Abstract
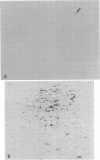
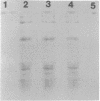
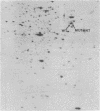
Protein B56.5 is a major Escherichia coli protein, originally identified on two-dimensional gels as an abundant cellular protein with unique regulation. The groE gene product is a bacterial protein essential for the assembly of many diverse bacteriophages. The ribosomal A-protein is a large, acidic protein of unknown function associated with isolated, washed ribosomes. On the basis of comigration in two-dimensional gels, oligopeptide map patterns, amino acid composition, immunological specificity, physical properties, and genetic analysis, protein B56.5 has now been shown to be the groE gene product and to be identical with the A-protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R. M., Reeh S., Pedersen S. Regulation of transcription factor rho and the alpha subunit of RNA polymerase in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2285–2288. doi: 10.1073/pnas.73.7.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product). Proc Natl Acad Sci U S A. 1978 Jan;75(1):131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A., Ikeuchi T., Matsumoto A., Yamamoto S. A novel adenosine triphosphatase isolated from RNA polymerase preparations of Escherichia coli. II. Enzymatic properties and molecular structure. J Biochem. 1976 May;79(5):927–937. doi: 10.1093/oxfordjournals.jbchem.a131160. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Ikeuchi T., Yura T. A novel adenosine triphosphatase isolated from RNA polymerase preparations of Escherichia coli. I. Copurification and separation. J Biochem. 1976 May;79(5):917–925. doi: 10.1093/oxfordjournals.jbchem.a131159. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Wittmann H. G. Ribosomal proteins. XV. Amino acid compositions of isolated ribosomal proteins from 30S and 50S subunits of Escherichia coli. Mol Gen Genet. 1970;109(4):292–297. doi: 10.1007/BF00267698. [DOI] [PubMed] [Google Scholar]
- Kratky O., Leopold H., Stabinger H. The determination of the partial specific volume of proteins by the mechanical oscillator technique. Methods Enzymol. 1973;27:98–110. doi: 10.1016/s0076-6879(73)27007-6. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Subramanian A. R. Protein S1 from Escherichia coli ribosomes: an improved isolation procedure and shape determination by small-angle X-ray scattering. Eur J Biochem. 1979 Apr 2;95(2):359–366. doi: 10.1111/j.1432-1033.1979.tb12973.x. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- McKie J. E., Brandts J. F. High precision capillary viscometry. Methods Enzymol. 1972;26:257–288. doi: 10.1016/s0076-6879(72)26014-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Wirth R., Smith M. W., Van Bogelen R. Selective synthesis of plasmid-coded proteins by Escherichia coli during recovery from chloramphenicol treatment. J Bacteriol. 1980 Jul;143(1):535–537. doi: 10.1128/jb.143.1.535-537.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Coy G. On the purification of DNA-dependent RNA polymerase from E. coli: removal of an ATPase. Can J Biochem. 1972 Feb;50(2):142–150. doi: 10.1139/o72-018. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Reeh S., Pedersen S., Friesen J. D. Biosynthetic regulation of individual proteins in relA+ and relA strains of Escherichia coli during amino acid starvation. Mol Gen Genet. 1976 Dec 22;149(3):279–289. doi: 10.1007/BF00268529. [DOI] [PubMed] [Google Scholar]
- SCHACHMAN H. K. THE ULTRACENTRIFUGE: PROBLEMS AND PROSPECTS. Biochemistry. 1963 Sep-Oct;2:887–905. doi: 10.1021/bi00905a001. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). I. Initial characterization. J Mol Biol. 1973 May 5;76(1):1–23. doi: 10.1016/0022-2836(73)90078-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Wittmann H. G. Sequence differences of Escherichia coli 30S ribosomal proteins as determined by immunochemical methods. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2283–2287. doi: 10.1073/pnas.68.9.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Haase C., Giesen M. Isolation and characterization of a growth-cycle-reflecting, high-molecular-weight protein associated with Escherichia coli ribosomes. Eur J Biochem. 1976 Aug 16;67(2):591–601. doi: 10.1111/j.1432-1033.1976.tb10725.x. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Wittmann-Liebold B., Geissler A. W., Stöffler G., Giesen M. Comparison of ribosomal protein S1 and the A-protein from Escherichia coli. Lack of structural or functional homology. FEBS Lett. 1979 Mar 15;99(2):357–360. doi: 10.1016/0014-5793(79)80991-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Visentin L. P., Hasnain S., Gallin W. Ribosomal protein S1/S1A in bacteria. FEBS Lett. 1977 Jul 15;79(2):258–263. doi: 10.1016/0014-5793(77)80799-0. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]