Abstract
In developing glomeruli, laminin α5 replaces laminin α1 in the glomerular basement membrane (GBM) at the capillary loop stage, a transition required for glomerulogenesis. To investigate domain-specific functions of laminin α5 during glomerulogenesis, we produced transgenic mice that express a chimeric laminin composed of laminin α5 domains VI through I fused to the human laminin α1 globular (G) domain, designated Mr51. Transgene-derived protein accumulated in many basement membranes, including the developing GBM. When bred onto the Lama5 −/− background, Mr51 supported GBM formation, preventing the breakdown that normally occurs in Lama5 −/− glomeruli. In addition, podocytes exhibited their typical arrangement in a single cell layer epithelium adjacent to the GBM, but convolution of glomerular capillaries did not occur. Instead, capillaries were distended and exhibited a ballooned appearance, a phenotype similar to that observed in the total absence of mesangial cells. However, here the phenotype could be attributed to the lack of mesangial cell adhesion to the GBM, suggesting that the G domain of laminin α5 is essential for this adhesion. Analysis of an additional chimeric transgene allowed us to narrow the region of the α5 G domain essential for mesangial cell adhesion to α5LG3-5. Finally, in vitro studies showed that integrin α3β1 and the Lutheran glycoprotein mediate adhesion of mesangial cells to laminin α5. Our results elucidate a mechanism whereby mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin α5 in the GBM.
Keywords: mesangium; cell adhesion; kidney glomerulus; integrin α3β1; transgenic mice
Introduction
Basement membranes are thin, sheetlike structures that abut many cell types, including epithelia, endothelia, muscle, fat, and peripheral nerve. They serve as extracellular barriers and as substrates for cellular interactions. Basement membranes are assembled through complex interactions among the major components: laminins, collagen IV, perlecan, and nidogens (Timpl, 1996). Of these components, it is well-known that laminins regulate various cellular functions such as adhesion, motility, growth, differentiation, and apoptosis through interactions with specific cell surface receptors (Aumailley and Smyth, 1998; Colognato and Yurchenco, 2000). All laminins are composed of three subunits, designated α, β, and γ chains. Five α, four β, and three γ chains have been identified. To date, 15 different laminin heterotrimers have been found to be synthesized and secreted by cells (Burgeson et al., 1994; Miner et al., 1997; Koch et al., 1999; Libby et al., 2000), although many more combinations are theoretically possible. Of the three laminin chain types, only the α chain has a large COOH-terminal globular (G)* domain consisting of a tandem array of five small laminin-type globular (LG) modules LG1 through LG5 (Timpl et al., 2000). These LG modules contain binding sites for β1 integrins and heparin, as well as for α-dystroglycan and the Lutheran blood group glycoprotein (Lu) in some isoforms (Talts and Timpl, 1999; Kikkawa et al., 2002). Therefore, laminin α chains play pivotal roles in laminin-mediated cellular functions.
Genetic inactivation of laminin α chains has also demonstrated that they have specific functions. Mutation in laminin α2 results in congenital muscular dystrophy (Helbling-Leclerc et al., 1995). Mutations in laminin-5 (α3β3γ2) subunits lead to junctional epidermolysis bullosa, a severe skin blistering disease (Pulkkinen and Uitto, 1999). Targeted deletion of laminin α4 leads to impaired microvessel maturation and aberrant localization of neuromuscular synaptic specializations (Patton et al., 2001; Thyboll et al., 2002). We have shown that mice lacking laminin α5 die during late embryogenesis with several developmental defects, including defects in neural tube closure, digit separation, placentation, and kidney and lung development (Miner et al., 1998; Miner and Li, 2000; Nguyen et al., 2002).
In the kidney, basement membranes serve both as structural barriers for tubular epithelia and as a component of the glomerular filter. The glomerular basement membrane (GBM) contains an atypical assortment of basement membrane protein isoforms, including laminin-11 (α5β2γ1) and collagen α3–α5(IV) (Miner, 1998, 1999). There are transitions in the basement membrane component isoforms that are deposited in the developing GBM (Miner, 1998). During glomerulogenesis, transition of laminin isoforms is especially drastic (Miner et al., 1997; Sorokin et al., 1997a). The nascent GBM initially contains laminin-1 (α1β1γ1) and laminin-8 (α4β1γ1), and laminin-10 (α5β1γ1) joins them at the S-shape stage. By the capillary loop stage, laminin-1 is eliminated from the GBM, and then laminin-9 (α4β2γ1) and laminin-11 (α5β2γ1) begin to accumulate. At maturity, only components of laminin-11 are detected in the GBM (Miner, 1998, 1999). We have previously shown that mice lacking laminin α5 exhibit avascular glomeruli associated with breakdown of the GBM during glomerulogenesis (Miner and Li, 2000). This defect correlates with failure of the developmental switch in laminin α chain deposition in which α5 replaces α1 in the GBM at the capillary loop stage. However, the specific role of laminin α5 in the glomerulus is still undefined.
To investigate domain-specific functions of laminin α5 in developing glomeruli, we analyzed transgenic mice that express chimeric laminin α chains: Mr51 is composed of laminin α5 domains VI through I fused to the human laminin α1 G domain; and Mr5G2 is composed of α5 domains VI through LG2 fused to human α1LG3-5. These chimeras were expressed on the genetic background of the laminin α5 knockout (Lama5 −/−). The developing kidney was analyzed by immunohistochemistry and transmission electron microscopy. We found that the adhesion of mesangial cells to the GBM via the G domain of laminin α5 plays a key role in capillary loop formation during glomerular development. In vitro studies suggested that integrin α3β1 and Lu are the receptors that mediate binding of mesangial cells to laminin α5.
Results
The developmental switch from laminin α1 to α5 during glomerular development
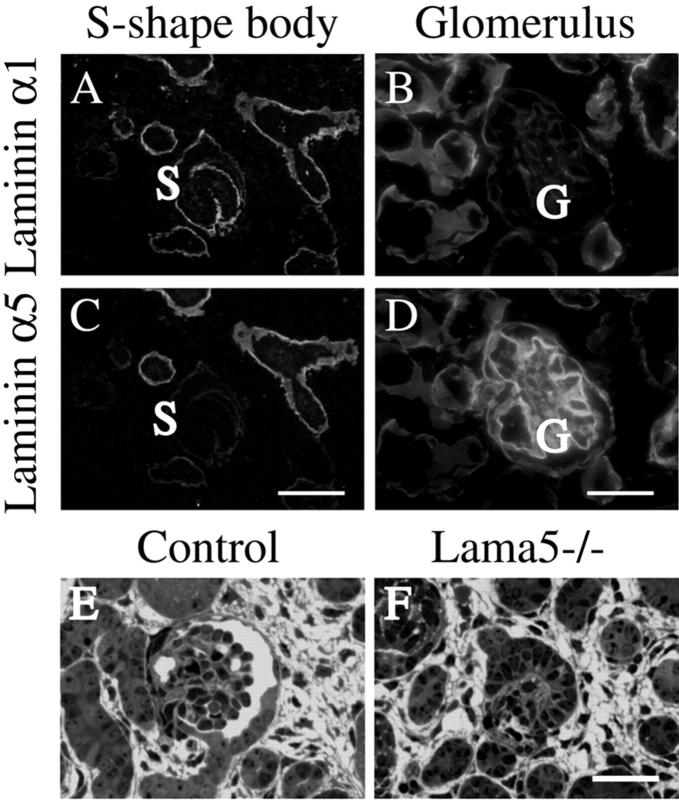
As described in previous papers, transitions in laminin isoform deposition are quite dynamic during kidney development and maturation of the GBM (Miner and Sanes, 1994; Miner et al., 1997; Sorokin et al., 1997a). A crucial developmental switch in laminin α chain deposition occurs in the GBM when the laminin α1 chain, which is predominantly expressed in basement membranes of the S-shape body, is replaced by laminin α5 in the capillary loop stage GBM (Fig. 1 , A–D). In Lama5 −/− mutant glomeruli, where this switch cannot occur, the kidney exhibits avascular glomeruli associated with GBM breakdown (Fig. 1, E and F). The GBM breaks down because laminin α1 is eliminated even in the absence of α5 expression, and without a compensating full-length laminin α chain, basement membrane structure cannot be maintained. As a result of GBM breakdown, the cells that comprise the glomerulus––podocytes, endothelial cells, and mesangial cell––are unable to maintain their proper positions adjacent to the GBM, resulting in failed glomerulogenesis (Miner and Li, 2000). This demonstrates the extreme importance of cell–matrix interactions during glomerulogenesis.
Figure 1.
Laminin α chain switching and its importance during glomerulogenesis. From the S-shaped to the capillary loop stage of glomerular development, the laminin α1 chain (A and B) is replaced by the laminin α5 chain (C and D) in the GBM, though α1 continues to be expressed by proximal tubules seen in B. (E and F) Targeted mutation of Lama5 prevented this developmental transition, resulting in GBM breakdown and failed vascularization of glomeruli. Sections shown are toluidine blue–stained plastic sections of E18.5 control and Lama5 −/− kidneys. S, S-shaped structure; G, glomerulus. Bars: (A and C) 100 μm; (B and D–F) 50 μm.
Expression of the chimeric laminin α chains, Mr51 and Mr5G2, in glomeruli
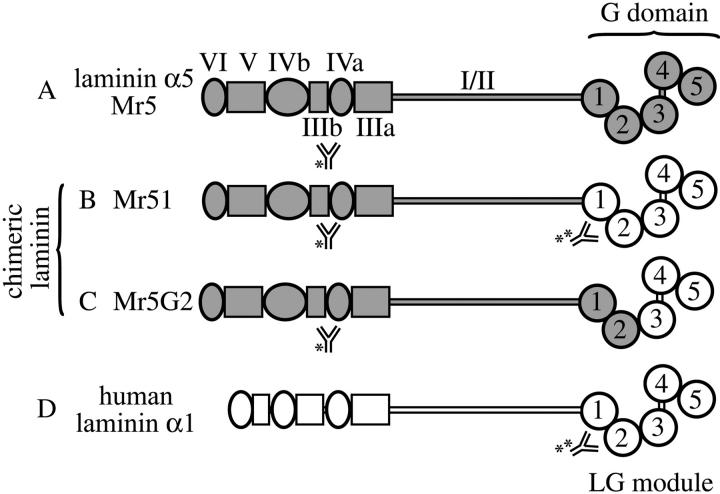
To begin to examine domain-specific functions of laminin α5, we produced transgenic mice expressing two different full-length chimeric laminin α chains. These encoded laminin α5 domains VI through I and VI through LG2 fused to the complete human laminin α1 G domain and α1LG3-5, designated Mr51 and Mr5G2, respectively (Fig. 2, B and C) . We chose to use the human rather than mouse α1 G domain because of the availability of mouse monoclonal antibodies specific for the human domain (Virtanen et al., 2000); thus, transgene-derived proteins could be specifically localized in transgenic mouse tissues. A transgene encoding the full-length mouse α5 chain, designated Mr5 (Fig. 2 A), served as a control. The widely active regulatory element miw (Suemori et al., 1990) was used to drive transgene expression. As described in our previous papers, transgene-derived laminin levels were significantly increased in heart and skeletal muscle (Moulson et al., 2001; Kikkawa et al., 2002). Crossing of the Mr5 transgene onto the Lama5 −/− background revealed that transgene-derived laminin α5 was deposited widely in basement membranes. Expression was sufficient to fully rescue all known Lama5 −/− embryonic defects in two independent lines, and the resulting Lama5 −/−; Mr5 mice are viable and fertile (unpublished observations). These results show that the miw regulatory element directs expression of the transgene in a manner sufficient to replace the missing endogenous α5 wherever it is necessary.
Figure 2.
Structure of wild-type and chimeric laminin chains. The domains present in full-length laminin α5 (A), in the chimeric laminin α chains (B and C), and in full–length human α1 (D) are shown. (B) Mr51 contains human laminin α1 G domain linked to domains VI through I of mouse laminin α5. (C) Mr5G2 contains laminin α5 domains VI through α5 LG2 fused to the human laminin α1LG3-5 domain. Anti–mouse laminin α5 (*) and anti–human laminin α1 LG1–2 (**) antibody epitopes are indicated.
Expression of the two chimeric laminins was also widespread, as determined by staining with the anti–human α1 LG1–2 and LG4–5 domain antibodies (Kikkawa et al., 2002; Fig. 3 ; unpublished data). The Mr51 and Mr5G2 transgenes were separately mated onto the Lama5 mutant genetic background, but the resulting Lama5 −/−; Mr51 and Lama5 −/−; Mr5G2 mice were not viable. Lama5 −/−; Mr51 embryos exhibited the same defects we initially reported for Lama5 −/− embryos (Miner et al., 1998), but the incidence of exencephaly was reduced from 60% to <10%. On the other hand, although defects in digit septation were still observed, the Mr5G2 transgene improved the growth of Lama5 −/− embryos, which are usually smaller than controls (Miner et al., 1998). This indicates that the chimeric proteins have limited functions, and that the G domain of α1 cannot fully compensate for the missing α5 G domain.
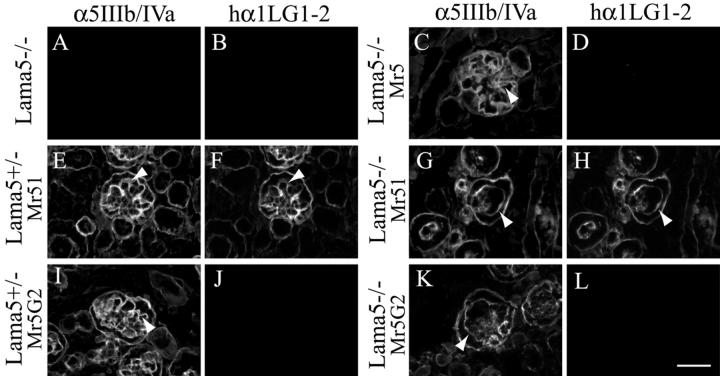
Figure 3.
Expression of the chimeric α chains, Mr51 and Mr5G2, in transgenic mice. Micrographs show E17.5 glomeruli from Lama5 −/− (A and B), Lama5 −/−; Mr5 (C and D), Lama5 +/−; Mr51 (E and F), Lama5 −/−; Mr51 (G and H), Lama5 +/−; Mr5G2 (I and J), and Lama5 −/−; Mr5G2 (K and L) embryos. Sections were doubly stained with an antiserum to domains IIIb/IVa of laminin α5 (A, C, E, G, I, and K) and a mouse monoclonal antibody specific for human laminin α1 LG1–2 (B, D, F, H, J, and L). Although the chimeric laminins were assembled into the GBM, Lama5 −/−; Mr51 and Lama5 −/−; Mr5G2 glomeruli exhibited distended capillaries (G, H, and K). Arrowheads indicate the GBM. Bar, 50 μm.
In fetal kidney, Mr51 and Mr5G2 were detected in the GBM on both the control and Lama5 −/− genetic backgrounds (Fig. 3, F–H and K), suggesting that incorporation of the chimeric laminin chains into the GBM might be capable of preventing the breakdown normally observed in Lama5 −/− glomeruli. There was no apparent effect of the chimeric laminins on Lama5 +/− control glomeruli (Fig. 3, E, F, and I), and the mice were healthy. However, the glomeruli of Lama5 −/−; Mr51 and Lama5 −/−; Mr5G2 embryos exhibited greatly reduced or absent capillary looping (Fig. 3, G, H, and K).
Identification of cell types in glomerular structures
To further investigate the effects of Mr51 and Mr5G2 on glomerulogenesis, we used cell type–specific antibodies to identify the three cell types found in glomeruli. Frozen sections of E17.5 control, Lama5 −/−, Lama5 −/−; Mr51, and Lama5 −/−; Mr5G2 kidneys were stained with antibodies to WT1, platelet endothelial cell adhesion molecule (PECAM), and desmin to label podocytes, endothelial cells, and mesangial cells, respectively (Fig. 4 , green), and doubly labeled with an anti–basement membrane antibody (Fig. 4, red). In the control, podocytes were observed in a single cell layer epithelium adjacent to the glomerular capillaries (Fig. 4 A), and mesangial cells, which provide tension to maintain the glomerular capillary loop structure, were found associated with endothelial cells in the interior of the glomerulus (Fig. 4 I). In the Lama5 −/− mutant, the podocytes were in disarray, and the endothelial cells and mesangial cells were extruded from glomerulus, as we showed previously (Miner and Li, 2000; Fig. 4, B, F, and J). In Lama5 −/−; Mr51 glomeruli, the transgene-derived chimeric laminin chain partially rescued the defects observed in the mutant. The podocytes were arranged in a single cell layer (Fig. 4 C), and the endothelial cells and mesangial cells were localized in the interior of the glomerulus, similar to the control (Fig. 4, G and K). These results suggest that the COOH-terminal portion of laminin α5 is dispensable for the assembly of the GBM and arrangement of podocytes. However, the great reduction in capillary looping (Fig. 4, C and K) is indicative of a mesangial cell defect because a similar phenotype has been observed in the total absence of mesangial cells in mice lacking either PDGF B or PDGF receptor β (Lindahl et al., 1998). Lama5 −/−; Mr5G2 glomeruli exhibited a very similar aberrant glomerular phenotype (Fig. 4, D, H, and L).
Figure 4.
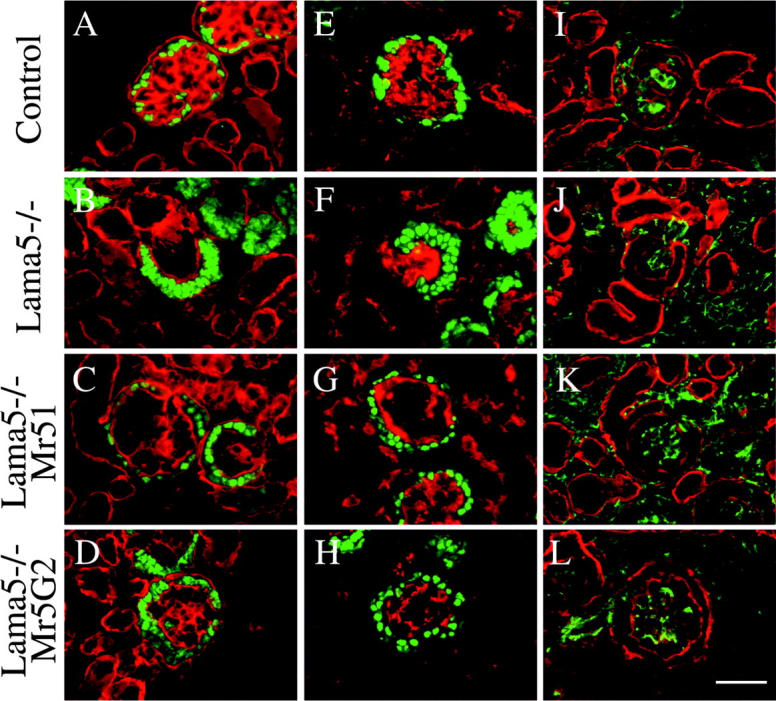
Identification and localization of cell types in developing glomeruli. E17.5 control, Lama5 −/−, Lama5 −/−; Mr51, and Lama5 −/−; Mr5G2 frozen kidney sections (as indicated) were stained with antibodies to WT1 to label podocytes (A–H, green), PECAM to label endothelial cells (E–H, red), and desmin to label mesangial cells (I–L, green). Basement membranes were stained with anti–laminin γ 1 monoclonal antibody (A–D, red) or anti–laminin-1 polyclonal antibody (I–L, red). In Lama5 −/−; Mr51 or Mr5G2 glomeruli, the podocytes were arranged properly in a single cell layer at the periphery (C and D), and the glomerular endothelial cells were properly localized (G and H). However, defective capillary loop formation was observed, despite the presence of mesangial cells (K and L). Bar, 50 μm.
Analysis of glomerular ultrastructure and podocyte gene expression
To further investigate the fine structure of the GBM and adjacent cells, we used transmission electron microscopy to visualize E17.5 control, Lama5 −/− and Lama5 −/−; Mr51 glomeruli (Fig. 5) . In the control, the GBM was clearly visible between podocytes and endothelial cells (Fig. 5, A and G). Mesangial cells were attached to the GBM at the bases of the capillary loops (Fig. 5 D); this allows them to maintain the glomerular capillary loop structure. In the Lama5 −/− glomerulus, the absence of laminin α5, coupled with the programmed disappearance of laminin α1, resulted in breakdown of the GBM by the late capillary loop stage (Fig. 5, B and E). In the Lama5 −/−; Mr51 glomerulus, the ultrastructure of the GBM was similar to that observed in control (Fig. 5, C and H), confirming that the transgene-derived chimeric laminin α chain must have been assembled into the GBM, as previously demonstrated by immunofluorescence (Fig. 3). This suggests that the short and long arm regions of laminin α5 are sufficient for directing incorporation of the chimera into the GBM. Although podocytes extended foot processes in both control and Lama5 −/−; Mr51 glomeruli (Fig. 5, A, C, G, and H), there appeared to be fewer processes in the latter. This may suggest that the α5 G domain has some role in inducing process extension. However, slit diaphragms, the delicate tight and adherens junctions–related structures that are required for glomerular filtration (Kerjaschki, 2001; Miner, 2002), were observed between many of those foot processes that did form (Fig. 5 H), indicating that this crucial aspect of podocyte differentiation was progressing appropriately. To investigate this in more detail, we used antibodies to slit diaphragm– and foot process–associated proteins. The results were essentially similar for the three genetic backgrounds; all podocytes expressed nephrin, CD2AP, podocin, and synaptopodin (unpublished data). The straightforward interpretation is that neither GBM composition nor its integrity is relevant for expression of these proteins. However, it is important to note that initiation of expression of these proteins has been shown to occur as early as the S-shape stage of glomerular development, when the GBM is structurally sound even in the Lama5 −/− background. Thus, we cannot rule out the possibility that podocyte interactions with the basement membrane at early stages are required for their proper differentiation and gene expression in vivo.
Figure 5.
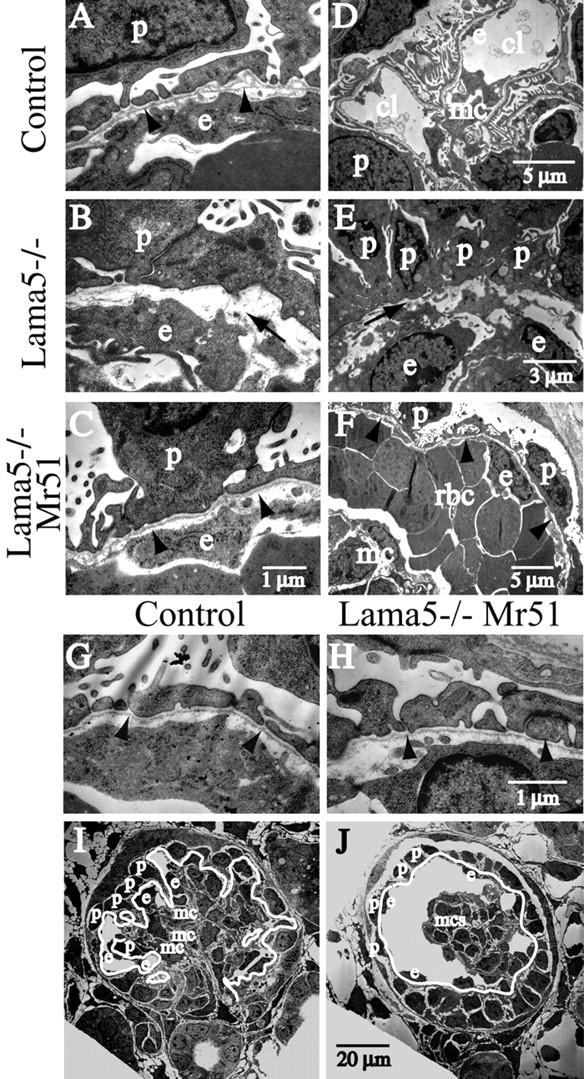
Ultrastructural analysis of glomeruli. Low and high magnification electron micrographs show E17.5 glomeruli from control, Lama5 −/−, and Lama5 −/−; Mr51 kidneys, as indicated. The normally continuous basement membrane underlying the podocytes in control (A and G, arrowheads) was disrupted in the Lama5 −/− mutant (B and E, arrows). The chimeric laminin Mr51 rescued assembly of the basement membrane and formation of podocyte foot processes on the Lama5 −/− genetic background (C and H, arrowheads). In the control (D and I), mesangial cells (mc) bound the GBM (I, white line) to maintain the capillary loop (cl) structure. In Lama5 −/−; Mr51 glomeruli (F and J), the ballooning of the capillaries, which were commonly filled with red blood cells (F, rbc), was associated with detachment of mesangial cells from the GBM (F, arrowheads; J, white line). However, podocytes (p) and endothelial cells (e) maintained their normal positions on either side of the GBM. To better present the structure of glomeruli, red blood cells were removed digitally in I and J.
Low power electron microscopic observations of Lama5 −/−; Mr51 glomeruli revealed distension of the glomerular capillaries and detachment of mesangial cells from the GBM (compare Fig. 5, F and J with Fig. 4, C and K). We conclude that the binding of mesangial cells to the α5 G domain, which is absent in Lama5 −/−; Mr51 glomeruli, is essential for maintenance of this normal capillary loop structure, an example of which is shown in the electron micrographs from control glomeruli (compare Fig. 5, D and I with Fig. 4 I). No such structure was ever observed in Lama5 −/− glomeruli (Fig. 5 E; unpublished data), which are extremely disorganized (Miner and Li, 2000).
Molecular composition of the Lama5 −/−; Mr51 glomerular basement membrane
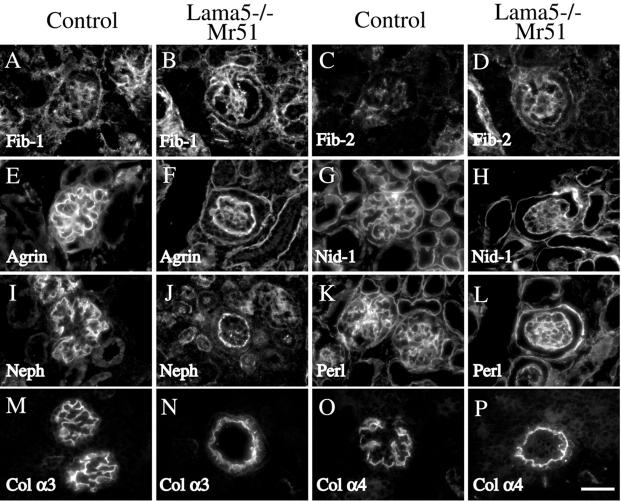
The distended capillaries observed upon substitution of Mr51 transgene–derived protein for endogenous α5 could result from secondary defects in basement membrane composition. For example, Kostka et al. (2001) recently reported that 10–40% of glomeruli in fibulin-1–deficient mice exhibit a similar capillary malformation. Thus, the distended capillaries in Lama5 −/−; Mr51 glomeruli could be secondary to the failure of fibulin-1 to incorporate into the GBM. To investigate whether deposition of fibulin-1 or other GBM components was altered in Lama5 −/−; Mr51 glomeruli, we stained E17.5 kidney sections from control and Lama5 −/−; Mr51 fetuses with a panel of antibodies to GBM proteins (Fig. 6 ; unpublished data). Fibulin-1 was detected in both the GBM and the mesangial matrix on the Lama5 −/−; Mr51 background, suggesting that the distended capillary loops were not secondary to the absence of fibulin-1. We also examined the expression of fibulin-2, agrin, nidogen-1/entactin-1, nephronectin, perlecan, and the collagen α3(IV) and α4(IV) chains, and in no case was there a significant difference between control and Lama5 −/−; Mr51 glomeruli (Fig. 6). Again, this suggested that the defects observed in Lama5 −/−; Mr51 glomeruli are not due to the disappearance of other GBM or mesangial matrix components. However, we did find greatly reduced levels of the laminin β2 chain (Fig. 7, E and G) , whereas laminin β1 was present in both control and Lama5 −/−; Mr51 maturing glomeruli (Fig. 7, A and C).
Figure 6.
Deposition of ECM proteins in control and Lama5 −/−; Mr51 glomeruli. Sections of E17.5 control and Lama5 −/−; Mr51 kidneys were stained with specific antibodies to fibulin-1 (A and B), fibulin-2 (C and D), agrin (E and F), nidogen-1/entactin-1 (G and H), nephronectin (I and J), perlecan (K and L), collagen α3(IV) (M and N), and collagen α4(IV) (O and P). The mutant GBM had a composition very similar to the control. Bar, 50 μm.
Figure 7.
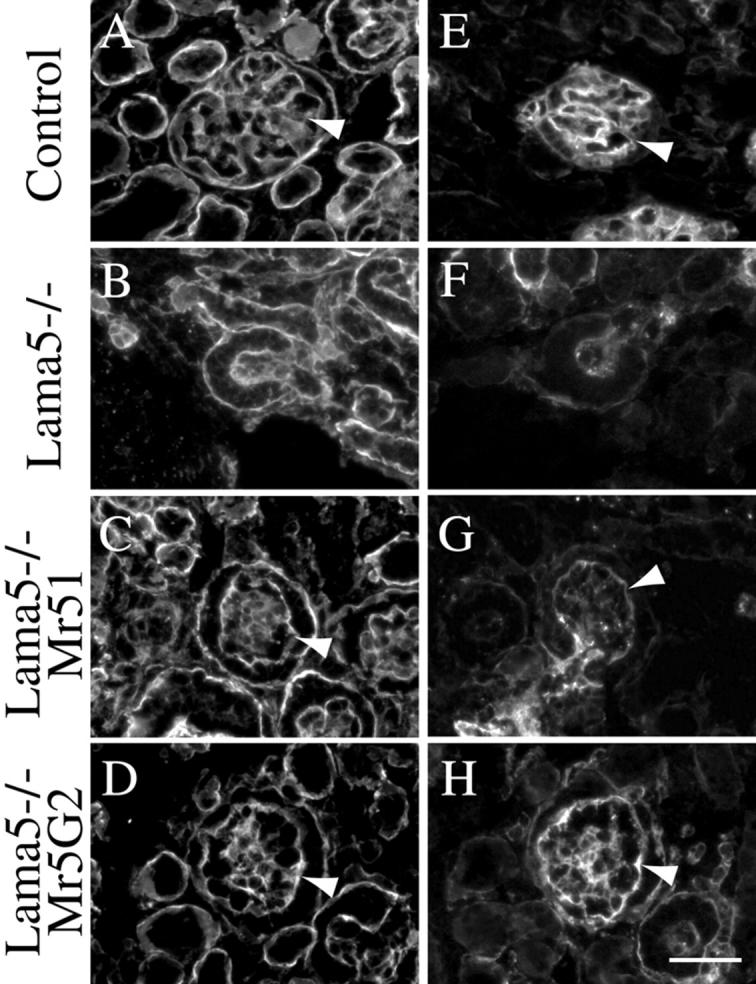
Expression of laminin β chains in glomeruli. Sections of E17.5 control (A and E), Lama5 −/− (B and F), Lama5 −/−; Mr51 (C and G) and Lama5 −/−; Mr5G2 (D and H) kidneys were stained with specific antibodies to laminin β1 (A–D) and β2 (E–H). Arrowheads indicate GBM. Laminin β2 was almost undetectable in Lama5 −/−; Mr51 GBM (G), but Mr5G2 increased β2 accumulation (H), though it was somewhat lower than that in the control (E). Bar, 50 μm.
Laminin β2 normally trimerizes with the laminin α5 and γ1 chains to form laminin-11, which is first detected at the capillary loop stage of glomerulogenesis (Miner and Sanes, 1994; Miner et al., 1997). The paucity of laminin β2 in Lama5 −/−; Mr51 GBM indicates either that β2 does not efficiently associate with the Mr51 chimera to form a trimer, or that the trimer forms but does not efficiently incorporate into the GBM. On the other hand, β2 did efficiently incorporate into the GBM in the presence of Mr5G2 (Fig. 7 H). Although little is known about how specificity of laminin trimerization or incorporation into basement membranes is determined, these results suggest that it may be encoded in the α chain G domain. Indeed, laminin α1 and β2 are only rarely found in the same basement membrane (unpublished observations), perhaps because the α1 G domain directs preferential assembly with β1. Nevertheless, the laminin-3 trimer (α1β2γ1) has been isolated from placenta (Champliaud et al., 2000), suggesting that whatever code exists could be cell type–specific. In any event, although laminin β2 knockout mice exhibit a congenital nephrotic syndrome (Noakes et al., 1995), there is no capillary loop distension similar to that observed here. Thus, these capillary loop defects cannot be ascribed to lack of either β2 or any other GBM component for which we have antibodies. The straightforward conclusion is that mesangial cells organize the glomerular capillary loops by adhering to the G domain of laminin α5 in the GBM, and that they are unable to adhere to the α1 G domain modules present in the Mr51 and Mr5G2 chimeras. These results point to α5LG3–5 as necessary for effective mesangial cell adhesion.
Adhesion of mesangial cells to laminins in vitro
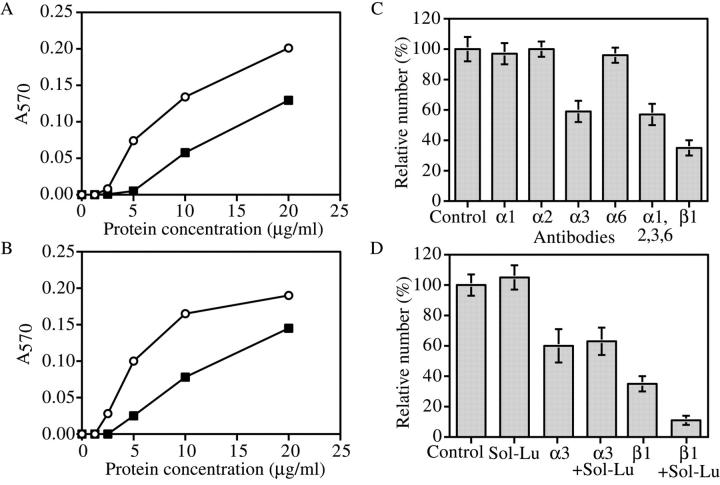
To further investigate interactions between mesangial cells and the laminin α5 G domain, we turned to in vitro adhesion assays that used primary human and rat mesangial cells and purified laminin preparations. We have not yet been able to isolate laminin trimers containing the chimeric α chains, and G domain preparations were not available, so we chose to use commercially available laminins. First, we compared the abilities of laminin-10/11 (α5β1/2γ1) and laminin-1 (α1β1γ1) to promote adhesion of mesangial cells. Quantitative analysis of both human and rat mesangial cell adhesion to surfaces coated with increasing concentrations of these proteins showed that laminin-10/11 had higher cell adhesion activity than laminin-1, especially at the lower protein concentrations (Fig. 8, A and B) . In addition, the cells spread less well on laminin-1 than they did on laminin-10/11 (unpublished data). These data provide an explanation as to why mesangial cells adhere poorly to GBM containing the α1 G domain (Fig. 5, F and J) but adhere well to normal GBM containing the α5 G domain (Fig. 5, D and I). We believe that these in vitro data using laminin trimers justify our conclusions concerning adhesiveness of G domains because in vivo mesangial cells adhere to wild-type α5 but not to Mr51, which contains α5 domains VI through I. Thus, adhesion to laminin-10/11 trimers is likely mediated primarily by the α5 G domain.
Figure 8.
Analysis of mesangial cell adhesion to laminins in vitro. 96-well plates were coated with increasing concentrations of laminin-1 (closed squares) or laminin-10/11 (open circles) and incubated with mesangial cells at 37°C for 1 h. Human (A) and rat (B) mesangial cells readily attached to laminin-10/11, but were significantly less adherent to laminin-1. (C) Identification of receptors mediating adhesion of mesangial cells to laminin-10/11. Human mesangial cells preincubated with function-blocking antibodies against the indicated integrin subunits were added to laminin-10/11–coated wells. Values are expressed as percentages of the number of cells adhering in the absence of monoclonal antibodies. Each column represents the mean of triplicate assays. Error bars show the standard deviation. Anti–integrin α3 and β1 antibodies partially inhibited the adhesion of human mesangial cells to laminin-10/11, indicating that integrin α3β1 is a primary receptor. (D) The effect of sol-Lu on adhesion of human mesangial cells to laminin-10/11. Sol-Lu had little inhibitory effect on its own, but cooperated with anti–integrin β1 antibodies to inhibit adhesion by ∼90%. This indicates that Lu cooperates with integrin α3β1 to mediate mesangial cell adhesion to laminin α5 in the GBM.
Identification of receptors mediating mesangial cell adhesion to laminin-10/11
We next wished to investigate the mechanism of mesangial cell adhesion to the α5 G domain. Cell adhesion to laminin-10/11 is mediated predominantly by the integrin family of adhesion receptors (Kikkawa et al., 1998, 2000). Therefore, we examined the effects of function-blocking monoclonal antibodies against various integrin subunits on human mesangial cell adhesion to laminin-10/11 (Fig. 8 C). Anti–integrin α3 and β1 antibodies significantly inhibited the adhesion of human mesangial cells to laminin-10/11. On the other hand, monoclonal antibodies to integrin α1, α2, and α6 had no effect on adhesion, even when combined with the anti–integrin α3 antibody (Fig. 8 C). Together, these results indicate that integrin α3β1 is one primary receptor that mesangial cells use to adhere to laminin-10/11.
Recently, it has been reported that Lu, a member of the Ig superfamily, is a potential nonintegrin receptor for the laminin α5 chain (Lee et al., 1998; Udai et al., 1998; Parsons et al., 2001). A splice variant of Lu present in humans that should also bind laminin α5 is known as basal cell adhesion molecule (El Nemer et al., 1998; Zen et al., 1999). In our previous studies, we showed that Lu is expressed on the surface of a subset of muscle and epithelial cells in diverse tissues adjacent to basement membranes containing the laminin α5 chain (Moulson et al., 2001). We have also identified Lu to be a specific receptor for laminin α5 via binding to the α5 G domain by using a recombinant form of soluble Lu (sol-Lu) that contains only the Lu extracellular domain; in addition, the presence of α5LG3 was required for binding (Kikkawa et al., 2002). Lu was coexpressed with integrin α3 on mouse mesangial cells in vivo (unpublished data). To examine whether Lu is also involved in adhesion of mesangial cells to laminin α5, sol-Lu was used in adhesion/inhibition assays (Fig. 8 D). In theory, sol-Lu should bind Lu binding sites on laminin α5 and prevent the Lu present on mesangial cells from interacting with α5. Although sol-Lu alone had no effect on mesangial cell adhesion to laminin-10/11, a significant inhibitory effect was observed when it was combined with the anti–integrin β1 antibody (Fig. 8 D). Thus, Lu may be a secondary receptor for adhesion of mesangial cells to laminin-10/11. In addition, the fact that sol-Lu did not enhance the anti–integrin α3 antibody inhibition suggests that there may be another integrin α subunit that is involved.
Discussion
We and others have previously shown that the laminin α5 chain is widely expressed in mice (Miner et al., 1995, 1997; Sorokin et al., 1997b). Based on knockout studies, laminin α5 has roles in several important developmental processes, including neural tube closure, digit septation, placentation, and lung and kidney development (Miner et al., 1998; Miner and Li, 2000; Nguyen et al., 2002). We found that most of the developmental defects are associated with basement membrane breakdown or discontinuity resulting from the absence of α5. Here, we have begun to investigate the role of laminin α5 using a combined transgenic/knockout approach, which effectively substitutes either all or part of the α5 G domain with analogous segments of α1. The result is expression of full-length chimeric laminin α chains capable of trimerizing with β and γ chains and incorporating into basement membranes. A similar strategy was previously used in vitro to map a synaptic basement membrane localization domain on the laminin β2 chain (Martin et al., 1995). This approach has allowed us to uncover a laminin α5 G domain–specific function in glomerulogenesis that might never have been found through traditional knockouts. It also demonstrates the feasibility of using widely expressed transgenes encoding altered basement membrane proteins to replace existing knocked out genes, effectively generating knockins without the use of further gene targeting in ES cells.
Expression of the Mr51, and presumably Mr5G2, chimeric α chains on the Lama5 −/− background was able to rescue the breakdown of the GBM (Fig. 5) that normally occurs in Lama5 −/− glomeruli when laminin α1 is eliminated (Miner and Li, 2000). The mechanism of α1 elimination is unknown; but if it is not purely transcriptional, then it must somehow be selective for α1 because α5 is not eliminated. It is likely that primary sequence differences or domain structural differences account for the selective elimination of α1. The G domain of α1, present in Mr51, could have carried a signal for elimination, but our results suggest this not to be the case because Mr51 was not eliminated. We are continuing to investigate this interesting issue using additional α chain transgenes.
The major defect in the Lama5 −/−; Mr51 and Lama5 −/−; Mr5G2 embryos was ballooning of the glomerular capillaries. This same defect was observed in mice lacking mesangial cells due to absence of PDGFB/PDGF receptor β signaling (Lindahl et al., 1998). However, in our case, mesangial cells were clearly present (Fig. 4, K and L, and Fig. 5 J), so we concluded that they must not be adhering properly to the GBM to maintain capillary looping. As the only known differences between normal and Lama5 −/−; Mr51/Mr5G2 GBMs are the G domain substitutions, and laminin α chain G domains have been shown to harbor recognition sites for numerous cell adhesion receptors (Colognato and Yurchenco, 2000), we hypothesized that mesangial cells normally adhere to the α5 G domain but were unable to adhere tightly to either the complete α1 G domain or to α1 LG3–5. Our in vitro studies confirmed that both human and rat mesangial cells adhere better to α5-containing laminins than to α1-containing laminin (Fig. 8, A and B).
With regard to mechanisms for mesangial adhesion to the α5 G domain, mesangial cells express several β1 integrins, including α1β1, α2β1, α3β1, α5β1, α6β1, and α8β1 (Gauer et al., 1997; Sterk et al., 1998; unpublished observations). Furthermore, immuno-EM studies have shown that β1-containing integrins are concentrated at the mesangial cell surface adjacent to the GBM and the mesangial matrix (Kerjaschki et al., 1989). Antibody inhibition studies demonstrated that integrin α3β1 plays a major role in mesangial cell adhesion to laminin-10/11 (Fig. 8). In support of this, the glomerular capillaries of Itga3 −/− kidneys are dilated (Kreidberg et al., 1996), suggesting a defect in mesangial adhesion to the GBM but the fact that the capillaries are not ballooned suggests that another receptor normally cooperates with α3β1 and is able to partially compensate in Itga3 −/− mesangial cells. We found that Lu is expressed on mouse mesangial cells and cooperates with β1 integrins to mediate adhesion in vitro (unpublished data; Fig. 8 D). The fact that Lu was found to be involved is consistent with the fact that Mr5G2 does not support capillary loop formation in vivo, because we have shown that sol-Lu does not bind Mr5G2 (Kikkawa et al., 2002). Lu mutant mice being generated in our laboratory will allow us to more directly address the function of Lu in glomerulogenesis.
An important issue to consider here is the relationship of mesangial cells with laminins in the mesangium, a non–basement membrane ECM, which mesangial cells secrete and in which they are embedded. Several different laminins are found in the mesangium, including substantial amounts of laminins-1 (α1β1γ1), -2 (α2β1γ1), and -10 (α5β1γ1), but others can be detected at lower levels (Miner, 1999). It has not been possible to determine the relative levels of these laminins, but one would suspect that, based on our findings, decreased levels of laminin-10 or increased levels of laminin-1, as might occur in disease states, could correlate with reduced adhesion of mesangial cells to the mesangial matrix. On the other hand, the fact that mesangial cells are almost totally surrounded by their matrix may make this issue irrelevant, as weaker adhesion may be tolerated, both in disease and in normal states. This would be in contrast to the relationship of mesangial cells to the GBM, with which they make contact only at the bases of the capillary loops. A more robust adhesion to the GBM may be necessary in this setting of limited contact in order to counteract the force of blood pressure. Therefore, interaction with the G domain of α5, normally the only α chain in the GBM, would ensure a tight adhesion.
Two other laminin mutant mice with kidney defects have been described. In mice lacking laminin β2, the β1 chain compensates and allows an ultrastructurally normal basement membrane to form. However, the glomerular filter fails as a barrier to plasma proteins, and the mice die at 3 wk of age with massive proteinuria (Noakes et al., 1995). No defects in capillary looping were observed, consistent with the fact that laminin α5, as part of laminin-10 (α5β1γ1), is present in the GBM (unpublished observations); thus, mesangial cells should still be capable of binding to the GBM and maintaining capillary looping. On the other hand, mice lacking the binding site for nidogen on laminin γ1 exhibit glomerular capillary aneurysms similar to the ballooned capillaries we have reported here. The aneurysms were associated with GBM discontinuities (Willem et al., 2002), and we suggest that these GBM defects prevent mesangial cells from adhering and maintaining the integrity of the capillary loops.
Integrin α3β1 is expressed basally on podocytes (Korhonen et al., 1990; Kreidberg et al., 1996), yet detachment of podocytes from the GBM in Lama5 −/− Mr51/Mr5G2 glomeruli, as occurred with mesangial cells, was not observed. There are two possibilities to explain this. First, integrin α3β1 on podocytes may serve primarily as a signal transducing receptor rather than as an anchoring one. Dystroglycan is also expressed on podocytes but not on mesangial cells (unpublished data), and together with Lu, this may be sufficient for adhesion of podocytes to the GBM. Second, podocytes and mesangial cells may be adhering only weakly to the chimeric laminins through integrin α3β1. This may be sufficient for long-term adhesion of podocytes to the GBM but not for mesangial cells. Capillary looping was evident in immature Lama5 −/−; Mr51/Mr5G2 glomeruli, but mesangial cell adhesion to the GBM was apparently too weak to counteract the force of blood pressure, leading to de-adhesion and capillary ballooning. In support of this is our finding that adhesion activity of laminin-10/11 (α5β1/2γ1) was stronger than that of laminin-1 (α1β1γ1) for both human and rat mesangial cells (Fig. 8, A and B).
In conclusion, the laminin α5 chain plays a crucial role in maintaining glomerular capillary loop structure. We mapped the adhesive site in vivo to the LG3–5 modules of the G domain. Adhesion of mesangial cells to the laminin α5 G domain is mediated by integrin α3β1 and Lu. Mesangial cells express contractile proteins and are similar to smooth-muscle cells. Their frequency and extent of contraction in response to vasoactive substances are thought to determine the glomerular filtration rate. It is interesting to speculate that defective interactions between mesangial cells and laminin α5 in the GBM may be a feature of diverse glomerulopathies in the adult kidney.
Materials and methods
Proteins and antibodies
Mouse laminin-1 (α1β1γ1) and human laminin-10/11 (α5β1/β2γ1) were purchased from Invitrogen. Soluble recombinant Lu extracellular domain (sol-Lu) was produced and characterized as described previously (Kikkawa et al., 2002). Monoclonal antibody against human laminin α1 LG1–2 (161EB7) has been described previously (Virtanen et al., 2000). Rat monoclonal antibody 8B3 to laminin α1 (Abrahamson et al., 1989) was a gift from Dr. D. Abrahamson (University of Kansas Medical Center, Kansas City, KS). Polyclonal antibodies against laminin-1 (Sanes et al., 1990) and agrin were gifts from Dr. J. Sanes (Washington University School of Medicine, St. Louis, MO). Rat monoclonal antibodies MAB1914 to laminin γ1, MAB1946 to entactin/nidogen-1, and MAB1948 to perlecan were purchased from CHEMICON International, Inc. Polyclonal antibodies against the following mouse laminins and fibulins were gifts from Drs. R. Timpl and T. Sasaki (Max-Planck Institute, Martinsried, Germany): domain VI of laminin α1, domain IV of laminin β1, domain IV of laminin β2, fibulin-1C, and fibulin-2 (Pan et al., 1993; Ettner et al., 1998; Sasaki et al., 2002). Rat monoclonal antibodies against collagen α3(IV) (H31) and α4(IV) (H43) chains have been described previously (Ninomiya et al., 1995). Rabbit antibody against nephronectin (Brandenberger et al., 2001) was a gift from Dr. L. Reichardt (University of California, San Francisco, San Francisco, CA). Rabbit antibody against domain IIIb/IVa of mouse laminin α5 was produced and characterized as described previously (Miner et al., 1997). Rat monoclonal antibody MEC 13.3 to PECAM was purchased from BD Biosciences. Rabbit antiserum sc-192 to Wilms' tumor protein (WT1) was purchased from Santa Cruz Biotechnology, Inc. Mouse monoclonal antibody D33 to desmin was purchased from DakoCytomation. Mouse monoclonal antibody to synaptopodin (Mundel et al., 1991) was a gift from Dr. P. Mundel (Albert Einstein College of Medicine, Bronx, NY). Rabbit antibodies to CD2AP, nephrin, and podocin have been described previously and were gifts from A. Shaw (Washington University School of Medicine), L. Holzman (University of Michigan Medical School, Ann Arbor, MI), and C. Antignac (Necker Hospital, Paris, France; Dustin et al., 1998; Holzman et al., 1999; Roselli et al., 2002). Monoclonal antibodies against human integrin α1 (FB12), α2 (P1E6), α3 (P1B5), and α6 (GoH3) were purchased from CHEMICON International, Inc.
Preparation of the chimeric laminin constructs
Preparation of the chimeric laminin α chains, designated Mr51 and Mr5G2, has been described in Kikkawa et al. (2002). Both chimeric laminin cDNAs were cloned into a modified version of the widely active expression vector miw, which contains the RSV LTR inserted into the chicken β-actin promoter (Suemori et al., 1990).
Generation of knockout and transgenic mice
Production of Lama5 mutant mice and of transgenic mice expressing a full-length laminin α5 transgene or the chimeric laminin transgenes has been described previously (Miner et al., 1998; Moulson et al., 2001; Kikkawa et al., 2002). Five independent Mr51 lines, all of which gave similar results, and one Mr5G2 line were produced.
Immunohistochemistry
For immunohistochemistry, mouse embryos from timed matings were frozen whole by immersing in OCT compound and quick-freezing in 2-methylbutane cooled in a dry ice ethanol bath. Sections were cut at 7 μm in a cryostat and air-dried. For staining, sections were blocked in 10% normal goat serum and incubated with primary antibody. All antibody incubations were in PBS containing 1% BSA, and all washes were in PBS. Secondary antibodies were conjugated to FITC (ICN Biomedicals) or Cy3 (CHEMICON International, Inc.). After several washes, sections were mounted in 90% glycerol containing 0.1× PBS and 1 mg/ml p-phenylenediamine. Sections were examined through a microscope (Eclipse E800; Nikon). Images were captured with a Spot 2 cooled color digital camera (Diagnostic Instruments) using Spot Software Version 2.1. Images were imported into Adobe Photoshop 5.0 and Adobe Illustrator 9.0 for processing and layout.
For semi-thin and thin sectioning, embryonic kidneys were fixed in 4% paraformaldehyde, 4% glutaraldehyde in 0.1 M cacodylate buffer and processed as described previously (Noakes et al., 1995). 2-μm sections were cut with a glass knife and stained with toluidine blue for light microscopy. Thin sections were cut with a diamond knife and stained with lead citrate plus uranyl acetate for transmission electron microscopy. Reagents were obtained from Polysciences Inc.
Cell culture and adhesion assay
Normal human mesangial cells were purchased from Cambrex Life Science Corporation. Cells were grown in MsGM medium supplied by Cambrex Life Science Corporation and used within seven passages. Primary rat mesangial cells were provided by Q. Yu and A.R. Morrison (Washington University School of Medicine). Cells were grown in DME supplemented with 20% FBS, 10 μg/ml insulin, 1 mM glutamate, and 1 mM sodium pyruvate (Invitrogen) and used within seven passages. Adhesion assays were performed as described previously (Kikkawa et al., 2000). In brief, 20 μg/ml of commercial laminin-10/11 was coated onto a 96-well plate (Nunc) at 37°C for 1 h. The wells were blocked with 1% BSA. Mesangial cells were trypsinized and allowed to recover in serum-free medium for ∼30 min and 100 μl of mesangial cells at 105 cells/ml in DME were added to the wells. After a 1-h incubation, the attached cells were stained with 0.2% crystal violet in 20% methanol for 10 min, 100 μl of 1% SDS was added to dissolve the cells, and absorbance was measured at 570 nm by VERSAmax (Molecular Devices). To identify the receptors for laminin-10/11, 10 μg/ml of monoclonal antibodies against different integrins and the recombinant sol-Lu protein were preincubated individually with mesangial cells in a volume of 50 μl of serum-free DME (5 × 103 cells/well) at room temperature for 15 min. The preincubated cells were transferred to plates coated with laminin-10/11 and incubated further at 37°C for 20 min. After incubation, the attached cells were stained with 0.2% crystal violet in 20% methanol for 10 min and counted under the microscope.
Acknowledgments
We thank Cong Li for technical assistance, the Mouse Genetics Core at Washington University School of Medicine for generating and caring for transgenic mice, Jacqueline L. Mudd for generating the Mr5 transgenic mice, Qing Yu and Aubrey Morrison for providing rat mesangial cells, Marilyn Levy for electron microscopy support, and Karl Tryggvason for supplying the human laminin α1 cDNA. We also thank Joshua Sanes, Louis Reichardt, Dale Abrahamson, Rupert Timpl, Takako Sasaki, Andrey Shaw, Lawrence Holzman, Corinne Antignac, and Peter Mundel for providing antibodies.
This work was supported by grants to J.H. Miner from the National Institutes of Health (P50DK045181 and R01GM060432) and in part by research grants from the March of Dimes (6-FY99-232 and 1-FY02-192).
Footnotes
Abbreviations used in this paper: G, globular; LG, laminin-type globular; GBM, glomerular basement membrane; Lu, Lutheran blood group glycoprotein; PECAM, platelet endothelial cell adhesion molecule; sol-Lu, soluble Lu.
References
- Abrahamson, D.R., M.H. Irwin, P.L. St. John, E.W. Perry, M.A. Accavitti, L.W. Heck, and J.R. Couchman. 1989. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J. Cell Biol. 109:3477–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley, M., and N. Smyth. 1998. The role of laminins in basement membrane function. J. Anat. 193:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger, R., A. Schmidt, J. Linton, D. Wang, C. Backus, S. Denda, U. Muller, and L.F. Reichardt. 2001. Identification and characterization of a novel ECM protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J. Cell Biol. 154:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson, R.E., M. Chiquet, R. Deutzmann, P. Ekblom, J. Engel, H. Kleinman, G.R. Martin, G. Meneguzzi, M. Paulsson, J. Sanes, et al. 1994. A new nomenclature for the laminins. Matrix Biol. 14:209–211. [DOI] [PubMed] [Google Scholar]
- Champliaud, M.F., I. Virtanen, C.F. Tiger, M. Korhonen, R. Burgeson, and D. Gullberg. 2000. Posttranslational modifications and beta/gamma chain associations of human laminin alpha1 and laminin alpha5 chains: purification of laminin-3 from placenta. Exp. Cell Res. 259:326–335. [DOI] [PubMed] [Google Scholar]
- Colognato, H., and P.D. Yurchenco. 2000. Form and function: The laminin family of heterotrimers. Dev. Dyn. 218:213–234. [DOI] [PubMed] [Google Scholar]
- Dustin, M.L., M.W. Olszowy, A.D. Holdorf, J. Li, S. Bromley, N. Desai, P. Widder, F. Rosenberger, P.A. van der Merwe, P.M. Allen, and A.S. Shaw. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 94:667–677. [DOI] [PubMed] [Google Scholar]
- El Nemer, W., P. Gane, Y. Colin, V. Bony, C. Rahuel, F. Galacteros, J.P. Cartron, and C. Le Van Kim. 1998. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 273:16686–16693. [DOI] [PubMed] [Google Scholar]
- Ettner, N., W. Gohring, T. Sasaki, K. Mann, and R. Timpl. 1998. The N-terminal globular domain of the laminin α1 chain binds to α1β1 and α2β1 integrins and to the heparan sulfate-containing domains of perlecan. FEBS Lett. 430:217–221. [DOI] [PubMed] [Google Scholar]
- Gauer, S., J. Yao, H.O. Schoecklmann, and R.B. Sterzel. 1997. Adhesion molecules in the glomerular mesangium. Kidney Int. 51:1447–1453. [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc, A., X. Zhang, H. Topaloglu, C. Cruaud, F. Tesson, J. Weissenbach, F.M. Tome, K. Schwartz, M. Fardeau, K. Tryggvason, et al. 1995. Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat. Genet. 11:216–218. [DOI] [PubMed] [Google Scholar]
- Holzman, L.B., P.L. John, I.A. Kovari, R. Verma, H. Holthofer, and D.R. Abrahamson. 1999. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56:1481–1491. [DOI] [PubMed] [Google Scholar]
- Kerjaschki, D. 2001. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Invest. 108:1583–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki, D., P.P. Ojha, M. Susani, R. Horvat, S. Binder, A. Hovorka, P. Hillemanns, and R. Pytela. 1989. A β1-integrin receptor for fibronectin in human kidney glomeruli. Am. J. Pathol. 134:481–489. [PMC free article] [PubMed] [Google Scholar]
- Kikkawa, Y., N. Sanzen, and K. Sekiguchi. 1998. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells: Laminin-10/11 mediates cell adhesion through integrin α3β1. J. Biol. Chem. 273:15854–15859. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., N. Sanzen, H. Fujiwara, A. Sonnenberg, and K. Sekiguchi. 2000. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1 and α6β4 integrins. J. Cell Sci. 113:869–876. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., C.L. Moulson, I. Virtanen, and J.H. Miner. 2002. Identification of the binding site for the Lutheran blood group glycoprotein on laminin α5 through expression of chimeric laminin chains in vivo. J. Biol. Chem. 277:44864–44869. [DOI] [PubMed] [Google Scholar]
- Koch, M., P.F. Olson, A. Albus, W. Jin, D.D. Hunter, W.J. Brunken, R.E. Burgeson, and M.F. Champliaud. 1999. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane–associated, laminin chain. J. Cell Biol. 145:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen, M., J. Ylanne, L. Laitinen, and I. Virtanen. 1990. The α1–α6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J. Cell Biol. 111:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka, G., R. Giltay, W. Bloch, K. Addicks, R. Timpl, R. Fassler, and M.-L. Chu. 2001. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol. 21:7025–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg, J.A., M.J. Donovan, S.L. Goldstein, H. Rennke, K. Shepherd, R.C. Jones, and R. Jaenisch. 1996. α3β1 integrin has a crucial role in kidney and lung organogenesis. Development. 122:3537–3547. [DOI] [PubMed] [Google Scholar]
- Lee, S.P., M.L. Cunningham, P.C. Hines, C.C. Joneckies, E.P. Orringer, and L.V. Parise. 1998. Sickel cell adhesion to laminin: potential role for the α5 chain. Blood. 92:2951–2958. [PubMed] [Google Scholar]
- Libby, R.T., M.F. Champliaud, T. Claudepierre, Y. Xu, E.P. Gibbons, M. Koch, R.E. Burgeson, D.D. Hunter, and W.J. Brunken. 2000. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J. Neurosci. 20:6517–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, P., M. Hellstrom, M. Kalen, L. Karlsson, M. Pekny, M. Pekna, P. Soriano, and C. Betsholtz. 1998. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 125:3313–3322. [DOI] [PubMed] [Google Scholar]
- Martin, P.T., A.J. Ettinger, and J.R. Sanes. 1995. A synaptic localization domain in the synaptic cleft protein laminin β2 (s-laminin). Science. 269:413–416. [DOI] [PubMed] [Google Scholar]
- Miner, J.H. 1998. Developmental biology of glomerular basement membrane components. Curr. Opin. Nephrol. Hypertens. 7:13–19. [DOI] [PubMed] [Google Scholar]
- Miner, J.H. 1999. Renal basement membrane components. Kidney Int. 56:2016–2024. [DOI] [PubMed] [Google Scholar]
- Miner, J.H. 2002. Focusing on the glomerular slit diaphragm: podocin enters the picture. Am. J. Pathol. 160:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J.H., and J.R. Sanes. 1994. Collagen IV α3, α4, and α5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J. Cell Biol. 127:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J.H., and C. Li. 2000. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 217:278–289. [DOI] [PubMed] [Google Scholar]
- Miner, J.H., R.M. Lewis, and J.R. Sanes. 1995. Molecular cloning of a novel laminin chain, α5, and widespread expression in adult mouse tissues. J. Biol. Chem. 270:28523–28526. [DOI] [PubMed] [Google Scholar]
- Miner, J.H., B.L. Patton, S.I. Lentz, D.J. Gilbert, W.D. Snider, N.A. Jenkins, N.G. Copeland, and J.R. Sanes. 1997. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell Biol. 137:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J.H., J. Cunningham, and J.R. Sanes. 1998. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol. 143:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulson, C.L., C. Li, and J.H. Miner. 2001. Localization of lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin α5 in vivo. Dev. Dyn. 222:101–114. [DOI] [PubMed] [Google Scholar]
- Mundel, P., P. Gilbert, and W. Kriz. 1991. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J. Histochem. Cytochem. 39:1047–1056. [DOI] [PubMed] [Google Scholar]
- Nguyen, N.M., J.H. Miner, R.A. Pierce, and R.M. Senior. 2002. Laminin α5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev. Biol. 246:231–244. [DOI] [PubMed] [Google Scholar]
- Ninomiya, Y., M. Kagawa, K. Iyama, I. Naito, Y. Kishiro, J.M. Seyer, M. Sugimoto, T. Oohashi, and Y. Sado. 1995. Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J. Cell Biol. 130:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes, P.G., J.H. Miner, M. Gautam, J.M. Cunningham, J.R. Sanes, and J.P. Merlie. 1995. The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat. Genet. 10:400–406. [DOI] [PubMed] [Google Scholar]
- Pan, T.C., T. Sasaki, R.Z. Zhang, R. Fassler, R. Timpl, and M.L. Chu. 1993. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J. Cell Biol. 123:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, S.F., G. Lee, F.A. Spring, T.N. Willig, L.L. Peters, J.A. Gimm, M.J. Tanner, N. Mohandas, D.J. Anstee, and J.A. Chasis. 2001. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind α5 chain-containing human laminin with high affinity. Blood. 97:312–320. [DOI] [PubMed] [Google Scholar]
- Patton, B.L., J.M. Cunningham, J. Thyboll, J. Kortesmaa, H. Westerblad, L. Edstrom, K. Tryggvason, and J.R. Sanes. 2001. Properly formed but improperly localized synaptic specializations in the absence of laminin α4. Nat. Neurosci. 4:597–604. [DOI] [PubMed] [Google Scholar]
- Pulkkinen, L., and J. Uitto. 1999. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 18:29–42. [DOI] [PubMed] [Google Scholar]
- Roselli, S., O. Gribouval, N. Boute, M. Sich, F. Benessy, T. Attie, M.-C. Gubler, and C. Antignac. 2002. Podocin localizes in the kidney to the slit diaphragm area. Am. J. Pathol. 160:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes, J.R., E. Engvall, R. Butkowski, and D.D. Hunter. 1990. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J. Cell Biol. 111:1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T., K. Mann, J.H. Miner, N. Miosge, and R. Timpl. 2002. Domain IV of mouse laminin β1 and β2 chains. Eur. J. Biochem. 269:431–442. [DOI] [PubMed] [Google Scholar]
- Sorokin, L.M., F. Pausch, M. Durbeej, and P. Ekblom. 1997. a. Differential expression of five laminin α (1-5) chains in developing and adult mouse kidney. Dev. Dyn. 210:446–462. [DOI] [PubMed] [Google Scholar]
- Sorokin, L.M., F. Pausch, M. Frieser, S. Kroger, E. Ohage, and R. Deutzmann. 1997. b. Developmental regulation of the laminin α5 chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 189:285–300. [DOI] [PubMed] [Google Scholar]
- Sterk, L.M., A.A. de Melker, D. Kramer, I. Kuikman, A. Chand, N. Claessen, J.J. Weening, and A. Sonnenberg. 1998. Glomerular extracellular matrix components and integrins. Cell Adhes. Commun. 5:177–192. [DOI] [PubMed] [Google Scholar]
- Suemori, H., Y. Kadokawa, K. Goto, I. Araki, H. Kondoh, and N. Nakatsuji. 1990. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous beta-galactosidase expression. Cell Differ. Dev. 29:181–186. [DOI] [PubMed] [Google Scholar]
- Talts, J.F., and R. Timpl. 1999. Mutation of a basic sequence in the laminin α2LG3 module leads to a lack of proteolytic processing and has different effects on β1 integrin-mediated cell adhesion and α-dystroglycan binding. FEBS Lett. 458:319–323. [DOI] [PubMed] [Google Scholar]
- Thyboll, J., J. Kortesmaa, R. Cao, R. Soininen, L. Wang, A. Iivanainen, L. Sorokin, M. Risling, Y. Cao, and K. Tryggvason. 2002. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 22:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl, R. 1996. Macromolecular organization of basement membranes. Curr. Opin. Cell Biol. 8:618–624. [DOI] [PubMed] [Google Scholar]
- Timpl, R., D. Tisi, J.F. Talts, Z. Andac, T. Sasaki, and E. Hohenester. 2000. Structure and function of laminin LG modules. Matrix Biol. 19:309–317. [DOI] [PubMed] [Google Scholar]
- Udai, M., Q. Zen, M. Cottman, N. Leonard, S. Jefferson, C. Daymont, G. Truskey, and M.J. Telen. 1998. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickel cell adhesion to laminin. J. Clin. Invest. 101:2550–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen, I., D. Gullberg, J. Rissanen, E. Kivilaakso, T. Kiviluoto, L.A. Laitinen, V.P. Lehto, and P. Ekblom. 2000. Laminin α1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp. Cell Res. 257:298–309. [DOI] [PubMed] [Google Scholar]
- Willem, M., N. Miosge, W. Halfter, N. Smyth, I. Jannetti, E. Burghart, R. Timpl, and U. Mayer. 2002. Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development. 129:2711–2722. [DOI] [PubMed] [Google Scholar]
- Zen, Q., M. Cottman, G. Truskey, R. Fraser, and M.J. Telen. 1999. Critical factors in basal cell adhesion molecule/lutheran-mediated adhesion to laminin. J. Biol. Chem. 274:728–734. [DOI] [PubMed] [Google Scholar]