Abstract
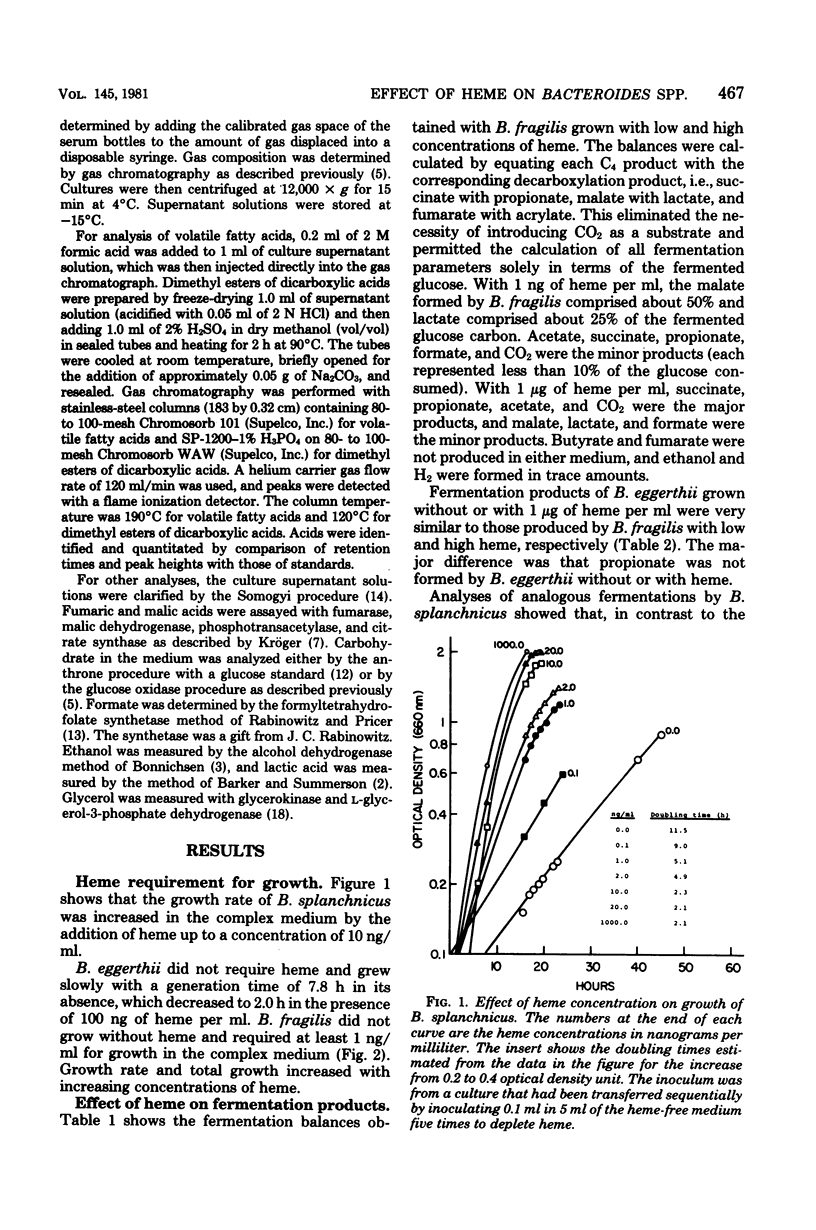
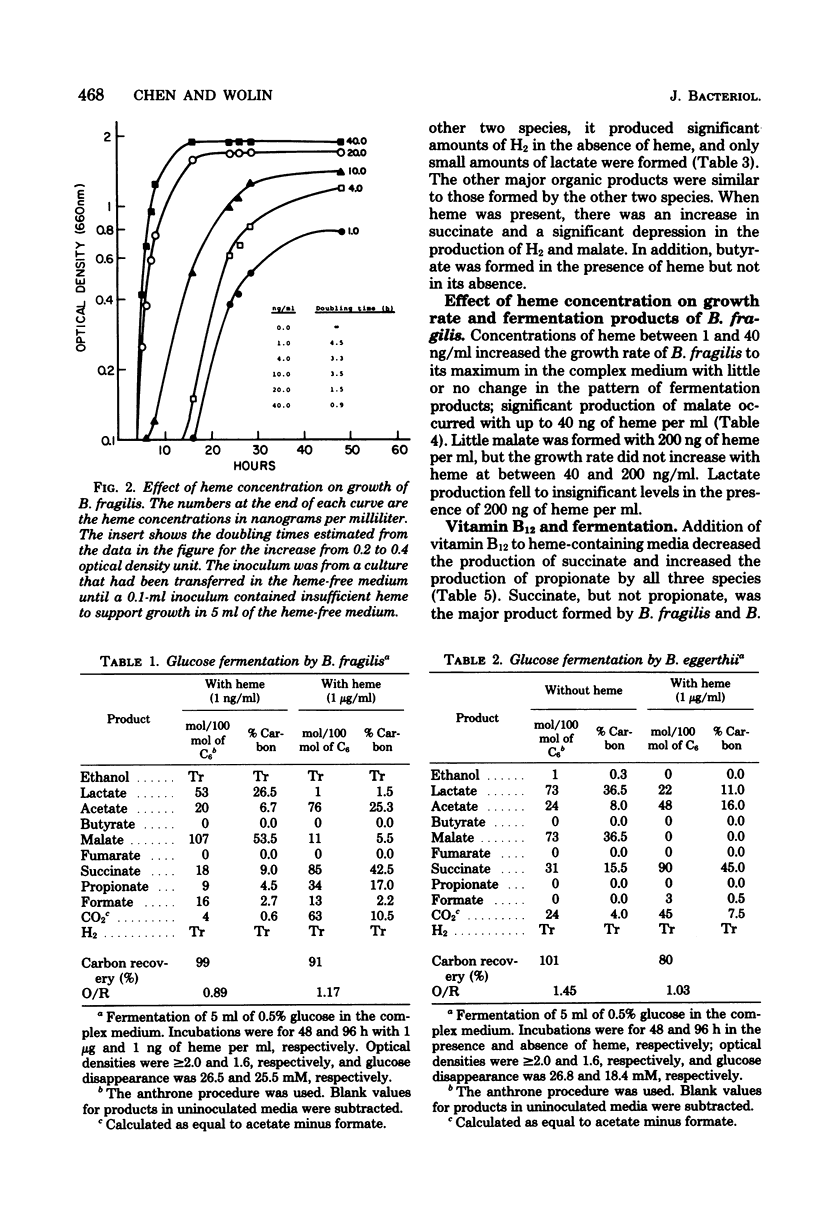
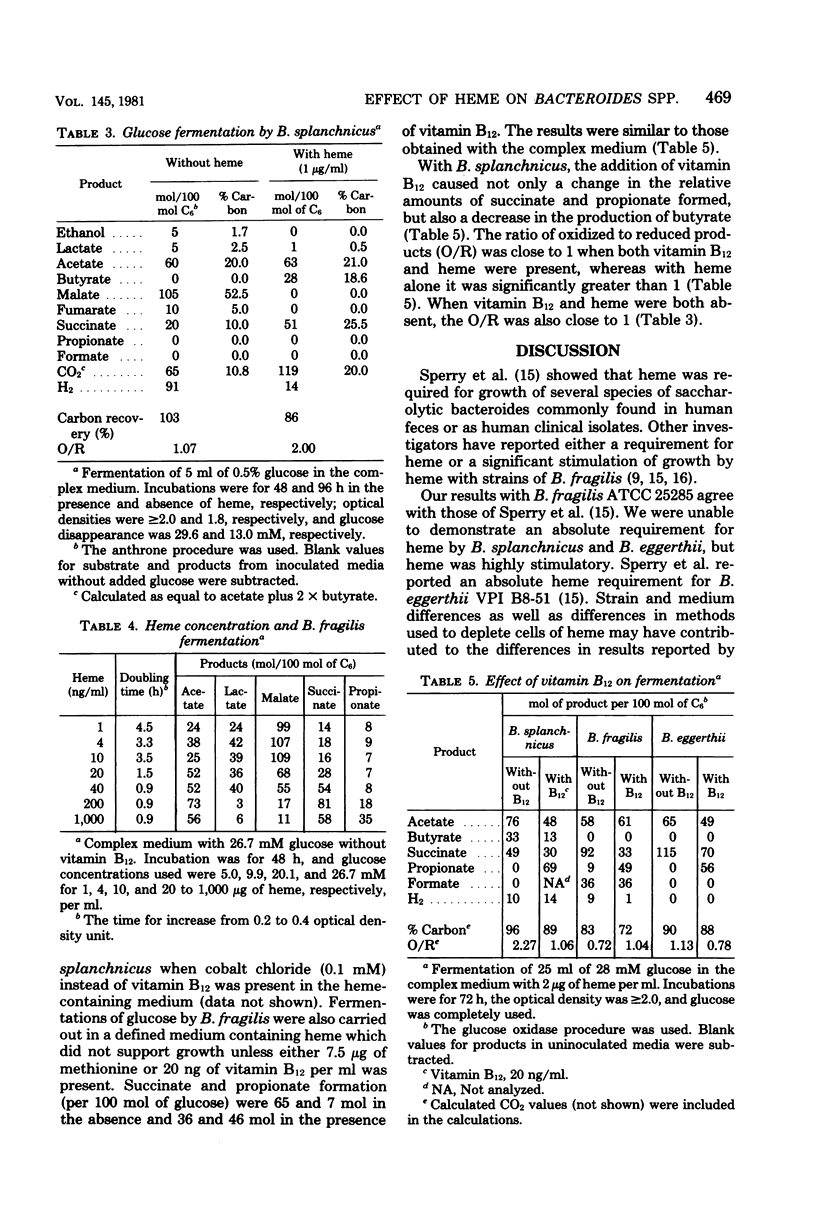
We examined the effects of heme on the growth and fermentations of Bacteroides species. Bacteroides fragilis ATCC 25285 required heme for growth and produced malate and lactate as major products of glucose fermentation when the concentration of heme was 1 ng/ml. With 1 microgram of heme per ml, malate was not formed, lactate production decreased, and succinate and acetate were the major fermentation products. B. eggerthii ATCC 27754 grew without heme, with the production of mainly malate and lactate from glucose. Its fermentation with 1 microgram of heme per ml was similar to that of B. fragilis grown with the same concentration of heme. B. splanchicus VPI 6842 grew without heme, with the production of mainly malate, acetate, and H2 from glucose. With 1 microgram of heme per ml, malate disappeared, H2 decreased significantly, and succinate, acetate, and butyrate were the major products. The addition of vitamin B12 to media containing 1 microgram of heme per ml caused all species to produce propionate at the expense of succinate and, with B. splanchnicus, also at the expense of butyrate. Thus, the concentration of heme and the presence of vitamin B12 significantly influenced the course of glucose fermentation by these bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Influence of CH4 production by Methanobacterium ruminantium on the fermentation of glucose and lactate by Selenomonas ruminantium. Appl Environ Microbiol. 1977 Dec;34(6):756–759. doi: 10.1128/aem.34.6.756-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen R. W., Oace S. M. Methylmalonic acid and vitamin B12 excretion of rats consuming diets varying in cellulose and pectin. J Nutr. 1978 Apr;108(4):640–647. doi: 10.1093/jn/108.4.640. [DOI] [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Fermentations by saccharolytic intestinal bacteria. Am J Clin Nutr. 1979 Jan;32(1):164–172. doi: 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Sperry J. F., Appleman M. D., Wilkins T. D. Requirement of heme for growth of Bacteroides fragilis. Appl Environ Microbiol. 1977 Oct;34(4):386–390. doi: 10.1128/aem.34.4.386-390.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


