Abstract
A subset of obese humans has relatively low plasma levels of leptin. This finding has suggested that in some cases abnormal regulation of the leptin gene in adipose tissue is etiologic in the pathogenesis of the obese state. The possibility that a relative decrease in leptin production can lead to obesity was tested by mating animals carrying a weakly expressed adipocyte specific aP2-human leptin transgene to C57BL/6J ob/ob mice (which do not express leptin). The transgene does not contain the regulatory elements of the leptin gene and is analogous to a circumstance in which the cis elements and/or trans factors regulating leptin RNA production are abnormal. The ob/ob mice carrying the transgene had a plasma leptin level of 1.78 ng/ml, which is ≈one-half that found in normal, nontransgenic mice (3.72 ng/ml, P < 0.01). The ob/ob animals expressing the leptin transgene were markedly obese though not as obese as ob/ob mice without the transgene. The infertility as well as several of the endocrine abnormalities generally evident in ob/ob mice were normalized in the ob/ob transgenic mice. However, the ob/ob transgenic mice had an abnormal response when placed at an ambient temperature of 4°C, suggesting that different thresholds exist for the different biologic effects of leptin. Leptin treatment of the ob/ob transgenic mice resulted in marked weight loss with efficacy similar to that seen after treatment of wild-type mice. In aggregate these data suggest that dysregulation of leptin gene can result in obesity with relatively normal levels of leptin and that this form of obesity is responsive to leptin treatment.
Many clinical studies have suggested the hypothesis that body weight is regulated by a “set point mechanism” (1–3). This hypothesis holds that individuals reach equilibrium at different weights. It is posited that when individuals are at their set point, compensatory mechanisms resist weight change in either direction. The observation that weight loss in both lean and obese subjects is associated with reduced energy expenditure supports the set point hypothesis as does the high recidivism rate among obese subjects who lose weight by dieting (4, 5).
A possible molecular basis for differences in weight among individuals has been suggested with the cloning of the ob gene and the identification of leptin (6–12). In principle, differences in leptin sensitivity and/or production of leptin could lead to differences in weight. It has been suggested that high plasma levels of leptin and/or increased levels of leptin RNA in obese subjects is indicative of leptin resistance (13–17). Indeed, 90–95% of obese humans have high leptin levels as do all forms of rodent obesity that have been analyzed (with the exception of leptin-deficient ob/ob mice). Treatment of several strains of obese rodents with leptin has confirmed that high leptin levels indicate complete or partial leptin resistance (18, 19).
A subset of obese humans have normal or relatively low leptin levels (≈5–10% of subjects) (13, 14). In these individuals, it has been postulated that a decreased rate of leptin production by adipose tissue is causal of the obese state. If true, a partial decrease in the activity of the leptin gene should result in obesity with normal leptin sensitivity. To test this hypothesis, transgenic mice expressing a weak leptin transgene were bred to ob/ob mice. Constitutive expression of leptin at a low level in the ob/ob transgenic mice resulted in a moderately obese phenotype that is less severe than that seen in standard C57BL/6J ob/ob mice. In addition, the ob/ob transgenic mice manifest some, but not all, of the abnormalities generally seen in C57BL/6J ob/ob mice (3). These data suggest that different thresholds exist for the different biologic responses elicited by quantitative differences in leptin concentration.
ob/ob mice expressing the transgene are quite obese with 30% body fat, a level 3-fold higher than that of wild-type mice. Treatment of these animals with low doses of leptin results in the loss of copious amounts of weight. These data have implications for the pathogenesis of human obesity and may indicate that the subset of individuals with low leptin obesity will respond robustly to leptin treatment.
METHODS
Transgene Construction and Production of Transgenic Mice.
A human leptin transgene was constructed by ligating the 5.4-kb aP2 promoter fragment to a 1-kb fragment of human leptin cDNA, followed by a simian virus 40 (SV40) polyadenylation signal (4, 5). Transgene DNA was injected into FVB/N embryos to produce transgenic mice. The transgenic mice were identified by PCR amplification of genomic DNA from their tail tips by using SV40 poly(A)-derived oligonucleotides simian virus F, 5′-TCTTTGTGAAGGAACCTTAC-3′, and Rous sarcoma virus, 5′-GGAATCTAAAATACACAAAC-3′, to produce a diagnostic PCR fragment of 233 bp. A transgenic founder with normal body weight and low plasma level of human leptin was bred to the C57BL/6 ob/+ mice and intercrossed. The transgene was backcrossed onto the C57BL/6J background for at least six generations before being studied.
Animal Maintenance and Analysis.
Animals were housed in groups of 1–5 on a 12-hr light/dark cycle on a standard chow diet and weighed weekly or daily during leptin infusion. All animals were typed by PCR for the presence of human leptin transgene as described above. The ob genotype was determined by PCR using oligonucleotides 5′-GCCATCCAGGCTCTCTGG-3′ and 5′-TGAGTTTGTCCAAGATGGACC-3′, with subsequent digestion of the PCR product with the DdeI restriction enzyme. Fertility was tested by housing each mouse with a proven breeder of the opposite sex for at least a week. Animals were considered fertile if a litter was subsequently born. Sensitivity to cold was tested by placing animals in individual cages without food and water in a cold room at a temperature of 4°C. Body temperatures were measured with a rectal thermometer every 1 hr.
Leptin Infusion and Analysis of Body Composition.
Transgenic ob/ob males were separated into individual cages with ad libitum access to a standard chow diet and water, and their body weights and food intakes were monitored daily until stabile. Daily diet intake was determined by weighing the food remaining in each cage each 24-hr period. Subcutaneous Alzet 14-day osmotic pumps filled with either PBS or recombinant murine leptin (pumping rate 400 ng/hr) were implanted as described (18). All pumps were replaced after 14 days at which time fresh pumps were placed for an additional 14 days. Blood was collected by intraocular bleeding into tubes containing EDTA and separated into plasma. Body composition analysis was performed as described (7).
Determination of Endocrine Parameters.
Plasma was collected, and 50 μl was assayed by using human or mouse leptin RIA kits (Linco Research Immunoassay, St. Charles, MO) to determine serum leptin concentrations. Human leptin concentration in plasma also was determined by ELISA using a polyclonal antibody against recombinant human leptin, which was cross-purified against mouse plasma. Plasma insulin was quantitated with a rat insulin RIA kit (Linco), corticosterone concentration was determined by using an RIA kit for rats and mice (ICN), and total T4 was measured with an RIA kit (Diagnostic Products, Los Angeles). Blood glucose was quantitated by using the SureStep Complete Blood Glucose Monitoring System (Johnson and Johnson, Milpitas, CA).
Reverse Transcription–PCR and Northern Blot Analyses.
Total RNA was prepared from mouse tissues by using RNAzol B reagent (Tel-Test, Friendswood, TX). RNA from white adipose tissue were subjected to reverse transcription–PCR analysis by using the specific primers described above. Northern analysis was performed on 20 μg of total RNA from each tissue. Blots were probed with PCR-labeled human leptin cDNA fragment using primers 5′-TGTCACCAGGATCAATGACA-3′ and 5′-TGGCAGCTCTTAGAGAAGGCC-3′. Blots were hybridized for 4 hr in Rapid-hyb buffer (Amersham) and exposed to Reflection autoradiography film (NEN) with a screen for 64 hr.
Determination of Adipose Cell Size and Number.
Parametrial, retroperitoneal, and subcutaneous fat pads were dissected from ob/ob and ob/+ transgenic and nontransgenic mice at 9–14 weeks of age as described (21). Tissues were washed with warm saline, and 100-mg samples from each tissue were prepared. Two representative samples from each fat pad were used to determine adipocyte size by extraction with chloroform-methanol. Two more samples from each fat pad were used for determination of adipocytes number by using fixation with osmium tetroxide as described (21). The number of fat cells was estimated by counting four aliquots from each sample and averaging the results. The values derived independently from each pair of samples from the same fat pad were considered accurate if they differed from each other by less then 10%.
RESULTS
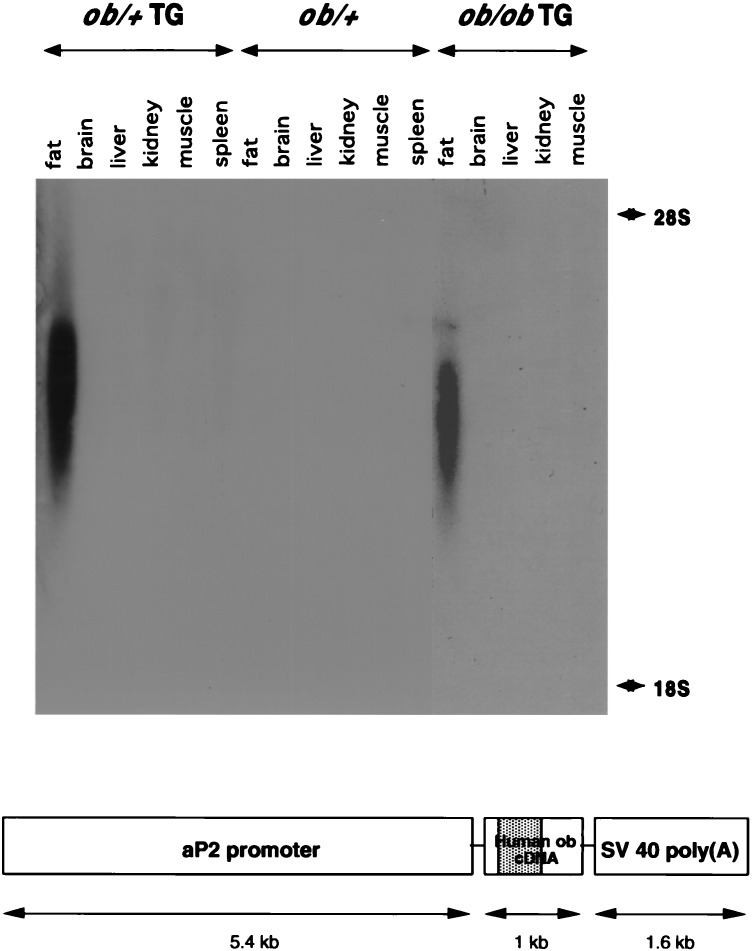
A transgenic founder mouse carrying a weakly expressed human leptin transgene was generated by ligating the fat-specific aP2 promoter to a human leptin cDNA with a simian virus 40 poly(A) site (Fig. 1, Lower). The 5.4-kb aP2 promoter leads to expression of transgenes specifically in adipose tissue (22, 23). A transgenic animal expressing low levels of human leptin was identified. The plasma level of human leptin in this founder animal was 1.5 ng/ml as determined by using an RIA specific for human leptin. This level is ≈50% lower than the plasma leptin levels in wild-type mice (3.72 ng/ml). The transgenic founder was bred to C57BL/6J ob/+ mice. ob/+ animals were identified by digesting a PCR product spanning the C57BL/6J ob/ob missense mutation with DdeI (24). The PCR product from the mutant gene is digested by DdeI, whereas the wild-type one is not (data not shown). The transgene was backcrossed onto the C57BL/6J ob/ob strain for at least six generations before any experiments were performed. ob/ob mice carrying the transgene were identified among the progeny of genetic crosses between ob/+ and ob/ob transgenic mice (ob/ob mice are generally infertile but the ob/ob transgenic mice breed normally, see below) (3, 25, 26). DNA was prepared from the progeny of this mating and used to assign genotype at the ob locus and to determine which of the mice carried the transgene. Four groups of mice were characterized: ob/+, ob/+ TG, ob/ob, and ob/ob TG (TG refers to the transgene).
Figure 1.
Human leptin transgene and its expression pattern. A transgenic mouse expressing a leptin transgene was generated. The construct is shown at the bottom. The founder was bred into the C57BL/6J ob/ob background for six or more generations. Four groups were studied: ob/+, ob/+ TG, ob/ob, and ob/ob TG. A Northern blot of total RNA from tissues of ob/+ and ob/ob transgenic and nontransgenic mice was probed with a labeled human leptin cDNA fragment. A positive signal was detectable only in white adipose tissue of transgenic mice. The probe did not detect RNA in any tissues of the nontransgenic mice.
The expression of the transgene was assessed by using Northern blots probed with a fragment of the human leptin gene (Fig. 1). Northern blots indicated that the transgene was expressed only in adipose tissue. Reverse transcription–PCR using primers derived from the simian virus 40 sequence at the 3′ end of the transgene RNA yielded similar results (data not shown). The signal intensity of the transgene on Northern blots was not different in the ob/+ TG and the ob/ob TG groups. The size of the transgenic RNA was ≈2.5 kb, which is substantially shorter than the 4.5-kb wild-type leptin transcript. Signals were not detected in mice that did not carry the transgene, indicating that the human leptin probe did not crossreact with the endogenous RNA in ob/+ TG mice. Although the RNA bands detected on Northern blots were rather diffuse, the bands detected by using a glyceraldehyde-3-phosphate dehydrogenase probe were not (data not shown). The basis for the variable size of RNAs expressed from the transgene is not clear but may indicate that the RNA is unstable. It has been suggested that RNA expressed from transgenes that do not include introns is often unstable.
The leptin levels of the four groups of mice were measured by using RIAs specific for either mouse or human leptin (Table 1). The plasma level of human leptin was equivalent in ob/+ TG and ob/ob TG mice (1.81 vs. 1.78 ng/ml, see Table 1 for values in males and females). Mouse leptin was not detected in ob/ob mice not carrying the transgene. The concentration of endogenous mouse leptin was not significantly different in the ob/+ TG mice vs. the ob/+ animals not carrying the transgene (3.17 ng/ml vs. 3.72 ng/ml, see Table 1 for values in males and females).
Table 1.
Phenotypic features of the ob/ob, ob/ob TG, ob/+, and ob/+ TG mice
| Males
|
Females
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ob/+ TG | ob/+ None | ob/ob TG | ob/ob None | ob/+ TG | ob/+ None | ob/ob TG | ob/ob None | |
| Plasma mouse leptin (ng/ml) | 2.80 | 3.49 | 0 | 0 | 3.60 | 4.04 | 0 | 0 |
| (±0.96) | (±1.89) | (±0) | (±0) | (±0.39) | (±2.65) | (±0) | (±0) | |
| Plasma human leptin (ng/ml) | 1.54 | 0 | 1.68 | 0 | 2.19 | 0 | 1.90 | 0 |
| (±0.42) | (±0) | (±0.48) | (±0) | (±0.34) | (±0) | (±0.60) | (±0) | |
| Blood glucose (mg/dl) | 162.9 | 171.8 | 144.8 | 265.2 | 134.7 | 147.3 | 150.9 | 228.4 |
| (±23.7) | (±14.2) | (±19.3) | (±74.9) | (±10.4) | (±20.1) | (±14.3) | (±51) | |
| Plasma insulin (ng/ml) | 0.70 | 1.49 | 2.42 | 28.62 | 0.52 | 0.61 | 1.60 | 7.55 |
| (±0.36) | (±0.90) | (±1.08) | (±14.19) | (±0.11) | (±0.17) | (±0.77) | (±7.27) | |
| Plasma corticosterone (ng/ml) | 132.3 | 94.6 | 85.7 | 248.1 | 107.3 | 122.9 | 152.2 | 210.3 |
| (±81.9) | (±41.7) | (±36.7) | (±58.0) | (±48.2) | (±57.8) | (±41.4) | (±45.8) | |
| Plasma thyroxine (μg/dl) | 3.52 | 3.23 | 4.00 | 3.35 | 3.67 | 3.88 | 4.97 | 3.54 |
| (±1.25) | (±0.85) | (±1.00) | (±0.53) | (±1.64) | (±0.58) | (±0.83) | (±0.44) | |
| Fertility (%) | 6/6 | 3/3 | 6/6 | 0/5 | 6/6 | 10/10 | 10/12 | 0/5 |
| (100%) | (100%) | (100%) | (0%) | (100%) | (100%) | (83%) | (0%) | |
| Litter size | 5.7 (±2.7) | 7.3 (±2.7) | 5.0 (±1.3) | 0 | ||||
| n | 11 | 9 | 6 | 0 | ||||
Data are mean (± SD); n = 5–11 per group for mouse leptin; n = 5–14 for human leptin; n = 5–13 for glucose and insulin; n = 7–13 for corticosterone; n = 6–11 for thyroxine.
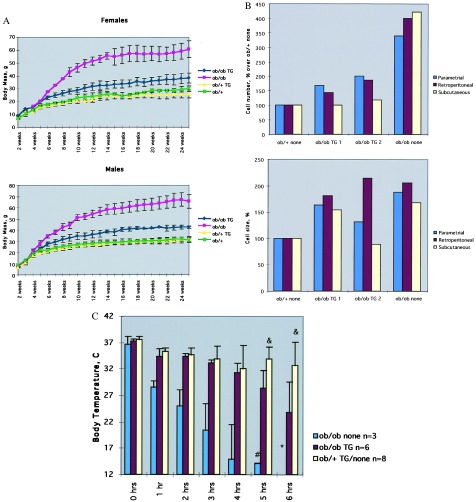
The growth curves of the four groups of animals was compared by measuring body mass every week (Fig. 2A). All animals were fed a standard chow diet. There was no difference in the growth rates between the ob/+ mice with and without the transgene. There was a marked difference however, among ob/ob TG mice, the ob/ob mice without the transgene, and wild-type mice. At all time points after 4 weeks, the ob/ob TG mice had an increased weight relative to wild-type mice but weighed less than the ob/ob mice not carrying the transgene (Fig. 2A). At 6 months the average weight of the ob/ob TG mice was 42.5 g in males and 38.2 g in females. The ob/ob males weighed 65.6 g and females weighed 60.7 g. The wild-type males weighed 31.5 g and the females weighed 25.9 g. Analysis of the body composition of the ob/ob TG indicated that they had ≈30% body fat, a ≈3-fold increase relative to the wild-type mice (see Fig. 3B). The percentage of fat of the ob/ob TG mice was 2-fold lower than that reported for ob/ob mice (7).
Figure 2.
Phenotype of ob/ob TG mice. (A) The body weight of the ob/ob TG, ob/+ TG, and ob/+ mice was measured every week. At all times past 4 weeks, ob/ob TG mice were significantly larger that the wild-type mice though not as heavy as ob/ob mice. All animals were fed a standard chow diet. Data for males and females are shown. (B) The adipocyte cell size was equivalent in the ob/ob TG and ob/ob mice but larger than that of wild-type mice. In contrast, the adipocyte cell number was increased ≈2.5-fold in the ob/ob mice. Thus the ob/ob TG have a hypertrophic form of obesity. (C) The response to an ambient temperature of 4°C was compared among the four groups. In contrast to wild-type mice, which maintain a normal core temperature, the ob/ob TG mice became hypothermic after 6 hr at 4°C. The ob/ob mice became hypothermic after 2 hr.
Figure 3.
Response of ob/ob TG mice to leptin treatment. Leptin (400 ng/ml) or PBS was delivered to groups of five ob/ob TG mice as a 28-day s.c. infusion by using Alzet osmotuic pumps. (A) The food intake and body weights of mice were measured daily. Data are shown as mean ± SD. Leptin treatment resulted in a marked decrease in food intake and body weight. (B) Leptin treatment of the ob/ob TG mice resulted in a decrease in body fat content from 30% to 3%. The treatment did not affect lean body mass or the amount of body water.
Obesity can be the result of hyperplasia and/or hypertrophy of adipocytes (27). The fat cell numbers and fat cell size were compared in several adipose tissue depots of ob/ob TG, ob/ob, and wild-type mice (Fig. 2B). As previously reported, ob/ob mice have both an increased number of fat cells and an increased fat cell size relative to wild type (21). In contrast, adipose tissue from the ob/ob TG mice had cells with a size similar to the ob/ob mice but contained ≈2.5-fold fewer cells. The ob/ob TG mice had a small increase in the number of fat cells as compared with the wild-type mice. These data indicate that the obesity of ob/ob TG mice is primarily a result of adipocyte hypertroply.
ob/ob animals manifest a number of abnormalities besides obesity, including infertility, severe insulin-resistant diabetes, abnormal thermoregulation, and hypercortisolemia (3, 25). Assays to test for the presence of these abnormalities were performed (Table 1). Although none of 10 ob/ob animals (five males, five females) were successfully bred, 6/6 ob/ob TG males and 10/12 ob/ob TG females yielded progeny in test matings. ob/ob mice were severely insulin resistant and diabetic with blood glucose in the range of 228–265 ng/dl and a markedly increased level of plasma insulin of 28.62 ng/ml. ob/ob TG mice had normal plasma glucose levels although plasma insulin was 2-fold elevated, indicating the presence of mild insulin resistance. Of interest, the ob/+ transgenic mice had lower plasma insulin compared with the nontransgenic groups, suggesting that they may be especially insulin sensitive. The hypercortisolemia evident in ob/ob mice also was normalized in the ob/ob mice carrying the transgene (248 ng/ml in ob/ob mice vs. 85.7 ng/ml in ob/ob TG mice).
Abnormalities in thermoregulation in response to a cold stress were evident in the ob/ob TG animals (Fig. 2C). Wild-type mice maintained a core temperature of 32.65 ± 4.42 after 6 hr at an ambient temperature of 4°C. As previously reported C57BL/6J ob/ob mice do not tolerate cold exposure and exhibited a marked decrease in core temperature after 2 hr at 4°C. ob/ob TG mice have an intermediate phenotype and showed a significantly decreased temperature after 4–6 hr at 4°C (32.6°C vs. 23.7°C, P < 0.02).
The efficacy of leptin treatment of the ob/ob TG mice was studied. Leptin (400 ng/hr) or PBS was infused s.c. into groups of ob/ob TG mice by using Alzet osmotic pumps (Fig. 3A). A s.c. leptin dose of 400 ng/hr is sufficient to markedly reduce body fat content in wild-type mice but is ineffective in leptin-resistant NZO and Ay mice (18). ob/ob TG mice treated with leptin consumed significantly less food relative to the PBS-treated group (83.4 g vs. 127.1 g, P < 0.001) and lost copious amounts of weight (Fig. 3A). Weight loss was specific for adipose tissue mass, and the amount of body fat fell from an average of 30.55% in the PBS groups vs. 3.36% in the treated group (Fig. 3B). Treatment of the ob/ob TG mice with leptin also was associated with a decrease in the plasma levels of glucose and insulin (glucose 116.8 ng/ml, insulin 0.29 ng/ml in the treated group; glucose 208.6 ng/ml, insulin 2.44 ng/ml in the PBS-treated group).
DISCUSSION
Differences in leptin sensitivity and/or leptin production have been suggested to play a role in the pathogenesis of obesity. The majority of human and rodent obesity is associated with hyperleptinemia, suggesting that in these cases leptin resistance is responsible for this condition (13–17). This conclusion is supported by the observed insensitivity to exogenous leptin in DIO, Ay, and NZO mice (18, 19). However, approximately 5–10% of obese humans have relatively normal leptin levels (i.e., below 10 ng/ml) (13, 14). It has been suggested that in these cases, obesity is a result of a relative decrease in the synthesis of leptin RNA and/or protein. If true, abnormal regulation of the leptin gene leading to constitutive expression of a small amount of leptin should result in obesity. Moreover this form of obesity should be remediable with leptin treatment.
The hypothesis that abnormal gene regulation can cause obesity was tested by breeding a weakly expressed leptin transgene. In these studies, the effect of the transgene, which is the only source of human leptin, was compared among animals with different ob genotypes. The choice of the transgene as leptin “delivery tool” rather than administration of exogenous leptin was justified by our interest in the phenotypic consequences of a slightly decreased leptin level in animals that expressed it beginning in the neonatal period continuing into adulthood. This approach allowed us to predict the likely phenotype of an individual with a “regulatory” defect that results in a constitutively decreased rate of leptin production. It would not have been feasible to implant an infusion pump continuously from the neonatal period onward, nor could we have reliably estimated the leptin levels that were achieved. In addition, the bioactivity of recombinant leptin may change over a long time and is not necessarily equivalent to that of leptin produced in vivo. Finally, pump insertion leads to a number of responses, stresses the animals, and invariably causes infections if maintained for the several months. Injections of leptin would be even less suitable than infusion pumps. Thus, the use of mice carrying leptin transgene was essential for the conduct of these studies.
ob/ob mice carrying the leptin transgene expressed relatively low levels of leptin and were markedly obese. In addition, many of the features of the ob phenotype were mitigated by the low level of leptin expressed from the transgene. Unlike ob/ob mice, the ob/ob TG animals are fertile, have relatively normal plasma corticosterone levels, and are nondiabetic (3, 25). Thus leptin circulating at a level of 1.5 ng/ml is sufficient to prevent these abnormalities. The ob/ob TG mice do exhibit mild insulin resistance as evidenced by the presence of mild hyperinsulinemia in the presence of normal glucose levels. This finding suggests that mild insulin resistance, which generally is associated with obesity, can be seen in the absence of hyperleptinemia.
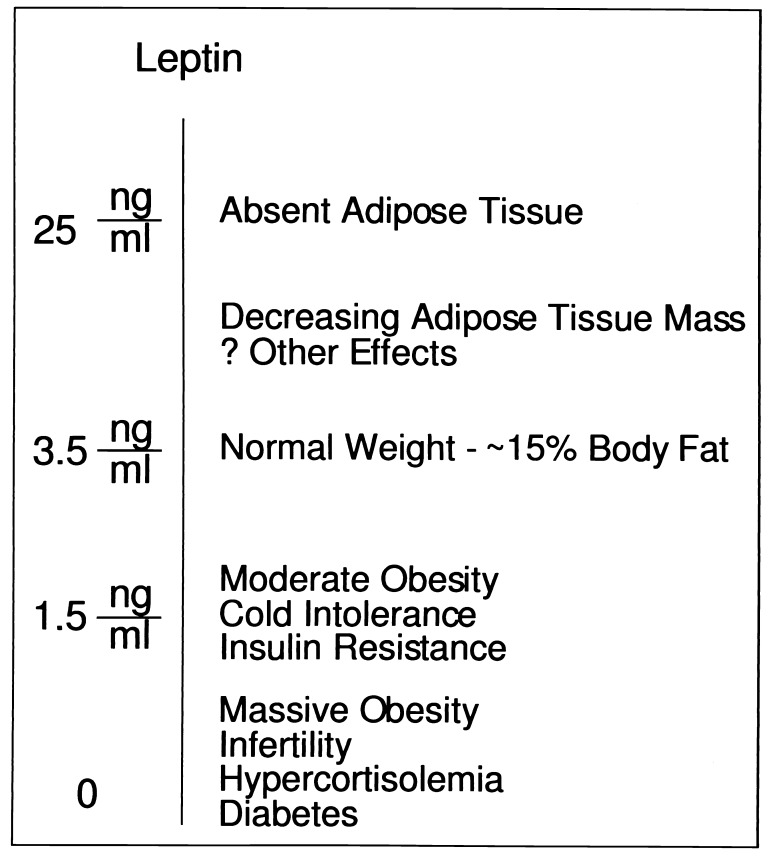
The ob/ob mice expressing the transgene did not respond normally to cold stress. This abnormality suggests that the threshold for the various responses to leptin are set at different levels (Fig. 4). Although the leptin level of 1.5 ng/ml expressed in the ob/ob TG mice is sufficient to correct several of the endocrine abnormalities of ob/ob mice, they are not adequate to completely normalize body weight and cold tolerance. Several lines of evidence suggest that the hypothalamus is an important site of leptin action (9, 10, 18, 28). The mechanisms by which the hypothalamus senses quantitative differences in leptin level are not understood. These data may indicate that neurons expressing the leptin receptor activate different pathways in response to different concentrations of leptin. Alternatively, the leptin receptor positive neurons that control body fat content and thermoregulation may be distinct from those that lead to infertility and hypercortisolemia in the complete absence of leptin.
Figure 4.
Responses to quantitative changes in leptin level. Different biological responses to leptin are observed at different plasma concentrations. The complete absence of leptin leads to a markedly abnormal phenotype. Slightly subnormal levels ameliorate many of the features of leptin-deficient animals but is associated with cold intolerance and moderate obesity. Finally, physiologic increases in leptin levels decrease body fat. The molecular basis for the different physiologic responses elicited at various leptin concentration is not known.
The ob/ob TG and ob/ob mice carrying the transgene also differed with respect to the cellularity of adipose tissue. Significant hyperplasia is not seen in the ob/ob TG mice, suggesting that partial leptin deficiency induces adipocyte hypertrophy. This finding is in contrast to complete leptin deficiency, which also leads to increased adipocyte proliferation and/or differentiation. Thus the low levels of leptin expressed in the ob/ob TG mice are apparently sufficient to blunt the hyperplasia of adipose tissue seen in the complete absence of leptin. The high potency of leptin administered intracerebroventricularly suggests that leptin’s effects on adipose tissue are likely to be controlled by efferent signals coming from the central nervous system (9, 18). Although the nature of this signal(s) is unclear, it has been suggested that leptin increases the activity of the sympathetic nervous system, which may mediate some of its effects on adipose tissue (29). The in vivo signaling mechanisms that regulate adipose cell proliferation and/or differentiation are largely unknown but may include PPARγ2 and its ligand as well as the recently cloned brown adipose tissue coactivator protein, PGC-1 (30, 31).
It has been previously reported that mutations in the leptin gene result in obesity in humans and rodents (6, 20, 32). The data presented here indicate that abnormal regulation of the leptin gene resulting in a quantitative decrease in leptin production also can cause obesity. These results have important implications for the subset of obese individuals who have relatively normal plasma levels of leptin and suggests that these individuals may have a relative decrease in the rate of leptin production. The molecular mechanisms that regulate leptin synthesis in relation to the amount of adipose tissue are not known. These data suggest the possibility that variation in the genes controlling the rate of leptin production can lead to differences in body weight. Further studies to identify the factors regulating leptin synthesis and secretion may be important for the elucidation of the pathogenesis of obesity in euleptinemic obese subjects. The robust response of the obese ob/ob TG mice to exogenous leptin suggests that obese individuals with low leptin levels may respond well to treatment with exogenous leptin. This possibility awaits the outcome of clinical trials now underway.
Acknowledgments
We thank Susan Korres for expert assistance in preparing this manuscript.
ABBREVIATION
- TG
transgene
References
- 1. Kennedy G C. Proc R Soc London Ser B. 1953;140:578–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 2.Leibelt R A, Ichinoe S, Nicholson N. Ann NY Acad Sci. 1965;131:559–582. doi: 10.1111/j.1749-6632.1965.tb34820.x. [DOI] [PubMed] [Google Scholar]
- 3.Coleman D L. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 4.Leibel R L, Rosenbaum M, Hirsch J. N Eng J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 5.Weigle D S. FASEB J. 1994;8:302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Proenca P, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 7.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 8.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 9.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 10.Stephens T W, Bashinski M, Bristow P K, Bue-Valleskey J M, Burgett S G, Hale H, Hoffmann J, Hsiung H M, Krauciunas A, Mackellar W, et al. Nature (London) 1995;377:530–534. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 11.Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weigle D S, Bukowski T R, Foster D C, Holderman S, Kramer J M, Lasser G, Lofton-Day C E, Prunkard D E, Raymond C, Kuijper J L. J Clin Invest. 1995;96:2065–2070. doi: 10.1172/JCI118254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maffei M, Halaas J, Ravussin E, Pratley R E, Lee G H, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern P A, Friedman J M. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 14.Considine R V, Sinha M K, Heiman M L, Kriauciunas A, Stephens T W, Nyce M R, Ohannesian J P, Marco C C, McKee L J, Bauer T L. N Eng J Med. 1996;334:324–325. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 15.Considine R V, Considine E L, Williams C J, Nyce M R, Magosin S A, Bauer T L, Rosato E L, Colberg J, Caro J F. J Clin Invest. 1995;95:2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonnqvist F, Arner P, Nordfors L, Schalling M. Nat Med. 1995;9:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton B S, Paglia D, Kwan A Y M, Deitel M. Nat Med. 1995;9:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 18.Halaas J L, Boozer C, Blair-West J, Fidahusein N, Denton D, Friedman J M. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Heek M, Compton D S, France C F, Tedesco R P, Fawzi A B, Graziano M P, Sybertz E J, Strader C D, Davis H R., Jr J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strobel A, Camoin T I L, Ozata M, Strosberg A D. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P R, Hirsch J. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- 22.Ross S R, Graves R A, Greenstein A, Platt K A, Shyu H L, Mellovitz B, Spiegelman B M. Proc Natl Acad Sci USA. 1990;87:9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves R A, Tontonoz P, Ross S R, Spiegleman B M. Genes Dev. 1991;5:428–437. doi: 10.1101/gad.5.3.428. [DOI] [PubMed] [Google Scholar]
- 24.Chung W K, Chua S C, Lee G H, Leibel R L. Obesity Res. 1997;5:183–185. doi: 10.1002/j.1550-8528.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 25.Lane P W, Dickie M M. J Hered. 1954;45:56–58. [Google Scholar]
- 26.Ingalls A M, Dickie M M, Snell G D. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch J, Gallian E. J Lipid Res. 1968;9:110–119. [PubMed] [Google Scholar]
- 28.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 29.Collins S, Kuhn C M, Petro A E, Swick A G, Chrunyk B A, Surwit R S. Nature (London) 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 30.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;43:1271–1278. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Puigserver P, Wu Z, Park C W, Graves R, Wright M, Spiegleman B M. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 32.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]