:670–682. [DOI] [PubMed] [Google Scholar]
- O'Toole, C.M., C. Arnoult, A. Darszon, R.A. Steinhardt, and H.M. Florman. 2000. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Mol. Biol. Cell. 11:1571–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrat, C., C. Serres, and P. Jouannet. 2000. The acrosome reaction in human spermatozoa. Biol. Cell. 92:255–266. [DOI] [PubMed] [Google Scholar]
- Peng, J.B., and M.A. Hediger. 2002. A family of calcium-permeable channels in the kidney: distinct roles in renal calcium handling. Curr. Opin. Nephrol. Hypertens. 11:555–561. [DOI] [PubMed] [Google Scholar]
- Primakoff, P., and D.G. Myles. 2002. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science. 296:2183–2185. [DOI] [PubMed] [Google Scholar]
- Rebecchi, M.J., and S.N. Pentyala. 2000. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80:1291–1335. [DOI] [PubMed] [Google Scholar]
- Rhee, S.G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan, E.R., T. Murase, and Q.X. Shi. 1994. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 266:1578–1581. [DOI] [PubMed] [Google Scholar]
- Rossato, M., F. Di Virgilio, R. Rizzuto, C. Galeazzi, and C. Foresta. 2001. Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Mol. Hum. Reprod. 7:119–128. [DOI] [PubMed] [Google Scholar]
- Runnels, L.W., L. Yue, and D.E. Clapham. 2002. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 4:329–336. [DOI] [PubMed] [Google Scholar]
- Sabala, P., M. Czarny, J.P. Woronczak, and J. Baranska. 1993. Thapsigargin: potent inhibitor of Ca2+ transport ATP-ases of endoplasmic and sarcoplasmic reticulum. Acta Biochim. Pol. 40:309–319. [PubMed] [Google Scholar]
- Schacht, J. 1978. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J. Lipid Res. 19:1063–1067. [PubMed] [Google Scholar]
- Shirakawa, H., and S. Miyazaki. 1999. Spatiotemporal characterization of intracellular Ca2+ rise during the acrosome reaction of mammalian spermatozoa induced by zona pellucida. Dev. Biol. 208:70–78. [DOI] [PubMed] [Google Scholar]
- Takenawa, T., T. Itoh, and K. Fukami. 1999. Regulation of phosphatidylinositol 4,5-bisphosphate levels and its roles in cytoskeletal re-organization and malignant transformation. Chem. Phys. Lipids. 98:13–22. [DOI] [PubMed] [Google Scholar]
- Tollner, T.L., A.I. Yudin, G.N. Cherr, and J.W. Overstreet. 2000. Soybean trypsin inhibitor as a probe for the acrosome reaction in motile cynomolgus macaque sperm. Zygote. 8:127–137. [DOI] [PubMed] [Google Scholar]
- Torbergsen, T. 2002. Rippling muscle disease: a review. Muscle Nerve. Suppl. 11:S103–S107. [DOI] [PubMed] [Google Scholar]
- Trevino, C.L., C.J. Serrano, C. Beltran, R. Felix, and A. Darszon. 2001. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 509:119–125. [DOI] [PubMed] [Google Scholar]
- Visconti, P.E., J.L. Bailey, G.D. Moore, D. Pan, P. Olds-Clarke, and G.S. Kopf. 1995. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 121:1129–1137. [DOI] [PubMed] [Google Scholar]
- Visconti, P.E., H. Galantino-Homer, X. Ning, G.D. Moore, J.P. Valenzuela, C.J. Jorgez, J.G. Alvarez, and G.S. Kopf. 1999. Cholesterol efflux-mediated signal transduction in mammalian sperm. β-Cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 274:3235–3242. [DOI] [PubMed] [Google Scholar]
- Walensky, L.D., and S.H. Snyder. 1995. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 130:857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, C.R., B.T. Storey, and G.S. Kopf. 1992. Activation of a Gi protein in mouse sperm membranes by solubilized proteins of the zona pellucida, the egg's extracellular matrix. J. Biol. Chem. 267:14061–14067. [PubMed] [Google Scholar]
- Wassarman, P.M. 1999. Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell. 96:175–183. [DOI] [PubMed] [Google Scholar]
- Wassarman, P.M., L. Jovine, and E.S. Litscher. 2001. A profile of fertilization in mammals. Nat. Cell Biol. 3:E59–E64. [DOI] [PubMed] [Google Scholar]